Introduction
Deferiprone is an effective oral iron chelator which plays a particularly important role in the removal of cardiac iron burden in thalassaemic patients with transfusional iron overload1–5. Agranulocytosis, of uncertain pathogenesis, is its most important and severe side effect, reported in less than 2% of cases. Only a few such cases have so far been described in detail6–11: of these, the most recently reported was a case of fatal secondary agranulocytosis occurring in a child affected by Blackfan-Diamond anaemia11.
In this report we describe a case of agranulocytosis due to deferiprone in a child with β-thalassaemia treated at our Centre.
Case description
A 5-year old girl with β°/β+ thalassaemia (cod39/IVS-I-110), on a chronic transfusion regimen since the age of 3, started chelation with desferrioxamine 25–35 mg/kg/day at the age of 4 years. Nevertheless, ferritin values progressively increased (median 1,700 ng/mL) and the liver iron overload, measured with a superconducting quantum interference device (SQUID), progressed from moderate to severe. At 5 years of age, the child was started on intensive, combined therapy consisting of desferrioxamine 30 mg/kg/day for 4–5 days/week and deferiprone 75 mg/kg/day, aimed at reducing the iron overload. The white blood cell (WBC) count, meticulously monitored once a week as recommended, showed steadily normal values of neutrophils (3,200–6,000/mm3). Seventy-five days after the start of deferiprone (4 days after a normal blood count showing a WBC count of 5,840/mm3, neutrophils: 2,800/mm3), the girl developed a fever up to 38.5–39 °C and an evident cough. The child was seen at home by the family paediatrician and given amoxicillin-clavulanate; deferiprone was discontinued. After 72 hours of fever with peaks reaching 40 °C, the girl came to our Centre for a clinical check-up. The blood count showed: haemoglobin 5.8 g/dL, WBC 1,880/mm3, neutrophils 40/mm3, platelets 529,000/mm3, and serum C-reactive protein (CRP) 8 mg/dL (normal values <0.5). Chest X-ray was negative.
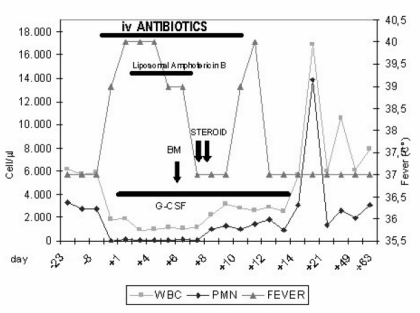
The child was admitted to hospital and started intravenous therapy with amikacin (20 mg/kg/day), ceftazidime (100 mg/kg/day) and subcutaneous filgrastim (human granulocyte colony-stimulating factor, G-CSF) 5 μg/kg/day (Figure 1).
Figure 1.
The patient’s clinical evolution: blood counts, fever and therapy.
The girl’s fever peaked up to 40 °C 3–4 times a day accompanied by shivering and responded poorly to paracetamol. After 2 days, vancomycin (40 mg/kg/die) was added. As the high fever persisted with progressively increasing values of CRP (up to 17 mg/dL) in spite of the very broad spectrum antibiotic therapy, on day 4 liposomal amphotericin B (2 mg/kg/day) was added (Figure 1). The results of blood cultures and tests for galactomannan antigen were negative; the main viral infections were excluded by specific serological tests for cytomegalovirus, Epstein-Barr virus and parvovirus B19.
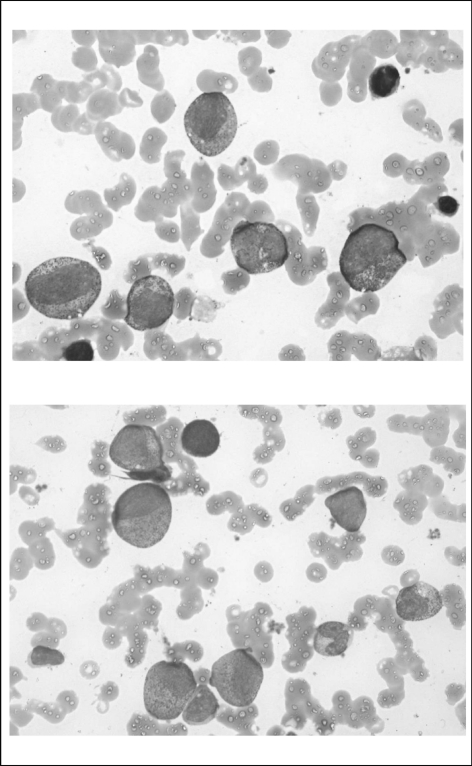
On day 6, bone marrow aspiration was performed. Cytomorphological examination showed an absence of atypical cells, normal cellularity with a block of myeloid differentiation at the stage of promyelocytes. Differentiation of erythroid lineage was normal, although some features of dyserythropoiesis were detectable. The number and morphological aspects of megakaryocytes were normal (Figure 2).
Figure 2.
Bone marrow on day +6 after admission. Images show normal cellularity with a block of myeloid differentiation at the stage of promyelocytes.
In vitro, clonogenic assay of mononuclear cells (MNC) from bone marrow, isolated on a Ficoll-Hypaque (Pharmacia, Piscataway, NY, USA) density gradient, showed completely normal proliferative ability of haematopoietic progenitor cells of both myeloid and erythroid lineages: 136 CFU-GM/105 MNC (range 35–190) and 80 BFU-E/105 MNC (range 30–95), respectively. On days 7 and 8, the patient presented with oedema of the lips and vomiting during infusion of liposomal amphotericin B, interpreted as an allergic reaction to the drug. For this reason, two consecutive doses of hydrocortisone (10 mg/kg) were administered intravenously and amphotericin B was discontinued.
The day after hydrocortisone administration, the child’s temperature returned to normal, with a progressive recovery of neutrophil counts (Figure 1).
On day 10, the child presented again with fever (3–4 peaks/day up to 40 °C), without significantly elevated inflammatory markers. On day 12, interpreting the fever as related to the massive broad spectrum antibiotic therapy, all antibiotics were discontinued and the girl became completely afebrile.
Therapy with G-CSF was continued until day 14, when the neutrophil count was 2,850/mm3 and the child was discharged from hospital. Administration of deferiprone was definitely discontinued; desferrioxamine was continued alone. At a 12-month follow-up the child is in good condition, maintaining neutrophil counts in the normal range.
Discussion
Agranulocytosis, a well-known, serious side effect of deferiprone, has been observed from 6 weeks to 21 months after the beginning of deferiprone administration12. Its frequency has been reported to range from 0.5 to 3.6% in different studies, while the frequency of neutropenia is up to 8.5%13–15. In a 4-year multicentre, prospective study of the safety and efficacy of deferiprone in patients with thalassaemia major during 531 patient-years by Cohen et al. in 200313, the rates of agranulocytosis (neutrophil <0.5x109/L) and milder forms of neutropenia (0.5–1.5x109/L) were 0.2 and 2.8 per 100 patient-years, respectively. The frequency of neutropenia was also described to be significantly higher in non-splenectomised patients aged less than 18 years15. Combined treatment has been associated with frequencies of agranulocytosis and neutropenia similar to those reported for deferiprone monotherapy16,17, although in a multicentre randomised trial18 the alternating chelation scheme with desferrioxamine and deferiprone did not result in agranulocytosis, whereas in the deferiprone group 3.5% of patients developed this side effect. Maggio et al.18 suggested that this reduction in the frequency of agranulocytosis during sequential treatment could be due to decreased exposure of the bone marrow to the drug. However, more data are needed to elucidate the mechanisms at the basis of this phenomenon and we are aware of ongoing studies on this topic.
The appearance of agranulocytosis in our child, as in the above-mentioned patient with Blackfan-Diamond anaemia, was abrupt11.
Four days after a normal blood count our patient developed a fever. Given the normal blood count found 4 days earlier, the patient was initially managed at home by a family doctor with oral antibiotics only, and agranulocytosis was demonstrated 2 days after the onset of fever. It is likely that neutropenia coexisted with the appearance of fever. In this instance, the previous blood count gave false reassurance.
We suggest that weekly blood counts, which are burdensome for both patients and health structures, might not be useful. Perhaps a blood count performed as soon as fever appears could be enough or even better to guarantee immediate and appropriate treatment for agranulocytosis.
A literature search revealed that although the risk of deferiprone-related agranulocytosis is well recognised, the pathogenic mechanism remains unknown. The effect does not seem to be dose-dependent. Various hypotheses have been extensively explored in a recent paper19. Depletion of iron and other ions (particularly copper), due to direct toxicity of the chelating drug on colony-forming units in the bone marrow, and an immune-mediated mechanism have been implicated.
In our case, the block of the myeloid lineage at the stage of promyelocytes, the normal functional capacity of hematopoietic progenitor cells in vitro, and the recovery of neutrophil counts just 1 day after the administration of steroids suggest an immune mechanism i.e. an immunological block during myeloid differentiation at the stage of promyelocytes. Unfortunately, we did not test for the presence of an inhibitor by incubating a normal donor’s haematopoietic mononuclear cells with the patient’s serum in vitro. Moreover, it should be considered that the recent treatment with G-CSF could have influenced the results of our morphological and functional tests. It would be interesting to investigate other cases of agranulocytosis due to deferiprone with specific studies on serum and bone marrow. It would also be relevant to collect data systematically on past and future cases of severe neutropenia occurring during deferiprone treatment. A better understanding of agranulocytosis, a rare but potentially fatal phenomenon, would lead to optimal use of deferiprone and would allow patients to benefit from treatment with this drug, which is recognised as an important and efficient chelating agent alone and in combination with desferrioxamine.
References
- 1.Anderson LJ, Wonke B, Prescott E, et al. Comparison of effects of oral deferiprone and subcutaneous desferioxamine on myocardial iron concentrations and ventricular function in beta-thalassemia. Lancet. 2002;360:516–20. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 2.Piga A, Gaglioti C, Fogliacco E, et al. Comparative effects of deferiprone and deferoxamine on survival and cardiac disease in patients with thalassemia major: a retrospective analysis. Haematologica. 2003;88:489–96. [PubMed] [Google Scholar]
- 3.Pennell DJ, Berdoukas V, Karagiorga M, et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–44. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 4.Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006;107:3733–7. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- 5.Modell B, Khan M, Darlison M, et al. Improved survival of thalassemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffbrand AV, Bartlett AN, Veys PA, et al. Agranulocytosis and thrombocytopenia in patient with Blackfan-Diamond anaemia during oral chelator trial. Lancet. 1989;2:457. doi: 10.1016/s0140-6736(89)90641-7. [DOI] [PubMed] [Google Scholar]
- 7.Al-Refaie FN, Wilkes S, Veys P, et al. Agranulocytosis in a patient with thalassaemia major during treatment with the oral iron chelator, 1,2-dimethyl-3-hydroxypyrid-4-one. Acta Haematol. 1993;89:86–90. doi: 10.1159/000204494. [DOI] [PubMed] [Google Scholar]
- 8.Al-Refaie FN, Wonke B, Hoffbrand AV. Deferiprone associated myelotoxicity. Eur J Haematol. 1994;53:298–301. doi: 10.1111/j.1600-0609.1994.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 9.Pati HP, Choudhry VP. Deferiprone (L1) associated neutropenia in beta thalassemia major: an Indian experience. Eur J Haematol. 1999;63:267–8. doi: 10.1111/j.1600-0609.1999.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 10.Sheikh-Taha M, Koussa S, Inati A, Taher A. Febrile neutropenia and hemorrhagic stroke in a thalassemia major patient. Hemoglobin. 2007;31:499–501. doi: 10.1080/03630260701641211. [DOI] [PubMed] [Google Scholar]
- 11.Henter JI, Karlén J. Fatal agranulocytosis after deferiprone therapy in a child with Diamond-Blackfan anaemia. Blood. 2007;109:5157–9. doi: 10.1182/blood-2007-02-065805. [DOI] [PubMed] [Google Scholar]
- 12.Olivieri MF. Long term therapy with deferiprone. Acta Haematol. 1996;95:37–48. doi: 10.1159/000203854. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AR, Galanello R, Piga A, et al. Safety and effectiveness of long term therapy with the oral iron chelator deferiprone. Blood. 2003;102:1583–7. doi: 10.1182/blood-2002-10-3280. [DOI] [PubMed] [Google Scholar]
- 14.Al-Refaie FN, Hershko C, Hoffbrand AV, et al. Results of long term deferiprone L1 therapy: a report by the International Study Group on Oral Iron Chelators. Br J Haematol. 1995;91:224–9. doi: 10.1111/j.1365-2141.1995.tb05274.x. [DOI] [PubMed] [Google Scholar]
- 15.Ceci A, Baiardi P, Felisi M, et al. The safety and effectiveness of deferiprone in a large-scale, 3 year study in Italian patients. Br J Haematol. 2002;118:330–6. doi: 10.1046/j.1365-2141.2002.03554.x. [DOI] [PubMed] [Google Scholar]
- 16.Daar S, Pathare AV. Combined therapy with desferrioxamine and deferiprone in β-thalassemia major patients with transfusional iron overload. Ann Hematol. 2006;85:315–9. doi: 10.1007/s00277-005-0075-z. [DOI] [PubMed] [Google Scholar]
- 17.Telfer PT, Warburton F, Christou S, et al. Improved survival in thalassemia major patients on switching from desferioxamine to combined chelation therapy with desferioxamine and deferiprone. Haematologica. 2009;94:1777–8. doi: 10.3324/haematol.2009.009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggio A, Vitrano A, Capra M, et al. Long-term sequential deferiprone-desferioxamine versus deferiprone alone for thalassemia major patients: a randomized clinical trial. Br J Haematol. 2009;145:245–54. doi: 10.1111/j.1365-2141.2009.07609.x. [DOI] [PubMed] [Google Scholar]
- 19.Pontikoglou C, Papadaki HA. Idiosyncratic drug-induced agranulocytosis: the paradigm of deferiprone. Hemoglobin. 2010;34:1–14. doi: 10.3109/03630269.2010.484791. [DOI] [PubMed] [Google Scholar]