Abstract
Tbx2 and Tbx3 are closely related members of the T-box family of transcription factors that are important regulators during normal development as well as major contributors to human developmental syndromes when mutated. Although there is evidence for the involvement of Tbx2 and Tbx3 in pancreatic cancer, so far there are no reports characterizing the normal expression pattern of these genes in the pancreas. In this study, we examined spatial and temporal expression of Tbx2 and Tbx3 in mouse pancreas during development and in the adult using in situ hybridization and immunohistochemistry. Our results show that Tbx2 and Tbx3 are both expressed in the pancreatic mesenchyme throughout development beginning at embryonic day (E) 9.5. In addition, Tbx2 is expressed in pancreatic vasculature during development and in epithelial-derived endocrine and ductal cells during late fetal stages, postnatal development and in adult pancreas. In contrast, Tbx3 is expressed in exocrine tissue in the postnatal and adult pancreas. Further our results demonstrate that Tbx2 and Tbx3 are expressed in tumor-derived endocrine and exocrine cell lines, respectively. These dynamic changes in the expression pattern of these transcription factors lay the foundation for investigation of potential roles in pancreas development.
Keywords: Tbx2, Tbx3, T-box, Pancreas, Islets
Tbx2 and Tbx3 are closely related members of the Tbx2 subfamily of the T-box transcription factor family that play essential roles in cell proliferation, cell fate and tissue identity during development (Papaioannou, 2001; Papaioannou and Silver, 1998). Tbx2 and Tbx3 share over 90% sequence identity in their DNA binding domain (Agulnik et al., 1996) and during embryonic development, Tbx2 and Tbx3 have overlapping expression patterns in many tissues (Chapman et al., 1996; Gibson-Brown et al., 1998). Targeted mutagenesis studies have revealed crucial roles for both genes during embryonic development. Mice homozygous for a Tbx2 null mutation show lethal cardiovascular defects and abnormal limb development (Harrelson et al., 2004; Suzuki et al., 2004). Mice homozygous for a Tbx3 null mutation die during midgestation with abnormalities in the yolk sac, limbs, mammary glands and heart (Davenport et al., 2003; Mesbah et al., 2008).
In addition to important roles in development, increasing evidence suggests a role for these two genes in a variety of cancers, including pancreatic cancers (Fan et al., 2004; Jacobs et al., 2000; Mahlamäki et al., 2002; Prince et al., 2004; Sinclair et al., 2002; Vance et al., 2005). Tbx2 was amplified in 50% of 31 pancreatic cell lines tested (Mahlamäki et al., 2002) and Tbx2 protein and RNA expression was found in human pancreatic tumors (Chen et al., 2008; Duo et al., 2009). There is also a report that Tbx3 is over expressed in metastatic endocrine neoplasm's (Hansel et al., 2004). As pancreatic cancer is the fourth leading cause of cancer death in the United States (Postier, 2003) a new biomarker could be valuable for early detection of the disease.
In recent years, several reports identified Tbx3 as one of the self renewal regulators of embryonic stem cells (ESC), as well as a factor increasing the germ-line competency of induced pluripotent stem cells (iPSC) (Galan-Caridad et al., 2007; Han et al., 2010; Ivanova et al., 2006). Thus, characterization of Tbx3 and the closely related Tbx2 in the developing pancreas could provide valuable information on the control of self renewal of pancreatic progenitor cells and the differentiation of cells from ES cells.
Although there is one report of Tbx3 expression in pancreatic mesenchyme at E 9.5 (Zhou et al., 2007), no detailed expression has been previously reported. In this study, we examine the spatial and temporal expression of Tbx2 and Tbx3 in mouse pancreas throughout development and in the adult using a combination of in situ hybridization (ISH), immunohistochemistry (IHC) and comparison of expression with known markers of differentiation. Our results show that Tbx2 and Tbx3 are both expressed in the pancreatic mesenchyme throughout development beginning at E9.5. In addition, Tbx2 is expressed in pancreatic vasculature during development and in epithelial-derived endocrine and ductal cells during late fetal stages, postnatal development and in the adult pancreas. In contrast, Tbx3 is expressed in exocrine tissue in the postnatal and adult pancreas. Additional studies demonstrate that Tbx2 and Tbx3 are expressed in tumor-derived endocrine and exocrine cell lines, respectively.
1. Results and discussion
1.1.Mesenchymal expression of Tbx2 and Tbx3 during budding and early morphogenesis of the pancreas
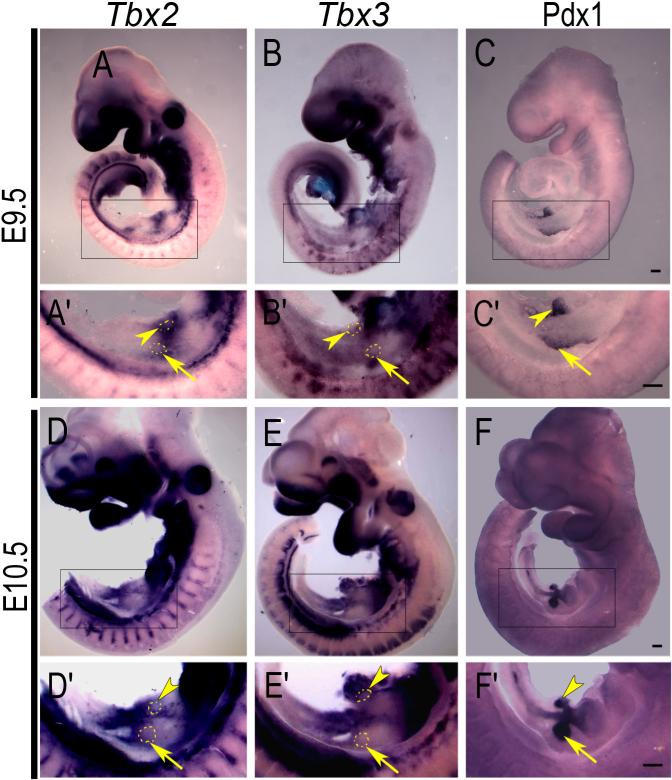
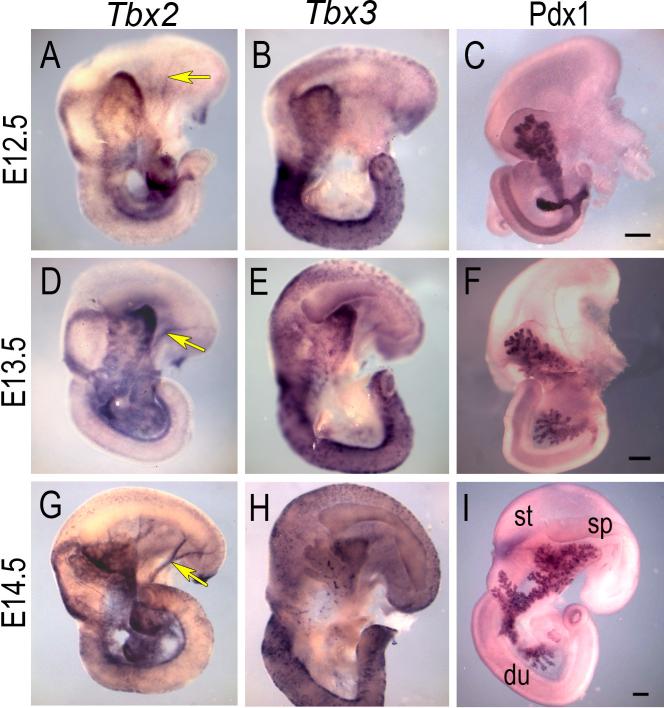
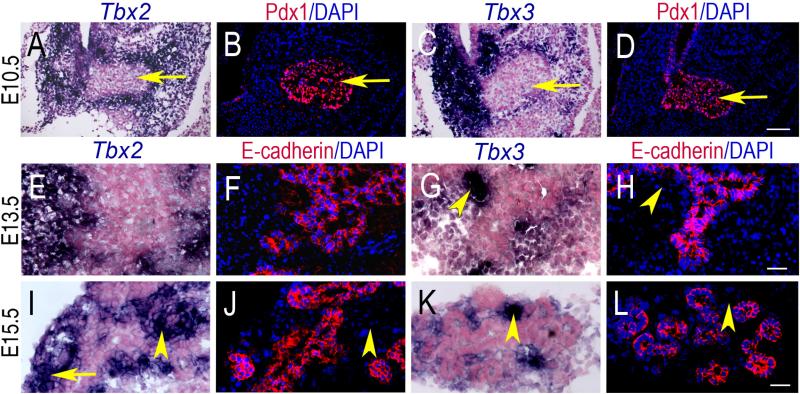
Tbx2 and Tbx3 expression was first detected in the developing pancreas at E9.5 and E10.5 in the mesenchyme surrounding the pancreatic buds, which are identified by the epithelial marker, Pdx1 (Fig. 1). During early pancreas morphogenesis at E12.5 and E13.5 a diffuse pattern of mesenchymal expression of Tbx2 and Tbx3 was observed (Fig. 2A-F) with the most intense expression at the dorso-lateral angle of the embryonic pancreas, which is composed mostly of mesenchymal cells. Expression is only in the mesenchyme and never in the epithelium, as shown by comparison with a known epithelial marker, Pdx1, in adjacent section at E10.5 (Fig. 3A-D). At E13.5, analysis of adjacent sections using probes for either Tbx2 or Tbx3 and anti-E-cadherin antibody, a marker of epithelium, confirmed that neither Tbx2 nor Tbx3 are expressed in the pancreatic epithelium (Fig. 3E-H). The pattern of mesenchymal expression, however, differs between the two genes: Tbx2 is expressed uniformly in the mesenchyme and in close contact with epithelial branches (Fig. 3E, F); Tbx3 is expressed around the epithelial branches but with intense localized expression at the bifurcation of branching tubes (Fig. 3G, H), indicating a potential role for Tbx3 in pancreatic epithelial branching morphogenesis.
Fig. 1.
Tbx2 and Tbx3 RNA expression at bud stages of pancreas development. Whole-mount ISH was performed for Tbx2 (A, D) and Tbx3 (B, E) on dissected whole embryos at E9.5 and E10.5. Pdx1 expression (C, F) was detected by whole-mount IHC to highlight pancreatic epithelium. Boxed areas in A-F show the pancreatic region at higher magnification in A'-F'. The approximate position of the epithelial buds are marked by dotted lines (A', B', D' and E'). Arrows indicate the dorsal pancreas and arrowheads indicate ventral pancreas. Scale bars in C, C , F and F = 200μm (A- F ).
Fig. 2.
Tbx2 and Tbx3 RNA expression in the developing pancreas, E12.5-E14.5. Whole-mount ISH of the pancreas (dorsal view) isolated with stomach (st), duodenum (du), and spleen (sp). Both Tbx2 (A, D, G) and Tbx3 (B, E, H) are expressed in the pancreatic mesenchyme. Pdx1 epithelial expression by whole-mount IHC is shown for comparison (C, F, I). Arrows show Tbx2 expression in the pancreatic vasculature (A, D, G). Scale bars in C, F and I = 200μm (A-I).
Fig. 3.
Tbx2 and Tbx3 RNA expression in sections of the developing pancreas, E10.5- E15.5. Tbx2 (A) and Tbx3 (C) are expressed in the pancreatic mesenchyme at E10.5 but not in the epithelium (arrows), as indicated by Pdx1 expression (B, D) in adjacent sections. At E13.5, Tbx2 (E) is expressed in the mesenchyme around the epithelial branching network, as indicated by E-cadherin (F) in adjacent sections. Tbx3 (G) shows localized expression (arrowhead) in the mesenchyme at epithelial bifurcations, shown by E-cadherin in adjacent sections (H). At E15.5, Tbx2 (I) is expressed mostly in the mesenchyme (arrowhead), but is also present in epithelial cell clusters (arrows) which are positive for E-cadherin (J). Tbx3 (K) maintains localized expression in the mesenchyme (arrowhead) and is not present in the epithelium as indicated by E-cadherin in adjacent sections (L). A, E, I, C, G, K are section ISH on frozen sections counterstained with Nuclear Fast Red. B, F, J, D, H, L are section IHC counterstained with DAPI. Scale bar in D = 50μm (A-D); scale bar in H, L =20μm (E-L).
1.2. Tbx2 and Tbx3 expression during mid and late stages of pancreas development
E14.5 marks the transition point of pancreas development when differentiation of the progenitor cells begins (Zhou et al., 2007). At this stage, Tbx2 is expressed throughout the pancreas (Fig. 2G) and, by E15.5, is expressed uniformly in the pancreatic mesenchyme in close contact with the epithelial branching network, marked by E-cadherin (Fig. 3I, J). In addition, Tbx2 expression was seen in a few clusters of epithelial cells, which were E-cadherin positive in adjacent sections (arrows, Fig. 3I, J). Tbx3, on the other hand, is expressed in a punctate pattern (Fig. 2H) in the mesenchyme at these stages (Fig. 3K, L).
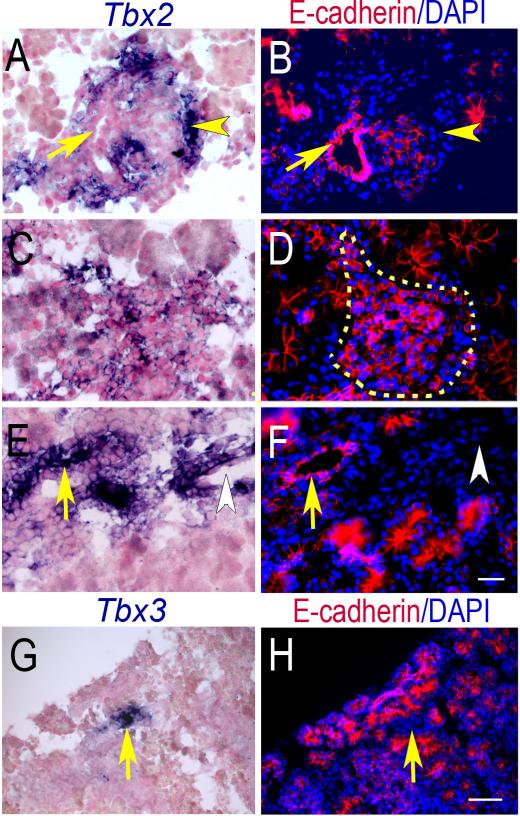
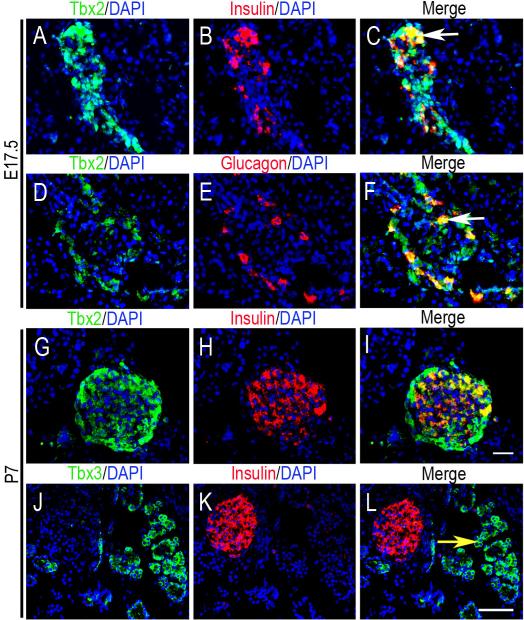
At E17.5, Tbx2 is expressed both in mesenchyme and epithelial-derived tissues. It is present in mesenchyme surrounding clusters of E-cadherin positive endocrine cells (Fig. 4A, B), and where well-formed islets are evident morphologically, Tbx2 is expressed in the endocrine cells (Fig. 4C, D). Double immunofluoresence (IF) using anti-Tbx2 with anti-insulin or anti-glucagon showed that Tbx2 is co-expressed in the insulin-positive cells and glucagon-positive cells of endocrine clusters at E17.5 (Fig. 5A-F), suggesting that Tbx2 might play a role in the maturation of major hormone producing cells. Some (Fig. 4E, F) but not all (Fig. 4A, B) E-cadherin-positive epithelial ducts show Tbx2 expression. In contrast, Tbx3 is expressed only in the mesenchyme with epithelial tissues completely devoid of signal (Fig. 4G, H).
Fig. 4.
Tbx2 and Tbx3 RNA expression at E17.5. Tbx2 is expressed in mesenchyme (yellow arrowheads) surrounding E-cadherin-positive developing islets (A, B) and morphologically distinct islets (C, D, dotted line). Tbx2 is expressed in some epithelial ducts (arrows in E, F) but not all (arrows in A, B) and is expressed E-cadherin-negative blood vessels (white arrowheads in E, F). Tbx3 is expressed only in E-cadherin-negative mesenchyme (G, H). Section ISH was performed on frozen sections counterstained with Nuclear Fast Red (A, C, E, G). Adjacent sections stained with anti-E-cadherin (B, D, F, H). Scale bar in F =20μm (A-F); scale bar in H=50μm (G-H).
Fig. 5.
Tbx2 and Tbx3 protein expression in epithelial derived tissues at late fetal and early postnatal stages. (A-F) Double IF staining on frozen sections at E17.5 shows Tbx2 is expressed in the islets in both insulin-positive cells and glucagon-positive cells (white arrows in C and F show double labeled cells in the merged images). Similar expression is seen at P7 (G-I). (J-L) Tbx3 expression is limited to exocrine tissue at P7 (yellow arrow in L). Scale bar in I = 20μm (AI); scale bar in L =50μm (J-L).
Tbx2 expression was also observed in pancreatic vasculature from E12.5 to E17.5 (Fig. 2A, D, G, arrows; Fig. 4E, F, arrowheads). Vessels were identified in sections at E17.5 as duct-like structures lined by endothelial cells or as structures lacking E-cadherin expression in adjacent sections (Fig. 4E, F, white arrowheads). The importance of vascular signals in pancreas development and differentiation has been noted (Lammert et al., 2001).
1.3. Tbx2 and Tbx3 expression during postnatal and in adult pancreas
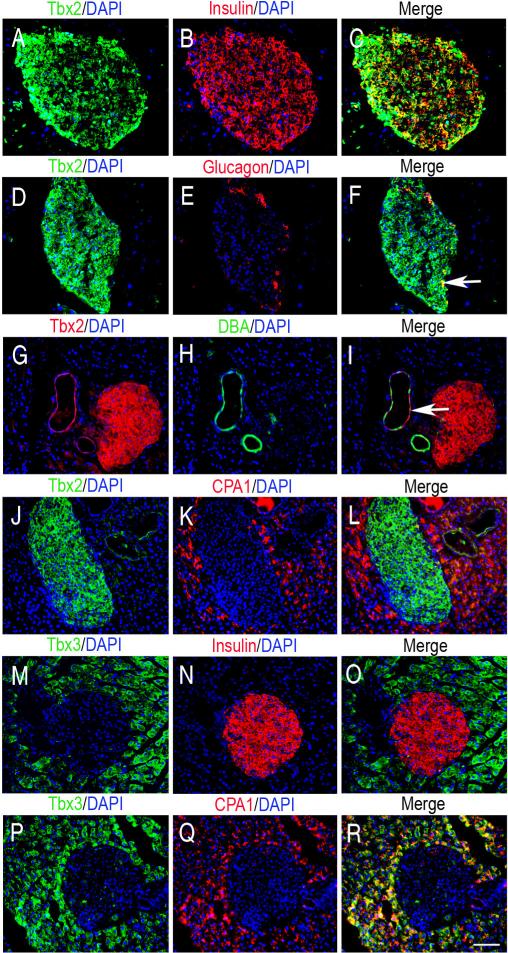
At postnatal day (P) 7, Tbx2 is expressed in the cytoplasm of islet cells, including the insulin-positive cells (Fig. 5G-I) and in pancreatic ducts (data not shown), whereas Tbx3 is not in the islets but is expressed in exocrine tissue (Fig. 5J-L). In the adult, Tbx2 is present in the islets in cells (Fig. 6A-C), glucagon-positive cells (Fig. 6D-F), Dolichos Biflorus Agglutinin (DBA)-positive ducts (Fig. 6G-I), but not in carboxypeptidaseA1 (CPA1)-positive exocrine tissue (Fig. 6J-L). Tbx3 is not expressed in the islet (Fig. 6M-O) but is expressed in exocrine tissue (Fig. 6P-R).
Fig. 6.
Tbx2 and Tbx3 protein expression in the adult pancreas. (A-L) Double IF staining on frozen sections shows Tbx2 expression in insulin-positive cells and glucagon-positive cells of islets (arrow F shows colocalization in merged images), in some DBA-positive pancreatic ducts (arrow in I), but not in CPA1-positive exocrine tissue. (M-R) Double IF staining shows Tbx3 expression is not in the insulin-positive cells but is present in the CPA1-positive exocrine tissue. Scale bar = 50μm.
1.4. Tbx2 and Tbx3 expression in pancreatic cell lines
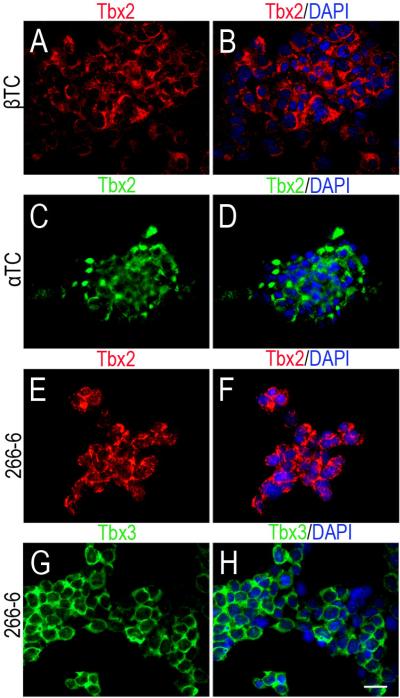
We investigated Tbx2 and Tbx3 protein expression in tumor-derived endocrine pancreatic cell lines TC and TC, which were derived from mouse insulinoma and glucagonoma and maintain adult differentiated and cell phenotypes, respectively (Efrat et al., 1988; Hamaguchi et al., 2003) and an exocrine cell line 266-6, derived from a pancreatic acinar cell tumor (Ornitz et al., 1987). Tbx2 is expressed in the cytoplasm of both endocrine cell lines (Fig. 7A-D) and in the exocrine cell line (Fig. 7E, F). Tbx3 is not expressed in βTC cells (data not shown) but was present in line 266-6 (Fig. 7G, H). These findings are consistent with our in vivo results in adult pancreas with the exception that Tbx2 is expressed in the 266-6 exocrine cell line, but not in exocrine tissue in normal pancreas. This Tbx2 expression may be due to the tumor origin of cells. In all three cell lines, both Tbx2 and Tbx3 are expressed in the cytoplasm rather than the nucleus, similar to postnatal and adult pancreases (Fig. 7A-H).
Fig. 7.
Tbx2 and Tbx3 protein expression in pancreatic cell lines. IF on cell lines shows Tbx2 is expressed in the insulinoma-derived β cell line, βTC (A, B), in the glucagonoma-derived cell line, αTC (C, D) and in the acinar tumor-derived line, 266-6 (E, F). Tbx3 is expressed in 266-6 (G, H). Scale bar = 20μm.
1.5. Conclusion
The dynamic changes in expression of Tbx2 and Tbx3 expression throughout pancreas development and in pancreatic tumor cell lines (summarized in Table 1) lay the foundation for investigation of potential roles of these transcription factor genes in pancreas development and their potential roles in pancreatic cancer.
Table 1.
Tbx2 and Tbx3 expression in mouse pancreas throughout development and in tumor cell lines.
| Gene | E9.5-E10.5 | E12.5-E14.5 | E15.5 | E17.5 | P7 | Adult | βTC | αTC | 266-6 |
|---|---|---|---|---|---|---|---|---|---|
| Tbx2 | Mesenchyme around epithelial buds | Mesenchyme, uniformly around epithelial branches, vessels | Mesenchyme, some epithelial cells | Mesenchyme, islet α and β cells, ducts, vessels | Islet α and β cells, ducts | Islet α and β cells, ducts | Yes | Yes | Yes |
| Tbx3 | Mesenchyme around epithelial buds | Mesenchyme, punctate | Mesenchyme, punctate | Mesenchyme | Exocrine tissue | Exocrine tissue | No | ND1 | Yes |
ND=not done
2. Experimental procedures
2.1. Animals, in situ hybridization and immunohistochemistry
Random bred ICR mice (Taconic, Germantown, NY) were used to generate embryos from E9.5 to E17.5, where noon on the day of the vaginal plug detection was designated E0.5. Embryos were dissected in cold phosphate buffer saline (PBS), fixed overnight in 4% paraformaldehyde, and washed two times in PBT (PBS containing Tween-20), dehydrated in 100% methanol and stored at -20°C until use. For whole-mount ISH and IHC at E9.5 and E10.5, embryos were partially dissected to expose the foregut for visualization of the pancreatic area. At E12.5-E14.5, pancreases were dissected out, along with the stomach, spleen and duodenum. Pancreases from P7 pups and two months old adults were collected for analysis. Samples for section ISH and IF were treated with 30% sucrose overnight following fixation, embedded in Tissue Tek OCT compound (Sakura Fine Technical Co, Ltd, Tokyo, Japan), and snap-frozen on dry ice in ethanol and stored at -80°C until use.
Sense and antisense digoxigenin labeled riboprobes for Tbx2 and Tbx3 (Chapman 1996) were generated by in vitro transcription in the presence of digoxigenin-labeled dUTP (Roche, Nutely, NJ). Standard procedures were used for whole-mount ISH (Wilkinson, 1992). For section ISH analysis, 10μm frozen sections were made of E10.5-E17.5 embryos. Section ISH was performed as described previously (Prado et al., 2004). Sections were counter stained with Nuclear Fast Red.
Whole-mount IHC with anti-Pdx1 antibody was performed using standard protocol (Davis, 1993). IF was performed on 10 μm frozen sections from whole embryos (E10.5-13.5) and pancreases ( E17.5, P7, and adult) using protocols as previously described (Begum et al., 2009). The antibodies used were as follows: guinea pig anti-human insulin, guinea pig anti-glucagon (both from Linco Research, St. Charles, MO), rabbit and goat anti-Pdx1 (a gift from Dr. Christopher Wright), rabbit anti-Tbx2 ( Bioworld Technology Inc, MN, USA), rabbit anti-Tbx3 (Invitrogen, CA, USA), goat anti-mouse carboxypeptidase A1 (R&D system, MN, USA), rat anti-E-cadherin (Sigma, St. Louis, MO), Fluorescin Dolichos Biflorus Agglutinin (Vector Laboratories, CA, USA), Alexa fluor donkey anti-rabbit (Molecular Probes Inc. Eugene, OR, USA), Texas redconjugated anti-guinea pig, Cy3 conjugated anti-rabbit, Cy3 conjugated anti-rat, and Cy3 conjugated anti-goat (all from Jackson Immuno Research, West Grove, PA). Western blot analysis of the Tbx2 and Tbx3 antibodies indicated that they each recognize as single band of the expected size (74 kDa and 79 kDa, respectively; Bioworld Technology, Inc. and Invitrogen product data sheets) (Smith et al., 2011). All sections were stained with 4', 6'-diamidine-2-phenylindole dihydrochloride (DAPI) for nuclear visualization. Images of whole mount samples were taken under bright field on a Nikon SMZ1500 microscope (Nikon, Japan). Sections for ISH and IF were examined with a Nikon MICROPHOT-FXA microscope (Nikon, Japan), and images were captured using NIS-Elements D3.10 software. Each ISH and IHC result shown represents a minimum of 4-6 samples.
2.2. Cell culture
Mouse pancreatic cell lines, βTC, αTC, and 266-6, were obtained from ATCC (Manassas, VA, USA). βTC was grown in DMEM (Invitrogen) and supplemented with 15% fetal bovine serum (FBS) (Hyclone, Utah, USA), αTC in RPM1 (Invitrogen) and supplemented with 10% FBS, and 266-6 in DMEM supplemented with 10% FBS. Cultures were grown at 37°C under humidified conditions in 5% CO2/95% air. For IF, cells were grown in chamber slides (NunC, NY, USA), fixed in 4% paraformaldehyde for 10 minutes, and following a wash with PBS, fixed cells were treated with antibodies. DAPI was used to visualize the nucleus in all IF analyses. IF staining was examined with a Nikon MICROPHOT-FXA microscope (Nikon, Japan), and images were captured using NIS-Elements D3.10 software.
Highlights.
We examined spatial and temporal expression of Tbx2 and Tbx3 in mouse pancreas.
Tbx2 and Tbx3 are expressed in the pancreatic mesenchyme throughout development.
Tbx2 and Tbx3 are expressed in adult pancreatic islet and acini respectively.
Tbx2 and Tbx3 are expressed in tumor-derived pancreatic cell lines.
Acknowledgments
We would like to thank Dr. Christopher Wright, (Vanderbilt University, Nashville, TN) for his generous gift of anti-Pdx1 antibody and Dr. Lori Sussel for helpful suggestions. This work was supported by the National Institutes of Health grants R37HD033082 (V. E. P.) and by a Pilot and Feasibility award from the Columbia University Diabetes and Endocrinology Research Center (DK63608).
Grant Sponsors National Institutes of Health; R37HD033082; P30DK63608
Abbreviations
- CPA1
carboxypeptidase A1
- DAPI
4’, 6’ –diamidine-2-phenylindole dihydrochloride
- DBA
Dolichos Biflorus Agglutinin
- E
embryonic
- ESC
embryonic stem cells
- IF
immunofluorescence
- IHC
immunohistochemistry
- iPSC
induced pluripotent stem cells
- ISH
in situ hybridization
- P
postnatal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agulnik SI, Garvey N, Hancock S, Ruvinsky I, Chapman DL, Agulnik I, Bollag R, Papaioannou V, Silver LM. Evolution of mouse T-box genes by tandem duplication and cluster dispersion. Genetics. 1996;144:249–254. doi: 10.1093/genetics/144.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S, Chen W, Herold KC, Papaioannou VE. Remission of Type 1 Diabetes after anti-CD3 antibody treatment and transplantation of embryonic pancreatic precursors. Endocrinology. 2009;150:4512–4520. doi: 10.1210/en.2009-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbxl-Tbx5, during early mouse development. Developmental Dynamics. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Chen P, Tian D, Liu M. The role of Tbx2 in pancreatic cancers and its regulation by Wnt/β-catenin signaling. Chinese-German Journal of Clinical Oncology. 2008;7:404–409. [Google Scholar]
- Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- Davis CA. Whole-mount immunohistochemistry. Methods in Enzymology. 1993;225:502–516. doi: 10.1016/0076-6879(93)25034-y. [DOI] [PubMed] [Google Scholar]
- Duo S, Tiao-dong T, Lei Z, Wei W, Hong-Li S, Xian-wei D. Expression and clinical significance of Tbx2 in pancreatic cancer. Asian Pacific Journal of Cancer Prevention. 2009;10:119–122. [PubMed] [Google Scholar]
- Efrat S, Linde S, Kofod H, Spector D, Delannoy M, Grant S, Hanahan D, Baekkeskov S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. PNAS. 1988;85:9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Huang X, Chen C, Gray J, Huang T. TBX3 and its isoform TBX3+2a are functionally distinctive in inhibition of senescence and are overexpressed in a subset of breast cancer cell lines. Cancer Research. 2004;64:5132–5139. doi: 10.1158/0008-5472.CAN-04-0615. [DOI] [PubMed] [Google Scholar]
- Galan-Caridad JM, Harel S, Arenzana T, Hou ZE, Doetsch FK, Mirny LA, Reizis B. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell. 2007;129:345–357. doi: 10.1016/j.cell.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson-Brown JJ, Agulnik SI, Silver LM, Papaioannou VE. Expression of T-box genes Tbx2-Tbx5 during chick organogenesis. Mechanisms of Development. 1998;74:165–169. doi: 10.1016/s0925-4773(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Utsunomiya N, Ryosaburo T, Yoshimatsu H, Sakata T. Cellular interaction between mouse pancreatic α -cell and β-cell lines: Possible contact dependent inhibition of insulin secretion. Experimental Biology and Medicine. 2003;228:1227–1233. doi: 10.1177/153537020322801020. [DOI] [PubMed] [Google Scholar]
- Han J, Yuan P, Yang H, Ihang J, Soh BS, Li P, Lim SL, Cao S, Tay J, Orlov YL, Lufkin T, Ng H-H, Tam W-L, Lim B. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DE, Rahman A, House M, Ashfaq R, Berg K, Yeo CJ, Maitra A. Met protooncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasms. Clinical Cancer Research. 2004;10:6152–6158. doi: 10.1158/1078-0432.CCR-04-0285. [DOI] [PubMed] [Google Scholar]
- Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, Papaioannou VE. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outlow tract during heart development. Development. 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–438. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ, Koh EY, Daley GQ, van Lohuizen M. Senescence bypass screen identifies Tbx2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nature Genetics. 2000;26:291–299. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton DA. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Mahlamäki EH, Bärlund M, Tanner M, Gorunova L, Höglund M, Karhu R, Kallioniemi A. Frequent amplification of 8q24, 11q, 17q and 20q-specific genes in pancreatic cancer. Genes, Chromosomes and Cancer. 2002;35:353–358. doi: 10.1002/gcc.10122. [DOI] [PubMed] [Google Scholar]
- Mesbah K, Harrelson Z, Théveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development. Circulation Research. 2008;237:743–750. doi: 10.1161/CIRCRESAHA.108.172858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Hammer RE, Messing A, Palmiter RD, Brinster RL. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987;238:188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE. T-box genes in development: from hydra to humans. International review of cytology. 2001;207:1–70. doi: 10.1016/s0074-7696(01)07002-4. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE, Silver LM. The T-box gene family. Bioessays. 1998;20:9–19. doi: 10.1002/(SICI)1521-1878(199801)20:1<9::AID-BIES4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Postier RG. The challenge of pancreatic cancer. The American Journal of Surgery. 2003;186:579–582. doi: 10.1016/j.amjsurg.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. PNAS. 2004;101:2924–9. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince S, Carreira S, Vance KW, Abrahams A, Goding CR. Tbx2 directly represses the expression of the p21WAF1 cyclin-dependent kinase inhibitor. Cancer Research. 2004;64:1669–1674. doi: 10.1158/0008-5472.can-03-3286. [DOI] [PubMed] [Google Scholar]
- Sinclair CS, Adem C, Naderi A, Soderberg C, Johnson M, Wu K, Wadum L, Couch VL, Sellers TA, Schaid D, Slezak J, Fredericksen Z, Ingle JN, Hartmann L, Jenkins RB, Couch FJ. Tbx2 is preferentially amplified in BRACA1- and BRACA2-related breast tumours. Cancer Research. 2002;62:3587–3591. [PubMed] [Google Scholar]
- Smith J, Mowla S, Prince S. Basal transcription of the human TBX3 gene, a key developmental regulator which is overexpressrd in several cancers, requires functional NF-Y and Sp1 sites. 2011 doi: 10.1016/j.gene.2011.07.013. Gene in press. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takeuchi J, Koshiba-Takeuchi K, Ogura T. Tbx genes specify posterior digit identity through Shh and BMP signaling. Developmental Cell. 2004;6:43–53. doi: 10.1016/s1534-5807(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Vance KW, Carreira S, Brosch G, Goding CR. Tbx2 is overexpressed and plays an important role in maintaining proliferation and suppresion of senescence in melanomas. Cancer Research. 2005;65:2260–2268. doi: 10.1158/0008-5472.CAN-04-3045. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. In situ hybridization: a practical approach. IRL press; Oxford: 1992. Whole mount in situ hybridization of vertebrate embryos. pp. 75–83. [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. multipotent progenitor domain guides pancreatic organogenesis Developmental. A. Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]