Abstract
Background
Iatrogenic anaemia caused by repeated blood sampling to monitor laboratory parameters can contribute, particularly in neonates, to the need for transfusion. “Point of care” laboratory equipment uses smaller amounts of blood for analytic determinations and could, therefore, help to prevent secondary anaemia. In this study we compared the results of haematological parameters measured using a standard laboratory method and using a “point of care” micromethod, with the aim of validating the use of this latter method in clinical practice in neonatology.
Materials and methods
One hundred and fifty venous or capillary blood samples were taken from full-term or premature neonates 2–4 hours or 48 hours after birth. Each sample was processed by a standard haematology analyser and another micromethod instrument. Bland-Altman plots were constructed for each parameter and intra-class coefficients of correlation were calculated in order to evaluate the concordance between the two analysers.
Results
The concordance between the data obtained with the two analysers, expressed as the intra-class correlation, was 0.98 for white blood cell count, 0.97 for haemoglobin concentration, 0.96 for haematocrit, 0.95 for mean red cell volume and 0.98 for platelet count. The micromethod produced overestimated mean values for the leucocyte count (+1.27; p<0.001), haematocrit (+1.80; p<0.001) and platelet count (+13.55; p<0.001).
Conclusions
Overall, the concordance between the values obtained with the two analysers was high for each of the parameters taken into consideration. In the case of haemoglobin and leucocytes, give the high intra-class correlation and lack of systematic overestimation of one method over another, the micromethod guarantees a correct evaluation; however, despite the high intra-class correlations for platelet counts, the systemic error seems to suggest that the micromethod cannot guarantee an appropriate evaluation of this parameter.
Keywords: point of care, transfusion trigger, neonatology, neonatal anaemia, neonatal thrombocytopenia
Introduction
The “critical” neonate and the neonate with a low birth weight represent categories of patients with high transfusion needs1. More than 50% of paediatric patients in a neonatal intensive care unit receive red blood cell transfusions2.
Anaemia in premature neonates depends on blood volume at birth, on the rate of erythropoiesis and the amount of blood loss due to blood sampling, haemorrhage or haemolysis. The decision to transfuse must only be taken after all possible strategies have been used to guarantee a higher total blood volume at birth, to optimise erythropoiesis, and to limit iatrogenic anaemia3. Besides drugs that promote erythropoiesis, the main strategies that can be used to increase total volaemia at birth in neonates are to postpone clamping the umbilical cord by 30–120 sec and to minimise the volume of blood taken for laboratory blood tests3. The role that iatrogenic anaemia plays in necessitating red blood cell transfusion in the neonatal period is not negligible4. Transfusion therapy is more frequently needed in neonates with a very low weight at birth (<1,500 g), in whom a sample of 1 mL of blood often represents more than 1% of the total blood volume5. The volume of venous blood taken for blood-chemistry tests is the main factor that alone can contribute to and worsen anaemia in premature neonates; indeed the mean volume of blood sampled for diagnostic laboratory tests can range from 1.1 to 3.5 mL/kg/die3–5. It is common that the cumulative blood loss due to specimens taken during the first week of life equals or exceeds the neonate’s circulating blood volume. It is, therefore, imperative to try to minimise the blood lost due to sampling by using, for example, diagnostic tests based on micromethods that require less blood and by not withdrawing more blood than strictly necessary for the analyses required3–5. In recent years, technological advances and improvements in laboratory medicine have made it possible to carry out some first level analyses directly in the ward in which the patient is being cared for.
The aim of the introduction of “point of care” (POC) analysers was to carry out some tests more quickly than was possible by sending the sample for analysis in the hospital’s laboratory. A POC analyser is defined as any instrument that enables a test to be performed outside the laboratory6–7. The blood cell count can now be determined using a POC analyser8–9. The advantages of using POC equipment are, above all, that they require only small amounts of blood, they reduce the risk of iatrogenic anaemia, and the results are available quickly, since the test is carried out directly in the ward in which the patient is being treated. This implies a notable reduction in the time between blood sampling, performance of the analysis and the therapeutic intervention10. Such a reduction in the “turn around time” is a notable strong point of POC instruments not only in emergency and urgent situations, but also in the routine management of patients.
Guidelines on the use of POC analysers in haematology strongly recommend that only automated instruments that use primary samples should be employed, while semi-automated instruments, which require a dilution of the blood sample during the pre-analytic stage, should not be used6.
The purpose of this study was to compare the values of haematological parameters obtained with the standard method in use at the haematology laboratory with those obtained using a micromethod-based POC instrument, with the aim of validating the possible introduction of such an instrument in daily clinical practice in the field of neonatology, in order to minimise the risk of iatrogenic anaemia.
Materials and methods
Patients
Capillary blood samples (91 cases) or venous blood samples (59 cases) were taken 2–4 hours or 48 hours after birth from 150 neonates born at term or prematurely.
Following approval from the Ethics Committee of the “San Giovanni Calibita” Fatebenefratelli Hospital (Rome), demographic data and perinatal and maternal information (type of delivery, gestational age, birth weight, Apgar score) were collected for the patients enrolled in the study. All blood samples were taken after having obtained informed consent from the parents of each of the patients enrolled.
Standard method and micromethod for measuring the blood count
All capillary and venous blood samples were processed in a blood analyser habitually used in our laboratory (SysmexXE2100 [TOA Medical Electronics, Kobe, Japan]) for the determination of the complete blood count and differential counts of leucocytes, reticolocytes and erythroblasts. Ten microlitres of each whole blood sample were also analysed with a POC instrument, ABX-MicrosCRP200 (Horiba Medical, Montpellier, France), which uses a primary sample for the rapid measurement of the blood count through an impedancemetric method.
The ABX-MicrosCRP200 is a compact, completely automated blood analyser capable of determining the following parameters within 70 seconds: white blood cell (WBC) count, red blood cell (RBC) count, haemoglobin (Hb) concentration, haematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration(MCHC), red cell distribution width (RDW), platelet count (PLT), plateletcrit (PCT), mean platelet volume (MPV), platelet distribution width (PDW), and the percentages and absolute counts of lymphocytes, monocytes and granulocytes.
All the samples were analysed within 4 hours of having been taken. Both the analysers were regularly calibrated and underwent quality controls, as described by the manufacturers.
Statistical analysis
The data obtained were analysed using SPSS software (SPSS Inc., Chicago, IL, USA). In order to determine the concordance between the two analysers, Bland-Altman plots were constructed for each parameter and the intra-class coefficients of correlation (ICC) were calculated.
Results
The study was conducted on 150 neonates (81 males and 69 females). Of these, 60% were delivered by Caesarean section and 71% had an Apgar score at 5 minutes between 9 and 10. The mean gestational age was 38 weeks (range, 25.4 to 41.6 weeks), the mean weight at birth was 2,900 g (range, 468 g to 4,270 g) (Table I). Fifteen percent of the neonates weighed less than 1,500 g at birth.
Table I.
Characteristics at birth of the population of neonates enrolled in the study.
| Weight (g) | Gestational age (weeks) | ||
|---|---|---|---|
| Males (n=81) | Mean | 2,768 | 37 |
| SD | 841.8 | 3.6 | |
| Minimum | 734 | 27.0 | |
| Maximum | 4,270 | 41.4 | |
| Females (n=69) | Mean | 2,516.7 | 36.5 |
| SD | 903.3 | 3.7 | |
| Minimum | 468 | 25.4 | |
| Maximum | 4,160 | 41.6 | |
| All (n=150) | Mean | 2,900 | 38 |
| SD | 877.2 | 3.7 | |
| Minimum | 468 | 25.4 | |
| Maximum | 4,270 | 41.6 |
Legend:
SD = standard deviation.
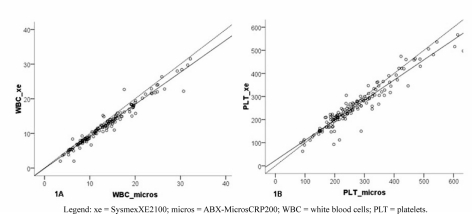
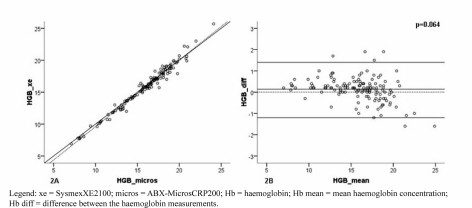
The concordance between the data obtained with the two analysers, expressed as the ICC (Table II), was 0.98 (95% CI: 0.95–0.99) for both the RBC count (Figure 1A) and the platelet count (95% CI: 0.96–0.98) (Figure 1B), 0.97 (95% CI: 0.96–0.98) for Hb concentration (Figure 2A), 0.96 for the Hct (95% CI: 0.93–0.98) and 0.95 for the MCV (95% CI: 0.93–0.96) (data not shown).
Table II.
Correlations between the results obtained with the standard method and the micromethod.
| 95% CI for the differences | Limits of concordance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | ICC | Mean | SD | SEM | Lower limit | Upper limit | p-value | Lower limit | Upper limit | External limits | |
| RBCx103/μL | 150 | 0.98 | 1.27 | 1.28 | 0.115 | 1.05 | 1.50 | 0.000 | −1.3 | 3.8 | 6% |
| RBC + NRBC | 150 | NC | 1.00 | 1.22 | 0.11 | 0.78 | 1.22 | 0.000 | −1.4 | 3.4 | 6% |
| Hb g/dL | 150 | 0.97 | 0.11 | 0.65 | 0.058 | −0.01 | 0.22 | 0.064 | −1.2 | 1.4 | 3% |
| Htc % | 150 | 0.96 | 1.80 | 2.82 | 0.255 | 1.30 | 2.31 | 0.000 | −3.8 | 7.4 | 5% |
| PLTx103/μL | 150 | 0.98 | 13.55 | 34.18 | 3.069 | 7.47 | 19.62 | 0.000 | −54.8 | 81.9 | 7% |
Legend:
ICC=intra-class correlation; Mean=difference between the means of the values; SD=standard deviation of the difference between the means of the values; SEM=standard error of the mean; p-value=p-value for the difference between the means; NRBC=erythroblasts; NC=not calculated.
Figure 1.
Concordance of the results obtained with the two different analysers for (A) white blood cell counts and (B) platelet counts.
Figure 2.
Concordance (A) and Bland-Altman correlation (B) between the results of haemoglobin concentration measured with the two different methods.
Overall, the difference between the mean values showed that the ABX-MicrosCRP200 analyser overestimated the RBC count (+1.27x103/μL; 95% CI: +1.05; +1.50; p<0.001), Hct (+1.80%; 95% CI: +1.30; +2.31; p<0.001) and platelet count (+13.55x103/μL; 95% CI: +7.47; +19.62; p<0.001) (Table II).
Our data did not show any statistically significant over- or under-estimation of Hb concentration (p=0.064) (Figure 2B, Table II) or MCV (data not shown).
The standard deviation of the difference between the values obtained with the two methods was 1.28 for the RBC count, 34.18 for the platelet count, and 0.65 for Hb concentration (Table II).
Conclusions
In recent years, POC instruments for the rapid determination of the blood count have provided considerable advantages for laboratory diagnostics. In the USA more than 20% of blood chemistry tests are now performed outside the hospital laboratory7. The Guidelines of the International Council for Standardization in Haematology (ICSH) express the hope that the technology and availability of POC for fast analysis of the blood count will become yet more widespread7.
The comparison between the haematology analysers conducted in this study was aimed at validating the possible introduction of the micromethod-based POC analyser into daily clinical practice, since this method uses a capillary sample of blood and, therefore, reduces the risk of iatrogenic anaemia among neonates.
The results obtained with the micromethod, compared with those of the conventional method, showed that the ABX-Micros analyser is sufficiently reliable for the measurement of Hb concentration, but that the RBC and platelet counts need to be interpreted with care.
The concordance between the two methods was high for each of the parameters considered.
The standard deviation of the difference between the means of the Hb concentration did not have an impact on the transfusion threshold1,11. In neonates, the administration of red cell concentrates is recommended when the neonate’s Hb concentration is below 10 g/dL, or below 12–13 g/dL in the first 24 hours of life or in the presence of cardiovascular or respiratory disease11.
In contrast, the standard deviation of the differences between the means of platelet count could be relevant as regards the evaluation of neonates with thrombocytopenia12. Platelet transfusion in neonates is currently not recommended if the platelet count is greater than 100,000/μL; if the platelet count is between 50,000/μL and 100,000/μL a transfusion should be carried out only in neonates with active bleeding; if the platelet count is between 30,000/μL and 50,000/μL, prophylactic transfusion should be considered in the following cases: (i) neonates with a birth weight 1,000 g in the first week of life, (ii) previous intraparenchymal/intraventricular haemorrhage (24–48 hours), (iii) presence of coagualation disorders, (iv) “critical” neonates, with sepsis or fluctuating blood pressure, (v) need to carry out invasive procedures. If the platelet count is below 20,000–30,000/μL, prophylactic transfusion should be considered in all cases12. Taking into account the transfusion threshold identified as optimal in the above mentioned recommendations, it is clear that a standard deviation of 34.1x103/μL in the difference between the mean values of platelet count could make a correct evaluation of the real count difficult, both for the purposes of deciding whether to give a transfusion, and for a correct estimate of the post-transfusion increase in platelet count.
The overestimation of the RBC count given by the ABX-MicrosCRP200 could be due, in part, to the interference of erythroblasts and could be clinically relevant, especially in leucopenic patients.
The ABX-MicrosCRP200 analyser’s ability to carry out a rapid blood count (70 seconds) can, however, be very important in a neonatal intensive care unit13. Indeed, the introduction of a POC analyser in a neonatal intensive care unit could lead to a reduction in iatrogenic anaemia among “critical” neonates10 and a consequent reduction of transfusion support with RBC4.
There are various advantages of using a POC system: the smaller blood sample (only 18 μL) necessary for the test, a significant reduction in the pre- and post-analytic error, immediate availability of results and, consequently, the possibility of rapid therapeutic intervention. These benefits largely outweigh any logistical-organisational problems that a laboratory must face (management of decentralised working sites and documentation, management and training of the staff carrying out the test) which could slow the spread of use of POC instruments in a hospital14.
Rapid measurement of haematological parameters (Hb and Hct) by POC analysers can increase safety and optimise the transfusion support also in adults during the intra-operative period15.
The recent recommendations of the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) on peri-operative transfusion in adult patients emphasised the need to exploit POC instruments provided that these are automated systems that do not require the dilution of the whole blood in the pre-analytic phase6,16.
Furthermore, a recent study in a large cohort of donors demonstrated that the use of a capillary blood test in pre-donation screening was an effective and safe approach in the process of selecting blood donors provided that the staff had sufficient dexterity in carrying out the capillary sampling17.
Our results are in accordance with those of other authors17–21, as far as regards the lack of significance of the overestimation of RBC counts and Hct by the micromethod. As far as regards the overestimation of the Hct, our data confirm already published information22–24 on the greater inaccuracy of the measurement of this parameter, which could be explained by the different technologies and methods used by the companies manufacturing blood counters and by the greater total variability (analytic and biological) compared to that of Hb. This is the reason why Hb is proposed as the main parameter to be used in clinical practice. For example, in 2008, the World Health Organization decided to consider Hb, and no longer Hct, as the most important parameter in the diagnosis of polycythemia vera25.
Compared to the findings of previously reported studies17–21, our results differ with regards to the platelet count, whose mean value was variably underestimated by the abovementioned studies both in neonates and in adult patients. This discrepancy could, hypothetically, be due to the fact that in our study we compared the performance of the two analysers on the same sample (venous or capillary) processing the same sample first on the POC instrument and then with the conventional analyser; this could have led to the formation of platelet aggregates in the samples of capillary blood.
Studies on larger populations of patients could clarify whether the differences observed between the performances of the two analysers for some parameters are due to poor standardisation of the technique of capillary blood sampling which, in a population of neonates, is still a critical phase.
It is to be hoped that, before introducing POC instruments such as the ABX-MicrosCRP200, each laboratory establishes the limits of acceptability of differences for each parameter between values obtained with the reference haematology laboratory analyser and the POC analyser. In fact, only in this way will it be possible to differentiate, in the population of patients (neonates or adults) in which the method is intended for use, which clinical decisions can be based on data obtained with the POC analyser from those for which it is necessary to use standard haematology analysers.
In conclusion, the data from this study, albeit collected in a relatively small number of patients, seem to demonstrate that the ABX-MicrosCRP200 analyser can definitely be of benefit as a POC analyser for initial screening evaluations in a neonatal intensive care unit, although it cannot completely replace a full blood count carried out using a traditional analyser.
Acknowledgements
The authors would like to thank Dr. Lidia Bonicalzi and Dr. Deborah Patti from Horiba ABX (Italy) for the scientific support supplied during this study.
References
- 1.Tripodi G, Antoncecchi S, Fanetti G, et al. Recommendations on transfusion therapy in Neonatology. Blood Transfus. 2006;4:158–80. doi: 10.2450/2015.0113-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 3.Bell EF. When to transfuse preterm babies. Arch Dis Child Fetal Neonatal Ed. 2008;93:F469–F473. doi: 10.1136/adc.2007.128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widness JA, Madan A, Grindeanu LA, et al. Treatment and prevention of neonatal anaemia. Neoreviews. 2008;9:526–33. doi: 10.1542/neo.9-11-e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nexo E, Christensen NC, Olesen H. Volume of blood removed for analytical purposes during hospitalization of low-birthweight infants. Clin Chem. 1981;27:759–61. [PubMed] [Google Scholar]
- 6.Briggs C, Guthrie D, Hyde K, et al. Guidelines for point-of-care testing: haematology. Br J Haematol. 2008a;142:904–15. doi: 10.1111/j.1365-2141.2008.07274.x. [DOI] [PubMed] [Google Scholar]
- 7.Briggs C, Carter J, Lee SH, et al. ICSH Guideline for worldwide point-of-care testing in haematology with special reference to the complete blood count. Int J Lab Hematol. 2008;30:105–16. doi: 10.1111/j.1751-553X.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 8.Grotto HZW, Borba R, Noronha JFA, Bertozzi LC. Measurement of C-reactive protein by Micros CRP: its use in an emergency department. Lab Hematol. 2002;8:1–5. [Google Scholar]
- 9.Cals JWL, Schot MJC, de Jong SAM, et al. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Ann Fam Med. 2010;8:124–33. doi: 10.1370/afm.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinds LE, Brown CL, Clark SJ. Point of care estimation of haemoglobin in neonates. Arch Dis Child Fetal Neonatal Ed. 2007;92:378–80. doi: 10.1136/adc.2006.107771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009;7:132–50. doi: 10.2450/2009.0005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makhoul IR, Smolkin T, Zinder O, et al. Fast bedside measurement of blood count and C-reactive protein in neonates with suspected late-onset sepsis. Acta Pædiatrica. 2005;94:960–3. doi: 10.1111/j.1651-2227.2005.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 14.Nichols JH. Point of care testing. Clin Lab Med. 2007;27:893–908. doi: 10.1016/j.cll.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Scottish Intercollegiate Guidelines Network. Perioperative blood transfusion for elective surgery. A national clinic guideline. Oct, 2001. [Last accessed on 25/03/2010]. Available at: http// www.sign.ac.uk/pdf/sign54.pdf.
- 16.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus. 2011;9:189–217. doi: 10.2450/2011.0075-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierelli L, Zennaro F, Patti D, et al. Evaluation of the analytical performances of a portable, 18-parameter hemometric system using capillary blood samples for blood donor enrolment. Vox Sang. 2010;98:145–50. doi: 10.1111/j.1423-0410.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 18.Daae LNW, Hallerud M, Halvorsen S. A comparison between haematological parameters in “capillary” and venous blood samples from hospitalized children aged 3 months to 14 years. Scand J Clin Lab Invest. 1991;51:651–4. doi: 10.3109/00365519109104576. [DOI] [PubMed] [Google Scholar]
- 19.Ozbek N, Gurakan B, Kayiran SM. Complete blood cell counts in capillary and venous blood of healthy term newborns. Acta Hematol. 2000;103:226–8. doi: 10.1159/000041056. [DOI] [PubMed] [Google Scholar]
- 20.Kayiran SM, Ozbek N, Turan M, Gurakan B. Significant differences between capillary and venous complete blood counts in the neonatal period. Clin Lab Haematol. 2003;25:9–16. doi: 10.1046/j.1365-2257.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 21.Schalk E, Heim MU, Koenigsmann M, Jentsch-Ullrich K. Use of capillary blood count parameters in adults. Vox Sang. 2007;93:348–53. doi: 10.1111/j.1423-0410.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacob G, Raj RR, Ketch T, et al. Postural pseudoanaemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80:611–4. doi: 10.4065/80.5.611. [DOI] [PubMed] [Google Scholar]
- 23.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123:333–7. doi: 10.1542/peds.2008-2654. [DOI] [PubMed] [Google Scholar]
- 24.Bosshart M, Stover JF, Stocker R, et al. Two different hematocrit detection methods: different methods, different results? BMC Research Notes. 2010;3:65–71. doi: 10.1186/1756-0500-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]