Introduction
Desmopressin (1-deamino-8-D-arginine vasopressin, DDAVP) is a synthetic analogue of the antidiuretic hormone vasopressin, which was originally developed for the treatment of diabetes insipidus1,2. Following its original clinical use in 1977, DDAVP rapidly emerged as the treatment of choice for patients with type 1 von Willebrand’s disease (VWD) and mild haemophilia A (factor VIII coagulant activity [FVIII:C] >5%)3–7. The widespread clinical use of this synthetic compound over the past 30 years has been mainly due to the fact that it is relatively inexpensive and safe, being associated with a very low rate of adverse reactions and no risk of transmission of blood-borne viral infections8. This latter aspect saved numerous patients with VWD and mild haemophilia A from the catastrophic consequences of human immunodeficiency virus (HIV) transmission with non-virus inactivated clotting factor concentrates prior to 19859.
The mechanisms of action of DDAVP are still incompletely understood, despite its extensive clinical use. Desmopressin induces a remarkable increase in the levels of plasma von Willebrand factor (VWF), FVIII:C, and tissue plasminogen activator (t-PA), and also exerts a vasodilatory effect. Desmopressin shortens the prolonged activated partial thromboplastin time (APTT) and the bleeding time. These effects probably result from increases in FVIII:C and VWF, both of which play a rate-accelerating role in these global tests of intrinsic coagulation and primary haemostasis10. The effects of desmopressin on VWF and t-PA, as well as its vasodilatory action, are explained by a direct effect on the endothelium, via activation of the endothelial vasopressin V2 receptor (V2R) and c-AMP-mediated signalling. This leads to exocytosis of VWF and t-PA from endothelial cell Weibel-Palade bodies where VWF and t-PA are stored11. The cellular mechanisms leading to the DDVAP-induced release of FVIII are, however, less certain. One hypothesis is that the close correlation between FVIII:C and VWF plasma levels could be explained by co-secretion, but a cell type that stores and secretes both proteins has not yet been identified as yet. Another reasonable hypothesis is that the effect of DDAVP on FVIII is mediated by indirect mechanisms, via VWF secretion, thereby making more binding sites for FVIII available within the VWF molecules11,12.
Desmopressin can be administered by intravenously (0.3 μg/kg diluted in 50–100 mL of isotonic saline and infused over 30 minutes), subcutaneously (0.3 μg/kg) or via the intranasal route (300 μg). The FVIII levels usually increase by 2- to 6-fold over baseline values but the response is not always linked to the basal FVIII:C levels13–18. For this reason and also considering that there is a high consistency of responses to separate DDAVP treatments, a test infusion/injection should be carried out in every patient to assess his/her level of response19. The level of the FVIII:C elicited after DDAVP administration peaks in the first hour while its half-life ranges between 5 and 8 hours, with inter-individual heterogeneity2. Decreased responsiveness after repeated administrations of DDAVP at 12–24 hour intervals has been reported in some patients. This phenomenon, known as tachyphylaxis, is thought to be due to FVIII:C depletion from cellular stores19,20.
Desmopressin has also been used successfully for the treatment of patients with autoantibodies against coagulation FVIII, a therapeutic use analysed in this article by systematically reviewing the published literature.
Search methods
We performed a search of MEDLINE, EMBASE, OVID, and SCOPUS using the following terms without time limits:
- “desmopressin”,
- “DDAVP”,
- “acquired inhibitors”,
- “acquired factor VIII inhibitors”,
- “acquired inhibitors and coagulation factors”,
- “autoantibodies and coagulation factors”,
- “anti-factor VIII antibodies”,
- “factor VIII autoantibodies”,
- “autoimmune factor VIII inhibitors”,
- “haemophilia and inhibitors”,
- “haemophilia and autoantibodies”, and
- “spontaneous inhibitors and factor VIII”.
Only articles or abstracts written in English were considered. The references of all retrieved studies and reviews were assessed for additional reports of clinical trials.
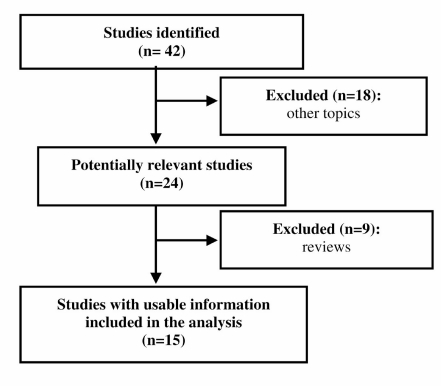
Figure 1 shows the flow-chart of the inclusion of the studies.
Figure 1.
Flow-chart of the inclusion of the studies.
Literature results and discussion
Acquired haemophilia A is an uncommon (incidence of 0.2–1.0 cases per 1 million persons per year) but potentially life-threatening clinical syndrome characterised by autoantibodies directed against functional epitopes of FVIII causing neutralisation of this clotting factor and/or its accelerated clearance from the plasma21–23. Acquired anti-FVIII inhibitors are distributed equally between sexes and have an typically biphasic age distribution, with a small peak between 20 and 30 years (mainly post-partum inhibitors) and a larger peak in patients aged 70–80 years old. In approximately 50% of cases FVIII autoantibodies occur in patients without concomitant diseases, while the remaining cases are associated with a variety of conditions, including pregnancy, autoimmune disorders, cancers and drugs24–27.
The clinical picture of acquired haemophilia is typically characterised by bleeding into the skin, muscles, soft tissues and mucous membranes, whereas haemarthroses, a typical manifestation of congenital haemophilia A, are unusual. Not rarely the haemorrhages in acquired haemophilia are serious or life-threatening, as in the case of cerebral haemorrhage or rapidly progressive retroperitoneal haematomas22. The diagnosis of acquired haemophilia is based on the demonstration of an isolated prolongation of the activated partial thromboplastin time (APTT), not corrected by incubating the patient’s plasma with equal volumes of normal plasma (mixing study), associated with low FVIII levels and evidence of a FVIII inhibitor (which can be titrated in Bethesda units [BU]), in a patient with no previous personal or family history of bleeding22. The treatment priorities of acquired haemophilia are to arrest the acute bleeding and to eradicate the FVIII autoantibody28,29. Acute bleeding episodes in patients with low-titre inhibitors can be treated using FVIII concentrates, whereas FVIII bypassing agents, such as activated prothrombin complex concentrates (APCC) or recombinant activated factor VII (rFVIIa), are effective for the treatment of those with high titre inhibitors. Inhibitor elimination may be achieved by using immunosuppressive agents such as corticosteroids alone or in association with cyclophosphamide29.
Second-line eradicating therapies include high-dose immunoglobulins, cyclosporine and rituximab6.
Following the first report on the use of DDAVP in non-haemophilic patients with acquired FVIII inhibitors in 1985, by De la Fuente and colleagues30, a number of other case reports or small case series on this subject have been published31–44. Di Bona and colleagues reported on 17 cases of acquired haemophilia A cases diagnosed at two Italian haemophilia centres between 1979 and 1995. Desmopressin was used successfully for minor haemorrhagic episodes in five patients with low-titre inhibitor and measurable FVIII:C levels. Although FVIII:C levels increased by approximately 6-fold over the mean basal level, the increment was short-lived and additional infusions at 24-hour intervals failed to elicit an increase of FVIII:C because of tachyphylaxis.
Three additional patients with acquired haemophilia A successfully treated with DDAVP for non-life threatening haemorrhages have been described by our group. One patient had a low-inhibitor titre, while the other two patients had a previous high-titre inhibitor which responded to immunosuppressive therapy thus allowing the use of DDAVP. All the three patients reported by Collins and colleagues responded to DDAVP, which was used to cover two minor invasive procedures and a minor bleeding episode. However, the largest experience is that reported by Nilsson and Lethagen36: in six of 11 patients with acquired haemophilia A treated with DDAVP, a satisfactory increase of FVIII:C levels and a haemostatic effect were obtained. By contrast, in the patients with high antibody titres and no measurable or very low FVIII:C concentrations, DDAVP had no effect. A literature review published by Mudad and Kane37, reporting on 22 cases of acquired haemophilia A treated with DDAVP revealed that in all 11 patients with initial FVIII:C levels greater than 5%, DDAVP induced a rise in FVIII:C levels to between 15 and 140%. The five patients in this group with inhibitor titres of less than 2 BU had the best responses with peak FVIII:C levels of greater than 80%. Responses were also documented in three of the ten patients with FVIII:C levels of less than 5%.
Our systematic analysis of the available literature, the largest published so far, has documented a total of 37 patients collected from 15 case reports published during the period 1985–2005 (see Table I). The male to female ratio was 1.3 (21 males and 16 females) while the median age was 65.5 years (range, 12–83 years). As regards the most frequent associated conditions, 11 of the 26 evaluable cases (42.3%) were idiopathic, while six cases (23.1%) occurred in the post-partum period and four cases (15.4%) were associated with bronchial asthma. In all cases DDAVP was used for the treatment of non-life-threatening haemorrhages or to cover minor surgical, invasive or dental procedures. In the great majority of the cases (28/32, 87.5%), DDAVP was administered intravenously at a dose of 0.3 μg/kg.
Table I.
The clinical use of desmopressin in acquired haemophilia A: analysis of the literature data.
| Author, yearRef. | Age/Sex | Associated condition | Reason for DDAVP use | Doses1, route/inhibitor titer2 | FVIII: C (%) | Concomitant anti- hemorrhagic therapy | Clinical outcome | |
|---|---|---|---|---|---|---|---|---|
| Pre- DDAVP | Post- DDAVP | |||||||
| De la Fuente, 198530 | 47/M | Idiopathic | Dental procedure | 0.3x1, IV/1.8 | 18 | 80 | EACA | Haemostatic efficacy |
| Vincente, 198531 | NR/F | Post-partum | DDAVP test | 0.4x1, IV/13 | 3 | 5 | No | No response |
| Hasson, 198632 | 64/M | Idiopathic | Dental procedure | 0.3x1, IV/0.6 | 51 | 130 | EACA | Haemostatic efficacy |
| Chistolini, 198733 | 60/M | Idiopathic | DDAVP test | 0.3x1, SC/1 | 6.5 | 110 | No | Haemostatic efficacy |
| Naarose-Abidi, 198834 | 75/F | Idiopathic | Haematuria | 0.3x1, IV/NR | 7 | 140 | No | Haemostatic efficacy |
| 20/F | Post-partum | Soft-tissue bleeding | 0.3x1, IV/4 | 1 | 18 | No | Haemostatic efficacy | |
| 27/F | Post-partum | Soft-tissue bleeding | 0.3x2, IV/NR | 2 | 27 | No | Haemostatic efficacy | |
| Muhm, 199035 | 12/M | Idiopathic | RPH, haemarthrosis, MH | 0.4x9, IV/4.6 | 15 | 90 | hFVIII | Haemostatic efficacy |
| 77/M | Asthma | Haematuria, SCH | 0.4x8, IV/267 | 2.9 | 15 | hFVIII | Haemostatic efficacy | |
| 73/M | Asthma | MH | 0.4x1, IV/444 | <1 | <1 | APCC | No response | |
| Nilsson, 199136 | 55/F | NR | NR | 0.3x17, IV/1:10,003 | 9.6 | 24.1 | NR | Haemostatic efficacy |
| 60/F | NR | NR | 0.3x1, IV/1:23 | 26 | 69 | NR | Haemostatic efficacy | |
| 76/F | NR | NR | 0.3x26, IV/1:103 | 5.5 | 16 | NR | Haemostatic efficacy | |
| 67/F | NR | NR | 0.3x2, IV/1:503 | 14 | 48.5 | NR | Haemostatic efficacy | |
| 68/M | NR | NR | 0.3x19, IV/1:1003 | 15.4 | 24.7 | NR | Haemostatic efficacy | |
| 69/F | NR | NR | 0.3x5, IV/1:23 | 9.8 | 23.6 | NR | Haemostatic efficacy | |
| 83/M | NR | NR | 0.3x1, IV/1:103 | <0.5 | 3.6 | NR | No response | |
| 71/M | NR | NR | 0.3x7, IV/1:1,003 | 1.1 | 4.8 | NR | No response | |
| 60/M | NR | NR | 0.3x1, IV/1:203 | 1.5 | 3 | NR | No response | |
| 80/F | NR | NR | 0.3x5, IV/1:213 | 2 | 8.8 | NR | No response | |
| 69/M | NR | NR | 0.3x4, IV/1:2,003 | <0.5 | <0.5 | NR | No response | |
| Mudad and Kane, 199337 | 80/F | Asthma, RA | GI bleeding | 0.3x1, IV/1.9 | 10 | 88 | No | Haemostatic efficacy |
| Vivaldi, 199338 | 82/M | Idiopathic | Cutaneous bleeding | 0.3x1, IV/15 | 6 | NR | APCC | Haemostatic efficacy |
| Di Bona, 199739 | 59/M | Diabetes, LAC | RPH, SCH, MH | 0.3x1, IV/14 | 4 | 30 | hFVIII, pFVIII, APCC | No response |
| 69/M | Idiopathic | MH, RPH | 0.3x4, IV/4 | 5 | 78 | hFVIII, pFVIII | Haemostatic efficacy | |
| 28/F | Post-partum | MH | 0.3x4, IV/140 | 12 | 20 | pFVIII | Haemostatic efficacy | |
| 70/M | MGUS | MH | 0.3x4, IV/3.6 | 5 | 40 | hFVIII | Haemostatic efficacy | |
| 59/F | Asthma | SCH | 0.3x1 IV/34 | 1 | 18 | hFVIII | Haemostatic efficacy | |
| Burnet, 200140 | 70/M | Quinine | NR | NR/3.4 | 2 | NR | TA | Haemostatic efficacy |
| Delgado, 200241 | 28/F | Post-partum | Vaginal bleeding, MH | NR/83 | 8 | NR | APCC | No response |
| Howland, 200242 | 38/F | Post-partum | Vaginal bleeding | 0.3x1 IV/2 | 16 | NR | TA | Haemostatic efficacy |
| Collins, 200443 | 41/M | Idiopathic | Cystoscopy | NR/7 | <1 | NR | No | Haemostatic efficacy |
| 51/M | Castlemann disease | Minor surgery | NR/35 | 3.5 | NR | No | Haemostatic efficacy | |
| 80/M | Idiopathic | SCH | NR/6 | 4 | NR | No | Haemostatic efficacy | |
| Franchini, 200544 | 75/F | Idiopathic | Rectosigmoidoscopy | 03.x5, SC/6 | 40 | NR | TA | Haemostatic efficacy |
| 45/M | Idiopathic | Haemarthrosis, MH | 0.3x3, SC/1 | 4 | 38 | No | Haemostatic efficacy | |
| 64/M | ATD | Conjunctival haemorrhage | 0.3x2, SC/2 | 16 | 54 | No | Haemostatic efficacy | |
Legend:
IV, intravenously; SC, subcutaneously; DDAVP, desmopressin; FVIII:C, factor VIII coagulant activity; EACA, epsilon aminocaproic acid; NR, not reported; RPH, retroperitoneal haemorrhage; hFVIII, human factor VIII concentrate; pFVIII, porcine factor VIII concentrate; APCC, activated prothrombin complex concentrate; SCH, subcutaneous haematoma; MH, muscle haematoma; RA, rheumatoid arthritis; GI, gastrointestinal; LAC, lupus anticoagulant; MGUS, monoclonal gammopathy of undetermined significance;TA, tranexamic acid; ATD, autoimmune thyroid disease.
μg/kg;
Bethesda Units;
The maximum dilution of inhibitor plasma that prolongs the clotting time of normal plasma is reported.
The median number of doses administered was 2 (range, 1–26 doses). The median inhibitor titre was 5.3 BU (range, 0–444 BU). The median FVIII:C level prior to DDAVP administration was 5% (range, 0–40%), while the median FVIII:C level following DDAVP administration was 24.7% (range, 0–140%). Thus, the use of DDAVP led to an approximately 5-fold increase of FVIII:C levels. In 15 of the 26 evaluable cases (57.7%), a concomitant haemostatic therapy was given. However, as antifibrinolytic agents were used only in two patients30,32, their role in improving the efficacy of DDAVP cannot be evaluated. Finally, DDAVP produced a haemostatic effect in 28 of 37 cases (75.7%).
In line with the results of the systematic review by Mudad and Kane37, the best responses (19/20 cases [95%] with an increase in FVIII:C levels between 8 and 133%) were observed in patients with basal FVIII:C levels higher than 5%. Similarly, all the 11 evaluable patients with inhibitor titres less than 5 BU responded to DDAVP. A comparison between patients who responded to DDAVP and those who did not showed statistically significant difference between the two groups with regards to both median inhibitor titre (4 BU versus 48.5 BU, P<0.001, Student’s t-test) and basal FVIII:C level (7% versus 1.5%, P<0.01, Student’s t-test). Thus, our systematic review shows that a low inhibitor titre (<5 BU) and a residual FVIII:C level greater than 5% were predictive of a clinical response to DDAVP in patients with acquired haemophilia A.
Conclusions
This analysis of the literature supports the role of DDAVP in the treatment of minor bleeding episodes or prophylaxis of minor surgical/invasive procedures in patients with acquired haemophilia A with a low inhibitor titre and measurable baseline levels of circulating plasma FVIII:C. Nevertheless, experience in using DDAVP in this setting is limited to a few case reports.
The data in the large multicentre European Acquired Haemophilia Registry (EACH2), which has collected information on more than 300 cases of acquired haemophilia, will help us to clarify the role of this agent in acquired haemophilia A.
References
- 1.Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first twenty years. Haemophilia. 2000;6(Suppl 1):60–7. doi: 10.1046/j.1365-2516.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M. The use of desmopressin as a hemostatic agent. Am J Hematol. 2007;82:731–5. doi: 10.1002/ajh.20940. [DOI] [PubMed] [Google Scholar]
- 3.Rodeghiero F, Castaman G, Mannucci PM. Clinical indications for desmopressin (DDAVP) in congenital and acquired von Willebrand disease. Blood Rev. 1991;5:155–61. doi: 10.1016/0268-960x(91)90032-8. [DOI] [PubMed] [Google Scholar]
- 4.Mannucci PM, Ruggeri ZM, Pareti FI, Capitanio A. 1-Deamino-8-d-arginine vasopressin: a new pharmacological approach to the management of haemophilia and von Willebrand’s diseases. Lancet. 1977;1:869–72. doi: 10.1016/s0140-6736(77)91197-7. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Favaloro EJ, Lippi G. Mild hemophilia A. J Thromb Haemost. 2010;8:421–32. doi: 10.1111/j.1538-7836.2009.03717.x. [DOI] [PubMed] [Google Scholar]
- 6.Franchini M, Lippi G. Acquired factor VIII inhibitors. Blood. 2008;112:250–5. doi: 10.1182/blood-2008-03-143586. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Zaffanello M, Lippi G. The use of desmopressin in mild hemophilia A. Blood Coagul Fibrinolysis. 2010 doi: 10.1097/MBC.0b013e32833c2bb5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Castaman G. Desmopressin for the treatment of haemophilia. Haemophilia. 2008;14(Suppl 1):15–20. doi: 10.1111/j.1365-2516.2007.01606.x. [DOI] [PubMed] [Google Scholar]
- 9.Sadler JE, Mannucci PM, Berntorp E, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost. 2000;84:160–74. [PubMed] [Google Scholar]
- 10.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245–53. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP) J Thromb Haemost. 2003;1:682–9. doi: 10.1046/j.1538-7836.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 12.Haberichter SL, Shi Q, Montgomery RR. Regulated release of VWF and FVIII and the biologic implications. Pediatr Blood Cancer. 2006;46:547–53. doi: 10.1002/pbc.20658. [DOI] [PubMed] [Google Scholar]
- 13.Mariani G, Ciavarella N, Mazzucconi MG, et al. Evaluation of the effectiveness of DDAVP in surgery and bleeding episodes in hemophilia and von Willebrand disease. A study of 43 patients. Clin Lab Haematol. 1984;6:229–38. doi: 10.1111/j.1365-2257.1984.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 14.de la Fuente B, Kasper CK, Rickles FR, Hoyer LW. Response of patients with mild and moderate hemophilia A and von Willebrand disease to treatment with desmopressin. Ann Intern Med. 1985;103:6–14. doi: 10.7326/0003-4819-103-1-6. [DOI] [PubMed] [Google Scholar]
- 15.Revel-Vilk S, Blanchette VS, Sparling C, et al. DDAVP challenge tests in boys with mild/moderate haemophilia A. Br J Haematol. 2002;117:947–51. doi: 10.1046/j.1365-2141.2002.03507.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodeghiero F, Castaman G, Mannucci PM. Prospective multicenter study on subcutaneous concentrated desmopressin for home treatment of patients with von Willebrand disease and mild or moderate hemophilia A. Thromb Haemost. 1996;76:692–6. [PubMed] [Google Scholar]
- 17.Seremetis SV, Aledort LM. Desmopressin nasal spray for hemophilia A and type I von Willebrand disease. Ann Intern Med. 1997;126:744–5. doi: 10.7326/0003-4819-126-9-199705010-00031. [DOI] [PubMed] [Google Scholar]
- 18.Leissinger V, Becton D, Cornell C, Cox Gill J. High-dose DDAVP intranasal spray (Stimate) for the prevention and treatment of bleeding in patients with mild haemophilia A, mild or moderate type 1 von Willebrand disease and symptomatic carriers of haemophilia A. Haemophilia. 2001;7:258–66. doi: 10.1046/j.1365-2516.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- 19.Nolan B, White B, Smith J, et al. Desmopressin: therapeutic limitations in children and adults with inherited coagulation disorders. Br J Haematol. 2000;109:865–9. doi: 10.1046/j.1365-2141.2000.02067.x. [DOI] [PubMed] [Google Scholar]
- 20.Lethagen S. Desmopressin in mild hemophilia A: indications, limitations, efficacy, and safety. Semin Thromb Hemost. 2003;29:101–6. doi: 10.1055/s-2003-37944. [DOI] [PubMed] [Google Scholar]
- 21.Delgado J, Yimenez-Yuste V, Hernandez-Navarro F, et al. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol. 2003;121:21–35. doi: 10.1046/j.1365-2141.2003.04162.x. [DOI] [PubMed] [Google Scholar]
- 22.Franchini M, Gandini G, Di Paolantonio T, et al. Acquired hemophilia A: a concise review. Am J Hematol. 2005;80:55–63. doi: 10.1002/ajh.20390. [DOI] [PubMed] [Google Scholar]
- 23.Franchini M, Targher G, Montagnana M, Lippi G. Laboratory, clinical and therapeutic aspects of acquired hemophilia A. Clin Chim Acta. 2008;395:14–8. doi: 10.1016/j.cca.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Boggio LN, Green D. Acquired hemophilia. Rev Clin Exp Hematol. 2001;5:389–404. doi: 10.1046/j.1468-0734.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 25.Solymoss S. Postpartum acquired factor VIII inhibitors: results of a survey. Am J Haematol. 1998;59:1–4. doi: 10.1002/(sici)1096-8652(199809)59:1<1::aid-ajh1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Bossi P, Cabane J, Ninet J, et al. Acquired haemophilia due to factor VIII inhibitors in 34 patients. Am J Med. 1998;105:400–8. doi: 10.1016/s0002-9343(98)00289-7. [DOI] [PubMed] [Google Scholar]
- 27.Cohen AJ, Kessler CM. Acquired inhibitors. Bailleres Clin Haematol. 1996;9:331–54. doi: 10.1016/s0950-3536(96)80067-9. [DOI] [PubMed] [Google Scholar]
- 28.Collins PW. Treatment of acquired hemophilia A. J Thromb Haemost. 2007;5:893–900. doi: 10.1111/j.1538-7836.2007.02433.x. [DOI] [PubMed] [Google Scholar]
- 29.Huth-Kühne A, Baudo F, Collins P, et al. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica. 2009;94:566–75. doi: 10.3324/haematol.2008.001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Fuente B, Panek S, Hoyer LW. The effect of 1-deamino 8 D-arginine vasopressin (DDAVP) in a nonhaemophilic patient with an acquired type II factor VIII inhibitor. Br J Haematol. 1985;59:127–31. doi: 10.1111/j.1365-2141.1985.tb02972.x. [DOI] [PubMed] [Google Scholar]
- 31.Vincente V, Corrales J, Miralles J, Alberca I. DDAVP in a non-haemophiliac patient with an acquired factor VIII inhibitor. Br J Haematol. 1985;60:585–6. doi: 10.1111/j.1365-2141.1985.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasson DM, Poole AE, de la Fuente B, Hoyer LW. Dental management of patients with spontaneous acquired factor VIII inhibitors. J Am Dent Assoc. 1986;113:633–6. doi: 10.14219/jada.archive.1986.0240. [DOI] [PubMed] [Google Scholar]
- 33.Chistolini A, Ghirardini A, Tirindelli MC, et al. Inhibitor to factor VIII in a non-haemophilic patient: evaluation of the response to DDAVP and the in vitro kinetics of factor VIII. A case report. Nouv Rev Fr Hematol. 1987;29:221–4. [PubMed] [Google Scholar]
- 34.Naarose-Abidi SM, Bond LR, Chitolie A, Bevan DH. Desmopressin therapy in patients with acquired factor VIII inhibitors. Lancet. 1988;1:366. doi: 10.1016/s0140-6736(88)91169-5. [DOI] [PubMed] [Google Scholar]
- 35.Muhm M, Grois N, Kier P, Stümpflen A, et al. 1-Deamino-8-D-arginine vasopressin in the treatment of non-haemophilic patients with acquired factor VIII inhibitor. Haemostasis. 1990;20:15–20. doi: 10.1159/000216100. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson IM, Lethagen S. Current status of DDAVP formulations and their use. Excerpta Medica. 1991;943:443–53. [Google Scholar]
- 37.Mudad R, Kane WH. DDAVP in acquired hemophilia A: case report and review of the literature. Am J Hematol. 1993;43:295–9. doi: 10.1002/ajh.2830430413. [DOI] [PubMed] [Google Scholar]
- 38.Vivaldi P, Savino M, Mazzon C, Rubertelli M. A hemorrhagic syndrome of the elderly patient caused by anti-factor VIII antibodies. Haematologica. 1993;78:245–8. [PubMed] [Google Scholar]
- 39.Di Bona E, Schiavoni M, Castaman G, et al. Acquired hemophilia: experience of two Italian centres with 17 new cases. Haemophilia. 1997;3:183–8. doi: 10.1046/j.1365-2516.1997.00102.x. [DOI] [PubMed] [Google Scholar]
- 40.Burnet SP, Duncan EM, Lloyd JV, Han P. Acquired haemophilia in South Australia: a case series. Intern Med J. 2001;31:556–9. doi: 10.1046/j.1445-5994.2001.00127.x. [DOI] [PubMed] [Google Scholar]
- 41.Delgado J, Villar A, Jimenez-Yuste V, et al. Acquired hemophilia: a single-center survey with emphasis on immunotherapy and treatment-related side-effects. Eur J Haematol. 2002;69:158–64. doi: 10.1034/j.1600-0609.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 42.Howland EJ, Palmer J, Lumley M, Keay SD. Acquired factor VIII inhibitors as a cause of primary post-partum haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2002;103:97–8. doi: 10.1016/s0301-2115(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 43.Collins P, Collins P, Macartney N, et al. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Br J Haematol. 2004;124:86–90. doi: 10.1046/j.1365-2141.2003.04731.x. [DOI] [PubMed] [Google Scholar]
- 44.Franchini M, Girelli D, Olivieri O, et al. Clinical heterogeneity of acquired hemophilia A: a description of 4 cases. Haematologica. 2005;90:ECR16. [PubMed] [Google Scholar]