Abstract
Active gibberellins (GAs) are endogenous factors that regulate plant growth and development in a dose-dependent fashion. Mutant plants that are GA deficient, or exhibit reduced GA responses, display a characteristic dwarf phenotype. Extragenic suppressor analysis has resulted in the isolation of Arabidopsis mutations, which partially suppress the dwarf phenotype conferred by GA deficiency and reduced GA-response mutations. Here we describe detailed studies of the effects of two of these suppressors, spy-7 and gar2–1, on several different GA-responsive growth processes (seed germination, vegetative growth, stem elongation, chlorophyll accumulation, and flowering) and on the in planta amounts of active and inactive GA species. The results of these experiments show that spy-7 and gar2–1 affect the GA dose-response relationship for a wide range of GA responses and suggest that all GA-regulated processes are controlled through a negatively acting GA-signaling pathway.
GA is essential for normal plant growth regulation (Hooley, 1994), and studies of GA-deficient and GA-response mutants have greatly advanced our understanding of GA action (Ross, 1994; Swain and Olszewski, 1996; Ross et al., 1997; Harberd et al., 1998). GA responses are dose dependent (Bethke and Jones, 1998). For example, GA-deficient mutant Arabidopsis hypocotyls are short in the absence of exogenous GA and become progressively longer with increasing doses of exogenous GA. As the GA dose increases further, the corresponding increase in hypocotyl length becomes less, and eventually the response reaches saturation, at which time further increases in GA dose cause little or no further increase in hypocotyl length (Jacobsen and Olszewski, 1993; Reed et al., 1996; Silverstone et al., 1997; Cowling et al., 1998).
The phenotype of the Arabidopsis gai mutant mimics the effects of GA deficiency: gai plants are dwarfed and have dark-green leaves (Koornneef et al., 1985; Peng and Harberd, 1993, 1997a; Wilson and Somerville, 1995; Peng et al., 1997; Harberd et al., 1998). However, unlike GA-deficient mutants, gai mutants cannot be restored to normal phenotype by application of GA (Koornneef et al., 1985; Wilson and Somerville, 1995; Peng and Harberd, 1997a) and accumulate bioactive GAs (GA1 and GA4) to a higher level than do wild-type plants (Talon et al., 1990a). Recent experiments have shown that in normal plants endogenous GA levels are homeostatically controlled via GA-mediated negative feedback regulation (Hedden and Croker, 1992). As a part of this process, the expression of a number of the genes encoding enzymes involved in GA biosynthesis is down-regulated by GA (Chiang et al., 1995; Phillips et al., 1995; Xu et al., 1995; Martin et al., 1996; Hedden and Kamiya, 1997; Cowling et al., 1998). This down-regulation is perturbed in gai (Talon et al., 1990a; Xu et al., 1995; Peng et al., 1997), thus explaining the increased endogenous GA levels in gai and indicating the involvement of GAI (the GAI gene product) in the homeostatic regulation of GA levels.
The GA responses of gai are saturated because the gai phenotype is unaffected by exogenous GA. However, the gai phenotype is affected by reductions in the endogenous GA level (Koornneef et al., 1985). ga1–1 mutants are severely dwarfed and GA deficient (Koornneef and van der Veen, 1980; Sun et al., 1992). gai ga1–1 double mutants are more severely dwarfed than gai and are similar in height to a ga1–1 single mutant (Koornneef et al., 1985). Treatment of gai ga1–1 plants with increasing doses of GA results in progressive increases in plant height, and a sufficiently large dose fully restores the height of gai ga1–1 plants to that of gai (Koornneef et al., 1985). Thus, gai plants are not completely GA insensitive and exhibit reduced GA responses (Wilson and Somerville, 1995; Cowling, 1997).
GAI encodes a protein that may act as a GA-controlled transcriptional regulator (Peng et al., 1997). gai encodes a mutant protein that lacks a 17-amino acid segment near the N terminus (Peng et al., 1997). In addition, RGA, another Arabidopsis gene involved in GA signaling, encodes a protein (RGA) whose amino acid sequence is closely related to that of GAI. GAI and RGA appear to have overlapping roles as negative regulators of GA signaling (Peng et al., 1997; Harberd et al., 1998; Silverstone et al., 1998).
The Arabidopsis spy mutations confer resistance to the plant-growth and GA-biosynthesis inhibitor PAC (Hedden and Graebe, 1985; Davis and Curry, 1991) and suppress the effects of GA deficiency (Jacobsen and Olszewski, 1993; Jacobsen et al., 1996). Thus, although the GA-deficient ga1–2 mutant requires exogenous GA for germination (Koornneef and van der Veen, 1980; Sun et al., 1992), spy-1 ga1–2 double-mutant seeds germinate in the absence of exogenous GA (Jacobsen and Olszewski, 1993). In addition, spy mutations can suppress the phenotype conferred by gai (Jacobsen et al., 1996). GA dose-response data suggest that spy mutants are not saturated in their GA responses (Jacobsen and Olszewski, 1993; Jacobsen et al., 1996). SPY encodes a tetratrichopeptide repeat protein (SPY) that has significant homology to animal O-linked N-acetylglucosamine transferase (Jacobsen et al., 1996; Robertson et al., 1998). The gar2–1 mutation also suppresses the gai phenotype and identifies a genetic locus (GAR2) that is distinct from SPY (Wilson and Somerville, 1995).
Previously, we described a model to explain the GA regulation of stem elongation (Peng et al., 1997; Harberd et al., 1998). According to this model, the GAI (wild-type gene), RGA, SPY, and GAR2 gene products are components or modulators of a signaling pathway that negatively regulates stem elongation and whose activity is opposed by GA. Here we describe further studies of the effects of the spy-7 and gar2–1 alleles on GA signaling. We wanted to (a) know whether spy-7 and gar2–1 affect the regulation of several different GA responses, in addition to their previously documented effects on the GA regulation of stem elongation; (b) test the effects of spy-7 and gar2–1 on endogenous GA levels; and (c) investigate the possibility of interactions between these two mutations by studying the effects of spy-7 and gar2–1 in single- and double-mutant combinations. The results of these experiments suggest that the negative regulation model of GA signaling, which was originally based on observations of stem elongation, is generally applicable to a wide range of GA responses. In addition, spy-7 and gar2–1 alter the GA dose-response relationship by reducing the amount of GA needed to elicit a given level of response.
MATERIALS AND METHODS
Plant Mutant Lines
All mutants described here were derived from Landsberg erecta (wild type). gai, ga4, and gai spy-7 (original name: gai gas1–1) were obtained as previously described (Peng and Harberd, 1993; Carol et al., 1995). gai gar2–1 an and spy-5 were obtained from R. Wilson (Wilson and Somerville, 1995) (an is a recessive mutation conferring narrow leaves and twisted siliques). gai gar2–1 AN was obtained from the F2 family of a gai gar2–1 an × gai GAR2 AN cross (confirmed by the absence of an segregation in the F3 generation). gai spy-7 gar2–1 was obtained from a gai spy-7 × gai gar2–1 cross. Approximately 3/16 of the F2 plants were pale green and slender (at least as tall as the wild type). These plants were either gai spy-7 gar2–1 homozygotes or homozygous for gai spy-7 and heterozygous for gar2–1. (gar2–1 is a dominant suppressor of the gai phenotype.) gai spy-7 gar2–1 homozygotes were used in all of the experiments described here.
To identify plants carrying gar2–1 in the absence of gai, gai gar2–1 plants were crossed with the wild type. F2 plants that were visually indistinguishable from the wild type were identified and their F3 progeny were further analyzed. F3 families that segregated dwarf (gai) plants were discarded. The remaining 10 F3 families contained only tall plants, which were all homozygous for GAI. Because GAR2 is unlinked to GAI, the plants in these families were all homozygous for GAR2, all homozygous for gar2–1, or segregating for GAR2/gar2–1 heterozygotes and homozygotes. Nine of these 10 F3 families exhibited PAC-resistant seed germination (data not shown), presumably conferred by gar2–1.
Plant Maintenance
Seeds were allowed to imbibe on moistened filter paper at 4°C for 7 d (to break dormancy) and then planted on “Arabidopsis mix” (2 parts Levington's M3 potting compost:1 part grit/sand). Plants were then grown in standard greenhouse conditions or in controlled environment chambers. The SD consisted of 10 h of fluorescent light supplemented with low-fluence-rate incandescent light. The LD was the same as for SD, except that the low-fluence-rate incandescent light was for an additional 8 h, resulting in an extended 18-h photoperiod) (Peng and Harberd, 1997b). In both LD and SD temperature and RH were constant at 20°C and 75%, respectively. For GA treatments plants and soil were sprayed once a week with 0.1 mm GA3.
For plants grown in sterile conditions, seeds were surface sterilized and sown on germination medium (Valvekens et al., 1988) supplemented (where appropriate) with GA3 or PAC, and then grown in an 18-h photoperiod (Peng and Harberd, 1993; Peng et al., 1997). Germination tests compared batches of seeds harvested at approximately the same time and were scored 7 d after sowing.
Measurement of Plant Growth and Chlorophyll Content
Adult plant heights and seedling chlorophyll contents were measured as previously described (Peng and Harberd, 1997a). Vegetative rosette radii were measured from photographs by marking the center of each rosette (apex) and measuring the distance from the apex to the most distant leaf edge. Flowering time was measured by counting rosette leaves.
Determination of Endogenous GA Content
Plants were grown in soil at 22°C (16-h photoperiod). Three- to four-week-old plants (five-leaf stage) were harvested and immediately frozen in liquid nitrogen. Samples (1 g fresh weight) were homogenized and extracted overnight in 20 mL of 80% methanol. [2H]GAs (17, 17-[2H2]GA; purchased from Prof. L. Mander, Canberra, Australia) were added as internal standards before extraction. After extraction each sample was reduced to aqueous phase in vacuo and diluted with 20 mL of water. The aqueous phase was adjusted to pH 2.8 with 1 m HCl and partitioned three times with equal volumes of ethyl acetate. The ethyl acetate extracts were combined, the water was frozen out, and the extracts were thereafter reduced to dryness in vacuo. The residue was dissolved in 2 mL of water, the pH adjusted to pH 8.0 with 1 m KOH, and applied to a preequilibrated QAE Sephadex anion-exchange column (30 × 10 mm i.d., Pharmacia). The column was washed with 15 mL of water, pH 8.0, prior to elution with 25 mL of 0.2 m formic acid, the eluate being run directly onto a preequilibrated 500-mg C18 ISOLUTE cartridge (Sorbent AB, V. Frölunda, Sweden), which was then eluted with 4 mL of methanol. The methanol eluate was dried and thereafter subjected to reversed-phase HPLC.
The HPLC system consisted of a pump (model 600, Waters) connected via an autosampler (model 717, Waters) to a 4-μm Nova-Pak C18 column (150 × 3.9 mm i.d., Waters). The mobile phase was a 20-min linear gradient of 20% to 100% methanol in 1% aqueous acetic acid at a flow rate of 1 mL min−1. Five fractions corresponding to the GAs of interest were dried, methylated with ethereal diazomethane, and after evaporation trimethylsilylated in 20 μL of dry pyridine/N,O-bis(trimethylsilyl)trifluoroacetamide/trimethylchlorosilane (50:50:1, v/v) at 70°C for 30 min. The derivatization mixture was then reduced to dryness and dissolved in dichloromethane. Samples were injected in the splitless mode into an HP 5890 gas chromatograph (Hewlett-Packard), fitted with a fused silica glass capillary column (30 m long, 0.25 mm i.d.), with a chemically bonded 0.25-μm DB-5MS stationary phase (J & W Scientific, Folsom, CA). The injector temperature was 270°C. The column temperature program varied depending on which GA was being analyzed. The column effluent was introduced into the ion source of a JMS-SX/SX102A mass spectrometer (JEOL). The interface temperature was 280°C and the ion source temperature was 250°C. The acceleration voltage was 10 kV, and ions were generated with 70 eV at an emission current of 500 μA. For quantification, samples were analyzed in either high resolution selected-ion monitoring mode or selected-reaction monitoring mode (Moritz and Olsen, 1995). For each of the GAs, calibration curves were recorded from 0.5 to 20 pg of GA with 5 pg of [2H2]GA as the internal standard. All data were processed by a JEOL MS-MP7010 data system.
RESULTS
spy-7 and gar2–1 Confer PAC-Resistant Seed Germination
gas1–1 is a recessive mutation that is unlinked to gai, and gai gas1–1 plants are less severely dwarfed and lighter green than gai plants (Carol et al., 1995). This phenotype closely resembles that of gai spy-5 plants (Wilson and Somerville, 1995), suggesting that spy-5 and gas1–1 might be allelic. To test this, we performed reciprocal crosses between gai gas1–1 and gai spy-5 and examined the F1 phenotype (Table I). As described above, spy alleles confer PAC-resistant seed germination (Jacobsen and Olszewski, 1993). Thus, gai spy-5 seeds exhibited PAC-resistant seed germination, although gai seeds did not (Table I). The gai gas1–1 seeds also exhibited PAC-resistant seed germination, as did F1 seeds obtained from the gai gas1–1 × gai spy-5 crosses (Table I). In addition, adult F1 plants were less green and less severely dwarfed than the gai plants (Table I). Approximately 30 F2 progeny were scored from each of five F1 plants obtained from each of the reciprocal gai gas1–1 × gai spy-5 crosses. All F2 plants were lighter green and taller than gai plants. Thus, gas1–1 and spy-5 are allelic, and gas1–1 was renamed spy-7 (Peng et al., 1997).
Table I.
gas1-1 and spy-5 are allelic mutations
| Genotype | Germination on
|
Adult Plant Phenotypeb | |
|---|---|---|---|
| Germination medium alonea | Germination medium + 0.1 mm PACa | ||
| Wild type | 22 (22) | 0 (21) | Normal |
| gai | 22 (22) | 0 (24) | Dwarf, dark-green |
| gai gas1-1 | 21 (21) | 14 (26) | Less dwarf, less dark-green than gai |
| gai spy-5 | 21 (21) | 17 (22) | Less dwarf, less dark-green than gai |
| gai spy-5 × gai gas1-1 (F1) | 20 (20) | 24 (29) | Less dwarf, less dark-green than gai |
| gai gas1-1 × gai spy-5 (F1) | 18 (20) | 13 (27) | Less dwarf, less dark-green than gai |
| gai SPY × gai spy-5 (F1) | 18 (18) | 1 (32) | Dwarf, dark-green |
Number of seeds germinated (number sown is in parentheses).
Phenotype of plants grown from seeds planted directly into soil.
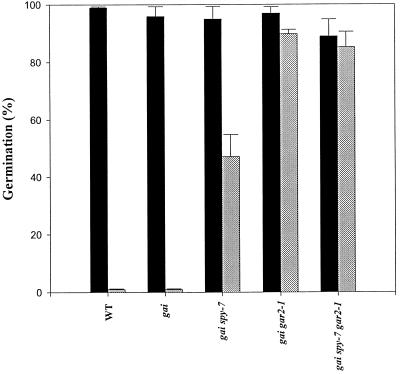
gar2–1 is a dominant, partial suppressor of the gai phenotype and is unlinked to either gai or spy (Wilson and Somerville, 1995; J. Peng and N.P. Harberd, unpublished data). To determine whether gar2–1 also confers PAC-resistant seed germination, the PAC resistances of wild-type, gai, gai spy-7, gai gar2–1, and gai spy-7 gar2–1 seeds were compared (Fig. 1). Although the wild type and gai failed to germinate, approximately 50% of gai spy-7 seeds achieved germination, consistent with spy-7 being a relatively mild spy allele (Peng et al., 1997). It is interesting that gai gar2–1 seeds are more PAC resistant than are gai spy-7 seeds (approximately 90% of the gai gar2–1 seeds germinated). The PAC resistance of gai spy-7 gar2–1 seeds was similar to that of gai gar2–1 (Fig. 1).
Figure 1.
Germination of wild-type (WT), gai, gai spy-7, gai gar2–1, and gai spy-7 gar2–1 seeds grown in the presence (gray bars) or absence (black bars) of 0.1 mm PAC. Germination was scored 7 d after moving the plates containing the seeds from the cold room. Results are presented as mean ± se of three separate experiments (for each sample, n = 10–22).
To determine the phenotype conferred by gar2–1 in the absence of gai, gai gar2–1 was backcrossed to the wild type and F2 and F3 phenotypes were analyzed (see Methods). These experiments showed that gar2–1 (in the absence of gai) confers PAC-resistant seed germination and a visible adult plant phenotype that is not obviously different from that conferred by the wild-type (GAR2) allele.
gai gar2–1 Plants Do Not Respond to Exogenous GA
gai spy-7 plants do not respond to exogenous GA (Carol et al., 1995). Therefore, although untreated gai spy-7 plants grow taller than gai plants, GA-treated gai spy-7 plants do not grow any taller than untreated controls. Thus, gai spy-7 plants, like gai plants, are saturated in their GA responses. However, the plant height at saturation is greater in gai spy-7 than it is in gai. We were interested in determining whether gai gar2–1 is also saturated in its GA responses.
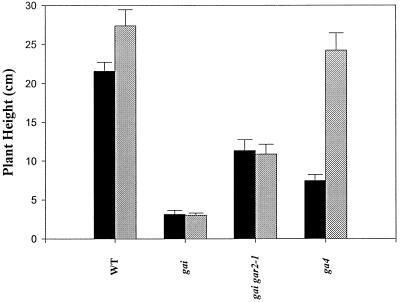
We compared the effects of exogenous GA on the growth of gai gar2–1 plants with its effects on the growth of gai and other controls (Fig. 2). As expected, wild-type plants showed an effect of GA3 on growth, ga4 (GA deficient; Talon et al., 1990b) plants exhibited a marked growth response, and gai plants exhibited no response. gai gar2–1 plants, although taller than gai plants, did not respond to exogenous GA. Thus, gai gar2–1, like gai spy-7 and gai, was saturated in its GA responses. As for gai spy-7, the plant height associated with the saturated GA responses of gai gar2–1 was greater than that of gai.
Figure 2.
Comparison of GA response of the wild type (WT), gai, and gai gar2–1. Adult heights (47 d after sowing) of GA3-treated (gray bars; 0.1 mm GA3) and control (black bars) plants are shown. Wild-type and ga4 (GA-deficient mutant) plants were taller after GA treatments, whereas gai and gai gar2–1 were unaffected. Plants were grown in an 18-h photoperiod. Results are presented as means ± se (n = 18–30).
gai spy-7 gar2–1 Plants Are Taller Than gai spy-7 or gai gar2–1
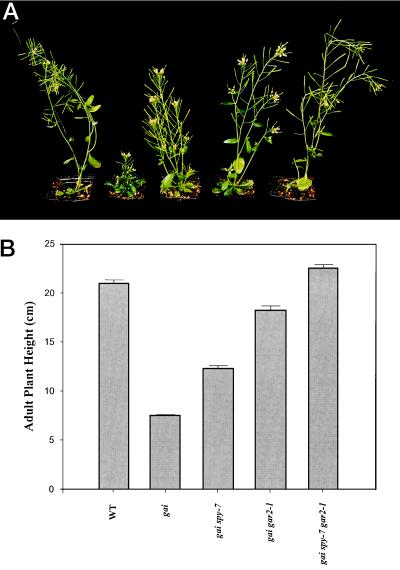
The heights of gai spy-7, gai gar2–1, and gai spy-7 gar2–1 plants were compared (Fig. 3; Peng et al., 1997). Although gai spy-7 and gai gar2–1 were both taller than gai, gai gar2–1 plants were significantly taller than gai spy-7 plants. The combination of spy-7 and gar2–1 dramatically suppressed the gai phenotype. gai spy-7 gar2–1 plants were at least as tall as the wild type (Fig. 3B; Peng et al., 1997). Thus, the combined suppressive effect of spy-7 and gar2–1 exceeded that of either allele alone.
Figure 3.
A, Photograph of (left to right) wild-type, gai, gai spy-7, gai gar2–1, and gai spy-7 gar2–1 plants. B, Comparison of adult plant heights (55 d after sowing). Plants were grown in an 18-h photoperiod. Results are presented as means ± se (n = 19–26).
spy-7 Affects Chlorophyll Accumulation
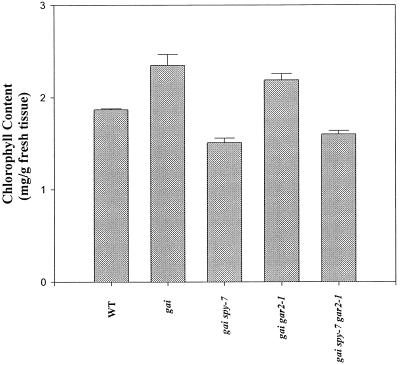
gai plants have an elevated chlorophyll content and are dark green (Peng and Harberd, 1997a). Somatic sector analysis indicates that this is a cell-autonomous trait (Peng and Harberd, 1997b). gai spy-7 and gai spy-7 gar2–1 plants were paler green than gai plants, but gai gar2–1 plants appeared to be as dark green as the gai plants (Fig. 3A; Peng et al., 1997). To quantify this observation, we determined the total chlorophyll content of the leaves of 21-d-old seedlings (Fig. 4). gai and gai gar2–1 had similar chlorophyll contents (approximately 25% and 20%, respectively, more chlorophyll than the wild type). On the other hand, the chlorophyll contents of gai spy-7 and gai spy-7 gar2–1 were lower than that of the wild type. gai spy-7 and gai spy-7 gar2–1 contained approximately 20% and 15%, respectively, less chlorophyll than the wild type (Fig. 4). Thus, spy-7, but not gar2–1, reduced leaf chlorophyll content in both gai spy-7 and gai spy-7 gar2–1 plants.
Figure 4.
Chlorophyll contents of leaves of wild type, gai, gai spy-7, gai gar2–1, and gai spy-7 gar2–1 (21-d-old plants, grown in an 18-h photoperiod). Results are presented as means ± se of three separate experiments.
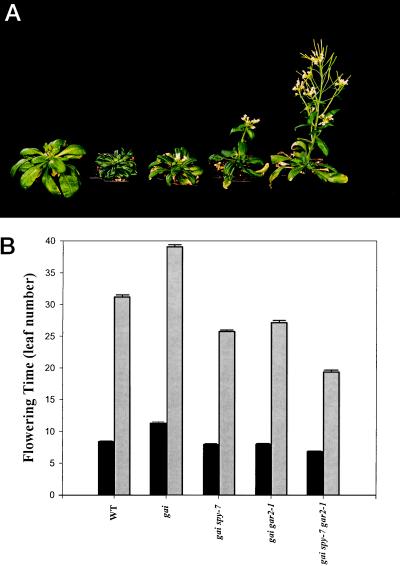
gai spy-7 gar2–1 Plants Flower Earlier Than gai spy-7 or gai gar2–1
GA regulates Arabidopsis flowering time (Langridge, 1957; Wilson et al., 1992; Putterill et al., 1995; Blázquez et al., 1998). For example, in SD the GA-deficient ga1–3 mutant does not flower and the flowering time of gai is greatly delayed (Wilson et al., 1992; Putterill et al., 1995). In experiments to determine whether spy-7 and gar2–1 affect the flowering time (LD and SD) of gai (Fig. 5) we found that in LD gai spy-7 and gai gar2–1 flowered at the same time as the wild type (eight to nine leaves), whereas gai flowered later. gai spy-7 gar2–1 plants flowered earlier than gai or the wild type in LD (Fig. 5B). In SD gai flowered much later than the wild type, gai spy-7 and gai gar2–1 flowered slightly earlier than the wild type, and gai spy-7 gar2–1 flowered much earlier than the wild type (Fig. 5). Thus, spy-7 and gar2–1 significantly suppress the LD- and SD-flowering responses of gai. Furthermore, the combined effect of spy-7 and gar2–1 exceeds that of either allele alone.
Figure 5.
A, Photograph of (left to right) wild type, gai, gai spy-7, gai gar2–1 and gai spy-7 gar2–1 grown (50 d after sowing) in SD. The wild-type and gai plants do not have open flowers, whereas suppressed gai plants, in particular gai gar2–1 and gai spy-7 gar2–1, have already flowered. B, Comparison of flowering times of the wild type (WT), gai, gai spy-7, gai gar2–1, and gai spy-7 gar2–1 under LD (black bars) and SD (gray bars). Numbers of rosette and cauline leaves that appeared prior to the opening of the first flower bud were counted. Results are presented as means ± se (n = 29–35).
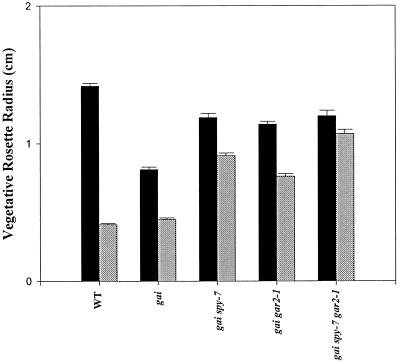
gai spy-7 gar2–1 Plants Are Strongly Resistant to the Growth-Retarding Effects of PAC
When grown on 1 nm PAC, wild-type seeds germinated and the seedlings developed into dwarf plants. When grown on 1 nm PAC plus 0.1 nm GA4, normal plants developed, suggesting that PAC-induced dwarfism is due to GA deficiency (Cowling, 1997). Stem elongation of gai spy-7 gar2–1 plants was more resistant to the dwarfing effects of PAC than was that of gai spy-7 or gai gar2–1 plants (Peng et al., 1997). We looked at the effects of 1 nm PAC on another measure of plant growth, the vegetative rosette radius (effectively a measure of leaf length) (Fig. 6). The rosette radii of PAC-treated wild-type plants were approximately 30% of those of untreated plants. PAC-treated gai plants had rosette radii that were about one-half of those of untreated gai plants (Fig. 6). The growth of gai spy-7 and gai gar2–1 plants was less severely inhibited by PAC than that of the wild type and gai. PAC-treated gai spy-7 and gai gar2–1 rosette radii were approximately 80% and 65%, respectively, of those of untreated plants. gai spy-7 gar2–1 plants displayed dramatic resistance to the inhibitory effects of PAC, and PAC-treated gai spy-7 gar2–1 rosette radii were about 90% of those of untreated plants (Fig. 6).
Figure 6.
Vegetative rosette radii of wild-type (WT), gai, gai spy-7, gai gar2–1, and gai spy-7 gar2–1 plants (30 d old) grown in the presence (gray bars) or absence (black bars) of 1 nm PAC. Results are presented as means ± se (n = 16–29).
PAC Treatments Reveal GA Responsiveness in gai spy-7 gar2–1 Plants
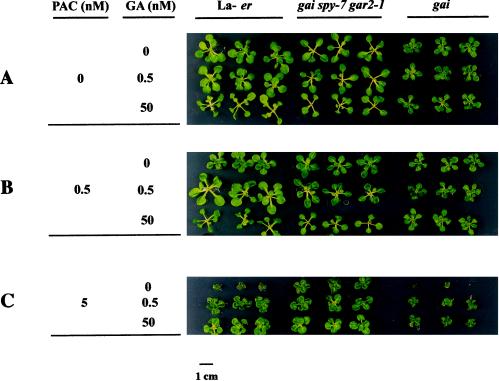
One possible explanation for the phenotypes conferred by spy-7 and gar2–1 is that they alter the GA dose-response relationship so that less GA is needed to elicit a given level of response than in SPY or GAR2 plants (see Discussion). The strong PAC resistance of gai spy-7 gar2–1 plants (Fig. 6) might have suggested that the combination of spy-7 and gar2–1 makes GA responses completely independent of GA dose. To address this point we tested the effects of combined treatments with PAC and GA3 on the growth of the wild type, gai, and gai spy-7 gar2–1. In these experiments we used increasing doses of PAC to cause increasingly severe reductions in endogenous GA level, and we used increasing doses of GA3 to promote the growth of PAC-treated plants. The results of these experiments are shown in Figure 7.
Figure 7.
Growth of wild-type, gai, and gai spy-7 gar2–1 plants on germination medium (Valvekens et al., 1988) containing different concentrations of PAC (A, 0 nm PAC; B, 0.5 nm PAC; and C, 5 nm PAC) and GA3. Seeds (15–18 seeds for each sample) were sterilized, placed on appropriate medium, chilled for 7 d at 4°C, and then grown at 23°C with an 18-h photoperiod. For each of the three genotypes, three representative plants (22 d old) are shown for each treatment.
The first of these experiments tested the effects of increasing concentrations of GA3 in the absence of PAC (Fig. 7A). The wild-type plants exhibited a typical dose-dependent GA response, with 50 nm GA3 eliciting a stronger response (elongated petioles and pale-green leaf color) than 0.5 nm GA3, whereas, as expected, the gai plants displayed no obvious response to exogenous GA (Fig. 7A; Koornneef et al., 1985; Carol et al., 1995). The gai spy-7 gar2–1 plants also displayed no obvious response to exogenous GA3. At all three GA3 doses, the gai spy-7 gar2–1 plants resembled wild-type plants treated with 50 nm GA3, having elongated petioles and pale-green leaves (Fig. 7A). Thus, although the phenotypes of gai (dwarf) and gai spy-7 gar2–1 (tall) are different, they are both saturated in their GA responses.
Additional experiments used two concentrations of PAC (low: 0.5 nm PAC, Fig. 7B; and high: 5 nm PAC, Fig. 7C) to reduce the endogenous GA levels in the wild type, gai, and gai spy-7 gar2–1. On high PAC (without exogenous GA) wild-type plants were very small, compact, and dark green (Fig. 7C). However, exogenous GA3 partially overcame the effects of high PAC on wild-type plants (Fig. 7C). For example, the mean rosette radius of wild-type plants on high PAC alone was 0.24 cm, whereas that of the wild type on high PAC, 50 nm GA, was 0.65 cm (the means of 8 and 10 plants, respectively, P < 0.01), a 1.7-fold increase in rosette radius. GA (0.5 nm) also partially overcame the dwarfism of wild-type plants grown on high PAC, but to a lesser extent than did 50 nm GA, demonstrating that GA reversed the effect of high PAC in a dose-dependent manner (Fig. 7C).
Wild-type plants grown on low PAC were also dwarfed compared with untreated controls (Fig. 7B), although not as dwarfed as the wild type grown (without exogenous GA) on high PAC. Again, exogenous GA overcame the effects of low PAC on wild-type plants in a dose-dependent fashion (Fig. 7B). For example, the petiole length of the wild type on low PAC, 50 nm GA (Fig. 7B), was very similar to that exhibited by the wild type on 50 nm GA alone (Fig. 7A) and much greater than that of wild-type plants on low PAC alone (Fig. 7B). GA (0.5 nm) also reduced the effect of low PAC on wild-type petiole length but to a lesser extent than did 50 nm GA (Fig. 7B).
On high PAC, gai plants were compact, dark green, and resembled the wild type grown in the same conditions (Fig. 7C). GA doses greatly reduced these effects (Fig. 7C). For example, the mean rosette radius of gai grown on high PAC alone was 0.25 cm, whereas that of gai grown on high PAC, 50 nm GA, was 0.40 cm (the means of two and seven plants respectively, P < 0.01), an approximate 70% increase in gai rosette radius. This is consistent with previous observations of GA responses in gai ga1–1 plants (see introduction; Koornneef et al., 1985). However, gai plants grown on low PAC showed no detectable growth response to exogenous GA (Fig. 7B). This is also consistent with observations that the gai phenotype is unaffected by exogenous GA and that GA responses are saturated in gai (Koornneef et al., 1985).
When grown on high PAC without exogenous GA, gai spy-7 gar2–1 plants were larger than comparably treated wild-type or gai plants (Fig. 7C), again demonstrating the relatively strong PAC resistance of gai spy-7 gar2–1. However, these plants were considerably more compact than were gai spy-7 gar2–1 plants grown in the absence of PAC (Fig. 7A). Furthermore, exogenous GA caused dose-dependent increases in the sizes of gai spy-7 gar2–1 plants grown on high PAC (Fig. 7C). The mean rosette radius of gai spy-7 gar2–1 plants grown on high PAC alone was 0.55 cm, whereas that of gai spy-7 gar2–1 plants on high PAC, 50 nm GA, was 0.70 cm (the means of 12 and 7 plants, respectively, P < 0.01), an approximate 30% increase in rosette radius. On low PAC, gai spy-7 gar2–1 plants were still compact relative to controls grown without PAC (Fig. 7, A and B). Again, exogenous GA caused dose-dependent promotion of the growth of gai spy-7 gar2–1 plants grown on low PAC. This is most clearly seen with respect to petiole elongation. Petioles of gai spy-7 gar2–1 plants on low PAC, 50 nm GA (Fig. 7B), were much longer than those of controls on low PAC, 0 nm GA (Fig. 7B), but are not obviously different from those of gai spy-7 gar2–1 plants grown without PAC or GA (Fig. 7A) or of wild-type plants on 0 nm PAC, 50 nm GA (Fig. 7A). GA (0.5 nm) also reduced the effect of low PAC on gai spy-7 gar2–1 petiole length but to a lesser extent than did 50 nm GA (Fig. 7B).
These observations show that the phenotype of gai spy-7 gar2–1 is GA dependent. When endogenous GA levels are reduced by PAC, gai spy-7 gar2–1 plants exhibit reduced growth. This effect of PAC is reversed in a dose-dependent manner by exogenous GA. In addition, these experiments indicate that gai and gai spy-7 gar2–1 plants, although having very visibly different plant phenotypes, are both saturated in their GA responses, whereas wild-type plants are not.
spy-7 and gar2–1 Suppress the Effects of gai on Endogenous GA Levels
We compared the endogenous GA contents of wild-type, gai, gai spy-7, gai gar2–1, and gai spy-7 gar2–1 plants. Previous experiments had shown that gai plants accumulated GA1, GA4, GA8, and GA34 (C19-GAs) to higher levels than the wild type, whereas the levels of some of the C20-GA precursors were reduced in gai (Talon et al., 1990a). Our results confirmed these findings (Table II). C20-GAs were at lower levels in gai than in the wild type (GA19, GA53), with particularly dramatic reductions in the levels of GA12 and GA24. C19-GA levels were elevated in gai, although the increases seen in our experiments are, on the whole, not as dramatic as previously reported. For example, in our experiments GA4 levels were approximately 5-fold elevated in gai with respect to the wild type (compare with the 20-fold increase previously reported; Talon et al., 1990a). We also confirmed that GA34, the 2β-hydroxylated (inactivated) product of GA4, was the most abundant GA in gai (among those GAs that were analyzed; Talon et al., 1990a).
Table II.
Quantification of endogenous GAs from wild type, gai, gai spy-7, gai gar2-1, and gai spy-7 gar2-1
| Endogenous GAs | Wild Type | gai | gai spy-7 | gai gar2-1 | gai spy-7 gar2-1 |
|---|---|---|---|---|---|
| ng g−1 fresh wt | |||||
| C20-GAs | |||||
| GA12 | 2.05 | 0.17 | 0.57 | 0.81 | 1.78 |
| GA53 | 0.34 | 0.11 | 0.20 | 0.30 | 0.31 |
| GA24 | 1.37 | 0.13 | 0.48 | 0.56 | 1.10 |
| GA19 | 0.52 | 0.23 | 0.40 | 0.47 | 0.35 |
| C19-GAs | |||||
| GA20 | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 |
| GA1 | 0.04 | 0.57 | 0.25 | 0.21 | 0.03 |
| GA9 | 0.19 | 0.55 | 0.25 | 0.41 | 0.15 |
| GA4 | 0.39 | 1.87 | 1.10 | 0.88 | 0.40 |
| GA34 | 0.36 | 2.36 | 1.19 | 0.91 | 0.24 |
Results are the means of two determinations.
gai plants accumulated higher than the wild-type levels of transcripts encoding the 20-oxidase enzyme of GA biosynthesis (Xu et al., 1995; Peng et al., 1997). spy-7 and gar2–1, in addition to suppressing the dwarfism conferred by gai, also suppressed the effects of gai on 20-oxidase transcript accumulation (Peng et al., 1997). Because gai had elevated C19-GA levels and reduced C20-GA levels, we were interested in seeing whether spy-7 or gar2–1 would also suppress the effects of gai on endogenous GA levels. As shown in Table II, GA levels in gai spy-7, gai gar2–1, and gai spy-7 gar2–1 were indeed different from those in gai. C19-GA levels (especially GA1, GA4, GA9, and GA34) were elevated in gai, partially elevated in gai spy-7 and gai gar2–1, and returned to approximately wild-type levels in gai spy-7 gar2–1. Similarly, C20-GA levels were reduced in gai, partially restored in gai spy-7 and gai gar2–1, and fully restored to wild-type levels in gai spy-7 gar2–1. Thus, endogenous GA levels in gai spy-7 gar2–1 plants were similar to those of the wild type, whereas those in gai spy-7 and gai gar2–1 were between those of the wild type and gai.
DISCUSSION
spy-7 and gar2–1 partially suppressed the dwarfism conferred by gai (Carol et al., 1995; Wilson and Somerville, 1995). Here we have shown that spy-7 and gar2–1 affected several different GA responses and that, for many of these responses, the effects of both alleles combined are greater than that of either allele alone (Peng et al., 1997). gai spy-7 gar2–1 plants are taller than gai spy-7 or gai gar2–1 and resemble wild-type plants that have received a large exogenous GA dose. These properties are common to some other GA-signaling mutations. For example, the pea la and crys alleles have stronger effects in combination than either allele has alone. la crys double mutants are tall irrespective of endogenous GA level and resemble GA-treated wild-type plants (Potts et al., 1985). Also, combinations of Arabidopsis rga and spy alleles suppressed the ga1–3 phenotype more effectively than either allele alone (Silverstone et al., 1997).
Our observations can be understood if spy-7 and gar2–1 both alter the GA dose-response relationship, as has already been established for other spy alleles. Dose-response experiments showed that spy ga1–2 requires a smaller GA dose than SPY ga1–2 to achieve equal hypocotyl length (Jacobsen and Olszewski, 1993; Jacobsen et al., 1996). We propose that the mutant alleles described in this paper (spy-7 and gar2–1) reduce the repression of GA signaling and alter the GA dose-response relationship in such a way that less GA (or GA signal) is required to elicit a given level of response in spy-7 and gar2–1 than in the wild type. Thus, the GAR2 gene product (like the SPY gene product; Jacobsen et al., 1996) may also be a negative regulator of GA signaling.
The GA dose-response concept can now be used to explain why gai spy-7 and gai gar2–1 plants are taller than gai plants. gai mutant plants are thought to be dwarfed because gai (the gai mutant gene product) represses GA signaling (Peng et al., 1997). Because spy-7 and gar2–1 mutants require less GA signal than SPY or GAR2 plants for a given level of response, gai spy-7 and gai gar2–1 plants are taller than gai. Furthermore, spy-7 gar2–1 double mutants require an even smaller signal for a given level of response than does either single mutant. Thus, gai spy-7 gar2–1 plants are taller than gai spy-7 or gai gar2–1.
Under normal conditions gai spy-7 gar2–1 plants do not respond to exogenous GA and grow as tall as wild-type plants. However, growth of gai spy-7 gar2–1 plants is inhibited by PAC, which is relieved by exogenous GA. Thus, growth of gai spy-7 gar2–1 plants is not completely independent of GA.
The gai mutant has elevated GA levels (Talon et al., 1990a; see Results). An alternative explanation for the action of spy-7 and gar2–1 in suppressing the visible phenotype of gai could have been that these mutations somehow restored responses to the elevated GA. However, we found that spy-7 and gar2–1 also suppressed the effects of gai on the GA level. gai spy-7 and gai gar2–1 had GA levels intermediate between gai and the wild type, whereas gai spy-7 gar2–1 had wild-type GA levels. Thus, spy-7 and gar2–1 do not increase the responses of gai to an elevated GA level.
The gai mutation affects all known Arabidopsis GA responses (Wilson and Somerville, 1995). Here we have shown that spy-7 and gar2–1 suppress the effects of gai on a wide range of these responses. Previously, we had outlined a model to explain the role of GAI and RGA (the GAI and RGA gene products) and associated signal transduction components in mediating GA responses (Peng et al., 1997; Harberd et al., 1998). This model proposed that GAI and RGA act as repressors of GA-regulated stem elongation and that the action of these repressors is opposed by GA. According to this model, the gai protein is a mutant repressor with reduced sensitivity to GA. The model also proposed that the SPY gene product either acts downstream of the GAI and RGA repressors or modulates their activities (Peng et al., 1997; Harberd et al., 1998). This model was initially elaborated on the basis of stem-elongation phenotypes. The data presented in this paper show that the model can be extended to explain GA responses in addition to stem elongation and is perhaps applicable to GA responses in general.
ACKNOWLEDGMENTS
We thank Inga-Britt Carlsson for technical assistance and Pierre Carol, George Coupland, Rachel Cowling, Caroline Dean, Thierry Desnos, Kati King, and Pilar Puente for helpful discussion.
Abbreviations:
- LD
long-day photoperiod
- PAC
paclobutrazol
- SD
short-day photoperiod
Footnotes
This work was made possible by a Biotechnology and Biological Sciences Research Council Core Strategic Grant to the John Innes Centre, by Agricultural and Food Research Council/Biotechnology and Biological Sciences Research Council Plant Molecular Biology grants PG208/520 and PG208/0600, by the European Commission DG XII Biotechnology Program (contract no. BIO4-96-0621), by the Foundation for Strategic Research, and by the Swedish Natural Science Research Council.
LITERATURE CITED
- Bethke PC, Jones RL. Gibberellin signaling. Curr Opin Plant Biol. 1998;1:440–446. doi: 10.1016/s1369-5266(98)80270-7. [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P, Peng J, Harberd NP. Isolation and preliminary characterization of gas1–1, a mutation causing partial suppression of the phenotype conferred by the gibberellin-insensitive (gai) mutation in Arabidopsis thaliana (L.) Heynh. Planta. 1995;197:414–417. doi: 10.1007/BF00202665. [DOI] [PubMed] [Google Scholar]
- Chiang H-H, Hwang I, Goodman HM. Isolation of the Arabidopsis GA4 locus. Plant Cell. 1995;7:195–201. doi: 10.1105/tpc.7.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling RJ (1997) Characterisation of gibberellin responses in Arabidopsis thaliana seedlings. PhD thesis, University of East Anglia, UK
- Cowling RJ, Kamiya Y, Seto H, Harberd NP. Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol. 1998;117:1195–1203. doi: 10.1104/pp.117.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TD, Curry EA. Chemical regulation of vegetative growth. Crit Rev Plant Sci. 1991;10:151–188. [Google Scholar]
- Harberd NP, King KE, Carol P, Cowling RJ, Peng J, Richards DE. Gibberellin: inhibitor of an inhibitor of . . .? BioEssays. 1998;20:1001–1008. doi: 10.1002/(SICI)1521-1878(199812)20:12<1001::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hedden P, Croker SJ. Regulation of gibberellin biosynthesis in maize seedlings. In: Karssen CM, van Loon LC, Vreugdenhil D, editors. Progress in Plant Growth Regulation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 534–544. [Google Scholar]
- Hedden P, Graebe JE. Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm and Malus pumila embryos. J Plant Growth Regul. 1985;4:111–122. [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes, and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Hooley R. Gibberellins: perception, transduction and responses. Plant Mol Biol. 1994;26:1529–1555. doi: 10.1007/BF00016489. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rign L, Zeevaart JAD. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant. 1985;65:33–39. [Google Scholar]
- Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heyhn. Theor Appl Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Langridge P. Effect of day-length and gibberellic acid on the flowering of Arabidopsis. Nature. 1957;180:36–37. [Google Scholar]
- Martin DN, Proebsting WL, Parks TD, Dougherty WG, Lange T, Lewis MJ, Gaskin P, Hedden P. Feedback regulation of gibberellin biosynthesis and gene expression in Pisum sativum L. Planta. 1996;200:159–166. doi: 10.1007/BF00208304. [DOI] [PubMed] [Google Scholar]
- Moritz T, Olsen J. Comparison between high-resolution selected ion monitoring, selected reaction monitoring and four-sector tandem mass spectrometry in quantitative analysis of gibberellins in milligram amounts of plant tissue. Anal Chem. 1995;67:1711–1716. [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP. Derivative alleles of the Arabidopsis gibberellin-insensitive (gai) mutation confer a wild-type phenotype. Plant Cell. 1993;5:351–360. doi: 10.1105/tpc.5.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP. Gibberellin deficiency and response mutations suppress the stem elongation phenotype of phytochrome-deficient mutants of Arabidopsis. Plant Physiol. 1997a;113:1051–1058. doi: 10.1104/pp.113.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP. Transposon-associated somatic gai-loss sectors in Arabidopsis. Plant Sci. 1997b;130:181–188. [Google Scholar]
- Phillips AL, Ward DA, Uknes S, Appleford NEJ, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P. Isolation and expression of three gibberellin-20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108:1049–1057. doi: 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts WC, Reid JB, Murfet IC. Internode length in Pisum. Gibberellins and the slender phenotype. Physiol Plant. 1985;63:357–364. [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The constans gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–858. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Reed JW, Foster KR, Morgan PW, Chory J. Phytochrome B affects responsiveness to gibberellins in Arabidopsis. Plant Physiol. 1996;112:337–342. doi: 10.1104/pp.112.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M, Swain SM, Chandler PM, Olszewski NE. Identification of a negative regulator of gibberellin action, HvSPY, in barley. Plant Cell. 1998;10:995–1007. doi: 10.1105/tpc.10.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ. Recent advances in the study of gibberellin mutants. Plant Growth Regul. 1994;15:193–206. [Google Scholar]
- Ross JJ, Murfet IC, Reid JB. Gibberellin mutants. Physiol Plant. 1997;100:550–560. [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T-p. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal-transduction pathway. Plant Cell. 1998;10:155–169. doi: 10.1105/tpc.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Mak PYA, Martínez EC, Sun T-p. The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics. 1997;146:1087–1099. doi: 10.1093/genetics/146.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T-p, Goodman HM, Ausubel FM. Cloning the Arabidopsis GA1 locus by genomic subtraction. Plant Cell. 1992;4:119–128. doi: 10.1105/tpc.4.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Olszewski NE. Genetic analysis of gibberellin signal transduction. Plant Physiol. 1996;112:11–17. doi: 10.1104/pp.112.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Koornneef M, Zeevaart JAD. Accumulation of C19-gibberellins in the gibberellin-insensitive dwarf mutant gai of Arabidopsis thaliana (L.) Heynh. Planta. 1990a;182:501–505. doi: 10.1007/BF02341024. [DOI] [PubMed] [Google Scholar]
- Talon M, Koornneef M, Zeevaart JAD. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci USA. 1990b;87:7983–7987. doi: 10.1073/pnas.87.20.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D, van Montagu M, van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis root explants using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Somerville CR. Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol. 1995;108:495–502. doi: 10.1104/pp.108.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-L, Li L, Wu K, Peeters AJM, Gage DA, Zeevaart JAD. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proc Natl Acad Sci USA. 1995;92:6640–6644. doi: 10.1073/pnas.92.14.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]