Abstract
Background
In Iran, cryoprecipitate is an important plasma product to provide coagulation factors such as factor VIII (FVIII) in patients with factor VIII deficiency. FVIII is one of the labile coagulation factors and as such is also used as a quality marker of fresh-frozen plasma and cryoprecipitate. It is, therefore, important to optimise plasma production in order to prevent a reduction of FVIII activity. In this study we assessed the effect of temperature, time and FVIII assay type on FVIII activity in cryoprecipitate produced in Iran.
Methods
Ninety-six whole blood units were kept at two different temperatures (48 units kept at 1–6 °C and 48 kept at 20–24 °C) for periods of 4, 6, 8 or 10 hours before plasma freezing. FVIII activity was then measured by both chromogenic and one-stage clotting assays.
Results
At both temperatures, FVIII activity in plasma prepared after 8 and 10 hours was lower than that in plasma prepared after 4 and 6 hours. A significant decrease of FVIII activity was not seen in samples kept for 4 and 6 hours. Compared to storage between 1–6 °C, storage at 20–24 °C appears to cause a reduction in FVIII activity. There was a significant difference in apparent FVIII activity measured by the one-stage clot-based and chromogenic assays.
Conclusion
In Iran, to improve cryoprecipitate quality, freezing should begin within 6 hours after donation and whole blood should be kept at 1–6 °C until the plasma can be frozen. In this study although a good correlation was seen between the results of the one-stage clot-based and chromogenic assays for measuring FVIII activity in cryoprecipitate, the absolute values were significantly different.
Keywords: FVIII activity, cryoprecipitate, time, temperature, FVIII assay type
Introduction
Although recombinant factor VIII (FVIII) concentrates are readily accessible and recommended for patients with congenital and acquired FVIII deficiency, in a developing country such as Iran, cryoprecipitate is still an important plasma product to provide a concentrated form of FVIII. FVIII is one of the labile coagulation factors and is also used as a quality marker of fresh-frozen plasma and cryoprecipitate. All steps of cryoprecipitate production should, therefore, be optimised in order to prevent a reduction of FVIII activity1–2.
Factors such as the time and temperature between donation and the start of the freezing process, rate office formation and eutectic point in plasma may affect FVIII activity3–4.
The results of a study reported by Carlebjork et al. showed that FVIII activity was stable in plasma kept for 2 hours at 22 °C between donation and freezing5. In a separate study, Sward-Nilson suggested that storage at room temperature for 6 hours caused a significant decrease in FVIII activity6. In another study, it was found that keeping blood at 1 to 6 °C for 8, 15 and 24 hours before freezing caused a reduction of FVIII activity7.
Although it is important to preserve FVIII activity, a number of studies have shown that high levels of FVIII activity are associated with a risk of thrombosis in patients with FVIII deficiency8–10. It is, therefore, also important to select the best methods for measuring FVIII activity in cryoprecipitate in order to estimate how many cryoprecipitate units a patient needs. Since there was no evidence about factors affecting the quality of cryoprecipitate in Iran, we investigated how different freezing times and temperatures influenced FVIII activity in cryoprecipitate and evaluated two different types of assay for measuring FVIII activity.
Material and methods
Blood collection
Ninety-six units of whole blood (450±45 mL) were collected from random donors and conserved with citrate-phosphate-dextrose adenine. Since there is a relationship between blood group and FVIII content, an equal number of group A, group B and group O donations were selected (32 A, 32 B, 32 O) to make sure the data were not biased. Although the prevalence of O blood group is higher than that of other blood groups, 32 units for each blood group were chosen based on calculations of statistical power indicating that these numbers would enable a 5% difference in mean results to be demonstrated.
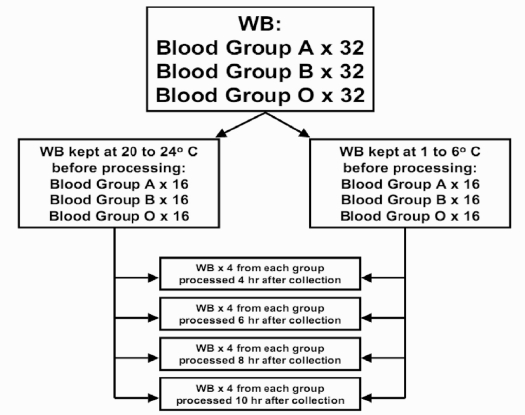
Immediately after collection, units of each blood group were divided into two groups: 16 whole blood units were cooled over about 30 minutes and stored refrigerated between 1 and 6 °C, while the other 16 units of each blood group were cooled to 20–24 °C without delay. The 16 units in each group were then divided into four groups: 4 units were kept for 4 hours, 4 units were stored for 6 hours, 4 units were kept for 8 hours and 4 units were stored for 10 hours before plasma freezing. The experimental design is shown in Figure 1.
Figure 1.
The design of the study. Ninety-six units of whole blood (WB) were separated into three groups according to blood type (32 A, 32 B, 32 O). Before separation and freezing of plasma, WB units of each blood type were divided into two groups: 16 WB units of each blood type were stored at 1 to 6 °C and 16 units of each blood type were kept at 20 to 24°C. For each storage temperature, the effects of four different storage periods (4, 6, 8 and 10 hours) were studied.
Plasma preparation
After subjecting whole blood units to each treatment described above, heavy-spin centrifugation with an automated blood component extractor system (LMB technologist GmbH, Schwaig, Germany) was used to extract the plasma which was frozen within 15 minutes of separation in a plasma blast freezer (Sina Ebtekar, Theran, Iran) and stored at –30 °C or colder. We prepared cryoprecipitate from the same whole blood donations. To prepare cryoprecipitate, plasma units were thawed at 4 °C. When the plasma had a slushy consistency, liquid plasma was separated from the cryoprecipitate by centrifugation using heavy-spin according to standard operating procedures and then stored at –20 °C or less.
Measurement of factor VIII activity
FVIII activity was measured in duplicate using both a chromogenic assay with reagents from Technochrom (Technoclone GmbH, Wien, Austria) on an STA instrument (Diagnostica Stago, Parsippany, NJ, USA) and a one-stage clotting assay with reagents from Diagnostica Stago, also on an STA instrument. Cryoprecipitate was thawed in a water bath at 37 °C before measurement of FVIII activity and all tests were performed within 1 month of collection of the initial blood product.
Statistical analysis
Statistical comparisons of mean factor VIII activity for the four times of plasma preparation were carried out using one way analysis of variance, while statistical comparisons of differences in mean FVIII activity for the two temperatures of storage were evaluated using an independent sample t-test. In addition paired t-tests were applied to compare the measurement of FVIII activity by the clotting and chromogenic assays. p values less than 0.05 were considered statistically significant.
Results
The effect of temperature of storage on factor VIII activity
In units with B blood group, the mean±standard deviation (SD) of FVIII activity was 94.4±13.86 and 83.06±9.53 IU/unit in units stored at 1 to 6 °C and those kept at 20 to 24 °C, respectively. In units with A blood group, the mean±SD of FVIII activity was 91.4±11.71 IU/unit in units stored at 1 to 6 °C and 84.25±9.92 IU/unit in those kept at 20 to 24 °C. In units with O blood group, the mean±SD of FVIII activity was 89.94±11.92 and 74.12±9.53 IU/unit in units stored at 1 to 6 °C and those kept at 20 to 24 °C, respectively.
For all blood groups, FVIII activity was significantly higher (p<0.05) in units stored at 1 to 6 °C than in those stored at 20 to 24 °C.
The effect of duration of storage on factor VIII activity
The whole blood units were stored for different times before the plasma was separated and frozen.
In units with B blood group, the mean±SD of FVIII activity and the 95% confidence interval (CI) for each storage period was as follows: 4 hours, 97.75±15.06 (85.15–110.35); 6 hours, 91.88±12.96 (81.03–102.72); 8 hours, 83.75±8.56 (76.59–90.91); 10 hours, 81.50±9.59 (73.48–89.52). In units with A blood group, the mean±SD of FVIII activity and 95% CI for each storage period was as follows: 4 hours, 97.38±13.25 (86.29–108.46); 6 hours, 90.25±8.1 (83.48–97.2); 8 hours, 83.38±8.01 (76.67–90.08); 10 hours, 79.12±7.45 (79.82–85.36). The corresponding values for units with O blood group were: 4 hours, 93.4±13.26 (82.28–104.5); 6 hours, 85.62±11.78 (75.77–95.48); 8 hours, 77±7.2 (70.97–83.03); 10 hours, 72.10±69 (63.18–81.07).
For all different blood groups, units that had been kept for 8 and 10 hours had lower FVIII activity than units stored for 4 and 6 hours before processing (p<0.05), but there was no statistical difference between samples kept for 4 or 6 hours.
The interaction of temperature and time on factor VIII activity
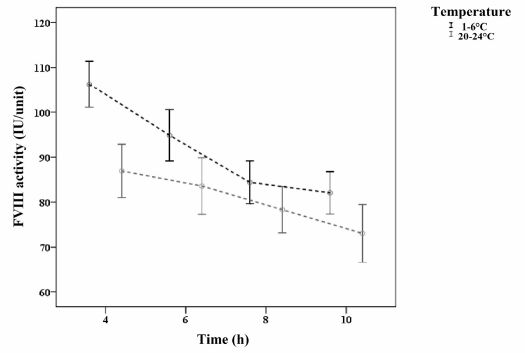
The interaction of temperature and time on FVIII activity was not significant. In other words, the effect of time on FVIII activity appeared to be independent of that of temperature (Figure 2).
Figure 2.
Trend of change of mean FVIII activity of units stored at different temperatures for different periods.
The effect of factor VIII assay type on measured levels of factor VIII activity
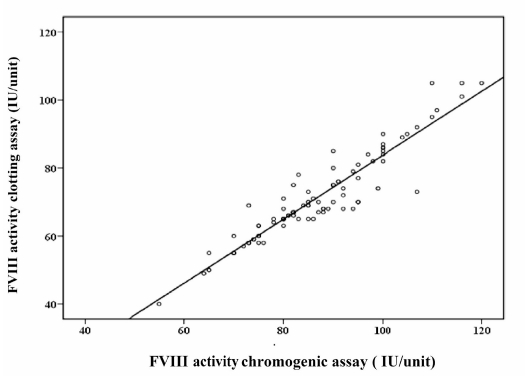
Samples were tested by each method to compare the ranges for each assay. The mean±SD of FVIII activity according to the chromogenic assay was 86.09±12.83 IU/unit, while the mean±SD FVIII activity measured by the one-stage clotting assay was 70.76±12.80 IU/unit. The difference between these two results was statistically significant. The correlation (r2) of values determined by the one-stage clot-based assay and the chromogenic assay was 0.94 (Figure 3).
Figure 3.
Comparison of FVIII activity measured by a chromogenic assay and a one-stage clot-based assay. The solid line indicates the Deming regression. The correlation coefficient (r2) for the clot-based assay versus the chromogenic assay is 0.94.
Discussion
Since cryoprecipitate is still an important plasma product used to provide FVIII concentrates in Iran, it is necessary to understand the influence of temperature and time from collection to freezing on FVIII activity in order to optimise its production. In this study we, therefore, kept units of whole blood at two different temperatures (1 to 6 °C or 20 to 24 °C) for 4, 6, 8 or 10 hours before separation and freezing of the plasma and evaluated the effect of these different temperatures and storage periods on the FVIII activity in cryoprecipitate. In addition, two different FVIII assays (chromogenic and clotting) were compared with regards to their measurement of FVIII activity in cryoprecipitate.
Our results showed that, for all different blood groups, the mean FVIII activity was higher for units stored at 1 to 6 °C than for those stored at 20 to 24 °C. We also found that the mean FVIII activity was lower for units kept for 8 and 10 hours before freezing than for units stored for 4 and 6 hours, whereas no significant differences were found between the FVIII activity in plasma extracted after 4 or 6 hours.
It has been shown before that the time and temperature taken to freeze plasma can affect the FVIII activity. Carlebjork et al. observed that FVIII activity was stable in plasma stored at 22 °C for 2 hours5. Smith et al. reported that storage at 1–6° C for 8, 15 and 24 hours before freezing led to a remarkable reduction of FVIII activity7. Sward-Nilson et al. found no difference in FVIII activity if the time before freezing was extended from 2 to 4 hours; however, if the delay was extended to 6 hours, there was a statistically significant loss of FVIII activity compared to that present after 4 hours of storage6. The same reduction in FVIII activity was shown in a study measuring the FVIII activity of plasma produced from whole blood stored at 4 °C for 20 to 24 hours. Cardigan and colleagues reported that keeping blood at 4 °C overnight led to a more than 20% loss of FVIII with respect to that present in plasma frozen within 8 hours of donation11. Furthermore, Serrano reported that FVIII and other factors exhibited loss of activity with increasing whole blood storage time. The loss of FVIII activity was close to 28%12. Based on the results of another study, Hughes et al. suggested that storage of whole blood in a warm environment for up to 72 hours may have less effect on FVIII activity13.
None of these studies compared the effect of two temperatures, namely 1 to 6 °C and 20 to 24 °C, on FVIII activity nor did they report on the interaction between time and temperature from donation to freezing. Although it is assumed that storing blood at 4 °C is not favourable to FVIII, we actually found that storage of whole blood for 4 or 6 hours at 1 to 6 °C before freezing resulted in cryoprecipitate of better quality than that produced from whole blood stored at 20–24 °C. There was, however, an apparently larger fall in FVIII activity in units stored at 1 to 6 °C than in those kept at 20 to 24 °C. We, therefore, evaluated the interaction of temperature and duration of storage on FVIII activity. This analysis showed that the effect of time on FVIII activity was independent of that of temperature; accordingly, the greater fall of FVIII activity in the former group (storage at 1 to 6 °C) compared to in the latter group (storage at 20 to 24 °C) was not statistically significant.
One limitation to our study was that we did not use cooling plates. The units of the second group should have been cooled quickly to room temperature by cooling plates and our lack of cooling plates might help to explain why we observed a loss of FVIII activity in units stored at 20–24 °C before plasma freezing while most other studies have not observed such a loss. The differences in FVIII activity between our study and others may also be related to differences in plasma preparation protocols. In addition, in our study, we did not consider samples at time zero to assess baseline FVIII activity. Therefore, after finishing the study, 24 units of blood group A, B and O were kept at 1 to 6 °C and 20 to 24 °C for 1 hour before freezing. Measuring the FVIII activity of these units, we found that the baseline FVIII activity was the same at both temperatures (115.6 ±4.9 and 114.5±4.1). Finally, it should be noted that we evaluated four different units of each blood type under different conditions, so our results must be interpreted with caution.
It is recommended that a chromogenic assay is used to measure FVIII activity in manufactured concentrates14,15 and a clot-based assay for plasma samples. A number of studies have shown the importance of using the chromogenic assay for measuring FVIII activity in concentrates. Chandler et al. demonstrated that the chromogenic FVIII activity assay was the optimal method, showing good precision, the best overall correlation with other assays, and no interference from heparin, low-molecular-weight heparin, lepirudin, or lupus inhibitors14. In another study Barrowcliffe reported that most manufacturers of concentrates use the chromogenic method, which is more precise and is the reference method of the European Pharmacopoeia and the International Society on Thrombosis and Haemostasis (ISTH)15. None of these studies compared results from chromogenic and clotting assays of FVIII activity in cryoprecipitate. We found that, although there was a good correlation between the results of the one-stage clot-based and chromogenic assays, the absolute values were significantly different. While the chromogenic assay provides greater consistency of data, this is not in itself a justification to use this assay instead of the one-stage clotting assay. In any case, more studies need to be performed in order to evaluate the suitability of chromogenic assays for measuring FVIII in cryoprecipitate.
In conclusion, our results demonstrate that, with regards to improving the quality of cryoprecipitate deriving from our plasma processing procedure in Iran, freezing should begin within 6 hours from donation and blood should be kept at 1–6 °C, rather than at 20 to 24 °C, before the start of plasma freezing. In this study, although a good correlation was seen between the results of one-stage clot-based and chromogenic assays for measuring FVIII activity in cryoprecipitate, the absolute values were significantly different.
Acknowledgements
This study was supported by Iranian blood transfusion organization. We thank Dr. Amini and Dr. Ahmadi Negad for their technical assistance.
References
- 1.Farrugia A. Plasma for fractionation: safety and quality issues. Haemophilia. 2004;10:334–40. doi: 10.1111/j.1365-2516.2004.00911.x. [DOI] [PubMed] [Google Scholar]
- 2.Carlebjork G, Blomback M, Pihlstedt P. Freezing of plasma and recovery of factor VIII. Transfusion. 1986;26:159–62. doi: 10.1046/j.1537-2995.1986.26286152906.x. [DOI] [PubMed] [Google Scholar]
- 3.Myllyla G. Factors determining quality of plasma. Vox Sang. 1998;74:507–11. doi: 10.1111/j.1423-0410.1998.tb05466.x. [DOI] [PubMed] [Google Scholar]
- 4.Hellstern P, Bach J, Haubelt H, et al. The impact of the intensity of serial automated plasmapheresis and the speed of deep-freezing on the quality of plasma. Transfusion. 2001;41:1601–5. doi: 10.1046/j.1537-2995.2001.41121601.x. [DOI] [PubMed] [Google Scholar]
- 5.Carlebjork G, Blomback M, Akerblom O. Improvement of plasma quality as raw material for factor VIII: C concentrates. Vox Sang. 1983;45:233–42. doi: 10.1111/j.1423-0410.1983.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 6.Sward-Nilsson AM, Persson PO, Johnson U, Lethagen S. Factors influencing factor VIII activity in frozen plasma. Vox Sang. 2006;90:33–9. doi: 10.1111/j.1423-0410.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith JF, Ness PM, Moroff G, Luban NL. Retention of coagulation factors in plasma frozen after extended holding at 1–6 °C. Vox Sang. 2000;78:28–30. doi: 10.1159/000031145. [DOI] [PubMed] [Google Scholar]
- 8.Koster T, Blann AD, Briet E, et al. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;21:152–5. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 9.Kamphuisen PW, Eikenboom JC, Vos HL, et al. Increased levels of factor VIII and fibrinogen in patients with venous thrombosis are not caused by acute phase reactions. Thromb Haemost. 1999;81:680–3. [PubMed] [Google Scholar]
- 10.Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. 2000;83:5–9. [PubMed] [Google Scholar]
- 11.Cardigan R, Lawrie AS, Mackie IJ, Williamson LM. The quality of fresh-frozen plasma produced from whole blood stored at 4 °C overnight. Transfusion. 2005;45:1342–8. doi: 10.1111/j.1537-2995.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 12.Serrano K, Scammell K, Weiss S, et al. Plasma and cryoprecipitate manufactured from whole blood held overnight at room temperature meet quality standards. Transfusion. 2010;50:344–53. doi: 10.1111/j.1537-2995.2009.02441.x. [DOI] [PubMed] [Google Scholar]
- 13.Hughes JD, Macdonald VW, Hess JR. Warm storage of whole blood for 72 hours. Transfusion. 2007;47:2050–6. doi: 10.1111/j.1537-2995.2007.01429.x. [DOI] [PubMed] [Google Scholar]
- 14.Chandler WL, Ferrell CH, Lee J, et al. Comparison of three methods for measuring factor VIII levels in plasma. Am J Clin Pathol. 2003;120:34–9. doi: 10.1309/C8T8-YNB4-G3W4-5PRF. [DOI] [PubMed] [Google Scholar]
- 15.Barrowcliffe TW, Raut S, Sands D, Hubbard AR. Coagulation and chromogenic assays of factor VIII activity: general aspects, standardization, and recommendations. Semin Thromb Hemost. 2002;28:247–56. doi: 10.1055/s-2002-32658. [DOI] [PubMed] [Google Scholar]