Summary
Despite the diverse biological pathways known to be regulated by ubiquitylation, global identification of substrates that are targeted for ubiquitylation has remained a challenge. To globally characterize the ubiquitin-modified proteome (ubiquitinome), we utilized a monoclonal antibody that recognizes diglycine (diGly) containing isopeptides following trypsin digestion. We identify ~19,000 diGly modified lysine residues within ~ 5000 proteins. Using quantitative proteomics we monitored temporal changes in diGly site abundance in response to both proteasomal and translational inhibition indicating both a dependence of on-going translation to observe alterations in site abundance and distinct dynamics of individual modified lysines in response to proteasome inhibition. Further, we demonstrate that quantitative diGly proteomics can be utilized to identify substrates for cullin-RING ubiquitin ligases. Interrogation of the ubiquitinome allows for not only a quantitative assessment of alterations in protein homeostasis fidelity, but also identification of substrates for individual ubiquitin pathway enzymes.
Introduction
The proteome is constantly remodeled to meet the changing environmental challenges of the cell. Protein degradation facilitated by the ubiquitin-proteasome system (UPS) is a major contributor to proteome remodeling. In this pathway, ubiquitin is activated and transferred to substrates via an E1-E2-E3 cascade (Ye and Rape, 2009). The chemical properties of the isopeptide bond formed between the C-terminal glycine in ubiquitin and the ε-amino group of lysine (Lys) residues in substrates provides a route for detection of ubiquitylated targets by mass spectrometry, as trypsinolysis of ubiquitin conjugates yields a characteristic “diGly remnant” due to cleavage of the C-terminal Arg-Gly-Gly sequence of ubiquitin (Peng et al., 2003).
It is convenient to consider two major classes of UPS targets: 1) regulatory substrates that undergo programmed ubiquitylation during a physiological process, and 2) quality control substrates that undergo ubiquitylation in response to misfolding, inappropriate complex formation, or aggregation. Signal-dependent regulatory ubiquitylation often results in the complete degradation of the target protein, eliminating its function. In contrast, quality control proteolysis generally affects only the fraction of protein that is defective. Errors in co-translational folding of proteins or translation may account for a significant fraction of the flux through the UPS (Schubert et al., 2000; Vabulas and Hartl, 2005).
It is currently thought that a wide cross-section of the proteome is subject to ubiquitin modification at some point during its lifetime. As such, there are two central challenges facing the field. First, a complete and quantitative description of the ubiquitinome – the array of proteins that are modified by the ubiquitin system as well as the actual site of modification – requires a means by which to detect, catalog, and quantify individual ubiquitylation events on proteins. Previous studies have focused on either the use of ubiquitin binding domains/antibodies or overexpression of epitope-tagged ubiquitin in an attempt to capture ubiquitylated proteins for identification by mass spectrometry (Danielsen et al., 2011; Matsumoto et al., 2005; Meierhofer et al., 2008; Peng et al., 2003; Tagwerker et al., 2006). However, the low occupancy of ubiquitylation challenges detection of endogenously modified proteins in the absence of overexpression of either ubiquitin or substrate. Advances in mass spectrometry and enrichment strategies, including affinity-capture of the diGly remnant, have assisted in the identification of a greater number of sites, with recent reports identifying 753 and 374 ubiquitylation sites (Danielsen et al., 2011; Xu et al., 2010). However, these studies relied upon exogenous expression of epitope-tagged ubiquitin, possibly subverting endogenous ubiquitin modification pathways. Despite these advances, the overall number of modification sites is small in comparison to the extent of acetylation and phosphorylation (Choudhary et al., 2009; Huttlin et al., 2010; Olsen et al., 2010), and it is unclear the extent to which ubiquitin overexpression affects the occupancy and specificity of ubiquitylation.
The second central challenge for the field is matching ubiquitylation targets with the vast array of ubiquitylation machinery encoded by eukaryotic genomes. The majority of substrates for E3s have been identified based on a physical interaction between the E3 and the substrate. While mutational analysis is most often used to identify candidate ubiquitylation sites in targets, this approach does not always provide a direct route to the actual sites of endogenous ubiquitylation in vivo, due to unmasking of cryptic sites and effects of overexpression.
Here, we employ an improved method for antibody-based capture of endogenous diGly-containing peptides to identify ~19,000 ubiquitylation sites in ~5000 proteins, and to quantitatively monitor temporal changes in the ubiquitinome in response to proteasome inhibition. This analysis reveals both increased ubiquitylation of proteasome targets and a loss of ubiquitin from a cohort of putatively monoubiquitylated proteins, presumably as a response to ubiquitin depletion. Surprisingly, detection of a large cross-section of the ubiquitinome upon proteasome inhibition requires on-going translation. We also demonstrate the utility of diGly capture for identification of cullin-RING ubiquitin ligase (CRL) substrates, and demonstrate that ubiquitin depletion promotes charging and transfer of the ubiquitin-like (UBL) protein NEDD8 by the ubiquitin conjugating machinery, thereby altering the targeting specificity of this UBL. Using a multi-classifier approach on merged quantitative data from multiple experiments, we categorize individual modified lysine residues into putative classes representing distinct functional outcomes. This dataset represents a powerful resource for the identification and classification of ubiquitin-modified lysine residues in both known UPS substrates and newly identified substrates allowing for facile future interrogation of individual site utilization.
Results
Characterization of the ubiquitinome in response to proteasome inhibition
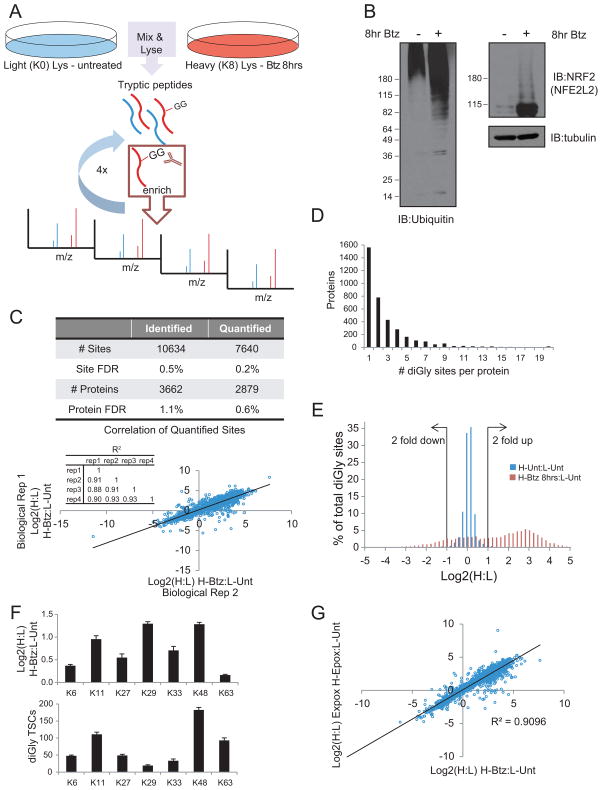
We combined a newly-developed monoclonal antibody that specifically recognizes the diGly remnant of ubiquitylated proteins resulting from trypsinolysis with metabolic labeling in cell culture to identify and quantify ubiquitylated proteins and their sites of modification on a global scale (Figure 1A, Supplemental Methods). Four biological replicate experiments were performed wherein HCT116 cells were metabolically labeled with heavy lysine (K8; Lys + 8 Da shift) and treated with 1μM bortezomib (Btz) for 8 hrs, and subsequently mixed with an equal amount of untreated cells cultured in light (K0) media (Figure 1A). As expected, both bulk ubiquitylated proteins and the well-characterized UPS substrate, NRF2 (also called NFE2L2) (Figure S1A) accumulated after Btz treatment (Figure 1B). Following mixing, cells were lysed in denaturing buffer to block deubiquitylating enzyme activity, and tryptic diGly-containing peptides were isolated by 4 sequential α-diGly immunoprecipitations for each biological replicate. In total, 10634 unique sites in 3662 proteins were identified in this experiment, of which 7640 unique sites in 2879 proteins were quantified (Figure 1C, Table S1,2, and Supplemental Methods). While a high correlation in the heavy-to-light (H:L) ratio of individual modified sites was observed between replicate experiments (Figure 1C), a substantial number of peptides were identified only in a single replicate experiment (Figure S1B). Multiple ubiquitylation sites were found in ~60% of the proteins identified, with a small fraction (~4%) containing >10 modified sites (Figure 1D). Interestingly, for those proteins containing multiple sites, all sites were not regulated similarly upon proteasome inhibition. In total, of the 962 proteins with three or more sites that were quantified, 13% contain individual sites that both increase and decrease by greater than 2-fold. For example, while 8 diGly-containing peptides were quantified for an alpha subunit of the 20S proteasome (PSMA4), 5 increased greater than 2-fold, two decreased greater than 2-fold, and one was unchanged in response to Btz (Table S2), consistent with the idea that not all ubiquitylation events on target proteins are regulated identically. In total, ~58% of the quantified sites increased in abundance by more than 2-fold in response to Btz treatment, whereas ~13% decreased more than 2-fold in abundance (Figure 1E). Consistent with previous results in yeast (Xu et al., 2009), the abundance of K11, K29, and K48 linkages were increased by greater than 2-fold after proteasome inhibition whereas the levels of K63 linkages were largely unaffected (Figure 1F).
Figure 1. Overview of diGly proteomic enrichment strategy.
A) Schematic overview of diGly peptide enrichment protocol.
B) Extracts from HCT116 cells treated as indicated were immunoblotted with α-ubiquitin, α-tubulin or α-NRF2.
C) The total number of diGly-modified lysines (sites) and proteins identified and quantified from four biological replicate experiments are shown with the determined false discovery rate. The correlation of the log2 ratios for all quantified peptides after 8hr Btz treatment between two biological replicates is plotted (inset: correlation coefficients).
D) Distribution of the sites per protein observed from diGly enrichment after 8hr Btz treatment.
E) H:L log2 ratios of all quantified diGly-containing peptides from untreated (blue bars) or 8hr Btz treated (red bars) cells.
F) Log2 ratio (top) and TSCs (bottom) of ubiquitin-derived diGly peptides after 8hr Btz treatment. Each bar represents the average log2 ratio or TSC from all quantified diGly peptides (Error bars: SEM, n=4).
G) Log2 ratios of H:L (treated:untreated) for sites quantified in both Btz and epoxomicin experiments.
The observed changes in site occupancy were not specific to Btz, as 92% of the quantified sties that increased by >2-fold with Btz also increased by more than 2-fold in response to a proteasome inhibitor with distinct active site specificity, epoxomycin (Figure 1G, Table S3), including ubiquitin linkages themselves and known ubiquitin modified proteins (Figure S1C,D). Further, the identified sites were not cell type specific as greater than 70% of sites identified in 293T cells were also identified in HCT116 cells (Figure S1E, Table S2).
Specificity of UBL usage in formation of the diGly proteome
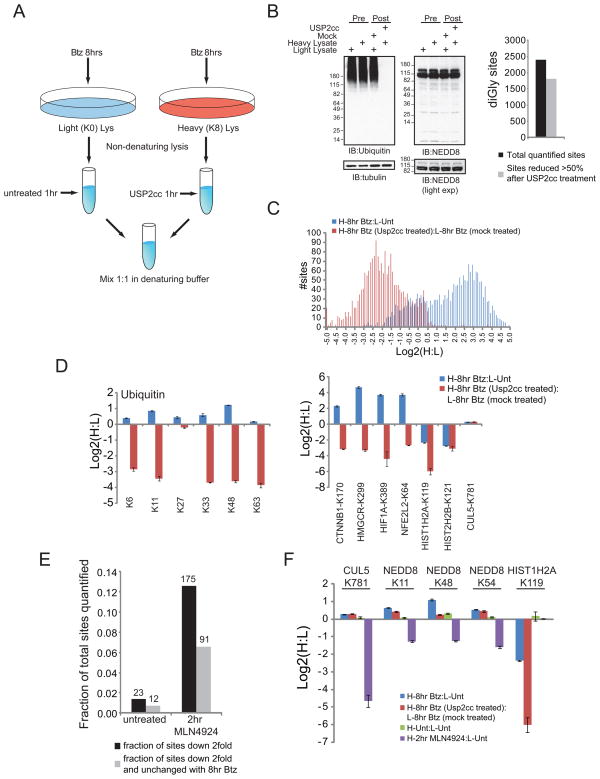
Like ubiquitin, two related UBLs - NEDD8 and ISG15 – contain C-terminal diGly motifs generated by trypsin cleavage, and therefore the diGly-modified proteome represents a composite of proteins modified by these 3 UBLs. However, consistent with ISG15 expression occurring only in response to interferon α/β (Skaug and Chen, 2010), it is undetectable in unstimulated HCT116 cells by immunoblotting but readily detectable upon interferon stimulation. Moreover, only 17 ISG15-derived diGly-modified peptide total spectral counts (TSCs) were detected in the more than 720,000 diGly-modified peptide TSCs observed in this study, suggesting ISG15 does not significantly contribute to the diGly-sites we observe (Figure S2). To distinguish the ubiquitin and NEDD8 proteomes, the catalytic domain of ubiquitin specific protease 2 (USP2cc) was used to strip ubiquitin from target proteins (Figure 2A). As expected, addition of USP2cc to extracts led to a complete loss of ubiquitin immunoreactivity, with no effect on NEDD8 immunoreactivity (Figure 2B) or the quantity of neddylated CUL5 (K781) (Figure 2D, right). Treatment with USP2cc prior to α-diGly enrichment resulted in at least a 50% reduction in the observed H:L ratio found with Btz treatment for 75% of quantified peptides (Figure 2B,C, S2C, Table S3), including known poly- and monoubiquitylated proteins (Figure 2D, right). Thus, the vast majority of observed diGly-modified peptides are the result of ubiquitylation as opposed to neddylation. With the exception of K27, all diGly-containing sites on ubiquitin itself were reduced more than 4-fold upon USP2cc treatment, largely confirming the linkage non-specificity of USP2cc (Figure 2D, left).
Figure 2. Determination of non-ubiquitin contribution to diGly sites.
A) Schematic of experiment.
B) Left, extracts from 8 hr Btz treated cells before and after mock or USP2cc treatment were immunoblotted with the indicated antibodies. Right, the total number of diGly sites (black bar) after Btz treatment and the number of sites in which their log2 ratio was decreased greater than 50% with USP2cc (grey bar).
C) Log2 ratios of all quantified diGly peptides from Btz treated cells either untreated (blue bars) or treated (red bars) with USP2cc.
D) Log2 ratios of all ubiquitin diGly sites (left) and representative known diGly modified proteins (right) from Btz treated heavy cells mixed with untreated light cells (blue bars) or light Btz treated, mock incubated and heavy Btz treated cells incubated with USP2cc post-lysis (red bars).
E) The fraction of the total quantified diGly sites with log2 ratios less than −1.0 (black bars) that were also unchanged after Btz treatment (grey bar) from cells treated as indicated.
F) Log2 ratios of representative peptides either known to be neddylated (CUL5) or putatively neddylated (NEDD8) from cells treated with Btz for 8 hours (blue bars), treated with Btz for 8 hours and subsequently treated with USP2cc post-lysis (red bars), untreated (green bars), or treated with MLN4924 for 2 hours (purple bars).
All error bars represent the SEM of multiple MS1 quantifications for the indicated site.
The finding that K27-linked ubiquitin was resistant to USP2cc, together with the potential protection of ubiquitin chains from cleavage by endogenous ubiquitin-binding proteins, suggested that this analysis may under-represent the ubiquitylated portion of the diGly-modified proteome. Therefore, as an alternative strategy we utilized the NEDD8 activating enzyme (NAE) inhibitor MLN4924 (Soucy et al., 2009) to estimate the portion of the diGly-containing proteome that is dependent upon NAE activity. Heavy, (K8) HCT116 cells were treated with MLN4924 for 2 hrs and mixed with untreated K0-labeled cells prior to lysis. Less than 2% of quantified peptides from untreated cells were decreased greater than 2-fold compared with 12% for MLN4924 treated cells (Figure 2E). The best characterized role for neddylation is the activation of the cullin-RING ubiquitin ligases (CRLs) by modification of a single conserved residue within the cullin subunit (Bedford et al., 2011). Thus, treatment with MLN4924 results in both the de-modification of neddylated proteins, and the inactivation of CRLs (Bennett et al., 2010; Soucy et al., 2009). This complicates the analysis because a reduction in the amount of a diGly-modified protein upon MLN4924 treatment could reflect either target neddylation, or ubiquitylation by a CRL. Because the level of endogenously neddylated cullins is insensitive to proteasome inhibition (Figure 2D CUL5_K781 site, S2A) we classified the diGly-containing proteome into: 1) peptides that either increased or decreased greater than 2-fold in response to 8 hr Btz treatment, indicating that they were modified by ubiquitin and 2) those that were unchanged. Of the peptides that decreased in response to 2 hr MLN4924 treatment, ~50% were unchanged with Btz treatment, suggesting that a maximum of 6% of the diGly-modified proteome is a result of NAE-mediated neddylation (Figure 2E). By way of example, the abundance of the known neddylation site K781 in CUL5 is unaffected by both Btz and USP2cc treatments, but was reduced greater than 16-fold upon 2hr MLN4924 treatment (Figure 2F). Three separate sites on NEDD8 itself followed this same trend, indicating that NEDD8 can either be poly-neddylated, or is ubiquitylated in a CRL-dependent manner (Figure 2F). In comparison, the known ubiquitin-modified site on Histone H2A is reduced by both Btz and USP2cc treatment but unaffected by MLN4924 addition (Figure 2F).
Temporal control of diGly site occupancy upon proteasome inhibition
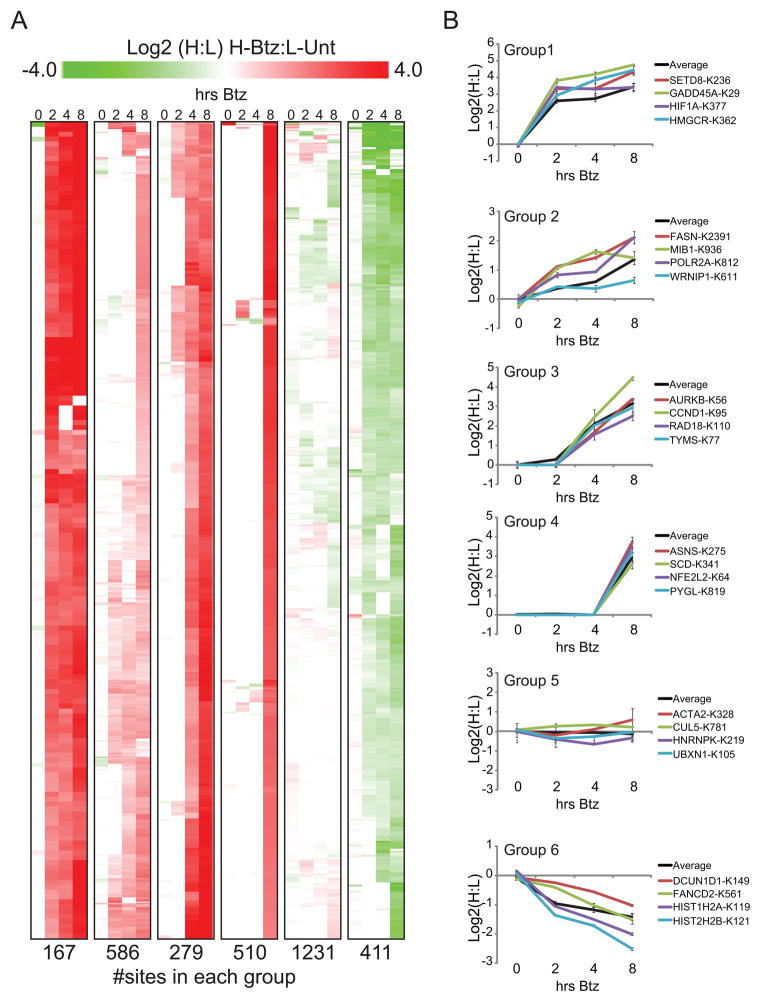
To begin to distinguish between regulatory ubiquitylation and quality control ubiquitylation, we classified ubiquitylation sites into those which accumulate rapidly upon proteasome inhibition, reflective of a short half-life regulatory protein targeted via a physiological response, and those that accumulate only after prolonged Btz-induced proteotoxic stress, indicative of a quality control substrate. Heavy (K8) Btz-treated HCT116 cells over an 8 hour time course were mixed with light (K0) untreated cells prior to diGly enrichment. Btz treatment resulted in a time-dependent increase in ubiquitin immunoreactivity as well as accumulation of the known UPS target HIF1α (Figure S1A, S3A). Each diGly-modified peptide was quantified at each time point, and only those sites which we quantified at least twice within a single time point or throughout the time-course (3184 sites) were selected for further analysis. As expected, the abundance of diGly peptides corresponding to K6, K11, K27, K33, and K48 from ubiquitin increased by ~2-fold after only 2 hours of Btz treatment (Figure S3B). In contrast, the diGly peptide corresponding to K63 on ubiquitin did not increase in abundance throughout the time course, consistent with its proteasome-independent role. Using Btz response curves, sites were separated into 6 groups using K-means clustering (Figure 3A). Greater than 72% of proteins had modified lysines that were placed within a single group (Figure S3C). However, some proteins contained lysines that fell into multiple groups, indicating heterogeneity among target lysines within the same protein in their response to Btz.
Figure 3. Quantification of diGly-modified peptides with increasing time of Btz treatment.
A) Cells were either untreated (K0) or treated with Btz for 0, 2, 4, or 8 hours (K8). Depicted is a heat map representing the hierarchical clustering of each peptide grouped using K-means clustering according to their log 2 H:L Btz response curves. The total number of peptides in each group is indicated.
B) Average log2 ratios of all peptides in each group with increasing time of Btz treatment (black line) and four representative peptides are depicted. Error bars: SEM of all peptide measurements in the group (black line) or multiple MS1 quantifications for the indicated peptide.
Group 1 sites (167 sites in 121 proteins) displayed the most immediate response to Btz treatment; a >2-fold increase in abundance after 2 hours, and further increased during the remainder of the time course (Figure 3A, Table S4). This group contained many proteins known to be regulated by the UPS, including 5 sites on HIF1α (a substrate of the CRL2VHL complex) and 2 sites on SETD8 (a substrate of the CRL4CDT2 complex) (Figure 3B, S1A, S3D). Groups 2 (586 sites in 414 proteins), 3 (279 sites in 193 proteins), and 4 (510 sites in 367 proteins) responded similarly to Btz treatment as group 1 members but with a smaller magnitude of change or delayed kinetics, including the known UPS targets RNA polymerase II (POLR2A) and cyclin-D1 (CCND1) (Figure 3B, S1A). A majority of sites within group 4 (510) were only observed at the 8 hr time point, indicating that either the protein was particularly low in abundance and needed 8 hours to accumulate to detectable levels (e.g. NFE2L2), or the protein was of high abundance and the modification is likely a response to the Btz-induced challenge to protein homeostasis (e.g. glycogen phosphorylase, PYGL) (Figure 3A,B). While just over half of the quantified sites increased in response to Btz, the largest group (1231 sites in 740 proteins) represents those sites whose abundance was unchanged in response to Btz (Group 5, Figure 3A,B) including the known neddylated protein CUL5. Interestingly, 411 sites in 273 proteins decreased in abundance after Btz treatment. Among the sites in group 6 were K119 on Histone H2A, K121 on Histone H2B, and K561 on FANCD2, which are all known to be monoubiquitylated (Figure 3B). Histones are deubiquitylated in response to proteasome inhibition and this has been suggested as a mechanism to recycle ubiquitin from monoubiquitylated proteins to be used for polyubiquitylation during protein homeostasis stress (Bennett et al., 2007; Dantuma et al., 2006). Our results indicate that deconjugation of ubiquitin from proteins in response to proteasome inhibition occurs on a much wider scale than previously appreciated. Overall, examination of the temporal response of individual sites can assist in characterizing sites as those arising from regulated versus quality control ubiquitylation events as proteins with diGly-modified sites in group 1 tended to be less abundant and those with sites in group 4 tended to more abundant when examining unfractionated cell lysates (data not shown). However, because some low abundance proteins that are known to be ubiquitylated in a regulated manner are present in group 4 (e.g. NFE2L2), it is not possible to utilize the kinetics of diGly accumulation alone to conclusively determine if the site arises from quality control versus regulated ubiquitylation.
Relationship between diGly modification and total proteome abundance
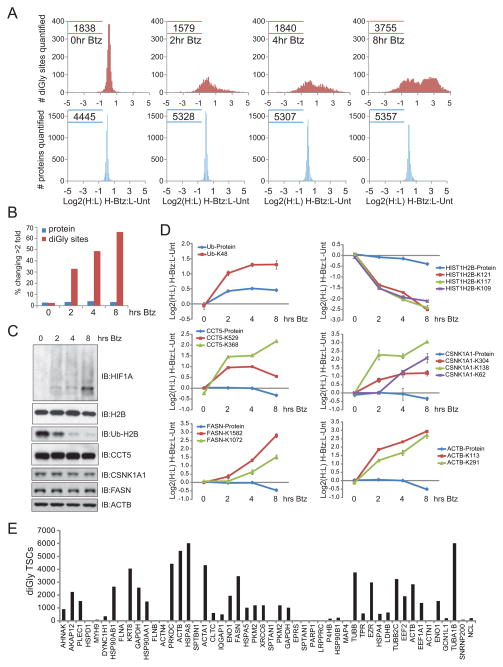
A hallmark of canonical UPS substrates is that, upon proteasome inhibition, their levels increase, reflecting their inherent instability. In contrast to expectation, we observed numerous Btz-inducible diGly-modified proteins not normally viewed as being overtly unstable. In order to examine the extent to which the behavior of the diGly-modified proteome correlated with changes in the abundance of the proteins from which they are derived, we performed LC-MS/MS on whole cell extracts from the same Btz time course samples used for Figure 3. In contrast to diGly-modified peptides, which substantially increase in the H:L ratio upon proteasome inhibition, the relative protein abundances did not change (Figure 4A,B, Table S4). Thus, large changes in the abundance of the ubiquitinome are not reflective of overt protein accumulation for the >4500 proteins that were quantified (Figure 4A,B). We confirmed this by immunoblotting for various proteins for which we observed diGly-modified peptides that increase by at least 2-fold after Btz treatment (Figure 4C, S4A). Expected changes in HIF1α levels were observed by immunoblotting, mirroring the increase in quantified diGly sites in HIF1α, but were unable to quantify HIF1α in the protein-based mass spectrometry experiment due to its low abundance, as was the case for many known low-abundance canonical UPS targets. Using an antibody that specifically recognizes only the ubiquitin-modified portion of Histone H2B, we validated loss of this species in response to Btz (Figure 4C) as observed by diGly proteomics (Figure 4D). Importantly, total histone H2B abundance was unchanged by immunoblotting, consistent with the absence of changes in total histone H2B levels by quantitative proteomics (Figure 4C,D). For the vast majority of diGly peptides whose abundance was significantly altered by proteasome inhibition, we did not detect similar changes in total protein abundance as measured by either immunoblotting or protein level quantitative mass spectrometry as seen with the chaperonin subunit CCT5, casein kinase 1α, fatty acid synthase, or β-actin (Figure 4C,D) and 20 other proteins (Figure S4A). This suggests that the diGly-modified portion of the protein population for a broad cross-section of proteins represents a distinct and most likely small fraction of the total protein pool.
Figure 4. Comparison of protein and ubiquitinome changes in response to proteasome inhibition.
A) The distribution of diGly site (red, top) or protein (blue, bottom) log2 ratios (H:L) from heavy cells treated with Btz for 0, 2, 4, or 8 hours. The protein level data was obtained by quantifying SCX fractionated whole cell extracts for each time point. Numbers indicate total number of quantified peptides.
B) Percentage of diGly-containing peptides (red) or proteins (blue) that change two fold or more in each Btz treated time point.
C) Immunoblot of extracts from the Btz time course.
D) Site and protein level changes during the Btz time course as quantified by mass spectrometry. The diGly-modified peptide that was quantified is indicated. All measurements represent the average log2 ratio for all quantified peptides with error bars representing the SEM.
E) TSCs for diGly-modified peptides derived from the 50 proteins with the largest protein level TSCs.
The finding that many highly abundant cellular “housekeeping” proteins are ubiquitylated, together with the observation that the diGly-modified proteome is distinct compared to the bulk protein population, led us to question whether there is a relationship between the propensity of a protein to be ubiquitylated and its overall abundance. If this were the case, one would expect the most often observed ubiquitylated proteins to also be the most abundant in extracts. Examination of diGly-modified TSCs derived from the 50 proteins with the largest protein TSCs from HCT116 cells revealed no correlation between protein abundance and diGly abundance (Figure 4E). In fact, two proteins within this group, despite being among the most abundant proteins in extracts, were never observed to be diGly-modified, indicating that protein abundance is not the sole determining factor regarding the propensity for a protein to be ubiquitylated. Further, we observed no correlation between the number of TSCs between diGly-modified peptides and the proteins to which these peptides mapped (Figure S4B). While TSCs can provide an estimate of protein abundance, there can be a non-linear relationship between TSCs and abundance due to various aspects of peptide-centric mass spectrometry. Thus, while there appears to be no relationship between protein abundance and diGly abundance we cannot rule this out without more careful determinations of absolute protein and diGly abundances.
The diGly proteome primarily represents a newly synthesized population of the proteome
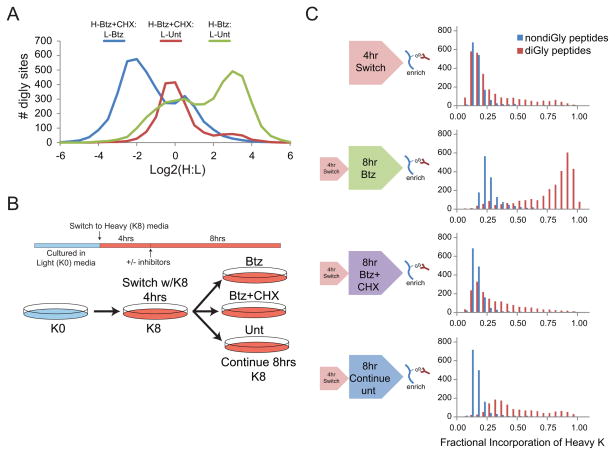
Previous studies suggest that between 6 and 30% of newly synthesized proteins are degraded by the ubiquitin system (Schubert et al., 2000; Vabulas and Hartl, 2005), presumably as a result of translational errors or inappropriate co-translational folding. These peptides can be utilized for immune surveillance functions and thus represent a particularly important fraction of the ubiquitylated proteome. Therefore, we sought to examine the extent to which formation of the ubiquitinome requires ongoing protein synthesis, either in the presence or absence of proteasome inhibition. K8-labelled HCT116 cells were treated with Btz in the presence or absence of the translational inhibitor cycloheximide (CHX). Consistent with previous results, proteasome inhibition resulted in the accumulation of a large portion of the diGly-modified proteome (log2 H:L > 1, Figure 5A, Table S5). However, simultaneous inhibition of both the ribosome and the proteasome resulted in a near ablation of all diGly peptide abundance increases seen with Btz alone (log2 H:L ~ 1, Figure 5A). Treatment of both K8 and K0 populations with Btz and inclusion of CHX with K8 cells resulted in 70% of all quantified proteins with a log2 ratio of less than 1 (Figure S5B, Table S5). Further, we observed that ~30% of diGly-modified peptides decreased greater than 2-fold upon treatment with CHX alone (Figure S5A, Table S5). Thus, a substantial fraction of the observable diGly-modified proteome requires ongoing protein synthesis to accumulate both with and without proteasome inhibition. This result does not indicate that the totality of sites observed using diGly capture arise from mistranslated proteins, as proteins known to be modified by ubiquitin in a regulatory manner, such as NRF2 and HIF1α, also required continued synthesis in order to accumulate in response to Btz to a level that they are observable by diGly proteomics (Table S5).
Figure 5. Observation of proteasome-dependent changes in diGly peptide abundance requires ongoing protein synthesis.
A) Distribution of log2 ratios from K8 cells treated with Btz for 8 hours (green line) or with Btz and CHX (red line) for 8 hours mixed with untreated K0 cells. Log2 ratios from K0, Btz treated cells mixed with K8 labeled cells treated with Btz and CHX (blue line).
B) Schematic of SILAC switching experiment.
C) The distribution of the fractional incorporation of the K8 label from each experiment for diGly-containing peptides quantified after diGly enrichment (red) or from total protein (blue).
As an independent test to validate the extent to which newly translated proteins are ubiquitylated, we performed a SILAC-switching study. HCT116 cells grown in K0 media were switched to K8 media for 4 hrs (4 hr Switch, Figure 5B) followed by either continued unperturbed growth in K8 media (continue) or in the presence of Btz with and without CHX (Figure 5B,C). DiGly-modified peptide and protein level data was obtained as described above. The fractional incorporation (FI), which is a measure of the proportion of peptides containing K8 Lys, was determined for each Lys-containing peptide for both diGly enriched samples and for total protein (Figure 5C). As expected, the FI of K8 increased over the duration of continuous growth in K8 media for diGly-containing peptides, indicating ubiquitylation of newly synthesized proteins. Strikingly, inclusion of Btz during continued growth in K8 media dramatically increased the FI of diGly-containing peptides and this increase was dependent upon continued protein synthesis (Figure 5C). We did observe an increase in the FI of total protein upon Btz treatment, consistent with previous studies (Yewdell and Nicchitta, 2006), but this increase was substantially smaller than what we observed in the diGly-modified peptide population (Figure 5C, Table S5).
Utilization of diGly proteomics to identify CRL substrates
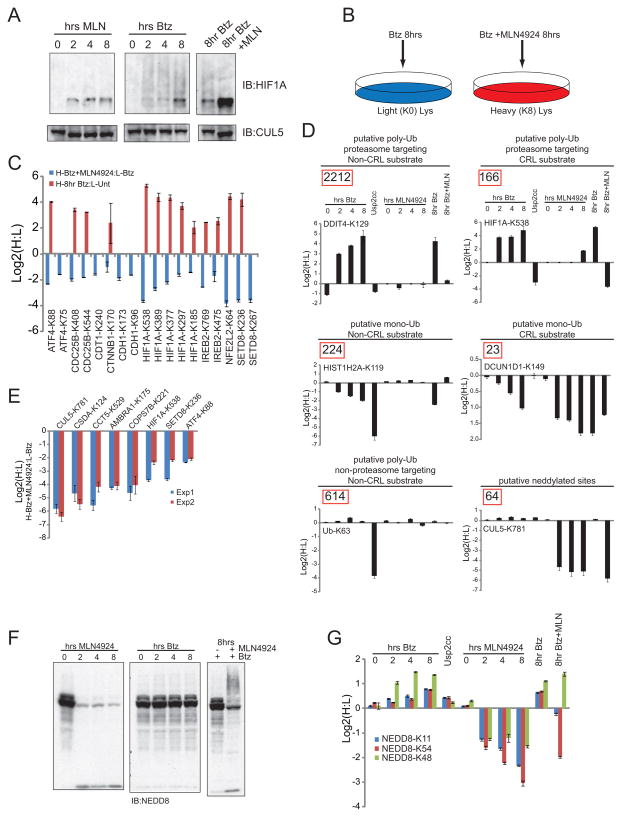
We next evaluated diGly capture as a strategy to identify targets of CRLs, the largest class of RING-dependent E3s ligases. As CRL activity requires cullin neddylation, we expected that acute inhibition of CRL activity by MLN4924 would lead to loss of accumulation of diGly containing peptides for CRL substrates normally found to accumulate in response to proteasome inhibition. Either Btz or MLN4924 stabilized the CUL2VHL target HIF1α in HCT116 cells, but only Btz promoted the formation of ubiquitin conjugates on HIF1α, a process that was blocked by addition of MLN4924 (Figure 6A). Thus, diGly sites whose Btz-dependent accumulation is blocked by MLN4924 are candidate CRL targets.
Figure 6. Utilization of diGly proteomics to identify ubiquitin-ligase substrates.
A) Extracts from cells treated as indicated were immunoblotted for HIF1α or CUL5.
B) Schematic of experimental setup for CRL substrate identification using the NEDD8 E1 inhibitor, MLN4924.
C) The log2 ratios for the indicated diGly peptides for Btz treated cells (red bars), or Btz and MLNL4924 treated K8 labeled cells mixed with Btz treated K0 cells (blue bars). Error bars represent the SEM of all diGly peptide ratios measured.
D) The log2 ratios for the indicated site across multiple experiments as indicated. The number inside of the red box indicates the total number of sites assigned to each diGly abundance profile.
E) The log2 ratios for the indicated diGly peptides from Btz and MLNL4924 treated K8 labeled cells mixed with Btz treated K0 cells between two replicate experiments.
F) Extracts from cells treated as indicated were immunoblotted with α-NEDD8 antibodies.
G) Log2 ratios corresponding to three diGly sites within NEDD8 from multiple experiments as indicated. All error bars represent the SEM of multiple MS1 quantifications for the indicated site.
K0 and K8 HCT116 cells were treated with Btz or both Btz and MLN4924, respectively, for 8 hrs (Figure 6B), followed by diGly proteomics. In total, we quantified 4001 diGly-modified peptides corresponding to ubiquitylation events utilizing many E3 ligases (Table S6). Substrates that are ubiquitylated in a CRL-independent manner were expected to accumulate equally in both the heavy and light labeled HCT116 cells resulting in a log2 ratio of 0, whereas CRL-dependent substrates will only accumulate ubiquitylated lysines in the light, Btz treated cells, resulting in a negative log2 ratio. As expected (Soucy et al., 2009), the vast majority, 92%, of diGly-modified sites were unaltered, suggesting that CRL inhibition did not globally alter Btz-dependent accumulation of the diGly-modified proteome. However, 253 diGly-modified sites decreased by more than 2-fold upon CRL and proteasome inhibition and 59 of these mapped to proteins either previously demonstrated to be CRL substrates or proteins that are known components of CRLs (e.g. substrate adaptors). Within this group, we observed 5 unique sites in HIF1α, 2 sites in SETD8, 2 sites in ATF4, and 2 sites in IREB2 that decreased by more than two fold with the combined MLN4924 and Btz treatment and had either increased more than 2 fold with Btz treatment alone or were unmeasured in the same experiment (Figure 6C, S1A).
Because MLN4924 treatment results in loss of not only ubiquitylated lysines on CRL substrates, but also potentially neddylated lysines, we classified each site into 6 categories by combining data from the Btz time course, a time course of MLN4924 treatment as well as the combined MLN4924 and Btz treatment (Figure 6D, S6A, Table S6). Sites (such as CUL5 K781 and 64 others) that failed to increase with proteasome inhibition, but decreased greater than two fold with MLN4924 treatment, were classified as potentially neddylated lysines. However, it is also possible that these substrates represent polyubiquitylated lysines that are not targeted for proteasomal degradation (e.g. UbK63-linked), but are dependent upon CRL activity. The largest group, representing proteasomal targets that are CRL-independent, contained 2212 diGly sites (including DDIT4_K129), that increased in response to Btz treatment and were unaffected by MLN4924 treatment (Figure 6D). Potentially monoubiquitylated sites, such as K119 in Histone H2A, are characterized by an observed decrease in abundance in response to Btz treatment, but were unchanged with MLN4924 treatment (Figure 6D). We also classified a group of diGly-modified sites as potentially polyubiquitylated in a manner unaffected by Btz such as K63 within ubiquitin itself (Figure 6D).
Modified lysines that increased in response to Btz but failed to increase in the presence of Btz and MLN4924, are likely derived from CRL substrates (Figure 6D). HIF1α falls within this category along with 166 other diGly sites in 106 proteins. Finally, 23 diGly sites having characteristics of monoubiquitylation appeared to be modified in a CRL dependent fashion (Figure 6D), including the CRL component DCN1, which promotes cullin neddylation, and is known to be monoubiquitylated.
We attempted to validate candidates by western blotting after MLN4924 treatment, but we observed no reliably quantifiable changes in overall protein levels for the majority of the tested candidates (Figure S4A). However, because our diGly data examines only the modified portion of the protein population, a population not directly interrogated by Western blotting (Fig 4A, C), we determined the reproducibility of identifying the relevant endogenously ubiquitylated candidate CRL substrates by repeating our diGly enrichment experiment. Consistent with the first experiment, we quantified 3033 diGly-modified sites in total, with only 229 sites decreased by greater than 2-fold after combined MLN4924 and Btz treatment. Of the putative CRL-modified lysines quantified in the first experiment, 133 were also quantified in the second experiment, 95 of which were also decreased two-fold in the second experiment, resulting in a greater than 70% reproducibility rate. We observed an excellent correlation of the quantified log2 ratios between the two experiments for both sites on known CRL targets, HIF1α, SETD8, and ATF4, but also novel sites on CCT5, AMBRA1, and CSDA (Figure 6E). Combining the two experiments, we identified 386 sites that were modified in a CRL-dependent manner, indicating that the quantitative diGly enrichment method can be used to identify novel substrates for ubiquitin ligases.
Altering free UBL levels leads to inappropriate E1 utilization
Overexpression of NEDD8 generates polymeric forms of NEDD8 and these chains appeared to increase in response to proteasome inhibition (Jones et al., 2008; Xirodimas et al., 2008). Examination of endogenous NEDD8 levels following Btz treatment revealed no changes in NEDD8 levels nor any appearance of slower migrating forms above the abundantly neddylated cullins (Figure 6F). In contrast, the amount of neddylated cullins is dramatically decreased upon MLN4924 treatment, as expected, with a concomitant increase in the amount of unconjugated NEDD8 (Figure 6F). However, simultaneous treatment of cells with Btz and MLN4924 led to the formation of more slowly migrating forms of endogenous NEDD8 (Figure 6F), indicating that these conditions promote utilization of NEDD8 in a manner not seen with proteasome inhibition alone. Using quantitative diGly proteomics, we confirmed that endogenous NEDD8 forms chains utilizing lysines 11, 48, and 53 (Figure 6G). Examination of the abundance of each modified Lys revealed that all three modified Lys residues do indeed accumulate in response to Btz, though only K48 accumulated by more than 2-fold. All three diGly-modified lysines within NEDD8 decreased in as little as 2 hrs after MLN4924 treatment, but each behaved uniquely in response to the combined MLN4924 and Btz treatments (Figure 6G). We observed that the Btz induced accumulation of K48 diGly-modified NEDD8 was insensitive to MLN4924 treatment which suggested, even in the absence of NAE activity, NEDD8 chains were formed most likely due to inappropriate charging by the ubiquitin E1 activating enzyme (UBA1). Indeed, previous in vitro studies indicate that NEDD8 can be activated by the ubiquitin activating enzyme UBA1 and transferred to a ubiquitin E2, but with reduced kinetics relative to the cognate enzymes (Whitby et al., 1998). Consistent with the use of UBA1 for NEDD8 activation in vivo under conditions of proteasome inhibition and ensuing ubiquitin depletion, treatment of cells with a pan-E1 inhibitor blocking the activity of both UBA1 and NAE (see Supplemental Methods) resulted in reversal of slowly migrating NEDD8 conjugates (Figure S6B).
Discussion
Expansion of diGly modified proteome
In this study, we have used a highly selective antibody recognizing the diGly remnant to provide a global analysis of the endogenous human ubiquinome, identifying ~19,000 sites in ~5000 proteins. Based on experiments utilizing USP2cc and inhibition of NAE-dependent neddylation, we conclude that >94% of the identified sites represent conjugation to ubiquitin, as opposed to NEDD8 or ISG15. As described below, this is likely an underestimate, given that under conditions of proteasome inhibition and ubiquitin depletion, NEDD8 can be utilized by the ubiquitin machinery and is likely transferred to canonical ubiquitination targets. Our collection of sites includes ~80% of the ~1000 previously identified sites (Figure S7A,B, Table S1). The absence of complete overlap likely reflects differences in cell types employed, under-sampling for low abundance or low occupancy sites, and differences in pre-enrichment strategies. Indeed, an examination of 4 different multiple myeloma cells lines revealed only ~50% overlap compared with 293T and HCT116 cells (Table S7, Supplemental Methods), consistent with the idea that ubiquitinomes will be distinct in more distantly related cell types. This work provides a powerful resource for investigators working on the ubiquitylation of single proteins, groups of proteins, or the global proteome and is readily accessible via the web portal at: https://gygi.med.harvard.edu/ggbase/.
A limitation of this approach is that it does not unambiguously reveal whether the target site was mono- or polyubiquitylated via one of 7 possible isopeptide-based chain-linkages. To address this limitation, we developed a multi-classifier approach based on known properties of chain linkage types to begin to categorize the observed diGly-modified sites into putative functional classes (Figure S6A). Sites that accumulate in response to Btz – the largest class in our dataset - likely represent polyubiquitylated forms involving principally K48 and K11 chain linkages, which are known to be targeted by the proteasome. In contrast, a substantial fraction of identified sites display no change in diGly abundance in response to Btz. Among diGly sites in ubiquitin itself, only the diGly-linked K63 peptide was insensitive to proteasome inhibition, raising the possibility that many of the diGly sites in our dataset could reflect anchor points for K63-linked chains. The previous immunological identification of K63 chains in unstimulated tissue culture cells (Newton et al., 2008), together with the large number of proteins (422) that fall into this category, indicates that K63-based signaling is likely occurring constitutively in cells without stimuli that are known to produce acute K63-linked polyubiquitylation of specific proteins (DNA damage, TNFα, and EGF) (Komander, 2009). Finally, a third category of sites are those whose diGly abundance decreases upon proteasome inhibition. Removal of ubiquitin from abundant monoubiquitylated proteins, such as histones has been suggested to occur in order to increase the free ubiquitin pool in response to acute ubiquitin depletion (Dantuma et al., 2006; Mimnaugh et al., 1997). Indeed, our study recapitulates this finding and further expands the number of putatively monoubiquitylated proteins and suggests that deubiquitylation of monoubiquitylated proteins is a broad response not limited to ubiquitylated histones. We cannot rule out the possibility that diGly-modified lysines that decrease in response to proteasome inhibition arise from K63-linked chains, as well as other linkages, or that some mono-ubiquitylated sites are unaltered in response to proteasome inhibition. Further studies are needed to examine if this response occurs under truly physiological conditions as well as to determine the signaling mechanism leading to such a response.
Does a ubiquitylation motif exist?
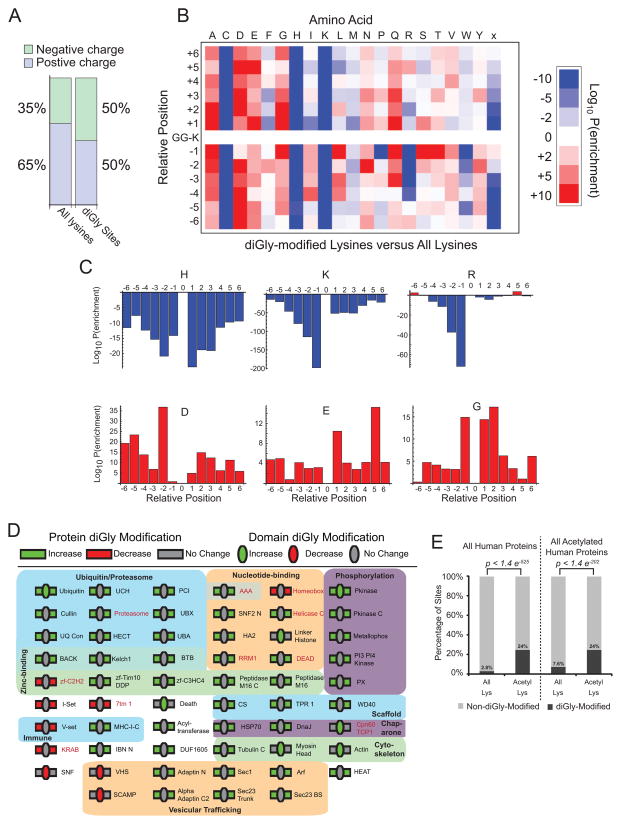
Previous studies using a limited number of diGly sites suggested absence of a sequence motif for ubiquitylation (Danielsen et al., 2011; Xu et al., 2010). However, because our study dramatically expanded the number of lysine residues demonstrated to be diGly-modified, we could analyze the structural nature of lysines utilized for ubiquitylation on a more global scale. As described in detail in Supplemental Methods, we find a pronounced shift toward a negative net local charge among surrounding residues (Figure 7A,B); 50% of diGly sites are found to be located in local regions with net negative charge, compared with 35% of lysines overall (Figure 7A). As expected, we observed significant over-representation of acidic residues surrounding sites of diGly modification, coupled with under-representation of basic residues (Figure 7A,B). In contrast to phosphorylation motifs, the most extreme cases of amino acid selection surrounding diGly sites are amino acids that are disfavored. Such patterns are more difficult to recognize, as existing tools such as MotifX (Schwartz and Gygi, 2005) have been designed specifically to detect motifs characterized by enriched amino acids. While a predictive sequence motif is not readily apparent, we find that diGly sites are preferentially located in regions characterized by depletion of Arg on the N-terminal side and Lys and His on both sides, with enrichment of acidic residues to a lesser extent (Figure 7C). We cannot exclude the possibility that the antibody enrichment technique used here is biased towards a particular sequence surrounding the diGly-modified lysine and this bias contributes to the weak depletion motifs described above. However, since we see no strong enrichment for any particular motif within our dataset, this possibility seems unlikely.
Figure 7. Local amino acid environment and domain preferences for diGly-modified peptides.
A) Fraction of all and diGly-modified lysines occurring in regions with positive and negative local charges at physiological pH.
B) Heat map (log(10) of the p-value) depicting significance of enrichment (red) or depletion (blue) for each amino acid within +/− six residues of each diGly site, evaluated using a Binomial test. ‘x’ represents the absence of an amino acid and was used when lysines and diGly sites fell within 6 amino acids of the N- or C-terminus of the protein.
C) The log(10) p-value associated with enrichment or depletion of selected amino acids near sites of diGly modification.
D) Pfam domains that were found to be enriched (green), disfavored (red), or unchanged (grey) on diGly-modified proteins anywhere within proteins containing the domain (rectangles) or within the domain itself (ovals). p < 0.01; Hypergeometric test, with Benjamini-Hochberg correction for multiple hypothesis testing. Domain names in red were also found to exhibit enriched or depleted levels of modification in a previous proteomic survey of lysine acetylation.
E) (Left) The fraction of diGly-modified lysines (black bar) and all non-diGly-modified lysines (grey bar) observed here compared to all lysines within the human proteome (left) and to acetylated-lysines previously described (right). (Right) The fraction of diGly-modified lysines (black bar) and all non-diGly-modified lysines (grey bar) observed here compared to all lysines observed in proteins demonstrated to be acetylated (left) and to acetylated-lysines (right). p-value calculated by the Binomial test.
Crosstalk between post-translational modifications
To investigate the influence of domain architecture on protein diGly modification, Pfam domains were mapped onto human sequences and observed diGly modifications were superimposed. The effects of each domain were assessed by comparing the frequency of diGly modifications with the overall lysine frequency, both within the domain in question, and anywhere within proteins containing each domain. Of the 58 Pfam domains associated with either increased or decreased levels of diGly modification, either within the domain itself or in the surrounding protein sequence, 14 were linked with the ubiquitin system, 8 with vesicle trafficking (a ubiquitin-dependent process), and 5 with protein phosphorylation (which often regulates ubiquitination) (Figure 7D). Moreover, of 3668 previously reported acetylated lysines (Choudhary et al., 2009), we found 878 to be sites of ubiquitylation as well (Figure 7E), suggesting the potential for large scale cross regulation of the two pathways akin to that previously seen with p53 (Benkirane et al., 2010).
Deciphering UPS enzyme substrate relationships
Given that mammalian cells express ~600 ubiquitin ligases, substrate identification is often a challenge. With appropriate tools for altering the abundance or activity of E3s, the diGly capture approach has the potential for direct identification of ubiquitylation sites in target proteins without the need for overexpression. As a proof of principle, we demonstrate that quantitative diGly-enrichment proteomics can be utilized to identify substrates for CRL-based E3s, including the identification of sites in the well known CRL targets ATF4, CDC25B, β-catenin (CTNNB1), CDT1, HIF1α, NFE2L2, and SETD8, and 271 additional candidate substrates. While replicate experiments revealed a 70% reproducibility rate, further studies are required to determine the specific CRL and signals responsible for targeting these candidate proteins in the absence of pharmacological inhibition of the proteasome.
Protein vs diGly paradox
The canonical view of protein ubiquitylation posits that the entire pool of a targeted protein become ubiquitylated and is subsequently degraded, such that incubation of cells with proteasome inhibitor results in the stabilization of the protein (usually in its unmodified form) which can be readily visualized by immunoblotting. While this is certainly the case for many of the known, short half-life, regulatory proteins whose turnover is signal dependent, our results suggest that the ubiquitylated portion of many proteins increases in response to proteasome inhibition in the absence of overt alterations in total protein levels. These results suggest a paradox; on one hand, proteasome inhibition leads to a dramatic increase in the abundance of many ubiquitylation events, as detected by diGly capture. On the other, the overall abundance of the vast majority of detectable proteins in cell extracts are themselves not largely effected by proteasome inhibition. One potential solution to this paradox is that a wide cross-section of the diGly-containing proteome does not represent conventional proteasome substrates, wherein the entire population of the protein is degraded in response to a particular signal. Instead, the data suggests that proteotoxic stress leads to substantial ubiquitylation of the proteome, but with low overall stoichiometry such that subsequent turnover of the modified pool is not easily observed within the precision of most immunoblotting techniques when examining the entire protein pool. We note that blotting for the unmodified form of H2B did not reveal any abundance changes consistent with the protein-level proteomic measurement while directly immunoblotting for the ubiquitin-modified portion of H2B recapitulated our diGly-proteomic data (Figure 2C). This suggests that only upon development of modification-specific antibodies, will it be possible to validate alterations in the ubiquitylated portion of proteins without using proteomic technologies. This is similar to what has been observed for protein phosphorylation as the advent of numerous phospho-specific antibodies has lead to an appreciation of the importance of low stoichiometric post-translation modifications on protein function.
Proteotoxic stress and miss-utilization of UBLs
We unexpectedly found that upon proteasome inhibition, there is an increase in the formation of poly-NEDD8 or mixed ubiquitin-NEDD8 chains. NEDD8 is most closely related to ubiquitin and polyneddylation occurs primarily via K48 in NEDD8. The formation of poly-NEDD8 conjugates is enhanced upon simultaneous inhibition of NAE and the proteasome, but is blocked upon simultaneous inhibition of UBA1. These findings, together with previous studies demonstrating activation of NEDD8 by UBA1 (Whitby et al., 1998) suggests that, in vivo, the steady-state abundance of free ubiquitin acts to limit inappropriate utilization of NEDD8 by UBA1. The specificity of ubiquitin and NEDD8 utilization is also strengthened by the fact that the majority of NEDD8 is conjugated to cullins at steady-state, and the low levels of free NEDD8 are unable to compete with high concentrations of free ubiquitin, thereby blocking inappropriate entry of NEDD8 into the ubiquitin conjugation pathway. Conditions which disturb this delicate balance, such as ubiquitin depletion as observed here, or NEDD8 overexpression may allow NEDD8 access to the ubiquitin machinery. Thus, further studies are necessary to understand whether the many putative NEDD8 substrates that have been reported based on overexpression (Jones et al., 2008; Xirodimas et al., 2008) reflect co-opting of the ubiquitin system.
The diGly-modified protein pool as unique indicator of proteome health
It has been suggested that 20–30% of newly synthesized proteins are ubiquitylated and targeted for degradation in the absence of amino acid starvation and this pool of degradation substrates are particularly important for immune surveillance (Yewdell and Nicchitta, 2006). Our studies indicate that, in order to accumulate to detectable levels, a substantial fraction of the diGly-modified sites that we observe both with and without proteasome inhibition need continued protein synthesis (Figure 5A, Table S5). Because diGly enrichment allows for the direct measurement of the ubiquitylated portion of putative quality control substrates, the abundance of these sites may serve as sensitive endogenous biomarkers to interrogate protein quality control fidelity in vivo. This may be particularly useful for the evaluation of proteome health in the context of various human pathologies associated with protein aggregation such as Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (Chiti and Dobson, 2006). Recent proteomic studies suggest that aggregation-prone proteins sequester newly synthesized proteins before they reach their native state (Olzscha et al., 2011). It is possible that these same unstable newly synthesized proteins also accumulate in an ubiquitylated form upon exposure to proteotoxic stress. Further, there is widespread interest in targeting enzymes within the UPS to treat human disease (Bedford et al., 2011; Cohen and Tcherpakov, 2010) and an evaluation of the diGly-modified proteome under physiological dosing concentrations and schedules for these drugs may reveal useful endogenous biomarkers to interrogate pathway function
Materials and Methods
An overview of experimental procedures is provided below. For complete details, see Supplemental Methods
Cell culture, immunoprecipitation, and mass spectrometry
HCT116 and 293T cells were grown in Lys- and Arg-free DMEM supplemented with FBS, Arg (85μg/ml), and either light (K0) or heavy (K8) Lys (50μg/mL). Cells were treated with Btz, MLN4942, and CHX at 1μM, 1μM, and 1μg/ml, respectively for the times indicated. After treatment, cells were mixed 1:1 by cell number and lysed in denaturing lysis buffer. Lysates were digested sequentially with LysC and trypsin followed by peptide desalting. Peptides were immunoprecipitated with α-diGly (generated against the sequence CXXXXXXK(GG)XXXXXX) coupled to protein A agarose beads for 1hr at 4°C. Eluted peptides were analyzed by LC-MS/MS on an LTQ Orbitrap Velos. Whole cell extracts (0.5mg) were digested, fractionated, and analyzed by LC-MS/MS (Huttlin et al., 2010). Where indicated, samples were treated with 10 μg of purified USP2cc.
Identification of peptides and proteins
MS/MS spectra searches (Sequest), target-decoy peptide filtering, and linear discriminant analysis was performed as described (Huttlin et al., 2010) with an initial 1% peptide-level false discovery rate and final protein-level false discovery rate of 1%. The RAW files will be deposited at www.proteomecommons.org. To view or search this dataset see https://gygi.med.harvard.edu/ggbase/. The localization of each diGly site was evaluated using a variation on the Ascore algorithm (Beausoleil et al., 2006), with minor modifications. Sites with scores >13 (p <0.05) were considered localized while others were discarded. Data across all experiments was used to generate a non-redundant list of all observed diGly sites.
Supplementary Material
Acknowledgments
We thank S. Sarraf for experimental assistance. This work was supported by NIH grants to J.W.H. and S.P.G and by Millennium Pharmaceuticals to J.W.H. E.J.B. is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG 1974-08).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkirane M, Sardet C, Coux O. Lessons from interconnected ubiquitylation and acetylation of p53: think metastable networks. Biochem Soc Trans. 2010;38:98–103. doi: 10.1042/BST0380098. [DOI] [PubMed] [Google Scholar]
- Bennett EJ, Rush J, Gygi SP, Harper JW. Dynamics of cullin-RING ubiquitin ligase network revealed by systematic quantitative proteomics. Cell. 2010;143:951–965. doi: 10.1016/j.cell.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. Global changes to the ubiquitin system in Huntington’s disease. Nature. 2007;448:704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Cohen P, Tcherpakov M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell. 2010;143:686–693. doi: 10.1016/j.cell.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Danielsen JM, Sylvestersen KB, Bekker-Jensen S, Szklarczyk D, Poulsen JW, Horn H, Jensen LJ, Mailand N, Nielsen ML. Mass spectrometric analysis of lysine ubiquitylation reveals promiscuity at site level. Mol Cell Proteomics. 2011;10:M110 003590. doi: 10.1074/mcp.M110.003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantuma NP, Groothuis TA, Salomons FA, Neefjes J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. J Cell Biol. 2006;173:19–26. doi: 10.1083/jcb.200510071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Wu K, Yang Y, Guerrero C, Nillegoda N, Pan ZQ, Huang L. A targeted proteomic analysis of the ubiquitin-like modifier nedd8 and associated proteins. J Proteome Res. 2008;7:1274–1287. doi: 10.1021/pr700749v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37:937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hatakeyama S, Oyamada K, Oda Y, Nishimura T, Nakayama KI. Large-scale analysis of the human ubiquitin-related proteome. Proteomics. 2005;5:4145–4151. doi: 10.1002/pmic.200401280. [DOI] [PubMed] [Google Scholar]
- Meierhofer D, Wang X, Huang L, Kaiser P. Quantitative analysis of global ubiquitination in HeLa cells by mass spectrometry. J Proteome Res. 2008;7:4566–4576. doi: 10.1021/pr800468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimnaugh EG, Chen HY, Davie JR, Celis JE, Neckers L. Rapid deubiquitination of nucleosomal histones in human tumor cells caused by proteasome inhibitors and stress response inducers: effects on replication, transcription, translation, and the cellular stress response. Biochemistry. 1997;36:14418–14429. doi: 10.1021/bi970998j. [DOI] [PubMed] [Google Scholar]
- Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- Schwartz D, Gygi SP. An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat Biotechnol. 2005;23:1391–1398. doi: 10.1038/nbt1146. [DOI] [PubMed] [Google Scholar]
- Skaug B, Chen ZJ. Emerging role of ISG15 in antiviral immunity. Cell. 2010;143:187–190. doi: 10.1016/j.cell.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- Tagwerker C, Flick K, Cui M, Guerrero C, Dou Y, Auer B, Baldi P, Huang L, Kaiser P. A tandem affinity tag for two-step purification under fully denaturing conditions: application in ubiquitin profiling and protein complex identification combined with in vivocross-linking. Mol Cell Proteomics. 2006;5:737–748. doi: 10.1074/mcp.M500368-MCP200. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science. 2005;310:1960–1963. doi: 10.1126/science.1121925. [DOI] [PubMed] [Google Scholar]
- Whitby FG, Xia G, Pickart CM, Hill CP. Crystal structure of the human ubiquitin-like protein NEDD8 and interactions with ubiquitin pathway enzymes. J Biol Chem. 1998;273:34983–34991. doi: 10.1074/jbc.273.52.34983. [DOI] [PubMed] [Google Scholar]
- Xirodimas DP, Sundqvist A, Nakamura A, Shen L, Botting C, Hay RT. Ribosomal proteins are targets for the NEDD8 pathway. EMBO Rep. 2008;9:280–286. doi: 10.1038/embor.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Paige JS, Jaffrey SR. Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat Biotechnol. 2010;28:868–873. doi: 10.1038/nbt.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 2009;10:755–764. doi: 10.1038/nrm2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol. 2006;27:368–373. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.