Abstract
Objective
To provide a preliminary assessment of obstetric healthcare provider opinions surrounding implementation of cell-free fetal DNA testing.
Methods
A 37-question pilot survey was used to address questions around the translation and use of non-invasive prenatal testing using cell-free fetal DNA. The survey was distributed and collected at a Continuing Medical Education course on obstetrics and gynecology.
Results
Of 62 survey respondents, 73% are female and 87% hold MD/DO degrees. Respondents generally agree that patients want prenatal diagnostic information to help make decisions about a pregnancy and that cell-free fetal DNA testing will encourage the testing of more patients for more conditions. However, there is an overall lack of knowledge or conviction about using this technology. Genetic counseling and professional society approval are deemed important to implementation whereas the possibility of direct-to-consumer testing and government regulation produce mixed responses. Respondents indicate that they are more likely to offer cell-free fetal DNA testing for chromosomal abnormalities and single-gene disorders, but are cautious with respect to determination of sex and behavioral or late-onset conditions.
Conclusion
Preliminary assessment indicates uncertainty among obstetric providers about the details of implementing cell-free fetal DNA testing and suggests expanded research on perspectives of this stakeholder group.
Keywords: Psychosocial; legal, and ethical implications; cell-free fetal DNA; cell-free fetal RNA; non-invasive prenatal diagnosis; healthcare provider perspectives; clinical translation
Introduction
After years of unsuccessful attempts at the analysis of fetal cells present in the maternal bloodstream, the groundbreaking discovery that fragments of fetal DNA, known as cell-free fetal DNA, also circulate in maternal blood opened the door to the possibility of comprehensive, non-invasive prenatal genetic testing (Lo et al., 1997). Over the past decade, innovative techniques for identification and analysis of this cell-free fetal DNA have brought us closer to an unobstructed view of “all of the information from the entire fetal genome” (Fan and Quake, 2010).
Indeed, the range of fetal genetic traits identifiable using this rapidly advancing technology appears limited only by technical constraints and our genomic knowledge, thus raising significant ethical, legal, and social concerns. Currently, commercial applications of cell-free fetal DNA testing include RhD blood group typing, offered through National Health Services laboratories in the United Kingdom, and fetal sex determination, available from direct-to-consumer companies in the United States and Canada (Wright, 2009). Moreover, large-scale validity studies for non-invasive, prenatal detection of trisomy 21 are ongoing and suggest that commercialization of non-invasive tests for aneuploidy is on the horizon (Chiu et al., 2011; Ehrich et al., 2011; Sehnert et al., 2011). Researchers have also demonstrated proof of principle for the use of cell-free fetal DNA to diagnose or exclude a number of single-gene disorders (Ding et al., 2004; Lun et al., 2008; Tsang and Lo, 2010). Perhaps most noteworthy, however, is the recent mapping of the entire fetal genome using fetal DNA sequencing and parental haplotypes (Fan and Quake, 2010; Lo et al., 2010).
Although current clinical use is limited, cell-free fetal DNA testing has the potential to revolutionize prenatal genetic testing; eventually, it may supplement or even supersede existing prenatal screening techniques and invasive diagnostic procedures. No technology offering such significant changes to accepted practice - diminished risks of miscarriage, earlier timing for use, and broader indications – can arrive without substantial practical and ethical concerns. Critical questions raised by this technology include: validity and clinical utility of testing; financial and clinical access, government regulation, the meaning and provision of informed consent, the implications of an easier ability to determine fetal traits – and particularly traits of little or no medical significance, and patient values and decision-making in the broader context of social and cultural perspectives (Benn and Chapman, 2009; Kent, 2008; Smith, Lombaard, and Soothill, 2006).
In order to address these concerns we must first have a thorough understanding of the beliefs, priorities, and risk perceptions of those with a stake in this technology. In particular, obstetrics providers are in a unique position to both educate patients and explore patient wishes in the healthcare setting in order to help them make decisions surrounding the appropriate use of reproductive technologies (Drazen, 2004). Because it will be under the discretion of providers when, where, how, and to whom cell-free fetal DNA testing is offered, it is essential to understand their values and attitudes before the technology becomes available (Reproduction and responsibility).
The purpose of this study is to provide a preliminary assessment of the opinions of obstetric healthcare providers surrounding non-invasive prenatal testing using cell-free fetal DNA. Using survey data, we may begin to understand the priorities and perceptions held by these individuals, who may represent the early adopters - or non-adopters - of this technology and are key to its successful introduction or exclusion.
Methods
Survey design
A 37-question survey was designed for providers of healthcare in obstetrics to address understanding of patient preferences surrounding prenatal testing, implications for decision-making, and the perceived impact of cell-free fetal DNA testing. The survey was grounded in concepts and questions posed in the literature and the practical experience of one of the authors, an obstetrician specializing in maternal-fetal medicine (MN). A short description of non-invasive prenatal testing using cell-free fetal DNA was provided. The survey included eight demographic questions, fifteen questions regarding prenatal genetic testing in general, and fourteen questions regarding cell-free fetal DNA testing. Twenty-five questions utilized the five-point Likert scale to assess degree of agreement or disagreement with provided statements.
Data collection
Paper copies of the survey were distributed to approximately 180 attendees at a conference entitled “Obstetrics and gynecology update: What does the evidence tell us?” in October 2010. Hosted by the University of California San Francisco, this Continuing Medical Education conference was targeted for a broad audience of practicing physicians and allied health professionals interested in recent advances in obstetrics and gynecology. Attendees at the conference represented 30 states and 3 countries; 57% were from California. Participants were not asked for their names or other identifying information.
The Stanford University Institutional Review Board and the University of California San Francisco Committee on Human Research approved this project.
Data analysis
Each collected survey was given a unique identifying code. Responses to surveys were entered into a spreadsheet and descriptive statistics were computed using the Predictive Analytics SoftWare Statistics SPSS program, version 18.0. Responses to Likert scale questions were separated into three categories of “agree or strongly agree”, “neither agree nor disagree”, and “disagree or strongly disagree”.
Results
Demographic characteristics
A total of 62 surveys were completed, yielding a 34% response rate. Some respondents did not answer all questions and thus sample size varies slightly by question. Demographic and practice characteristics of respondents are summarized in Table 1.
Table 1. Demographic characteristics of respondents.
| Total (n=62)* | |
|---|---|
|
| |
| Gender | |
| Female | 73% (45) |
| Male | 27% (17) |
|
| |
| Age | |
| <35 | 13% (8) |
| 36-45 | 35% (22) |
| 46-55 | 26% (16) |
| 56-65 | 15% (9) |
| >65 | 6 % (4) |
|
| |
| Practitioner type | |
| MD/DO | 87% (54) |
| CNM/NP/RN | 11% (7) |
|
| |
| Currently practices in United States | |
| Yes | 89% (55) |
| No | 10% (6) |
|
| |
| Years of practice | |
| <5 | 15% (9) |
| 6-10 | 21% (13) |
| 11-15 | 18% (11) |
| 16-20 | 15% (9) |
| 21-25 | 10% (6) |
| 26-30 | 10% (6) |
| >30 | 10% (6) |
|
| |
| Type of practice† | |
| Private practice | 47% (29) |
| Within HMO | 19% (12) |
| Public clinic/hospital | 21% (13) |
| Academic clinic/hospital | 13% (8) |
| Other | 3% (2) |
|
| |
| Primary patient insurance type† | |
| Private | 65% (40) |
| Medicare/Medicaid | 40% (25) |
| Self-pay | 3% (2) |
Values reported as % (n). Responses to some questions were missing; thus totals may not add to 100%.
Respondents may select more than one answer; thus totals may add to more than 100%.
Females comprise 73% of respondents. Respondents span a wide range of ages with a mean of 47.3 ± 11.1 years: 13% are 35 years or younger, 35% are 36 to 45 years, 26% are 46 to 55 years, 15% are 56 to 65 years, and 6% are 65 years or older.
Of all respondents, 87% hold MD or DO degrees and 11% hold NP, RN, or CNM (certified nurse-midwife) degrees. Eighty-nine percent are currently in practice in the United States. Respondents have practiced an average of 16.2 ± 10.6 years; while 15% have been in practice for five years or fewer, 30% have been practicing for more than twenty years. These providers practice in a variety of settings, including 47% in private practice, 19% within an HMO, 21% in a public clinic or hospital, and 13% in an academic clinic or hospital. A majority of respondents, 65%, categorize the health insurance of their patients as primarily private, 40% identify Medicare or Medicaid as the primary type of insurance for their patients, and 3% state that their patients pay costs out-of-pocket (respondents could select more than one option).
Patient and provider preferences surrounding prenatal testing
Sixty percent of respondents believe that most patients want as much diagnostic information about their pregnancy as possible. Meanwhile, 44% posit that there are strong social pressures on patients to obtain prenatal testing (see Figure 1). Notwithstanding perceived patient preferences, 16% of respondents do not feel that patients should receive all available diagnostic prenatal genetic tests upon their request, as opposed to the 73% who believe all patient requests for diagnostic tests should be fulfilled.
Figure 1. Respondents' agreement with statements.
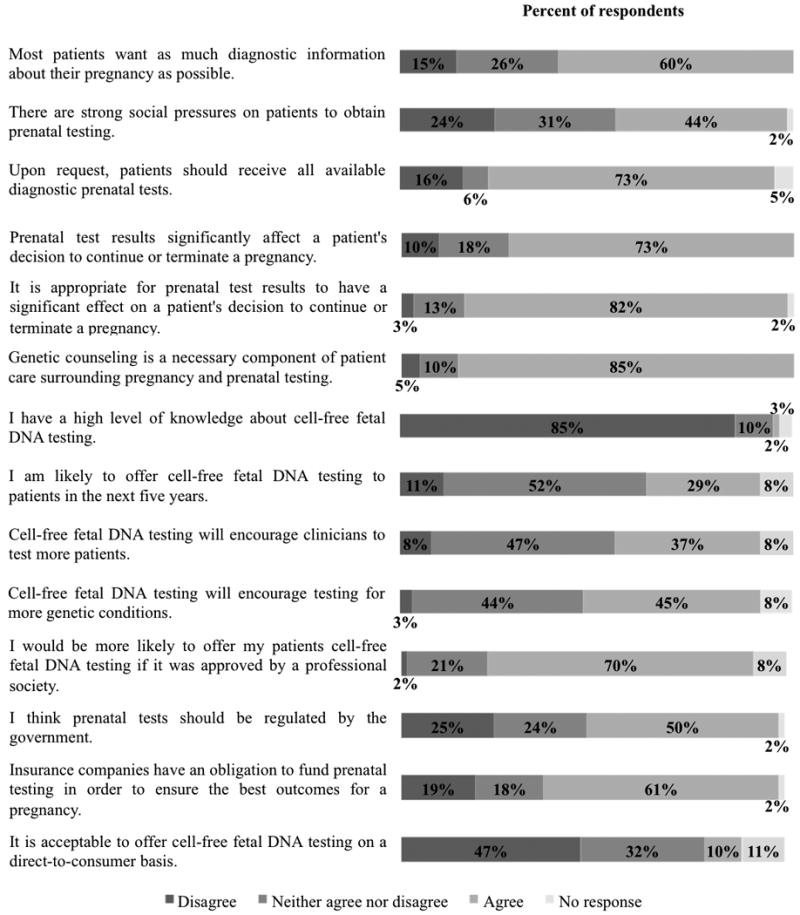
A majority of respondents, 73%, agree that the results of prenatal tests significantly affect a patient's decision whether to continue or terminate a pregnancy, although 10% think there is no considerable consequence of test results for patient decisions. An even greater percentage of respondents, 82%, judge this strong impact of prenatal test results on decision-making to be appropriate, whereas only 3% do not. Whether or not patients and providers agree on acceptable uses for prenatal test results, most respondents, 85%, believe that genetic counseling is a necessary component of prenatal genetic testing and patient care during pregnancy.
Perceived impact of cell-free fetal DNA testing
Eighty-five percent of respondents do not report a high level of knowledge about cell-free fetal DNA testing. Regardless, 29% respond that they are likely to offer this type of testing within the next five years, although 52% are ambivalent about the possibility. Approximately one-third of respondents believe testing using cell-free fetal DNA will encourage providers to test a greater number of patients; likewise, 45% think the availability of this technology will inspire testing for more genetic conditions.
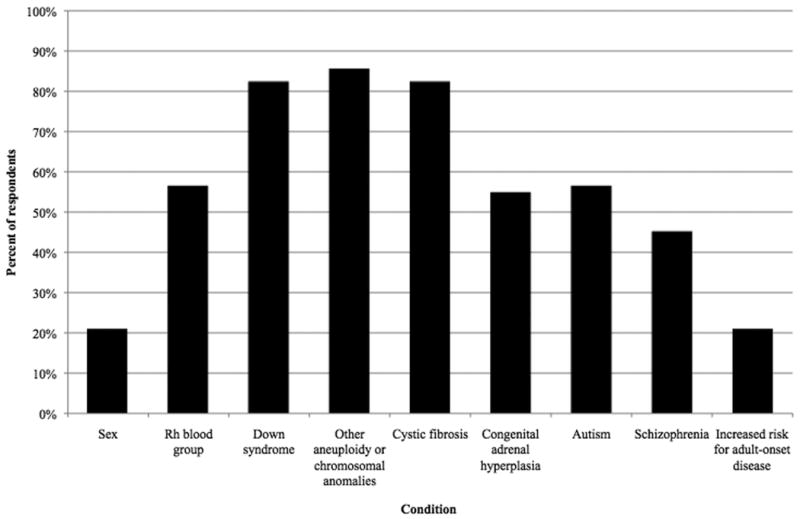
Respondents were probed regarding the specific genetic conditions for which they would offer cell-free fetal DNA testing, if these currently (mostly) hypothetical tests were to become available. While only 21% would offer sex testing and 56% would offer Rh blood group typing, a respective 82% and 85% would offer prenatal testing for Down syndrome and other chromosomal aneuploidies or anomalies (see Figure 2). Eighty-two percent would offer cystic fibrosis testing, whereas 55% would offer testing for congenital adrenal hyperplasia. Similarly, 56% of respondents would find it acceptable to offer prenatal genetic testing for autism, but a minority, 45%, would offer schizophrenia testing. Lastly, 21% would offer prenatal genetic tests for increased risk for adult-onset disorders.
Figure 2. If made available, for which conditions would you offer pregnant patients cell-free fetal DNA testing?
Practical considerations for cell-free fetal DNA testing
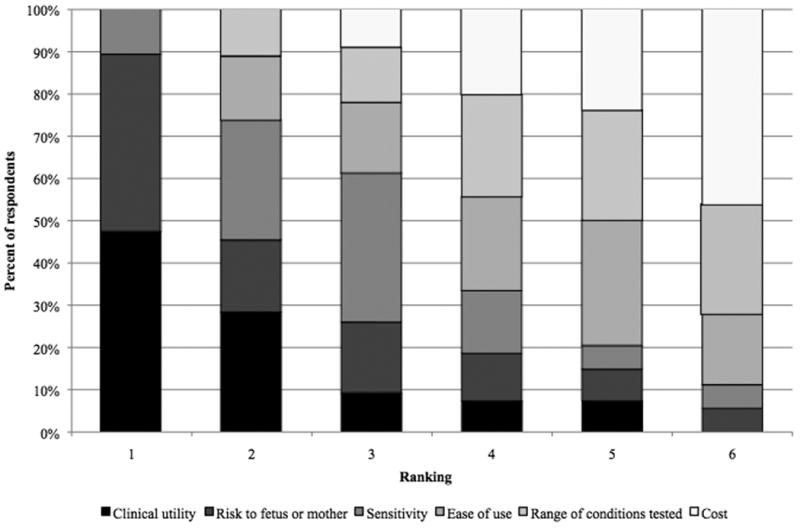
Respondents were asked to rank six aspects of prenatal genetic testing in order of their importance, with 1 being most important (see Figure 3). Clinical utility received a mean ranking of 1.98, followed by risk to fetus or mother with a ranking of 2.41 and test sensitivity with a ranking of 2.93. Ease of use, range of conditions, and cost have respective mean rankings of 4.17, 4.43, and 5.07. Of all respondents, 48% believe clinical utility to be the most important facet of testing although 43% say risk to fetus or mother is most important. Forty-six percent think cost is least important.
Figure 3. Rank the following aspects of prenatal genetic testing in order of their importance to you.
Respondents agree upon a number of means to successfully address these practical concerns about cell-free fetal DNA testing. Seventy percent of respondents suggest that they would follow the guidance of professional societies such as the American Congress of Obstetricians and Gynecologists in deciding whether to offer this testing. Approximately half believe that prenatal genetic tests should be regulated by a government agency. While a majority of respondents, 61%, think insurance companies have an obligation to pay for prenatal tests to ensure the best pregnancy outcomes, only 10% regard it as acceptable to offer cell-free fetal DNA testing on a direct-to-consumer basis. Forty-seven percent of respondents are not comfortable with direct-to-consumer cell-free fetal DNA testing.
Discussion
Integrating patient and provider values into prenatal testing
The attitudes of providers towards cell-free fetal DNA testing will be particularly important considering the pivotal role healthcare providers play, both deliberately and inadvertently, in the outcomes of patient decision-making (Anderson, 1999). As might be expected, obstetric healthcare providers are divided over whether patients desire more or less information about their pregnancy. This split may be due to genuinely differing information needs of unique patient populations, but likely also reflects uncertainty on the part of the providers as to what type of information and what level of detail patients actually desire and how this information might be used. Because of its non-invasiveness, and the decreased risk of obtaining fetal genetic data, cell-free fetal DNA testing may provide substantially larger volumes of information to a greater number of patients, making it imperative that the information needs of patients are explicitly addressed before consent is obtained.
Respondents nearly unanimously agree that it is appropriate for prenatal test results to play a significant role in patient decisions to continue or terminate a pregnancy, suggesting substantial value assigned by providers to the knowledge acquired from testing and comfort with therapeutic abortion on grounds of genetic conditions. Providers in this study also indicate a clear perception that patients face strong social pressures to undergo testing and that test results should have substantial impact on patient decision-making. Additional caution may be necessary to ensure that these opinions do not have undue influence on patient decisions to receive testing or continue or terminate a pregnancy.
In light of existing evidence that providers find communication of prenatal test results challenging, especially when termination is an option, guidelines should be developed that describe comprehensive, patient-centered means of presenting clinical options, outcomes, and implications of cell-free fetal DNA testing (Garel, Gosme-Seguret, Kaminski, and Cuttini, 2002). Those surveyed largely agree that genetic counseling is a necessary aspect of prenatal care, and this may be a significant means to ensure patient understanding and realization of wishes in the clinical setting, but also may represent a departure from standard practice for many physicians. The demand for counseling should be satisfied in part by trained genetic counselors, but due to their limited numbers, basic genetics education for all healthcare professionals will be essential (ACOG Committee Opinion No. 10; Biesecker, 1998). Educational materials, including innovative strategies such as computer decision-assisting tools, that offer relevant information about the options and significance of non-invasive prenatal testing in a clear and unbiased manner may also aid in patient decision-making. Education for both healthcare providers and patients is critical when a new technology such as cell-free fetal DNA testing becomes available in clinical practice.
Successfully introducing cell-free fetal DNA testing
Because of the limited scope of current clinical use of cell-free fetal DNA testing, it is not surprising that few respondents report a high level of knowledge of this technique. Given the uncertainty of the providers surveyed as to whether they will offer this type of testing in the coming years, it may be necessary to offer all providers comprehensive information about this technology and address the concerns of the wider provider population before the technology is further developed and introduced. Moreover, as our results suggest that cell-free fetal DNA testing will encourage testing of more patients and for more genetic conditions, ethical and practical concerns surrounding existing prenatal genetic testing will only be amplified if not preemptively addressed. In this study, providers prioritize clinical utility, test sensitivity, and risk to the fetus and mother as the most important aspects of testing.
Clinical utility and test sensitivity might be addressed through regulation of cell-free fetal DNA testing by a government agency, although only half of respondents agree with the prospect of such regulation. Previous studies have demonstrated reluctance on the part of healthcare providers for government regulation of assisted reproductive technologies, while emphasizing the importance of professional practice and ethics guidelines in provider decisions surrounding new reproductive technologies (Keye and Bradshaw, 2004). Indeed, a majority of respondents indicate that they would be more likely to offer cell-free fetal DNA testing if it was approved by a professional society.
Given the non-invasive nature of cell-free fetal DNA testing, concerns over risks of miscarriage are eliminated. Other negative outcomes may be mediated as described above, by ensuring providers are aware of patient values and risk perceptions during patient-provider interactions and decision-making. Genetic counseling will also play a critical role in the proper implementation of this technology, as it appears to be a highly desired factor in prenatal genetic testing settings and particularly before cell-free fetal DNA testing due to its potential as a one-step testing approach. The existing two-step framework - prenatal screening followed by invasive diagnostic testing - presents an opportunity for patients to receive detailed education and counseling about the range of possible diagnoses and their implications before diagnostic tests are performed. As non-invasive cell-free fetal DNA testing will, in theory, be diagnostic and not require confirmatory testing, counseling would need to occur before any testing is undertaken.
Respondents ranked cost as the least important facet of prenatal testing, perhaps because most express a belief that insurance companies have an obligation to fund testing and thus patients or providers would rarely bear the costs. Direct-to-consumer provision of cell-free fetal DNA testing is generally deemed inappropriate by respondents; assuming direct-to-consumer genetic testing in the United States continues in a largely unsupervised fashion, the unwillingness of providers to support such approaches may correspond to their clear prioritization of clinical utility and accuracy, neither of which is stringently regulated at the current time.
Despite advances over the past decade, further research and development of cell-free fetal DNA technology will be necessary to enable the implied intention of providers to offer a broad scope of non-invasive tests. Of the two tests that are currently available, RhD blood group typing is somewhat supported but sex testing is largely unwelcome by the providers we surveyed. As a general rule, providers express the strongest preference towards testing for those disorders that are currently recommended by professional societies, including Down syndrome and cystic fibrosis (ACOG Practice Bulletin No.77). However, given that providers in this study indicate a strong intention to comply with the recommendations of professional societies, the fact that one-fifth would offer sex determination testing is surprising, given its proscription (except in circumstances of sex-linked disease) by the medical community (ACOG Committee Opinion No. 360; Sex selection and preimplantation genetic diagnosis). Down syndrome and other aneuploidy tests are regarded the most favorably by respondents; this perspective is supported by recent studies demonstrating accuracy and reliability of cell-free fetal DNA testing for aneuploidy (Chiu et al., 2011; Ehrich et al., 2011; Sehnert et al., 2011). This finding is also in accordance with professional society recommendations to offer universal Down syndrome testing.
The majority of respondents also indicate a willingness to offer testing for cystic fibrosis, as is currently recommended, and for congenital adrenal hyperplasia. These are both single-gene disorders for which development of cell-free fetal DNA testing is less imminent although likely to be feasible (Ding et al., 2004; Lun et al., 2008; Tsang and Lo, 2010). Testing for autism and schizophrenia produced mixed responses from providers, but prenatal genetic tests for these conditions are also significantly less feasible given limited understanding of phenotypes and the complex contributions of genetic and environmental factors (Burmeister, 2006). Perhaps unsurprisingly, prenatal tests for increased risk of adult-onset diseases, for which there are few clinically useful tests even for adults, did not find positive reception from respondents.
With the recent mapping of the fetal genome and the expectation of non-invasive prenatal whole-genome sequencing, healthcare providers may soon be asked to deliberate over offering prenatal tests for an essentially unlimited range of genetic traits (Fan and Quake, 2010; Lo et al., 2010). Unlike with existing technologies, the rapid expansion of fetal traits that can be tested may force providers to make unprecedented decisions about the appropriate uses of this technology, to offer direction to patients about these expanded applications, and to establish professional guidelines as to which uses of cell-free fetal DNA testing are acceptable. Given the potential availability of testing for adult-onset, behavioral, or non-medical conditions, as well as genetic variations of uncertain clinical significance, where will they draw the line?
Directions for future research
This generalizability of these findings to diverse populations of healthcare providers is limited both by the study sample size and potential sampling bias. This study represents a small convenience sample of participants from a geographically-limited population keen to stay informed of advances in obstetric practice. While respondents to this survey represent providers from diverse healthcare settings, ages, and practice durations, females. physicians (as opposed to nurses and other allied health professionals), and California residents were overrepresented. Of note, previous studies have demonstrated marked differences in the nature of provider-patient conversations and decisional dynamics between male and female obstetricians as well as between obstetricians and nurse-midwives (Bernhardt et al., 1998; Geller and Holtzman, 1995). The low response rate to this survey, 34%, also may limit the generalizability of these findings by introducing response bias.
This pilot study has explored the opinions of obstetric healthcare providers surrounding cell-free fetal DNA testing based on questions previously raised in the literature and calls for more comprehensive investigation of these perspectives. Large-scale, nation-wide surveys might demonstrate whether the findings of this study are representative, while elucidating possible differences in opinion between demographic subgroups of providers. Furthermore, in-depth interviews and focus groups may provide a more textured understanding of the origins, details, and depth of the values and opinions as expressed by providers in this study. Finally, a comprehensive and iterative assessment of all stakeholder perspectives will be indispensable to the successful integration of social values with the consideration of the role of cell-free fetal DNA technology in prenatal care.
What's already known about this topic?
Non-invasive prenatal genetic testing using cell-free fetal DNA is becoming available for a broadening range of fetal traits and may raise significant practical and ethical concerns for obstetric healthcare providers.
What does this study add?
This study offers a preliminary assessment of provider attitudes towards this testing and presents some of their opinions and uncertainties surrounding its successful implementation.
Acknowledgments
This work was supported by NIH grant P50 HG003389 (Center for Integrating Ethics and Genetic Research).
References
- American Congress of Obstetricians and Gynecologists, Committee on Ethics. ACOG Committee Opinion No. 360: Sex selection. Obstet Gynecol. 2007;109:475–478. doi: 10.1097/00006250-200702000-00063. [DOI] [PubMed] [Google Scholar]
- American Congress of Obstetricians and Gynecologists, Committee on Ethics and Committee on Genetics. ACOG Committee Opinion No. 410: Ethical issues in genetic testing. Obstet Gynecol. 2008;111:1495–1502. doi: 10.1097/AOG.0b013e31817d252f. [DOI] [PubMed] [Google Scholar]
- American Congress of Obstetricians and Gynecologists, Committee on Practice Bulletins and Committee on Genetics; Society for Maternal-Fetal Medicine, Publications Committee. ACOG Practice Bulletin No. 77. Screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007;109:217–227. doi: 10.1097/00006250-200701000-00054. [DOI] [PubMed] [Google Scholar]
- American Society of Reproductive Medicine, Ethics Committee. Sex selection and preimplantation genetic diagnosis. Fertil Steril. 1999;72:595–598. [PubMed] [Google Scholar]
- Anderson G. Nondirectiveness in prenatal genetics: patients read between the lines. Nurs Ethics. 1999;6:126–136. doi: 10.1177/096973309900600205. [DOI] [PubMed] [Google Scholar]
- Benn PA, Chapman AR. Practical and ethical considerations of noninvasive prenatal diagnosis. JAMA. 2009;301:2154–2156. doi: 10.1001/jama.2009.707. [DOI] [PubMed] [Google Scholar]
- Bernhardt BA, Geller G, Doksum T, et al. Prenatal genetic testing: content of discussions between obstetric providers and pregnant women. Obstet Gynecol. 1998;91:648–655. doi: 10.1016/s0029-7844(98)00011-8. [DOI] [PubMed] [Google Scholar]
- Biesecker B. Future directions in genetic counseling: practical and ethical considerations. Kennedy Inst Ethics J. 1998;8:145–160. doi: 10.1353/ken.1998.0009. [DOI] [PubMed] [Google Scholar]
- Burmeister M. Genetics of psychiatric disorders: a primer. Focus. 2006;4:317–326. [Google Scholar]
- Chiu RWK, Akolekar R, Zheng YWL, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. Br Med J. 2011;342 doi: 10.1136/bmj.c7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Chiu RWK, Lau TK, et al. MS analysis of single-nucleotide differences in circulating nucleic acids: application to noninvasive prenatal diagnosis. Proc Natl Acad Sci U S A. 2004;101:10762–10767. doi: 10.1073/pnas.0403962101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen JM. Inserting government between patient and physician. N Engl J Med. 2004;350:178–179. doi: 10.1056/NEJMe038225. [DOI] [PubMed] [Google Scholar]
- Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:e1–11. doi: 10.1016/j.ajog.2010.12.060. [DOI] [PubMed] [Google Scholar]
- Fan HC, Quake SR. In principle method for noninvasive determination of the fetal genome. Nature Precedings. 2010 doi: 10.1038/npre.2010.5373.1. [DOI] [Google Scholar]
- Garel M, Gosme-Seguret S, Kaminski M, Cuttini M. Ethical decision-making in prenatal diagnosis and termination of pregnancy: a qualitative survey among physicians and midwives. Prenat Diagn. 2002;22:811–817. doi: 10.1002/pd.427. [DOI] [PubMed] [Google Scholar]
- Geller G, Holtzman NA. A qualitative assessment of primary care physicians' perceptions about the ethical and social implications of offering genetic testing. Qual Health Res. 1995;5:97–116. [Google Scholar]
- Kent A. Non-invasive prenatal diagnosis: public and patient perceptions. Semin Fetal Neonatal Med. 2008;13:109–112. doi: 10.1016/j.siny.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Keye WR, Jr, Bradshaw KD. A survey of the practices and opinions of the domestic members of the American Society for Reproductive Medicine. Fertil Steril. 2004;82:536–542. doi: 10.1016/j.fertnstert.2003.12.054. [DOI] [PubMed] [Google Scholar]
- Lo YMD, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- Lo YMD, Chan KCA, Sun H, et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001720. [DOI] [PubMed] [Google Scholar]
- Lun FMF, Tsui NBY, Chan KCA, et al. Noninvasive prenatal diagnosis of monogenic diseases by digital size selection and relative mutation dosage on DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008;105:19920–19925. doi: 10.1073/pnas.0810373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The President's Council on Bioethics. Reproduction and responsibility: the regulation of new biotechnologies. Washington, D.C.: 2004. [Google Scholar]
- Sehnert AJ, Rhees B, Comstock D, et al. Optimal detection of fetal chromosomal abnormalities by massively parallel DNA sequencing of cell-free fetal DNA from maternal blood. Clin Chem. 2011;57 doi: 10.1373/clinchem.2011.165910. [DOI] [PubMed] [Google Scholar]
- Smith RP, Lombaard H, Soothill PW. The obstetrician's view: ethical and societal implications of non-invasive prenatal diagnosis. Prenat Diagn. 2006;26:631–634. doi: 10.1002/pd.1476. [DOI] [PubMed] [Google Scholar]
- Tsang JCH, Lo YMD. Biology and diagnostic applications of cell-free fetal nucleic acids in maternal plasma. In: Kikuchi Y, Rykova EY, editors. Extracellular nucleic acids. Springer Berlin Heidelberg; Germany: 2010. pp. 147–166. [Google Scholar]
- Wright C. Cell-free fetal nucleic acids for non-invasive prenatal diagnosis: Report of the UK expert working group. PHG Foundation; United Kingdom: 2009. [Google Scholar]


