Abstract
The suprachiasmatic nucleus (SCN) has several structural characteristics and cell phenotypes shared across species. Here, we describe a novel feature of SCN anatomy that is seen in both hamster and mouse. Frozen sections through the SCN were obtained from fixed brains and stained for the presence of immunoreactivity to neuronal nuclear protein (NeuN-IR) using a mouse monoclonal antibody which is known to exclusively identify neurons. NeuN-IR did not identify all SCN neurons as medial NeuNIR neurons were generally not present. In the hamster, NeuN-IR cells are present rostrally, scattered in the dorsal half of the nucleus. More caudally, the NeuN-IR cells are largely, but not exclusively, scattered inside the lateral and dorsolateral border. At mid- to mid-caudal SCN levels, a dense group of NeuN-IR cells extends from the dorsolateral border ventromedially to encompass the central subnucleus of the SCN (SCNce). The pattern is similar in the mouse SCN. NeuN-IR does not co-localize with either vasopressin-, cholecystokinin- or vasoactive intestinal polypeptide-IR. In the hamster SCNce, numerous cells contain both calbindin- and NeuN-IR. The distribution of NeuN-IR cells in the SCN is unique, especially with regard to its generally lateral location through the length of the nucleus. The distribution of NeuN-IR cells is not consistent with most schemas representing SCN organization or with terminology referring to its widely accepted subdivisions. NeuN has recently been identified as Fox-3 protein. Its function in the SCN is not known, nor is it known why a large proportion of SCN cells do not contain NeuN-IR.
Keywords: SCN, circadian, anatomy, hamster, mouse
1. Introduction
Behavioral circadian rhythms are dependent upon the oscillatory activity of the suprachiasmatic nucleus (SCN) (Ralph et al., 1990; Silver et al., 1996a). The clock-like function of the SCN is thought to be derived from the oscillatory properties of individual cells linked together as a multicellular system (Welsh et al., 2010) . In fact, the system properties can overcome deficits in the molecular clockworks (Liu et al., 2007).
The system of interconnected neurons is gradually being explored (Drouyer et al., 2007; Kriegsfeld et al., 2004; LeSauter et al., 2002). Historically, a major focus has concerned the identification of cells defined by an immunohistochemically specific phenotype (van den Pol and Tsujimoto, 1985). Many, if not most, of the cells in the SCN contain at least one neuromodulatory peptide and are distributed in characteristic groups. These groups are fairly discrete, but do overlap. For example, vasopressinimmunoreactive (VP-IR) neurons are spread across the front pole of the SCN in a distribution that curves dorsomedially as it extends caudally (see (Morin et al., 2006)). Quite distinct from the group of VP cells is the set of vasoactive intestinal polypeptide (VIP) -IR cells which are distributed as a rounded pyramidal group in the centro-ventral part of the nucleus (Abrahamson and Moore, 2001; Moore et al., 2002; Morin et al., 2006; Silver et al., 1999). The locations of VP- and VIP-IR neurons tend to be conserved in eutherian mammals (Cassone et al., 1988) . The presence of such specific cell groupings focused attention on the possibility that they might have equally specific functions.
Initial physiological and behavioral tests of function related to cell type utilized the Brattleboro rat, a naturally occurring mutant unable to synthesize VP. Despite the fact that these rats have seemingly normal circadian rhythms (Peterson and Moore, 1980), interest in the functions of the various cell groups has persisted, as has the interest in identifying novel cell types in the SCN. Thus, one continuing and evolving perspective about the SCN expects function to be associated with cell neuromodulator identity and the associated, arguably specific, locations of those cells in the SCN. This view incorporates the spatial organization of SCN-afferent axon terminals and their associated functions, especially those arriving from the retina, the median raphe nucleus and the intergeniculate leaflet, to elaborate the general idea of a structure-function relationship based on the intrinsic organization of the SCN(Morin and Allen, 2006).
One reason that this perspective persists is the fact that the research focus has been on cell groups that overlap little or not at all. Thus, the SCN can be divided into several fairly discrete sectors according to cell phenotype: the VP region, the VIP region, a central region containing gastrin releasing peptide (GRP) neurons that overlaps somewhat with the more ventral VIP region, and a dorsolateral region where the cells do not have a known neuropeptide content (see (Morin and Allen, 2006; Morin et al., 2006) for a review.) This simplified description exists for rat, mouse and hamster, although it is not without its factual and theoretical difficulties (Morin, 2007). In the hamster, the central SCN region (SCNce) containing GRP cells is also identified by the location of substance P-, calretinin- and calbindin-IR (CalB-IR) neurons (Morin and Allen, 2006).
The present anatomical studies were borne of a need to re-evaluate the SCN in order to understand the consequences of chemical SCN lesions. Toward this end, cells were evaluated with histological markers for neurons or astrocytes. In particular, one antibody utilized was against neuronal nuclear protein (NeuN) which is found exclusively in neurons (Mullen et al., 1992). This protein has recently been identified as Fox-3 (Kim et al., 2009). NeuN-IR identifies the neurons of nearly all brain regions, although there are several locations in which such identification is not complete (cerebellar Purkinje cells, olfactory mitral cells, substantia nigra reticulata, among others) (Kumar and Buckmaster, 2007; Mullen et al., 1992)). In the SCN, NeuN-IR revealed a novel distribution of cells not consistent with the simple organizational description provided above and also failed to label a substantial proportion of SCN neurons. The present results, plus those from other recent studies involving different cell markers, suggest that the appreciation of SCN organization is substantially dependent upon the lens through which it is viewed and more complicated than suggested by simple models.
2. Results
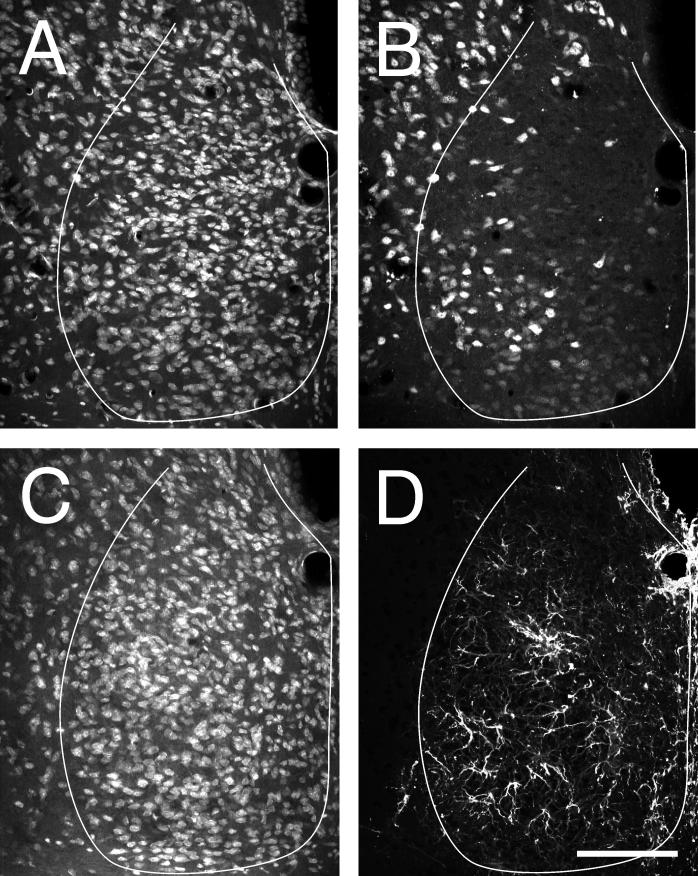
The unusual distribution of NeuN-IR cells is readily visible in the mid-caudal SCN level (Fig. 1A-C). All or nearly all cells are visualized with Nissl stain. In contrast, NeuNIR identifies only a subset of the Nissl-stained cells. These are moderately dense in the area corresponding to the SCNce and in a zone extending dorsally through the dorsolateral SCN. In adjacent hypothalamus, all or nearly all neurons are labeled by NeuN-IR. In medial SCN, NeuN-IR cells are almost completely absent, except for a few scattered cells.
Figure 1.
Coronal sections cut through the mid-caudal hamster SCN showing (A) NeuroTrace Nissl staining , (B) NeuN-IR, (C) Nissl staining, and (D) GFAP-IR. The SCN profile is outlined in white. All NeuN-IR cells were also positive for NeuroTrace Nissl. No cell labeled with GFAP-IR was stained, with certainty, by NeuroTrace Nissl. Bar = 100 μm.
Hamster SCN co-stained for Nissl substance and GFAP-IR revealed a moderately dense astrocytic fiber plexus throughout the nucleus. The fibers apparently extend from a small number of astrocyte cell bodies (Fig. 1D-F). These are sparse and could not be clearly identified as containing Nissl stain.
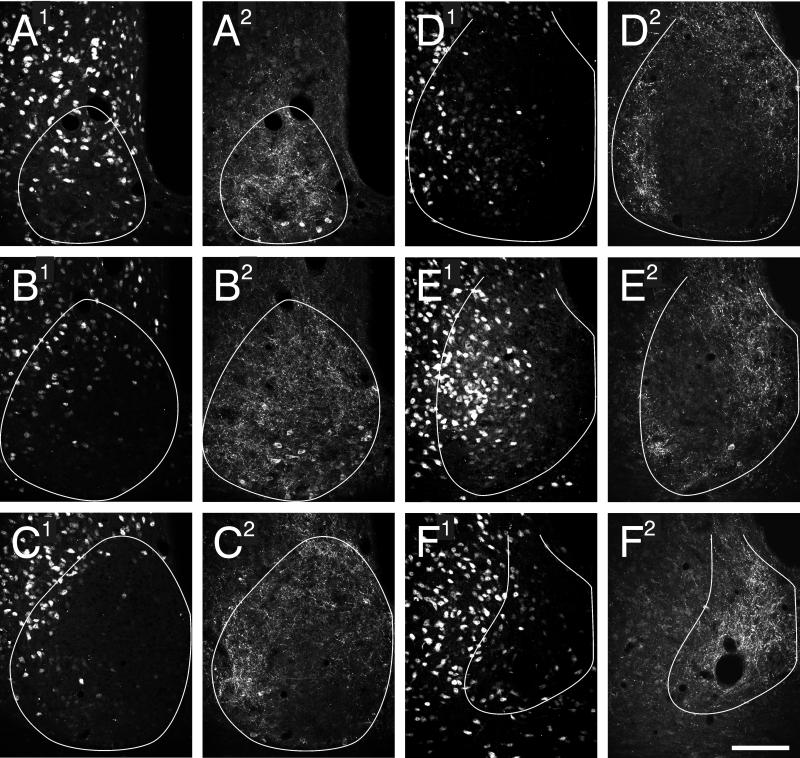
The complete SCN distribution of NeuN-IR in the hamster does not extend uniformly through the nucleus (Fig. 2). At the rostral pole (Fig. 2A), there is distributional overlap of NeuN-IR cells with CCK-IR cells. However, at the next caudal SCN level (Fig. 2B), the NeuN-IR cells are sparse in the lateral SCN and virtually absent from the medial SCN. The region of general NeuN-IR cell absence is occupied by CCK-IR cells and fibers. As the SCN extends caudally (Fig. 2C-E), the CCK-IR cells are found in dorsomedial and far ventrolateral locations, becoming exclusively dorsomedial in the most caudal part of the nucleus (Fig. 2F). In contrast, cells containing NeuN-IR become abundant in a region encompassing the centrolateral and dorsolateral SCN. This is particularly evident in the caudal third of the nucleus. The density and distribution of NeuN-IR cells in the dorsolateral SCN is contiguous with, and indistinguishable from, the population present in adjacent hypothalamus (i.e., there is no evident landmark among the NeuN-IR that separates SCN from non-SCN cells). Where the distributions of CCK- and NeuN-IR cells overlap, there is no co-localization of immunoreactivity.
Figure 2.
Hamster SCN in 6 rostral to caudal (A-F) coronal sections stained for NeuN- (A1-F1) and CCK-IR (A2-F2). There is no co-localization of NeuN- and CCK-IR in any cells. The SCN border is outlined in white. Bar = 100 μm.
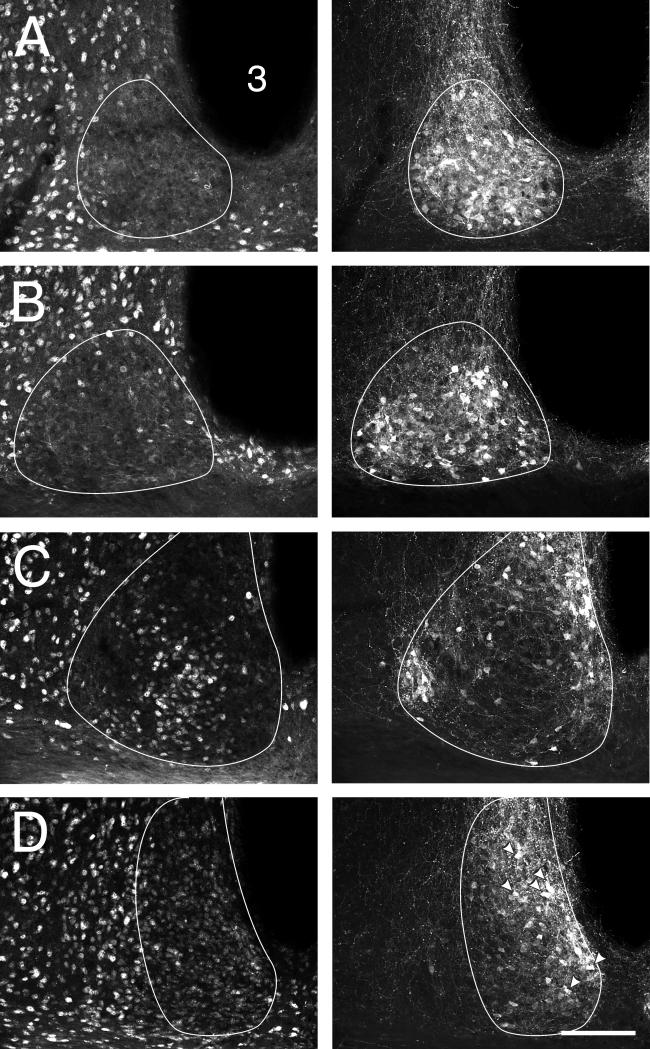
The more ventrolaterally located cluster of NeuN-IR cells found in the mid-caudal hamster SCN encompasses the SCNce as characterized by the presence of CalB-IR neurons (Fig. 3A,D). Many, but not all, of the CalB-IR cells both inside and outside the SCNce region exhibit co-localized NeuN-IR. In the SCN, such co-localization is evident in about half the CalB-IR neurons irrespective of exact location.
Figure 3.
Coronal sections through the mid-caudal hamster SCN showing double label in neurons immunoreactive to(A) NeuN and (B) CalB in the SCNce and for (C) NeuN and (D) VIP-IR. All cells in (B) that show clear co-localization (arrowheads) of CalB- and NeuN-IR. No cells co-localize NeuN- and VIP-IR and the distributions overlap very little. The SCN border line is outlined in white. Bars = 100 μm.
VIP-IR neurons are abundant across the ventral mid-rostral hamster SCN. At this level (Fig. 3C,D), the NeuN-IR cells are generally not apparent in the central or medial part of the nucleus. Rather, they are found in a loose distribution extending from dorsal to ventral just inside the lateral SCN border. The ventral extension of this distribution overlaps with the region containing VIP-IR cells without evident co-localization.
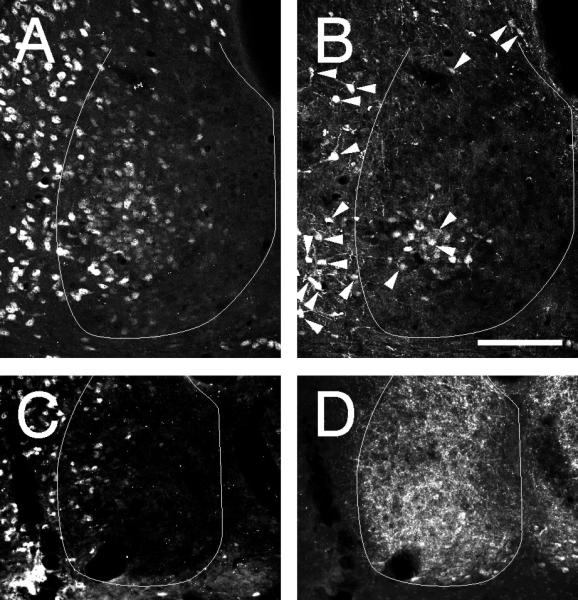
In the mouse, the distribution of NeuN-IR cells is also not uniform throughout the SCN. At the rostral pole, such cells are generally absent with the same region filled with abundant VP-IR neurons (Fig. 4A). More caudally (Fig. 4B,C), there are many NeuN-IR cells in a distribution that extends from the dorsolateral border to the ventral border with a broader cluster in the central area. NeuN-IR cells outside the SCN are abundant and where the SCN-intrinsic cell distribution abuts the SCN-extrinsic population, there is no obvious indication of a distinction between them and no indication of an SCN border. In most of the mouse SCN, cells immunoreactive for VP are found in regions not containing NeuN-IR. As a consequence, there can be no co-localization of antigens. However, this generalization is not true caudally where both NeuN-IR and VP-IR cells are abundant dorsomedially (Fig. 4D). Here, both VP- and NeuN-IR are commonly co-localized (Fig. 4D Right). Similar results for VP- and NeuN-IR distributions were found in the hamster (not shown).
Figure 4.
Distributions of NeuN- (left column) and VP-IR (right column) cells in 4 rostral (A) to caudal (D) levels of mouse SCN. Co-localization of NeuN- and VP-IR only in level D where the two distributions overlap (arrowheads in (D, right column) indicate some of the cells with co-localization). 3 = third ventricle. Bar = 100 μm.
3. Discussion
Analysis of hamster and mouse SCN anatomy using an antibody against a purported neuron-specific protein, NeuN, provides a novel perspective on organization of the nucleus comprising the master circadian clock. NeuN-IR cells are densely distributed in a large proportion of the SCN. This region includes much of the centro-lateral SCN and the central SCN, and extends dorsally to include the dorsolateral part of the nucleus. This pattern is evident in mouse and hamster (present data) and there may be a similar pattern in the rat SCN (Geoghegan and Carter, 2008).
3.1 SCN Neuroanatomy
Across mammalian species, the SCN has been shown to include a wide variety of immunohistochemically identified cell groups. The extent of this variability contrasts with the singular SCN function as master circadian clock (Morin and Allen, 2006). Generally, there are two constant anatomical features to the nucleus. First, VIP-IR neurons are present in a smoothed pyramid-like formation with its base spread across the ventral part of the mid-anterior nucleus. Second, a more widely distributed set of VP-IR neurons extends across the rostral pole of the SCN, then dorsomedially and caudally into the caudal dorsomedial SCN (e.g., (Morin et al., 2006)). VP-IR cells also tend to be scattered elsewhere in the SCN, particularly in the mouse. CCK-IR cells have a distribution similar to that for VP-IR neurons, but are also found ventrolaterally (LeSauter et al., 2002; Silver et al., 1999). CCK has been less widely studied than VP and the extent to which it is present in the SCN of diverse species is not known. In the context of the present results, the presence of CCK- (hamster) and VP-IR (mouse and hamster) cells is generally associated with an absence of NeuN-IR cells in the same area. There are exceptions, however. As an example, the more caudal, dorsomedial area in which VP-IR cells are found in the mouse also contains numerous NeuN-IR neurons. The presence NeuN-IR cells therefore does not necessarily preclude the presence of VP-IR cells. Further, the presence of NeuN-IR in specific cells does not preclude the simultaneous presence of VP-IR as evidenced by cellular co-localization of the two reaction products in the area in which the two cell types overlap.
Consistent with these observations, NeuN-IR neurons in the rat SCN are concentrated ventrally, away from the VP-IR cells in the dorsomedial region (Geoghegan and Carter, 2008). Additional between-species comparisons are difficult because direct tests for NeuN co-localization with other typical neuropeptides have not been performed in the rat or mouse SCN. Nevertheless, NeuN-IR cells distribute in the same region as doublecortin (DCX) -IR neurons and the two antigens are modestly co-localized. DCX-IR is present in all GRP-IR neurons (although not all DCX-IR cells contain GRP-IR) and a small percentage of VIP-IR neurons of the rat SCN (Geoghegan and Carter, 2008). DCX has no known function in the SCN, but is thought to regulate the migration of neurons via the promotion of neurite extension in perinatal animals (Gdalyahu et al., 2004; Moores et al., 2006).
Several species also have a central subnucleus in the SCN (SCNce) characterized by the presence of GRP-IR neurons, and often other neuropeptides (Mikkelsen et al., 1991; Silver et al., 1999; Smale et al., 1991; Smale and Boverhof, 1999; Tanaka et al., 1997). GRP-IR cells occupy this region in mouse, hamster and rat (Abrahamson and Moore, 2001; Moore et al., 2002; Morin and Allen, 2006; Schilling and Nurnberger, 1998). NeuN-IR cells fully encompass the SCNce, here identified by the presence of CalB-IR in the hamster, and there is modest co-localization of the two antigens. NeuN-IR is found in nearly all cells adjacent to the SCN. Cells immunoreactive to both NeuN- and CalB-IR and to each antigen separately are common within the SCNce and in adjacent hypothalamus.
The hamster SCNce, characterized by the presence of GRP-, SP-, CalB- and CALR-IR neurons (Morin and Allen, 2006), sits within dense plexes consisting of NPY-, ENK-, neurotensin- and GABA-IR terminals arriving from the thalamic intergeniculate leaflet via the geniculohypothalamic tract (Morin and Blanchard, 2001). The SCNce receives modest serotonergic input from the median raphe nucleus, with a dense plexus of such innervation present in an arc curving medially from dorsomedial to ventrolateral (Meyer-Bernstein and Morin, 1996). The third major source of SCN afferents are the melanopsin-containing, intrinsically photoreceptive ganglion cells of the retina(Belenky et al., 2003; Gooley et al., 2001; Hattar et al., 2006; Morin et al., 2003). This terminal plexus innervates virtually all the SCN, although density varies substantially with SCN region (Hattar et al., 2006; Morin et al., 2006; Muscat et al., 2003). In the hamster, the densest input from the contralateral retina generally corresponds to the SCN region containing most of the NeuN-IR cells.
The region dorsal and dorsolateral to the SCNce generally does not contain VP or VIP neurons, but does contain cells identifiable by GRP-induced FOS protein and p-ERK expression (Antle et al., 2005; Lee et al., 2003). In the context of the present data, this area appears to be substantially equivalent to the portion of the NeuN-IR cell distribution dorsal to the SCNce.
In anatomical studies, the focus tends to be on the stained cells and their locations. However, regions devoid of such cells may be of equal importance. In the present studies, there are areas not containing cell clusters identifiable by either NeuNIR or by any other marker that was tested. One such place is the ventromedial SCN in the middle SCN sections (Fig. 2C,D, negative for both NeuN- and CCK-IR, hamster and Fig. 4C, negative for both NeuN- and VP-IR, mouse).
The present data add to the still-emerging picture of SCN intrinsic anatomy, with the immunoreactivity to NeuN demonstrating several novel features. Foremost of these is the apparent restriction of NeuN-IR to a unique distribution within the SCN. Only a subset of all SCN neurons is identified by NeuN-IR, with the large majority of the subset found in a region corresponding closely to a area described as containing neurons rhythmically expressing the Magel2 gene (Kozlov et al., 2007). The ventral portion of the Magel2 gene expression region appears to extend well into the area known to contain large numbers of GRP-IR cells (Abrahamson and Moore, 2001; Silver et al., 1999), i.e., the putative homolog of the hamster SCNce.
The distribution of cells immunoreactive to the recently discovered third member of the globin family, neuroglobin (Ngb), has been described in the rat SCN (Hundahl et al., 2010). Although the distribution of Ngb-IR cells has unique features, it shares certain characteristics with the distribution NeuN-IR cells. Most notably, the Ngb-IR cells are present laterally from dorsal to ventral and there is very little co-localization with VP. The Ngb-IR cell distribution differs significantly from that for NeuN-IR cells in that most of the Ngb-IR neurons are found in two distinct, but connected, clusters. One is dorsal to, and one overlapping with, the region of GRP-IR cells ventrally. Ngb-IR substantially co-localizes with GRP-IR. Notably, the region of GRP-IR cells in the rat, as in the mouse, may be homologous to the hamster SCNce.
NeuN-IR appears to identify only neurons in the SCN and not astrocytes (present data and (Lindley et al., 2008)). Elsewhere in the brain, NeuN-IR is not found in any form of glia (Mullen et al., 1992). It appears to be neuron-specific, but fails to identify all neurons. Exceptions similar to those observed here have been documented in diverse brain regions and cell types. NeuN-IR is absent from cerebellar Purkinje cells, olfactory bulb mitral cells, Cajal-Retzius neocortical cells and neurons of the inferior olive, among others (see (Kumar and Buckmaster, 2007) for a more complete list). Furthermore, within a specific brain region, there may be differences between species with respect to the identity of cells in which NeuN-IR is present (Kumar and Buckmaster, 2007). In the SCN, the regions relatively devoid of NeuN-IR cells could be either the result of diminished signal resulting from reduced antigenicity (Unal-Cevik et al., 2004) or from differences in normal physiology, possibly related to prolonged cell depolarization (Lind et al., 2005), that might yield reduced protein.
Recent research identifying NeuN protein as Fox-3 has provided a clue as to its molecular function (Kim et al., 2009). Fox-3 is thought to be a neuron-specific alternative splicing regulator (Kim et al., 2009; Kim et al., 2011). The significance of Fox-3 for normal brain function is not known nor is there any knowledge concerning its function in the SCN, or why its distribution is limited to a particular sector of the SCN.
3.2 Nomenclature and SCN
We have previously suggested that widely accepted “divisions” of the SCN may be more apparent than real (Morin and Allen, 2006; Morin, 2007). The most common of these have been labeled the “core”/“shell” or the “ventrolateral”/“dorsomedial” regions. In addition, a variety of other SCN regions have been generated after application of different defining criteria or different environmental procedures. Here we show that large numbers of NeuN-IR cells are found in both a dorsal “shell” area and an area consistent with part of the SCN “core.” As described above, this is also true for the distributions of cells expressing the Magel2 gene or containing Ngb-IR. In the alternative terminology, the distributions of these three cellular markers are found both in the “dorsomedial” and “ventrolateral divisions” of the SCN. We strongly prefer use of neuroanatomical descriptors that indicate the cell locations relative to morphological features, such as the midline, optic chiasm or SCN boundary, rather than those that vary from lab to lab according to local criteria.
The question of functional subdivisions has become one of substance, especially with the advent of tools that enable detection of gene expression. For example, cells expressing Per1 gene activity are present throughout the entire SCN, but light-induced Per1is found in a smaller area (Shigeyoshi et al., 1997; Yan et al., 1999). Not until recently has there been consideration of the possibility that the area of light-induced Per1 expression could be, at least in part, the product of photoperiod history (Edelstein et al., 2003; Lee et al., 2009; Schwartz et al., 2009; Watanabe et al., 2006; Yan et al., 2010) or gonadal activity (Karatsoreos et al., 2011). In addition to the foregoing, size of a nominal “region” (e.g., that containing cells with VP gene expression) may vastly expand to include almost the entire SCN, depending upon the time of day (Hamada et al., 2004). Thus, activity of particular SCN cell groups appears to be plastic and subject to considerable stimulus-induced change. The data imply that “divisions” of the SCN are dynamic, rather than static (Morin, 2007). The present data support the view that the borders of putative SCN divisions are determined largely by the nature of the lens through which individual investigators examine the available evidence.
4. Experimental Procedure
Adult, outbred, male hamsters (Lakeview/Charles River) and male C57BL6/J were individually housed in 45 L × 20 W × 20 H cm clear polycarbonate cages equipped with corncob bedding. The prevailing photoperiod was LD14:10, with food and water continuously available. All procedures were in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of Stony Brook University.
4.1 Histology
To obtain brains for histology, animals were anesthetized with pentobarbital and perfused transcardially with physiological saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, with sodium m-periodate (0.01 M) and l-lysine (0.075 M) added. Brains were removed and postfixed overnight at 4°C, cryoprotected in 20% sucrose. Prior to sectioning, each brain was blocked and cut at 30 μm in the coronal plane through the SCN on a freezing stage microtome. Tissue sections were collected in 0.01M phosphate buffered saline (PBS)-azide (pH 7.4). The series of free-floating sections were incubated with a mixture of two primary antisera from different animals (Table 1) for 48-72 hours at 4°C. The sections were then washed for 2 hrs with several changes of PBS, before being incubated in a mixture of secondary antisera comprised of donkey anti-mouse Texas Red and donkey anti-rabbit or donkey anti-guinea pig CY2, (dilution 1:200; Jackson ImmunoResearch, West Grove PA) for 2 hrs at 20°C. After processing, sections were mounted on gelatin-coated slides, air dried, and coverslipped with Krystalon (Diagnostic Systems, Inc., Gibbstown, NJ). Digital black and white images of the SCN in each section were obtained using a Zeiss Axiocam on an Axioplan 2 microscope equipped for epifluorescence). Images were saved in 24 bit RGB pseudocolor, TIFF formatted files and adjusted for contrast, brightness and intensity with Corel Photo-Paint 12 software (Corel Corp., Ottawa, Ontario, Canada).
Table 1.
Antisera and their documented usage in hamster brain tissue.
| Antigen | Host | Manufacturer | Dilution |
|---|---|---|---|
| aCalB | rabbit | Chemicon, Temecula, CA | 1:50 |
| bCCK | rabbit | DiaSorin, Stillwater, MN | 1:500 |
| cGFAP | rabbit | DAKO, Carpinteria, CA | 1:1000 |
| dNeuN | mouse | Chemicon, Temecula, CA | 1:100 |
| eNPY | rabbit | Peninsula, Laboratories, San Carlos, CA | 1:1000 |
| fVIP | rabbit | Peninsula, Laboratories, San Carlos, CA | 1:500 |
| gVP | guinea pig | Peninsula, Laboratories, San Carlos, CA | 1:500 |
4.2 Antisera and stains
Green NeuroTrace Nissl (Invitrogen, Carlsbad, CA; stock diluted 1:800) was used to stain Nissl substance. DAPI (Invitrogen, Carlsbad, CA; 3 μM) was used to stain cell nuclei. The hamster SCN contains cells and axon terminal fields immunoreactive for calbindin (CalB), vasoactive intestinal polypeptide (VIP), and vasopressin (VP) with distributions that have been well-documented using a variety of antisera (e.g., (Card and Moore, 1984; Silver et al., 1996b)). With the exception of a monoclonal antibody against neuronal nuclear protein (NeuN), all antisera employed in these studies have been in regular, successful use with hamster tissue (Table 1). The NeuN antibody is now known to recognize the Fox-3 antigen (Kim et al., 2009). A series of tissue sections from at least two different brains was processed for each of the various combinations (see Results) of immunohistochemical staining.
Highlights.
Neuronal nuclear protein (NeuN) is present in roughly half of suprachiasmatic nucleus (SCN) neurons.
Their distribution is unique among all neuronal phenotypes described in the SCN.
The NeuN-IR does not co-localize with vasopressin-, cholecystokinin- or vasoactive intestinal polypeptide-IR.
NeuN-IR does co-localize with calbindin-IR in some, but not all, cells of the SCN central subnucleus in the hamster.
The NeuN-IR cell distributions are similar in hamster and mouse.
5. Acknowledgements
Supported by NIH grants NS22168 and NS061804 to LPM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Research. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Antle MC, Kriegsfeld LJ, Silver R. Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J Neurosci. 2005;25:2447–2454. doi: 10.1523/JNEUROSCI.4696-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J.Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Morin LP. Ontogeny of radial glia, astrocytes and vasoactive intestinal peptide immunoreactive neurons in hamster suprachiasmatic nucleus. Dev.Brain Res. 1995;86:48–56. doi: 10.1016/0165-3806(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13:390–396. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Speh JC, Card JP. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. Journal of Biological Rhythms. 1988;3:71–91. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of suprachiasmatic nucleus neurons to light and dark adaptation: relative contributions of melanopsin and rod-cone inputs. Journal of Neuroscience. 2007;27:9623–9631. doi: 10.1523/JNEUROSCI.1391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein K, De la Iglesia HO, Mrosovsky N. Period gene expression in the suprachiasmatic nucleus of behaviorally decoupled hamsters. Brain Res.Mol.Brain Res. 2003;114:40–45. doi: 10.1016/s0169-328x(03)00130-x. [DOI] [PubMed] [Google Scholar]
- Gdalyahu A, Ghosh I, Levy T, Sapir T, Sapoznik S, Fishler Y, Azoulai D, Reiner O. DCX, a new mediator of the JNK pathway. EMBO J. 2004;23:823–832. doi: 10.1038/sj.emboj.7600079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan D, Carter DA. A novel site of adult doublecortin expression: neuropeptide neurons within the suprachiasmatic nucleus circadian clock. BMC.Neurosci. 2008;9:2. doi: 10.1186/1471-2202-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Watanabe M, Fedorkova L, Shen H, Ungers G, Rutishauser U. Dynamic regulation of polysialylated neural cell adhesion molecule in the suprachiasmatic nucleus. Neuroscience. 2003;117:203–211. doi: 10.1016/s0306-4522(02)00817-5. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neuroscience. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Hamada T, Antle MC, Silver R. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. European Journal of Neuroscience. 2004;19:1741–1748. doi: 10.1111/j.1460-9568.2004.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundahl CA, Hannibal J, Fahrenkrug J, Dewilde S, Hay-Schmidt A. Neuroglobin expression in the rat suprachiasmatic nucleus: colocalization, innervation, and response to light. J Comp Neurol. 2010;518:1556–1569. doi: 10.1002/cne.22290. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Butler MP, LeSauter J, Silver R. Androgens Modulate Structure and Function of the Suprachiasmatic Nucleus Brain Clock. Endocrinology. 2011 doi: 10.1210/en.2010-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. The Journal of biological chemistry. 2009;284:31052–61. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kim YC, Adelstein RS, Kawamoto S. Fox-3 and PSF interact to activate neural cell-specific alternative splicing. Nucleic acids research. 2011;39:3064–78. doi: 10.1093/nar/gkq1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov SV, Bogenpohl JW, Howell MP, Wevrick R, Panda S, Hogenesch JB, Muglia LJ, Van Gelder RN, Herzog ED, Stewart CL. The imprinted gene Magel2 regulates normal circadian output. Nat.Genet. 2007;39:1266–1272. doi: 10.1038/ng2114. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J.Comp Neurol. 2004;468:361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Neuron-specific nuclear antigen NeuN is not detectable in gerbil subtantia nigra pars reticulata. Brain Research. 2007;1142:54–60. doi: 10.1016/j.brainres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nature Neuroscience. 2003;6:111–112. doi: 10.1038/nn1006. [DOI] [PubMed] [Google Scholar]
- Lee ML, Swanson BE, De la Iglesia HO. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus. Current Biology. 2009;19:848–852. doi: 10.1016/j.cub.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Kriegsfeld LJ, Hon J, Silver R. Calbindin-D 28K cells selectively contact intra-SCN neurons. Neuroscience. 2002;111:575–585. doi: 10.1016/s0306-4522(01)00604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phophorylated antigen with discrete subcellular localization. Journal of Neuroscience Research. 2005;79:295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- Lindley J, Deurveilher S, Rusak B, Semba K. Transforming growth factor-alpha and glial fibrillary acidic protein in the hamster circadian system: daily profile and cellular localization. Brain Research. 2008;1197:94–105. doi: 10.1016/j.brainres.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, III, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant EG, Morin LP. The hamster circadian rhythm system includes nuclei of the subcortical visual shell. Journal of Neuroscience. 1999;19:10482–10493. doi: 10.1523/JNEUROSCI.19-23-10482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. Journal of Neuroscience. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Larsen PJ, O'Hare MMT, Wiegand SJ. Gastrin releasing peptide in the rat suprachiasmatic nucleus: An immunohistochemical, chromatographic and radioimmunological study. Neuroscience. 1991;40:55–66. doi: 10.1016/0306-4522(91)90174-m. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- Moores CA, Perderiset M, Kappeler C, Kain S, Drummond D, Perkins SJ, Chelly J, Cross R, Houdusse A, Francis F. Distinct roles of doublecortin modulating the microtubule cytoskeleton. EMBO J. 2006;25:4448–4457. doi: 10.1038/sj.emboj.7601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Moore RY. Intergeniculate leaflet and suprachiasmatic nucleus organization and connections in the hamster. Visual Neuroscience. 1992;8:219–230. doi: 10.1017/s095252380000287x. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH. Organization of the hamster paraventricular hypothalamic nucleus. Journal of Comparative Neurology. 1993;332:341–357. doi: 10.1002/cne.903320307. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH. Neuromodulator content of hamster intergeniculate leaflet neurons and their projection to the suprachiasmatic nucleus or visual midbrain. Journal of Comparative Neurology. 2001;437:79–90. doi: 10.1002/cne.1271. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet and visual midbrain: bifurcation and melanopsin immunoreactivity. Journal of Comparative Neurology. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res.Rev. 2006 doi: 10.1016/j.brainresrev.2005.08.003. In Press. [DOI] [PubMed] [Google Scholar]
- Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137:1285–1297. doi: 10.1016/j.neuroscience.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Morin LP. SCN organization reconsidered. Journal of Biological Rhythms. 2007 doi: 10.1177/0748730406296749. in press. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Muscat L, Huberman AD, Jordan CL, Morin LP. Crossed and uncrossed retinal projections to the hamster circadian system. J.Comp Neurol. 2003;466:513–524. doi: 10.1002/cne.10894. [DOI] [PubMed] [Google Scholar]
- Peterson GM, Moore RY. Selective effects of kainic acid on diencephalic neurons. Brain Research. 1980;202:165–182. [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Schilling J, Nurnberger F. Dynamic changes in the immunoreactivity of neuropeptide systems of the suprachiasmatic nuclei in golden hamsters during the sleep-wake cycle. Cell Tissue Res. 1998;294:233–241. doi: 10.1007/s004410051173. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Wotus C, Liu T, Friesen WO, Borjigin J, Oda GA, De la Iglesia HO. Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc.Natl.Acad.Sci.U.S.A. 2009;106:17540–17545. doi: 10.1073/pnas.0906382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996a;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak RK, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport. 1996b;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- Silver R, Sookhoo AI, LeSauter J, Stevens P, Jansen HT, Lehman MN. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. Neuroreport. 1999;10:3165–3174. doi: 10.1097/00001756-199910190-00008. [DOI] [PubMed] [Google Scholar]
- Smale L, Blanchard JH, Moore RY, Morin LP. Immunocytochemical characterization of the suprachiasmatic nucleus and the intergeniculate leaflet in the diurnal ground squirrel, Spermophilus lateralis. Brain Research. 1991;563:77–86. doi: 10.1016/0006-8993(91)91517-5. [DOI] [PubMed] [Google Scholar]
- Smale L, Boverhof J. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. Journal of Comparative Neurology. 1999;403:190–208. doi: 10.1002/(sici)1096-9861(19990111)403:2<190::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hayashi S, Tamada Y, Ikeda T, Hisa Y, Takamatsu T, Ibata Y. Direct retinal projections to GRP neurons in the suprachiasmatic nucleus of the rat. Neuroreport. 1997;8:2187–2191. doi: 10.1097/00001756-199707070-00020. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Research. 2004;1015:169–174. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Naito E, Nakao N, Tei H, Yoshimura T, Ebihara S. Bimodal clock gene expression in mouse suprachiasmatic nucleus and peripheral tissues under a 7-hour light and 5-hour dark condition. Journal of Biological Rhythms. 2006 doi: 10.1177/0748730406295435. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu.Rev.Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003;73:400–409. doi: 10.1002/jnr.10655. [DOI] [PubMed] [Google Scholar]
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience. 1999;94:141–150. doi: 10.1016/s0306-4522(99)00223-7. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R, Gorman M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J Biol.Rhythms. 2010;25:19–27. doi: 10.1177/0748730409352054. [DOI] [PMC free article] [PubMed] [Google Scholar]