Abstract
Bone marrow-derived mesenchymal stem cells (BMDMSCs) have been targeted for use in enhancement of bone healing; and their osteogenic potential may be further augmented by genes encoding bone morphogenetic proteins (BMP’s). The purpose of this study was to compare the effect of genetic modification of human and equine BMDMSCs with BMP-2 or 7 or BMP-2 and 7 on their osteoblastogenic differentiation in the presence or absence of dexamethasone. The BMDMSCs were harvested from the iliac crest of 3 human donors and tuber coxae of 3 equine donors. Monolayer cells were genetically modified using adenovirus vectors encoding BMP-2, -7 or both and cultured in the presence or absence of dexamethasone. Expression of BMPs was confirmed by enzyme linked immunosorbent assay. To evaluate osteoblastic differentiation, cellular morphology was assessed every other day and expression and secretion of alkaline phosphatase (ALP), as well as expression levels of osteonectin, osteocalcin, and Runx2 were measured for up to 14 days.
Human and equine BMDMSCs showed a capacity for osteogenic differentiation regardless of genetic modification or dexamethasone supplementation. Dexamethasone supplementation was more important for osteoblastogenic differentiation of equine BMDMSCs than human BMDMSCs. Genetic modification of BMDMSCs increased ALP secretion with AdBMP-2 homodimer having the greatest effect in both human and equine cells compared to AdBMP 7 or AdBMP 2/7. BMP protein elution rates reached their maximal concentration between day 4 and 8 and remained relatively stable thereafter, suggesting that genetically modified BMDMSCs could be useful for cell-based delivery of BMPs to a site of bone formation.
Keywords: Bone Marrow Derived Mesenchymal Stem Cells, Genetic Modification, BMP-2, BMP-7, Heterodimer
Introduction
Bone Morphogenetic Proteins (BMPs) are growth factors important for skeletal development and bone growth and are classified amongst the transforming growth factor beta (TGF-β) superfamily.1–5 The benefits of BMP supplementation appear to occur early in healing, with more rapid bone formation and maturation and an earlier increase in mechanical strength compared with controls.6–7 A dose effect with BMP-2 and -7 homodimers has also been identified, with higher doses resulting in more bone formation and faster bone healing; however, complete healing was not dose dependent.8–14 While many studies have focused on the bone forming activity of recombinant BMP homodimers and/or expression of a single BMP gene, in bone and other organs, two or more BMP genes are often co-expressed in the same tissue.1–5, 15–18 Because of these findings, several groups including our own, have investigated BMP heterodimers and have shown that they are more potent than their respective homodimers.19–23 Such studies suggest that BMP-2 and BMP-7 heterodimers may be particularly attractive for clinical BMP gene transfer applications. However, the effects of homodimer genetic modification has not been compared to heterodimer genetic modification in human or equine bone marrow-derived mesenchymal stem cells nor have the effects of dexamethasone been compared in these cells when genetically modified with BMP homo or heterodimers.
Mesenchymal stem cells are multipotential cells capable of differentiating into multiple types of connective tissue cells.24 Bone marrow-derived mesenchymal stem cells (BMDMSCs) are an autologous source of cells that can be harvested by aspiration, isolated, and expanded in culture with minimal loss of differentiation potential.25 The capacity of BMDMSCs to differentiate into osteoblasts, has been well documented;26–29 therefore, BMDMSCs have been targeted for use in enhancement of bone healing. In this context, studies suggest that the osteoblastogenic capacity of BMDMSCs can be further augmented by delivery of genes encoding BMP’s.22
In addition to BMP’s, dexamethasone is a known osteoblastogenic supplement and has been demonstrated to support early osteoblastic differentiation in vitro.30 Treatment of BMDMSCs with dexamethasone alone can stimulate in vitro bone formation.24,31–35 However, this effect of dexamethasone varies considerably among species.32–35 Human BMDMSCs have previously been shown to require dexamethasone treatment to undergo osteoblastic differentiation and to produce alkaline phosphatase (ALP), a hallmark for distinguishing osteoblastic cells in culture.33,34 A recent study suggests equine BMDMSCs behave similarly to human BMDMSCs in the presence of dexamethasone in terms of osteoblastogenesis but the effects of dexamethasone on BMP 2 or 7 (homodimer or heterodimer)-expressing genetically modified cells have not been reported.36
The purpose of this study was to compare the effect of BMP-2, 7 and 2/7 genetic modification in human and equine BMDMSCs undergoing osteoblastogenic differentiation in the presence or absence of dexamethasone supplementation. We hypothesized that 1) combined expression of BMP-2/7 would be more efficacious than single BMP gene transfer to increase osteoblastogenic differentiation in human and equine BMDMSCs, 2) dexamethasone supplementation would not be necessary for AdBMP induced osteogenesis in either species, and 3) there would be a species difference between human and equine BMDMSCs in terms of osteoblastogenic differentiation with regards to BMP genetic modification and dexamethasone supplementation.
Materials and Methods
Cell Harvest and Culturing
Bone marrow aspirates were obtained aseptically from the iliac crest of 3 human donors (26-year-old male, and 26- and 23-year-old female) and tuber coxae of 3 equine donors (3- and 4-year-old male, and 6-year-old female), processed and cultured as previously described.37,38 The Institutional Animal Care and Use Committee and Institutional Review Board approved all procedures. Primary cells derived from the bone marrow were designated as passage 0 and each subsequent subculture of BMDMSCs was considered one further passage. All cells were cryogenically preserved at concentrations of 2 million cells/ml and stored for less than 6 months in liquid nitrogen prior to experimentation.
Identification of Appropriate Adenovirus dose
For dose-response studies, human and equine BMDMSCs were transduced with AdBMP-2, AdBMP-7 or Ad encoding the marker gene β-galactosidase (AdLacZ) at 1) 50,000 viral particles per cell (vpc), 2) 100,000 vpc, 3) 200,000 vpc, and 4) 400,000 vpc. Following transduction the following was assessed: a) BMP-2 or BMP-7 protein concentration in the media, b) cell viability, c) cell morphology, and d) transduction efficiency based on percentage β-galactosidase staining. All BMP genetically modified cells were compared to AdLacZ and untransduced control cells. Outcomes in human and equine cells were compared to determine whether species-specific differences to the reaction of adenovirus may be present.
Transduction
Second passage human and equine cells were thawed and seeded at 50% confluence in 48-well culture dishes (Corning, Corning, NY) supplemented α-MEM and allowed to grow two days (~70–80% confluence) prior to transduction. Cells were rinsed in 1x PBS and transduction occurred in serum-free DMEM with 25 mM HEPES and PSA for 4 hours. Cells were incubated with 1) AdBMP-2 (200,000 vpc), 2) AdBMP-7 (200,000 vpc), 3) AdBMP-2/7 (100,000 vpc of each AdBMP-2 and -7), 4) adenovirus-reporter gene construct encoding bacterial β-galactosidase (AdLacZ at 200,000 vpc), or 5) a negative control (no virus, transduction media alone). After transduction, media containing virus was removed, and cells were cultured in Media 1) supplemented DMEM, Media 2) supplemented DMEM with 170 μM ascorbic acid (Sigma, St. Louis, MO) and 5 mM β-glycerol phosphate (Sigma, St. Louis, MO), and Media 3) Dex-DMEM with 170 μM ascorbic acid, 5 mM β-glycerol phosphate, and 10−9 M dexamethasone (Sigma, St. Louis, MO). The assigned media was changed every other day for 14 days. Therefore, each treatment group was cultured in 3 different medias.
Cell Morphology Screening
Cells were monitored every other day by inverted light microscopy and cell morphology was scored on days 0, 2, 4, 6, 8, 10, 12, and 14 to assess cell health by a single examiner unaware of treatment groups as previously described.39
Transduction Efficiency
Transduction efficiency was assessed by staining cells using the in situ β-galactosidase staining kit (Stratagene, La Jolla, CA) on days 0, 8, and 14. Media from all cells was collected at days 0, 4, 8, 10, and 14 and stored at −20°C prior to testing for BMP production by enzyme linked immunosorbent assays (ELISAs) for BMP-2 and BMP-7 (R & D Systems, Minneapolis, MN).
Mineralization
Production of ALP was measured in the culture media and cell lysate by ELISA (Sigma, St. Louis, MO). Cells were also stained for ALP (Sigma kit # 85-L, St. Louis, MO) and mineral, calcium or calcium salts using Von Kossa stain (Von Kossa Kit, Sigma, St. Louis, MO) and scored on days 0, 8, and 14 by a single examiner as previously described.39
Gene Expression of Osteogenic Markers with Quantitative PCR
Cells were harvested at days 0, 8, and 14 for RNA extraction which was performed using RNeasy Mini Kit (Qiagen, Valencia, CA). RNA quantity and quality were measured (ND-1000 spectrophotometer, Nanodrop, Fisher, Pittsbugh, PA) prior to transcription of cDNA using 100 ng of total RNA and oligo(dT) primers with the SuperScript III first-strand synthesis kit (Invitrogen, Carlsbad, CA). Primers for house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and osteoblast marker genes ALP, osteocalcin (OCN), osteonectin (OSTN), and runt-related transcription factor-2 (Runx2) were designed to homologous regions of equine and human sequences. Quantitative real-time PCR was performed in as previously described.38 Relative quantitation of gene expression was determined using comparative CT method (Applied Biosystems, Foster City, CA) and calibrated to the highest delta CT value for each gene.
Statistical Analysis
Categorical data were analyzed using a Fisher’s Exact test (PROC FREQ, SAS Institute, Cary, NC). Continuous data were analyzed using an ANOVA (The GLIMMIX Procedure, SAS Institute Inc, Cary, NC) following log transformation. Species (Human or Equine), treatment (AdBMP-2, AdBMP-7, AdBMP-2/7, AdLacZ, negative control), media (media 1, media 2, and media 3), and day were used as class variables. Species nested within treatment, media, and day was used as the random variable. Data were analyzed using several models to evaluate the association between dependent variables (BMP and ALP protein production, and gene expression of osteogenic markers) and fixed effects (treatment, media, and day). When individual comparisons were made, least square means (LSM) was used and P<0.05 was considered significant. Values are reported as LSM ± standard error of the mean (SEM).
Results
Appropriate Adenoviral dose
The optimal adenoviral dose was identified as 200,000 vpc based on the BMP-2 and -7 protein production, absence of cell toxicity and a transduction efficiency (β-galactosidase-positive cells) that was between 50% and 90% in the preliminary study and is similar to previous reports.40
BMP Protein Production
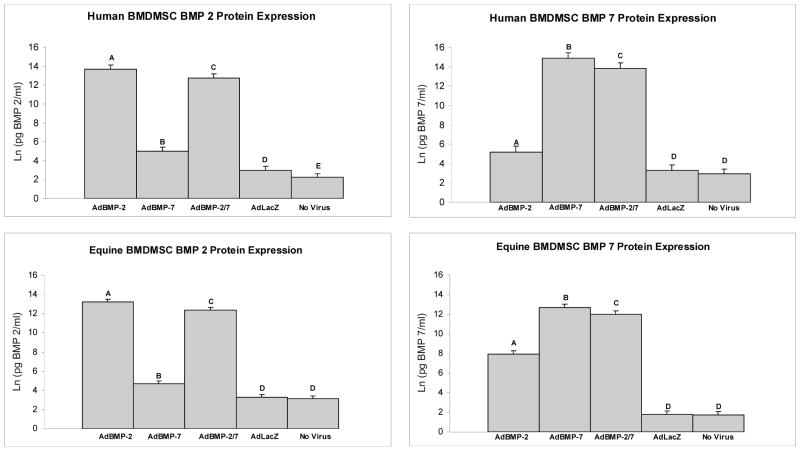
After transduction with the optimized dose, human BMDMSCs treated with AdBMP-2 and AdBMP-2/7 demonstrated a 4.5 (1.7×106 pg/ml) and 4.3 (8.5×105 pg/ml) fold increase in BMP-2 protein compared to controls, respectively (p<0.0001, both comparisons) (Figure 1). Human BMDMSCs treated with AdBMP-7 and AdBMP-2/7 demonstrated a 4.5 (3.0×106 pg/ml) and 4.2 (8.7×105 pg/ml) fold increase in BMP-7 protein compared to controls, respectively (p<0.0001, both comparisons) (Figure 1). BMP-2 and -7 protein elution rates reached their maximal concentration by day 4 and 8 and remained relatively stable thereafter (p<0.0001). Equine BMDMSCs treated with AdBMP-2 and AdBMP-2/7 demonstrated a 4.1 (9.9×105 pg/ml) and 3.8 (5.0×105 pg/ml) fold increase in BMP-2 protein compared to controls, respectively (p<0.0001, both comparisons) (Figure 1). Equine BMDMSCs treated with AdBMP-7 and AdBMP-2/7 demonstrated a 7.1 (1.1×106 pg/ml) and 6.7 (4.2×106 pg/ml) fold increase in BMP-7 protein compared to controls, respectively (p<0.0001, both comparisons) (Figure 1). The fold increase represents log transformed data and the averages represent raw data. The BMP-2 and -7 protein elution rates reached their maximal concentration by day 4 and remained relatively stable thereafter (p<0.0001). Media groups did not significantly influence BMP protein production in either human or equine BMDMSCs.
Figure 1.
Protein elution rates measured with an ELISA for BMP-2 (left) and BMP-7 (right) for each of the 5 different human (top) and equine (bottom) BMDMSCs treatment groups. BMDMSCs treated with AdBMP’s exhibited BMP protein levels higher than controls. Different letters indicate significant (P<0.05) differences among groups.
Osteoblastogenic Differentiation
Cell Morphology and Staining
Human BMDMSCs transduced with AdBMP-2, AdBMP-2/7 or AdLacZ demonstrated a significantly higher cell morphology score compared to human BMDMSCs transduced with AdBMP-7 alone and negative control, respectively (p<0.0001) suggesting the lowest toxicity for AdBMP-7 and control (data not shown). Equine BMDMSCs transduced with AdBMP-2, and -7 alone or in combination demonstrated a significantly lower cell morphology score compared to those transduced with AdLacZ (positive) or negative control, respectively (p<0.0001), suggesting the lowest toxicity for AdBMP genetic modification (data not shown). Equine BMDMSCs cultured in media supplemented with dexamethasone demonstrated a higher cell morphology score compared to other media groups, respectively (p<0.0001), suggesting increased osteoblastogenic differentiation (data not shown).
Alkaline Phosphatase Expression and Secretion
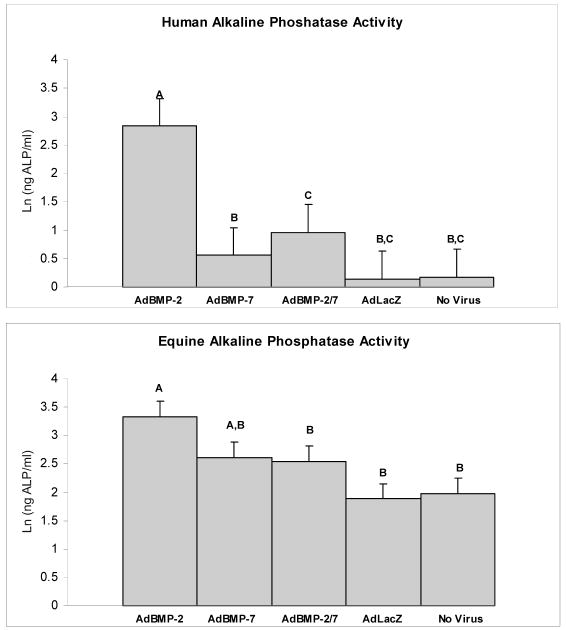
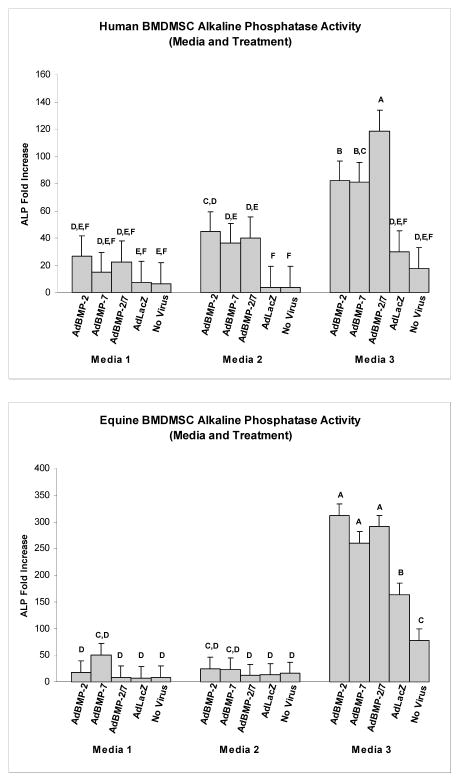
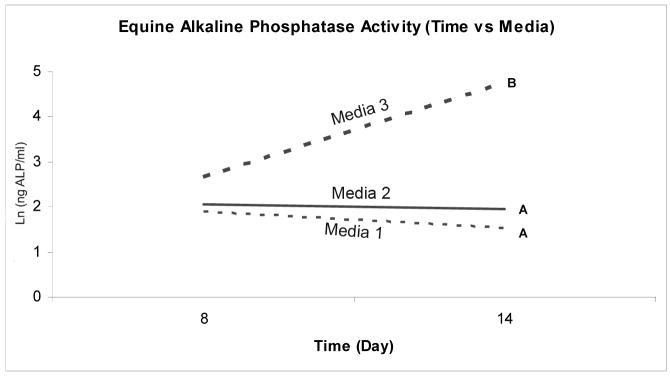
Expression and secretion of ALP, an important enzyme for matrix mineralization, was used as one parameter of osteoblastic differentiation. Genetic modification of human BMDMSCs with AdBMP-2, AdBMP-7, and AdBMP-2/7 resulted in a 4.5, 3.9, and 5.3-fold increase in ALP gene expression compared to AdLacZ-treated or naïve controls, respectively (p<0.0001). Human BMDMSCs treated with AdBMP-2 demonstrated a 14-fold increase in ALP secretion compared to controls (p<0.0001, Figure 2). Human BMDMSCs cultured in media supplemented with dexamethasone demonstrated a 3.1-fold increase in ALP mRNA levels compared to cells cultured in media that did not contain dexamethasone regardless of genetic modification (p<0.0001); however, no increase in ALP protein secretion could be detected in this group. The combination of genetic modification of human BMDMSCs cultured in media supplemented with dexamethasone demonstrated the highest levels of ALP mRNA (p<0.0001) (Figure 3). Genetic modification of equine BMDMSCs with AdBMP-2, AdBMP-7, and AdBMP-2/7 demonstrated a 2.5, 2.3, and 2.1-fold increase of demonstrated ALP mRNA levels compared to controls, respectively (p<0.0001), and ALP protein level was increased to a similar level (1.7-fold increase) compared to controls in AdBMP-2 treated cells (p=0.003, Figure 3). Equine BMDMSCs cultured in media supplemented with dexamethasone demonstrated a 12.2-fold increase in ALP mRNA levels compared to media that did not contain dexamethasone regardless of genetic modification (p<0.0001), and this correlated with a 1.95-fold increase in ALP protein (p<0.0001). As with human cells the combination of genetic modification of equine BMDMSCs cultured in media supplemented with dexamethasone demonstrated the highest levels of ALP mRNA (p=0.05) (Figure 3). The ALP protein levels continued to increase in cells cultured in dexamethasone for 14 days; whereas, ALP protein levels remained relatively constant or showed a decline in the cells cultured in the media not supplemented with dexamethasone (p=0.0002) (Figure 4). Genetically modified cells had higher ALP and Von Kossa scores compared to controls, suggesting increased mineralization capacity (Figure 5).
Figure 2.
Alkaline phosphatase activity for genetically modified human (top) and equine (bottom) BMDMSCs for each of the 5 different treatment groups. AdBMP-2 elicits the greatest effect on ALP production. Different letters indicate significant (P<0.05) differences among groups.
Figure 3.
Alkaline phosphatase mRNA levels for human (top) and equine (bottom) BMDMSCs for each of the 5 different treatment groups in the 3 different cell culture medias. The combination of dexamethasone and genetic modification with AdBMP’s demonstrated the highest levels of ALP mRNA. Different letters indicate significant (P<0.05) differences among groups.
Figure 4.
Alkaline phosphatase activity for equine BMDMSCs for the 3 different cell culture media as a function of time. ALP protein levels show a dramatic increase in cells cultured in dexamethasone (media 3) containing media on day 14. Different letters indicate significant (P<0.05) differences among groups.
Figure 5.
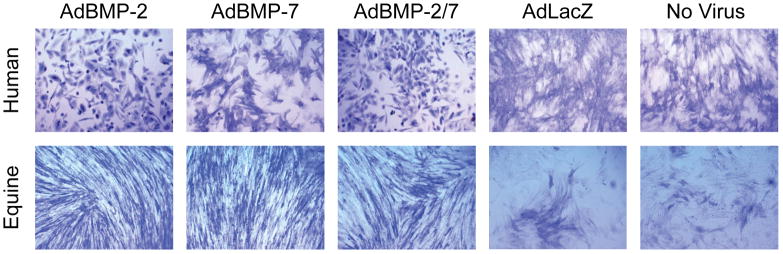
Appearance of human (top) and equine (bottom) BMDMSCs in monolayer for each of the 5 different treatment groups stained for alkaline phosphatase on day 14. Human BMDMSCs treated with AdBMP-2 and AdBMP-2/7 demonstrated osteogenic cell morphology compared to other treatment groups. Original magnification, 200x.
Gene Expression
Gene expression levels for OCN, OSTN and Runx2 were also assessed as measures of osteoblastic differentiation. In human BMDMSCs 1.7, 1.2, and 1.2-fold increases of OCN, OSTN and Runx2, respectively, were detected on day 14 compared to day 8 (p=0.02, p=0.0001, p=0.03) regardless of genetic modification or dexamethasone treatment. Similarly, expression levels for OCN, OSTN, and Runx2 in equine BMDMSC demonstrated a 1.9, 1.2, and 2.4 -fold increase on day 14 compared to day 8, respectively (p=0.02, p=0.05, p=0.0002). In both human and equine BMDMSCs dexamethasone supplementation increased OSTN mRNA levels by 1.3 (p=0.0001) and 1.4-fold (p=0.005), respectively, regardless of genetic modification.
Discussion
In this in vitro cell culture study, genetic modification of BMDMSCs with AdBMPs enhanced ALP expression and secretion with AdBMP-2 appearing to have the greatest effect in both human and equine BMDMSCs. Furthermore, dexamethasone supplementation appeared to be important for the osteoblastogenic differentiation of both genetically modified and naive equine BMDMSCs but not human BMDMSC in which increased Runx2 expression and BMP stimulation of ALP were evident regardless of dexamethasone treatment. Upon Ad-mediated gene transfer, BMP protein expression peaked by day 4 and 8 and remained relatively stable thereafter, until day 14 suggesting that transfer of genetically modified cells into a healing defect 4 days after transfection might be a good strategy for prolonged, high level growth factor delivery.
The capacity of BMDMSCs to differentiate into osteoblasts has been well documented.26–29 Our gene expression data is consistent with these data and show that human and equine BMDMSCs are capable of osteoblastic differentiation regardless of genetic modification or the presence of dexamethasone supplementation. Runx2 is an essential transcription factor for osteoblastic differentiation while ONC is a terminal marker for mature osteoblasts.40 Regardless of treatment or media groups, an increase in Runx2 and ONC gene expression was detected between day 8 and 14 suggestive of osteoblastogenic differentiation in both human and equine BMDMSCs during that time period. These data suggest that the BMDMSCs used in this study may already have been committed to the osteoblast phenotype; this degree of commitment may have diminished possible effects of BMP’s and dexamethasone in our study.
One surprising finding was the relative lack of BMP mediated osteoblastogenic differentiation in the equine BMDMSCs compared to human BMDMSCs. Based on gene expression and immunohistochemistry data for ALP, human BMDMSC responded to BMP’s with dexamethasone having an additive effect. This is in contrast to equine BMDMSCs, which only responded to BMP’s in the presence of dexamethasone. Our finding that human BMDMSCs did not require dexamethasone to increase ALP expression is in contrast to Jorgensen et al.41 who reported increased ALP activity in young healthy human BMDMSCs cultured in dexamethasone supplemented media as well as an additive effect when BMP-2 was combined with dexamethasone. Recently, Stolzing et al.42 identified an age-related decline in ALP activity showing a 40% and 60% reduction in ALP activity in adult (19–40 years old) and aged (>40 years old) BMDMSCs compared to young BMDMSCs (7–18 years old) cultured in dexamethasone. Our study population consisted of individuals over 26 years of age. A larger and significant effect of dexamethasone may have been identified if a younger population was used. The requirement for dexamethasone supplementation for increased ALP expression and secretion in equine BMDMSCs is consistent with previously published work.36 In addition, we report here that dexamethasone has effects that are additive to BMP gene transfer in equine BMDMSCs.
Another surprising finding was that combined AdBMP-2 and -7 (heterodimer) gene transfer did not have a greater effect than using a single AdBMP (homodimer). Previously, we have shown that this strategy results in the secretion of BMP-2/7 heterodimers, which have a significantly greater potency for osteoblastogenic differentiation of murine C2C12 myoblast cells23 and for forming bone in rats in vivo.22 Another group has also shown the superior potency of BMP-2/7 heterodimers in osteoblastic differentiation of C3H10T1/2 and in ectopic bone formation.40 Absence of a heterodimer effect in our studies may represent a species specific differences; alternatively, myoblasts may be specifically sensitive to BMP-2/7 heterodimers whereas BMDMSCs are not. Furthermore, it remains to be investigated whether in vivo effects of BMP-2/7 heterodimer which are evident in rodents occur in horses and/or humans. In our study, there appeared to be a synergistic effect when combining BMP-2 and -7 together in terms of the respective protein production as 100,000 vpc of each vector resulted in as high BMP-2 and -7 levels as 200,000 vpc of either one alone. In the preliminary dose titration studies identifying the appropriate adenoviral dose, we identified a significant difference between 100,000 vpc and 200,000 vpc for single BMP protein production. Therefore, we believe that this is related to a synergistic effect seen with the combined group and not over saturation of the BMDMSCs with adenovirus.
Of the BMPs tested, AdBMP-2 appeared to have the greatest effect on ALP protein production in both human and equine BMDMSCs. ALP is an important indicator of mineralization capacity and its expression is a characteristic of osteoblastic cells.33,34 Our findings are consistent with previous studies that have identified BMP-2’s ability to induce a more robust response in terms of osteogenic differentiation of BMDMSCs compared to other BMP’s. 22,23,39,40 Whereas all BMPs increased ALP gene expression, significant ALP increase was detected by ELISA only after genetic modification to over express BMP-2. The lack of direct correlation of the gene expression and cell staining data with the ELISA for ALP protein may be related to a limitation of the ALP assay; however, this is unlikely as a significant difference was detected within the AdBMP treated groups. In some instances a difference in mRNA data and protein data exists as we observed in this study and we speculate that the degree of ALP transcription appeared to be greater than ALP translation. It is our opinion that the protein data carries more relevance because the protein is what is ultimately biologically active regardless of the degree of mRNA expression. Although AdBMP-2 significantly increased ALP protein production in equine BMDMSCs, the magnitude of the effect of dexamethasone on ALP protein production was larger than that seen with AdBMP-2. This finding may be related to our use of an adenoviral vector encoding the human BMP gene. The homology between human and equine BMP-2 or 7 is greater than 90%;43 however, protein from the target animal species has been shown to yield better results at lower doses compared with protein derived from another species.43,44 Future studies using Ad vectors encoding equine genes will address this question, and will aid in developing clinical strategies for equine patients specifically.
In conclusion, overexpressin of BMPs by BMDMSCs enhanced the expression and secretion of ALP in equine and human BMDMSCs undergoing osteoblastogenic differentiation, with BMP-2 having a greater effect than BMP-7 or BMP-2/7. Furthermore, dexamethasone supplementation was required for the osteoblastogenic differentiation of both genetically modified and naive equine BMDMSCs but not human BMDMSCs. Finally, our hypothesis that there would be species differences between human and equine BMDMSCs in regards to BMP genetic modification and dexamethasone effects was confirmed. Human BMDMSCs appear to be more influenced by genetic modification by AdBMP-2; whereas greater osteoblastogenic differentiation of equine BMDMSCs could be attributed to the presence of dexamethasone. BMP protein expression data suggest that the ideal time to transfer these cells to a healing defect may be between day 4 and 8 after transduction when they are secreting the highest amount of BMP protein. In the equine cells, the ALP activity continues to increase through day 14 in the presence of dexamethasone supplementation, therefore the addition of dexamethasone in the matrix surrounding these cells may be important when delivering them to a healing bone defect. To the best of our knowledge, this is the first study to compare BMP homodimer to heterodimer effects in human and equine BMDMSCs when cells are genetically modified to produce these proteins. Although no effect of combined AdBMP-2 and -7 genetic modification was detected on the BMDMSCs in this in vitro study, future studies examining an in vivo model may show enhanced healing in horses or humans, just as we have previously shown in rats.22 Such a finding, in combination with these studies would suggest that other local cells such as myoblasts may be the target of BMP-2/7 heterodimer stimulation, and that the BMDMSCs are more suited to be the delivery vehicle for the factor, rather than the target cell.
Acknowledgments
The research was funded by Colorado State University CRC grant at the Orthopaedic Research Laboratory at CSU. The authors would like to thank Cristin Keohan and Michele Cheever for their assistance with the human BMDMSCs. The authors would also like to thank Dr. Renny Franceschi for providing the AdBMP-7 vector.
References
- 1.Urist MR. Bone formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Wang EA, Rosen V, Cordes P, et al. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci USA. 1998;85:9484–9488. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wozney JM, Rosen V, Cekeste AJ, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 4.Sampath TK, Coughlin JE, Whetstone RM, et al. Bovine osteogenic protein is composed of dimmers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily. J Biol Chem. 1990;265:13198–13205. [PubMed] [Google Scholar]
- 5.Ozkaynak E, Rueger DC, Drier EA, et al. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 1990;9:2085–2093. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook SD, Wolfe MW, Salkeld SL, et al. Effect of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates. J Bone Joint Surg. 1995;77A:734–750. doi: 10.2106/00004623-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Blokhuis TJ, den Boer FC, Bramer JAM, et al. Biomechanical and histological aspects of fracture healing stimulated with osteogenic protein-1. Biomaterials. 2001;22:725–730. doi: 10.1016/s0142-9612(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang JC, Kanim LE, Yoo S, et al. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Bone Joint Surg Am. 2003;85:905–911. doi: 10.2106/00004623-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Yaska AW, Lane JM, Fellinger EJ, et al. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2) J Bone Joint Surg. 1992;74A:659–670. [PubMed] [Google Scholar]
- 10.Cook SD, Baffes GC, Woolfe MW, et al. Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin Orthop Rel Res. 1994;301:302–312. [PubMed] [Google Scholar]
- 11.Lee SC, Shea M, Battle MA, et al. Healing of large segmental defects in rat femurs is aided by RhBMP-2 in PLGA matrix. J Biomed Mater Res. 1994;28:1149–1156. doi: 10.1002/jbm.820281005. [DOI] [PubMed] [Google Scholar]
- 12.Bostrom M, Lane JM, Tomin E, et al. Use of bone morphogenetic protein-2 in the rabbit ulnar nonunion model. Clin Orthop Rel Res. 1996;327:272–282. doi: 10.1097/00003086-199606000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Zegzula HD, Buck DC, Brekke J, et al. Bone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2) J Bone Joint Surg. 1997;79A:1778–1779. doi: 10.2106/00004623-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ohura K, Hamanishi C, Tanaka S, et al. Healing of segmental bone defects in rats induced by a B-TCP-MCPM cement combined with rhBMP-2. J Biomed Mater Res. 1999;44:168–174. doi: 10.1002/(sici)1097-4636(199902)44:2<168::aid-jbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen tyupes of human bone morophogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Lyons KM, Hogan BL, Robertsons EJ. Colocalization of BMP-7 and BMP-2 RNAs suggest that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri T, Boorla S, Frendo JL, et al. Skeletal abnormalities in doubly heterozygous BMP-4 and BMP-7 mice. Dev Genet. 1998;22:340–348. doi: 10.1002/(SICI)1520-6408(1998)22:4<340::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Solloway MJ, Robertson EJ. Early embryonic lethality in BMP-5; BMP-7 double mutant mice suggest functional redundancy within the 60A subgroup. Development. 1999;126:1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- 19.Aono A, Hazama M, Notoya K, et al. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun. 1995;210:670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- 20.Hazama M, Aono A, Ueno N, et al. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Commun. 1995;209:859–866. doi: 10.1006/bbrc.1995.1578. [DOI] [PubMed] [Google Scholar]
- 21.Isreal DI, Nove J, Derns KM, et al. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13:291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W, Rawlins BA, Boachie-Adjei O, et al. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res. 2004;19:2021–2032. doi: 10.1359/JBMR.040821. [DOI] [PubMed] [Google Scholar]
- 23.Zhu W, Kim J, Cheng C, et al. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39:61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Lange C, Schroeder J, Stute N, et al. High-potential human mesenchymal stem cells. Stem Cells Dev. 2005;14:70–80. doi: 10.1089/scd.2005.14.70. [DOI] [PubMed] [Google Scholar]
- 26.Barry FP, Murphy JM, English K, et al. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–265. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 27.Boskey A, Paschalis E, Binderman I, et al. BMP-6 accelerates both chondrogenesis and mineral maturation in differentiating chick limb-bud mesenchymal cell cultures. J Cell Biochem. 2002;84:509–519. [PubMed] [Google Scholar]
- 28.Musgrave DS, Bosch P, Ghivizzani S, et al. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 29.Thies RS, Bauduy M, Ashton BA, et al. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318–1324. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 30.Blum J, Parrott M, Mikos A, et al. Early osteoblastic differentiation inducted by dexamethasone enhances adenoviral gene delivery to marrow stromal cells. J Ortho Res. 2004;22:411–416. doi: 10.1016/j.orthres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Otto WR, Rao J. Tomorrow’s skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. cell Prolif. 2004;34:97–110. doi: 10.1111/j.1365-2184.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osyczka AM, Diefenderfer DL, Bhargave G, et al. Different effects of BMP-2 on marrow stromal cells and human and rat bone. Cells Tissues Organs. 2004;176:109–119. doi: 10.1159/000075032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diefenderfer DL, Osyczka AM, Garino JP, et al. Regulation of BMP-induced transcription in cultured human bone marrow stromal cells. J Bone Joint Surg AM. 2003;85(suppl 3):19–28. doi: 10.2106/00004623-200300003-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diefenderfer DL, Osyczka AM, Reilly GC, et al. BMP responsiveness in human mesenchymal stem cells. Connect Tissue Res. 2003;44(suppl 1):305–311. [PubMed] [Google Scholar]
- 35.Jaiswal N, Haynesworth S, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 36.Stewart AA, Byron CR, Pondenis HC, et al. Effect of dexamethasone supplementation of chondrogenesis of equine mesenchymal stem cells. Am J Vet Res. 2008;69:1013–1021. doi: 10.2460/ajvr.69.8.1013. [DOI] [PubMed] [Google Scholar]
- 37.Centeno CJ, Busse D, Kisiday JD, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–353. [PubMed] [Google Scholar]
- 38.Kisiday JD, Kopesky PW, Evans CH, et al. Evaluation of adult equine bone marriow-and adipose-drived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322–331. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 39.Zachos TA, Shields KM, Bertone AL. Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 or -6. J Orthop Res. 2006;24:1279–1291. doi: 10.1002/jor.20068. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M, Zhao Z, Koh JT, et al. Combinatorial gene therapy for bone regeneration: cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J Cell Biochem. 2005;95:1–16. doi: 10.1002/jcb.20411. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen NR, Henriksen Z, Sorensen OH, et al. Dexamethasone, BMP-2, and 1,25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids. 2008;69:219–226. doi: 10.1016/j.steroids.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson OS, Urist MR, Dawson EG, et al. Bone repair induced by bone morphogenetic protein in ulna defects in dogs. J Bone Joint Surg. 1986;68B:635–642. doi: 10.1302/0301-620X.68B4.3733844. [DOI] [PubMed] [Google Scholar]
- 44.Heckman JD, Boyan DB, Aufdemorte TB, et al. The use of bone morphogenetic protein in the treatment of non-union in a canine model. J Bone Joint Surg. 1991;73A:750–765. [PubMed] [Google Scholar]