Abstract
Toxoplasma is a highly successful parasite that establishes a life-long chronic infection. To do this it must carefully regulate immune activation and host cell effector mechanisms. Here we review the latest developments in our understanding of how Toxoplasma counteracts the host’s immune response, and in some cases provokes it, through the use of specific parasite effector proteins. An emerging theme from these discoveries is that Toxoplasma effectors are master regulators of the pro-inflammatory response, which elicits many of the host’s toxoplasmacidal mechanisms. We speculate that combinations of these effectors present in certain Toxoplasma strains work to maintain an optimal parasite burden in different hosts to ensure parasite transmission.
The immune response to Toxoplasma gondii
Toxoplasma gondii is an obligate intracellular parasite that can invade and replicate in almost all nucleated cells of warm-blooded animals [1]. It has a world-wide geographic distribution and is known to infect many species of birds and mammals, including approximately one third of humans [2]. Although the majority of infected healthy individuals have no symptoms, in immunocompromised people or in congenitally infected individuals infection can cause severe disease or even death often caused by damage to the brain, eyes or other organs [2].
Toxoplasma host cell invasion is an active, parasite-driven process [3] that leads to the formation of a specialized non-fusogenic compartment, termed the parasitophorous vacuole (PV) [4]. The invasion process is accompanied by a sequential discharge of parasite proteins from apical secretory organelles called micronemes, rhoptries and dense granules [5]. Proteins secreted from the micronemes are involved in the initial attachment and invasion, while dense granule and rhoptry proteins convert the host cell into a suitable environment for parasite growth by modulating a variety of host processes [6]. It is perhaps not surprising that many of the most polymorphic proteins in the Toxoplasma genome are secreted factors that interact with the host cell [7]. Over the past five years it has become increasingly clear that these effectors manipulate host resistance mechanisms at multiple points along the pro-inflammatory pathway (Figure 1). Host control of Toxoplasma depends on the production of the pro-inflammatory cytokine interleukin 12 (IL-12) [8], which is produced by macrophages and dendritic cells (DCs) in response to Toll like receptor (TLR) recognition of molecular structures broadly conserved across microbial species (Box 1) (recently reviewed in Ref. [9]). IL-12 in turn activates NK and T cells to secrete interferon γ (IFNγ) [10]. The latter activates effector mechanisms for intracellular elimination of Toxoplasma, including the activation of interferon-regulated GTPases (IRGs, see Glossary) [11, 12], induction of reactive nitrogen intermediates [13], tryptophan degradation in human cells [14], and autophagy [15, 16] (Figure 1). The inflammasome (Box 1) has recently gained attention, as defects in this pathway are associated with uncontrolled parasite growth [17, 18]. Inflammasome activation culminates in the release of IL-1 family members, including the pro-inflammatory cytokines IL-1β and IL-18. When produced in excess, pro-inflammatory cytokines end up damaging the host [19–21], showing that a delicate balance between pro- and anti-inflammatory signals is necessary to guarantee survival of both the host and parasite. Our recent understanding of how Toxoplasma effectors determine virulence and their mechanism of action reveal that Toxoplasma effectors are specifically aimed at modulating inflammatory pathways, which in turn dictate parasite burden and disease.
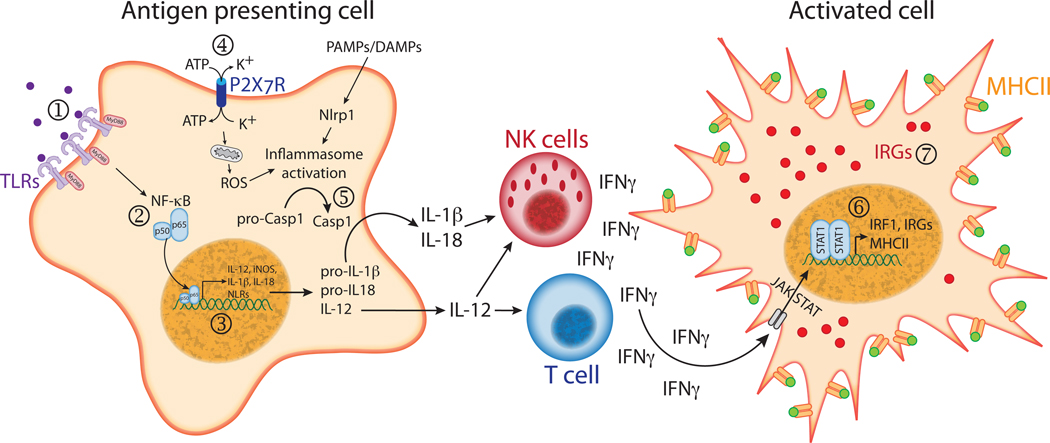
Figure 1. Host cell responses that can be modulated by Toxoplasma gondii.
(1) Toll-like receptors (TLRs) are activated upon recognition of pathogen associated molecular patterns (PAMPs). The main TLR ligand identified in T. gondii is a parasite profilin-like protein (TgPRF) that can bind to and activate TLR11 [59, 92]. Toxoplasma is also armed with molecules of glycosylphosphatidylinositol anchors (GPI) and glycoinositolphospholipids (GIPLs) that can be recognized by TLR2 and TLR4 [93]. (2) TLR engagement triggers MyD88-dependent signaling pathways that culminate with the activation of NF-kB. However, T. gondii strains that express the active form of the dense granule protein GRA15 are able to directly activate NF-κB through a MyD88-independent mechanism. (3) NF-κB activation leads to transcription of a series of pro-inflammatory genes, including genes for IL-1β, IL-12, IL-18, induced nitric oxide synthase (iNOS) and some NOD-like receptors (NLRs). Nevertheless, parasite ROP16 is able to suppress the IL-12 response of infected macrophages stimulated with the TLR agonists [41] and to inhibit NF-κB transcriptional activity [42], possibly due to its ability to phosphorylate and activate STAT3/6 [41], which dampens TLR-induced cytokine production. Parasite induced MAPK signaling pathways also modulate IL-12 production [94], and there is evidence that T. gondii ROP38 may regulate MAPK function [7]. (4) Binding of ATP to the purinergic receptor P2X7 and the subsequent efflux of intracellular K+ leads to activation of the inflammasome [52, 53]. Although it is not known if Toxoplasma infection affects P2X7R function, the parasite secretes nucleoside triphosphate hydrolases (NTPases) that could possibly control extracellular levels of ATP. (5) Inflammasome stimulation activates caspase-1, which cleaves the proforms of IL-1β and IL-18 generating bioactive cytokines. Both IL-1β and IL-18 receptors activate NF-κB and MAPK signaling and subsequent pro-inflammatory cytokine production. Toxoplasma is known to induce IL-1β and IL-18 secretion, both of which serve to amplify IFNγ production by NK cells [80, 81]. It remains to be elucidated if the parasite can directly activate the inflammasome or modulate caspase-1 activity. (6) IFNγ binding to its receptor triggers the JAK/STAT pathway, leading to phosphorylation of STAT1. Phosphorylated STAT1 then dimerizes and translocates to the nucleus, leading to transcription of interferon-stimulated genes, including the transcription factor IRF1, class II MHC and interferon regulated GTPases (IRGs). Yet, Toxoplasma infected cells display a marked inhibition of STAT1 dependent transcription [60], and parasite secreted kinase ROP18 can phosphorylate and inactivate IRGs (7), preventing their accumulation on the parasitophorous vacuole membrane and protecting the parasite from IRG-dependent intracellular killing [28, 29]. Abbreviations: IRF1, interferon regulatory factor 1; JAK, Janus kinases; MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription; ROS, reactive oxygen species.
Box 1. Innate immune requirements for Toxoplasma infection.
The innate immune system is armed with a variety of receptors that recognize structures conserved among microbial species or released by damaged cells, named pathogen-associated or damage-associated molecular patterns (PAMPs or DAMPs) [71]. One class of these receptors, the Toll-like receptor (TLR) family, was initially claimed to be a major player in innate immune recognition of protozoan parasite infections, including toxoplasmosis [72]. Mice deficient in MyD88, an adaptor protein necessary for the function of all TLRs but TLR3 [71], display a complete loss in acute resistance to systemic and oral infection with T. gondii which was hypothesized to be due to defective IL-12 production [73, 74]. The primary source of IL-12 during systemic murine infection with Toxoplasma is dendritic cells (DCs) [75]. However, T cell expression of MyD88 is essential for resistance to Toxoplasma, and injection of IL-12 in mice that lack MyD88 in T cells does not rescue susceptibility [76]. Similarly, although 100% mortality is achieved in mice that lack MyD88 in their DCs, they die three weeks later than MyD88−/− animals following infection. Furthermore, none of the TLR single or double knockout mice tested to date are very susceptible to intraperitoneal (i.p.) Toxoplasma infection [77], suggesting another cell type (non-DC or -macrophage), and MyD88 associated receptors (non-TLR) might be similarly necessary for early host resistance to Toxoplasma.
The IL-1 family members could possibly fulfill this requirement. The IL-1 response requires the activation of a family of cytoplasmic innate NOD-like receptors (NLRs) that recognize different classes of DAMPs and PAMPs altogether (reviewed in Refs. [52, 53]). Some members of the NLR family, including Nlrp1, Nlrp3 and Nlrc4 (Ipaf), are known to be involved in the activation of caspase-1 through the formation of large multimolecular complexes called inflammasomes [52, 53]. Caspase-1 proteolytically converts the proforms of IL-1β, IL-18 and IL-33 into the bioactive cytokines [78]. Both IL-1β and IL-18 receptors use MyD88 as an adaptor, leading to NF-κB and MAPK activation, and subsequent IL-12 production by antigen-presenting cells [79]. In the presence of IL-12, both IL-1β and IL-18 potentiate NK-cell production of IFNγ during T. gondii infection [80, 81]. Notably, IL-18 deficient animals experienced less morbidity and intestinal pathology after oral infection [21], suggesting that parasite-induced IL-18 contributes to the immunopathology. IL-33 also appears to be produced upon infection with T. gondii [82]. This cytokine is produced mainly by fibroblasts and endothelial cells, and its receptor also transduces signal through a MyD88-dependent pathway, but instead of inducing pro-inflammatory cytokines, it drives a Th2-type immune response [78]. Animal knockouts for the IL-33 receptor protein ST2 develop greater brain pathology after T. gondii infection due to augmented parasite burden and increased production of IFNγ, TNFα and induced nitric oxide synthase (iNOS) [82]. Thus, it is clear that the ability of the parasite to initiate both pro- and anti-inflammatory innate immune responses (through IL-1β and IL-18 or IL-33, respectively) can determine the fate of the host.
Strain-specific modulators of host immune responses
The majority of Toxoplasma strains found in North America and Europe can be grouped into three main clonal lineages (types I, II and III) that differ genetically by 1% or less [22] (Box 2). Most of what is known regarding the immune response against Toxoplasma is based on data obtained from laboratory mice infected with parasites from these three haplogroups. In laboratory mice, type I strains are categorically lethal, with an LD100 = 1, whereas the LD50 of type II and III strains are ~103 and 105, respectively [22, 23]. Genetic mapping of the virulence of F1 progeny (Toxoplasma is a haploid organism) derived from type I × II, I × III and II × III crosses [24–26] has identified the genetic loci that control this phenotype, and subsequent experiments have identified the causative genes within these loci. It is important to note that these analyses could not identify non-polymorphic Toxoplasma genes that determine virulence. Furthermore, it is currently unknown whether the identified polymorphic effectors are operative in species other than laboratory mice, as will be discussed below.
Box 2. Population structure of Toxoplasma.
Toxoplasma is unique among the apicomplexans in that tissue cysts generated in intermediate hosts are infectious to other intermediate hosts. Therefore sex in its definitive host, members of feline species, is not obligatory. Moreover, because Toxoplasma is haploid and a single strain can generate both micro- and macrogametes, self mating is very likely as the vast majority of intermediate hosts harbor cysts from just a single strain. However, in the rare occasion a feline gets infected with two distinct Toxoplasma strains sexual recombination can occur, and up to 100 million highly stable oocysts can be generated. Animals ingesting these oocysts can subsequently function to select the most successful of these genotypes. As discussed in this review an important part of this selection is likely determined by both the exact allelic combination of Toxoplasma effectors and the specifics of the immune system of the host species. In Europe and North America the majority of isolates from humans and domesticated animals belong to three clonal lineages, named type I, II and III [23]. Genotypes not belonging to these three main lineages are predominant in South America [83–85] and are also often isolated from non-domesticated animals [86]. Recently, a phylogenetic analysis of multiple Toxoplasma strains from different continents clustered the strains into 11 distinct haplogroups, including the type I, II and III strains. Remarkably, the three main clonal lineages, as well as many of the less common ‘atypical’ strains, appear to be the result of mixing of just four ancestral genotypes, resulting in limited allelic polymorphism among these strains [87].
Part of the variability of disease outcome in human infections may also be tied to the type of strain that causes the infection. In North America and Europe most human cases are due to type II strains. Type III strains appear to be more common in animals, and in general are not associated with disease while a relative oversampling of type I strains has been observed in severe congenital infections and in AIDS patients (reviewed in Ref. [88]). Interestingly, in South America Toxoplasma can cause disease, especially ocular disease, in otherwise healthy individuals resulting in a high prevalence of ocular toxoplasmosis [89, 90]. There is some evidence that this is associated with specific strains, mainly present in South America. Also in North America, only type I or atypical strains were found in non-immunosuppressed patients suffering from severe ocular toxoplasmosis [84, 91]. Thus different Toxoplasma strains seem to cause different pathology both in mice and humans. The molecular basis for these differences in mice is slowly being unraveled but remains largely unexplored in humans and other animal species. As indicated in this review the vast majority of our current knowledge of Toxoplasma’s interaction with the host immune system comes from infections of mice with the Euro American strain types. Certainly new and interesting biology will be discovered when the interaction between the non-canonical Toxoplasma strains and the immune system is studied.
ROP18
Genetic mapping of virulence in F1 progeny from a I × III cross identified a single locus which encodes a rhoptry protein kinase, ROP18 [24, 25]. ROP18 belongs to the ROP2 family of Toxoplasma kinases, and rapidly co-localizes to the parasitophorous vacuole membrane (PVM) following infection [24, 27]. Type III strains express extremely low levels of ROP18 due to an extra 2.1 kb sequence 85 bp upstream of the ATG start codon, and addition of a type I copy of the ROP18 locus to the type III strain (III+ROP18I) makes it as virulent as type I parasites [24]. Type III+ROP18I parasites have an increased replication rate in human foreskin fibroblasts (HFFs) [27] and are protected from IFNγ-mediated killing by mouse macrophages [28]. ROP18 can phosphorylate the nucleotide binding site of the switch loop 1 of Irga6 and Irgb6, mouse p47 IRGs which coat the PVM and are crucial for IFNγ mediated killing of Toxoplasma [28, 29]. Phosphorylation of the critical threonine residues (T102 and T108) destabilizes Irga6 by preventing subunit oligomerization and GTP hydrolysis, which in turn inhibits IRG accumulation on the PVM and protects the parasite from being destroyed [30]. Indeed, type I Δrop18 parasites have reduced numbers and delayed virulence in mice compared to the type I strain, but still kill 100% of infected mice [28, 29]. Type II parasites failed to significantly phosphorylate these p47 GTPases in IFNγ activated macrophages [29], which is surprising because ROP18 is functionally expressed in this strain and type II ROP18 can confer virulence to a type III strain. Thus it is likely that ROP18 works in concert with another polymorphic Toxoplasma effector (i.e. inactive in type II) to destabilize IRGs at the PVM. It is important to mention that humans have only two orthologous p47 GTPases, IRGC and IRGM, and both lack GAS and ISRE elements in their promoters, so they are not induced by IFNγ [31]. A splice isoform of human IRGM, IRGMd, localizes to the mitochondria and induces organelle fission and autophagy [32], which inhibits the intracellular survival of Mycobacterium tuberculosis [33]. Whether human IRGM has a role in Toxoplasma killing and is manipulated by ROP18, or whether ROP18 has another target in human cells is unknown.
Along this line, it was recently shown that ROP18 can phosphorylate the host transcription factor ATF6β, which is subsequently targeted for degradation via the proteasome, resulting in reduced ATF6β-mediated gene expression [34]. Importantly, ATF6 orthologs are present in humans, raising the possibility that expression of different ROP18 alleles by Toxoplasma strains may also be relevant for the development of human disease. Interestingly, ATF6β deficient mice died faster after infection with type I Δrop18 parasites, with kinetics similar to wild type mice infected with the type I strain, suggesting a role for the ROP18-mediated ATF6β degradation in Toxoplasma virulence. Although it is not clear how ATF6β affects parasite virulence, the observation that dendritic cells from ATF6β deficient mice have a reduced ability to elicit IFNγ production by CD8+, but not CD4+ T cells suggests that ATF6β may play a pivotal role in controlling the MHCI antigen presentation pathway. This is possibly through ATF6β modulation of expression of ER-associated degradation (ERAD) system components, which have been shown to be necessary for cross-priming of CD8+ T cells [34, 35]. The N-terminal portion of ROP18 (N2), which mediates the interaction with ATF6β, was necessary for full virulence of ROP18, suggesting a role for this region and binding of ATF6β. However, interpretation of this result is difficult because this N-terminal region has also been shown to be essential for ROP18 localization to the PVM [36]. Therefore, abrogated ΔN2-ROP18 PVM localization could result in reduced protection against PVM localized IRGs. Further analysis of ROP18’s interaction with its ligands Irga6, Irgb6 and ATF6β, will shed light on its precise mechanism during Toxoplasma infection.
ROP5
ROP5 represents a cluster of tandem, duplicated polymorphic pseudokinases that dictate virulence in mice [37]. ROP5 accounts for approximately 90% of the variance in virulence between F1 progeny derived from the I × II and II × III crosses [25, 26, 37]. Types I, II and III strains have divergent isoforms present in different numbers, although three major isoforms (A, B and C) appear to exist in each strain [37]. The virulent isoforms are expressed in the virulent type I and avirulent III strains, suggesting that ROP5 requires at least another factor not present in the type III strain to elicit virulence in mice. Although type III strains complemented with ROP18I are as virulent as type I strains, ROP5 contributes to virulence in strains that do not express ROP18 [25, 37], suggesting an independent mode of action. Strikingly, knockout of the entire ROP5 locus makes the highly virulent type I strain avirulent, as 106 parasites do not kill any mice [26, 37]. Apparently a single copy of ROP5 is enough to partially restore virulence in the Δrop5 type I strain, as complementation with one copy of ROP5 (from type III strains) is able to partially restore virulence. Different ROP5 isoforms vary in the magnitude of their effect. Whereas 20% of infected mice survive a dose of 103 parasites transgenically expressing two copies of allele ROP5AIII, Toxoplasma expressing one copy of ROP5AIII and ROP5BIII are 100% lethal [37].
While it is clear that ROP5 is a major virulence determinant for T. gondii strains, its function remains to be determined. ROP5 lacks the catalytic ‘HRD’ motif that is critical for phosphotransferase activity [38], and although polymorphisms in ROP5 cluster in the pseudokinase domain, none of the variants have a predicted active catalytic site. Nevertheless, the ROP5 catalytic loop contains an ‘HGB’ motif (‘B’ denoting any basic residue) that is conserved between other Toxoplasma pseudokinases [39]. Complementation of ROP5 knockout parasites with a mutant copy of ROP5AIII in which the basic residue (Arg) in its HGB motif was replaced by an acidic residue (Asp) only partially restored parasite virulence, suggesting that the conserved ‘pseudokinase motif’ has functional significance [39]. Differences between alleles are also found in substrate recognition domains of the kinase [26], suggesting that ROP5 variants might have different binding partners, which could possibly be relevant for virulence.
ROP5 does not seem to affect invasion, PV formation, nutrient acquisition, parasite replication or egress, as no detectable growth phenotype was observed during in vitro cultivation of Δrop5 parasites [26, 37]. Since ROP5 is secreted during Toxoplasma invasion and associated with the cytosolic face of the PV [40], it may serve as a scaffold or an adaptor protein, bridging together enzymes and substrates that modulate cell signaling pathways, perhaps even associating with other parasite effector proteins. The presence of tandem copies of different alleles of ROP5 might also affect the ability of the parasite to adapt to hosts other than mice.
ROP16
Whereas the aforementioned virulence factors have not been shown to affect the host transcriptional response, polymorphisms in the rhoptry kinase ROP16 and the dense granule protein GRA15 (see below) together account for approximately 50% of the transcriptional response differences of HFFs or mouse macrophages infected with type II or III strains [41–43]. ROP16 was inferred as a virulence determinant by mapping virulence QTLs of the II × III F1 progeny and was the major determinant controlling the transcriptional response differences of HFFs to type II and III infections [41]. Subsequent bioinformatic analyses indicated that ROP16 might influence the JAK/STAT pathway, and indeed, in vitro studies determined that type I and III ROP16, but not type II ROP16, can maintain constitutive activation of STAT3 and STAT6. Recently, direct (Tyr 641) tyrosine phosphorylation of recombinant STAT6 by recombinant ROP16 was clearly observed [44]. Similarly, STAT3 Tyr705, but not the Ser727 residue, was phosphorylated by ROP16 in an in vitro kinase assay [45]. Interestingly, a single ROP16 polymorphism at position 503 (Leu to Ser) renders type II ROP16 unable to efficiently activate STAT3. In silico modeling of this mutation predicts that the serine residue would narrow the cavity of the ROP16 kinase pocket and possibly weaken interactions with its substrate [45]. The region between amino acids 220 and 303 is required for ROP16 binding to STAT3 by immunoprecipitation [45] and represents one of the most polymorphic regions of ROP16 (http://toxodb.org/). It is possible that ROP16 has other targets since it is found in the nucleus of the host cell, and many of the genes regulated by ROP16 lack known STAT transcription factor binding elements. Following oral infection in susceptible mice, type II strains that transgenically express the type III or I versions of ROP16 quell intestinal inflammation which normally occurs following infection with the parental type II strain [43]. How ROP16 affects virulence is currently unclear. However, ROP16 can suppress the IL-12 response of infected macrophages stimulated with the Toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS) [41] and inhibit NF-κB transcriptional activity [42]. Whether this inhibition reflects the ability of ROP16 to activate STAT3, a known inhibitor of NF-κB activation, remains to be determined. A down-stream consequence of STAT6 activation in infected macrophages is the induction of the alternative activation program [43], which importantly, can inhibit pro-inflammatory responses.
GRA15
Like many pro-inflammatory cytokines, IL-12 synthesis requires the activity of the transcription factor NF-κB [46]. NF-κB modulation by Toxoplasma has been the focus of several studies (reviewed in Ref. [47]). Important strain-specific differences in NF-κB signaling were observed in murine bone marrow-derived macrophages and peritoneal exudate cells; type II parasites were shown to induce higher levels of NF-κB activation and IL-12 production, compared to type I strains [48]. These findings were recently expanded upon by showing that type III parasites are also weak inducers of NF-κB and strain differences in NF-κB activation can be reproduced in a variety of murine, human and rat cell lines [42]. Using the F1 progeny derived from the type II × III cross, the locus responsible for this difference was mapped and found to encode a secreted dense granule protein named GRA15 [42]. Type II GRA15 mediates RelA/p50 NF-κB heterodimer translocation into the host nucleus, ultimately activating transcription of genes involved in pro-inflammatory responses. Proof that GRA15 is indeed responsible for strain-specific NF-κB activation was obtained by generating a variety of GRA15II knockout and transgenic parasite strains and observing that NF-κB activation followed the expression of GRA15II in these strains. In fact, HeLa cells transfected with GRA15II induced the activation of host NF-κB demonstrating that GRA15II does not require other Toxoplasma effectors or the PVM to mediate NF-κB activation [42]. Furthermore, type II GRA15 knockout parasites induced significantly less IL-12 production, both in vitro and in vivo, and have a growth advantage when compared with wild type parasites, most likely through reduced induction of IFNγ [42]. The host cell interaction partner of GRA15 has not yet been identified, but its ability to activate NF-κB requires the IKK complex, TRAF6 and is independent of MyD88, signifying the direct activation of this pathway is independent of TLR recognition.
ROP38
Based on genome-wide expression profiling of tachyzoites, ROP38 gene expression was reported to be considerably higher in the type II and III strains (eightfold) when compared to the type I strain [7]. ROP38 is a putative functional kinase with a predicted signal peptide, and was observed both inside rhoptries and associated with the PVM [7]. A type I strain was generated with an additional copy of ROP38 under the control of the β-tubulin promoter; thus expression of ROP38 became similar to that observed in the type III strain. Transgenic parasites displayed a markedly reduced ability to upregulate host gene expression in vitro when compared to the wild type strain, suggesting that ROP38 may have an inhibitory effect on host cell transcription [7]. Although several of the affected genes are modulated by mitogen-activated protein kinase (MAPK) signaling cascades, a direct correlation between ROP38 and MAPK function remains to be evaluated.
Other Toxoplasma proteins that influence virulence
Another set of proteins that are potentially important for determining Toxoplasma virulence are the secreted nucleoside triphosphate hydrolases (NTPases). These enzymes are immunogenic antigens in both humans and mice [49, 50], and exist in two isoforms [51]. Virulent strains of Toxoplasma have both NTPase-I and II isoforms, while nonvirulent strains have only isoform II [51]. The role of these enzymes in immune modulation has not been investigated. It is known that binding of ATP to the purinergic receptor P2X7 and the subsequent efflux of intracellular K+ are necessary for the activation of the Nlrp3 inflammasome and assembly of the pyroptosome [52, 53]. Activation of the P2X7 receptor in vitro triggers the elimination of intracellular parasites by infected macrophages [18, 54], possibly through induction of pyroptosis [55]. Based on this data it is reasonable to infer that the T. gondii NTPases [51] can be tools secreted by the parasite to dampen inflammasome activation, thereby inhibiting pyroptosis-mediated parasite killing and reducing levels of the pro-inflammatory cytokines IL-1β and IL-18.
Based on the assumption that not all virulence factors are polymorphic, a forward genetic screen of a library of insertional mutants was used to find determinants that subvert host effector mechanisms. From these efforts a patatin-like phospholipase protein, that localized to an unidentified structure within the parasite cytoplasm, protected Toxoplasma from the degradative effects of nitrogen oxide (NO) in activated macrophages [56]. Similarly, a transmembrane protein expressed on the outer membrane of Toxoplasma also inhibited the toxoplasmacidal effects of NO and promoted cyst formation [57]. Other factors that promote cyst formation include the GRA6 and GRA4 dense granule proteins, possibly through their effect on the nanotubular network present inside the PVM [58]. Furthermore, in a search for parasite factors responsible for IL-12 induction in murine DCs, a stimulatory molecule from parasite extracts was isolated and identified as a profilin-like protein, TgPRF, which is recognized by TLR11 [59]. TgPFR, is conserved between type I, II and III strains (http://toxodb.org/), which suggests that activation of DCs by profilin is not involved in strain-specific modulation of immune responses. Nevertheless, because profilin is an intracellular actin-binding protein that likely gets released after destruction of intracellular parasites, for example by the IRGs, strain differences in resistance to host toxoplasmacidal activities might result in relative differences of profilin release and subsequent differences in TLR11 stimulation and immune activation.
Toxoplasma effectors modulate pro-inflammatory responses
So how might the aforementioned Toxoplasma effectors work to achieve chronic infection? At a glance, it appears that most of these effectors are either directly involved with inhibiting downstream toxoplasmacidal mechanisms of IFNγ or at least capable of manipulating Th1 responses through regulation of IL-12 (Figure 1). Added to these observations is the known phenomenon that cells infected with Toxoplasma cannot be stimulated with IFNγ to activate STAT1 [60], an IFNγ-activated transcription factor that induces many of the genes involved with killing Toxoplasma (iNOS, IRGs, autophagy, etc.). This inhibition is independent of the strain type and the parasite factors involved have remained elusive. Although the mechanism of ROP5 remains unclear, ROP5 likely controls some aspect of the host’s toxoplasmacidal mechanisms since Δrop5 strains are rapidly cleared from the host. Inhibition is not the only mode of inflammatory regulation by Toxoplasma, as GRA15II and TgPFR actually provoke the IL-12 response.
Therefore, different combinations of parasite factors that inhibit IFNγ-induced toxoplasmacidal mechanisms, such as ROP18 [28], as well as effectors that modulate signaling pathways which regulate cytokine production, such as STAT3/6 activation by ROP16 [41], NF-κB activation by GRA15 [42] and MAPK activation by ROP38 [7], could have profound implications for Toxoplasma-induced pathologies and strain-specific differences in parasite burden (Figure 2). Why certain strains have unique combinations of effectors that promote or inhibit inflammation remains an important and unresolved question, but likely reflects limitations of the mouse model and our narrow understanding of the driving forces that determine strain selection in nature (see below).
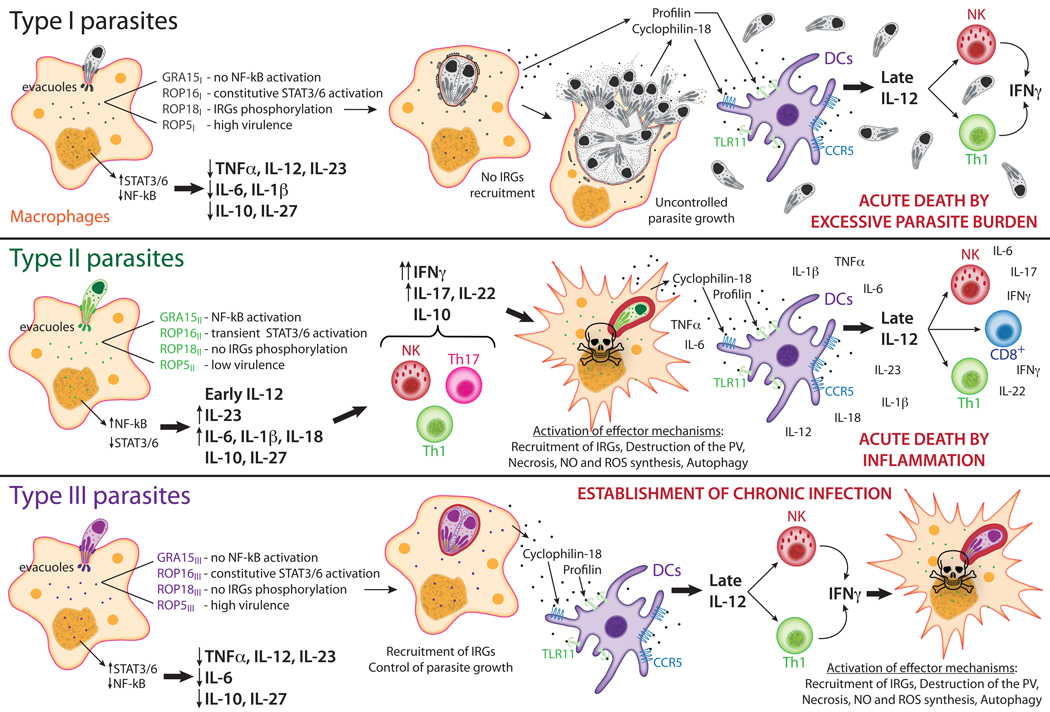
Figure 2. Overview of how Toxoplasma strains modulate host immune pathways.
Modulation of host cell signaling pathways requires the secretion of numerous parasite proteins from specialized secretory organelles called dense granules and rhoptries. At early time points, infection with type I parasites does not activate pro-inflammatory responses. The type I (RH strain) allele of GRA15 results in a truncated and non-functional protein, allowing a ‘silent’ infection without activation of NF-κB [42]. On the other hand, ROP16I induces sustained activation of STAT3 and STAT6, dampening the production of IL-12, IL-1β and IL-6 [41]. Together with the ability to reduce pro-inflammatory cytokine production, type I parasites express ROP5 alleles associated with high virulence [26, 37], and ROP18I phosphorylates IRGs blocking their recruitment to the PV, which is required for parasite destruction, permitting unrestricted parasite growth [28, 29]. Conserved parasite proteins secreted by infected cells, profilin and cyclophylin-18, are recognized by DCs via TLR11 and CCR5 respectively, leading to late NF-κB activation and production of IL-12, which in turn activates NK and T cells to secrete IFNγ [59, 95]. However, type I parasites also prevent activation of DCs [96], and by the time that the pro-inflammatory response kicks in, host survival is already compromised due to uncontrolled parasite burden. Type II parasites are very effective in activating an early response. These parasites express the active form of GRA15, which activates NF-κB in the infected cells [42], and a less functional form of ROP16, which leads to a transitory activation of STAT3/6 [41]. As a consequence there is a massive production of pro-inflammatory cytokines early after infection. The environment induced by the parasite modulates activation of several T cell subtypes, mainly directing the response towards a Th1 type [97]. Aspects of the Th17 response to Toxoplasma seem to have opposite effects on host survival, mainly an IL-23 driven IL-22 response by CD4 T cells has a negative effect [98], while signaling through the IL-17 receptor can have a beneficial effect by lowering parasite burden [99]. Intracellular parasite growth is controlled due to expression of an avirulent form of ROP18, which does not block the recruitment of IRGs to the PV [28, 29], and type II parasites also express ROP5 alleles associated with low virulence [26, 37], but susceptible animals die of severe ileitis [69]. Like type I, type III secreted GRA15 and ROP16 do not activate NF-κB and induce a sustained activation of STAT3/6 respectively, limiting the initial production of pro-inflammatory cytokines [41, 42]. Nevertheless, these parasites express an inactive ROP18, being unable to avoid intracellular killing mediated by IRGs [28, 29]. In this case, late production of IL-12 by DCs triggers a Th1-type response that is sufficient to control parasite burden and induce cyst formation, leading to a chronic infection. CCR5, C-C chemokine receptor type 5; DCs; dendritic cells; GRA, dense granule protein; IRG, interferon-regulated GTPase; NK, natural killer cells; NO, nitric oxide; PV, parasitophorous vacuole; ROP, rhoptry protein; STAT, signal transducer and activator of transcription; ROS, reactive oxygen species, TLR11, Toll-like receptor 11.
Concluding remarks
Although in the last decade there was a great advance in understanding how T. gondii modulates immune responses in the mouse model, little is known regarding the role of strain-specific virulence factors in other hosts. Toxoplasma’s world-wide distribution and its ability to chronically infect multiple animal species, including birds and mammals, raises the question of how this unicellular organism manages to control immune responses of such different species. In order to survive and propagate itself Toxoplasma has to convert to the encysted bradyzoite stage, meaning that high virulence leading to killing of its host before chronic infection is not advantageous from an evolutionary point of view. It has been argued that different Toxoplasma strains and their effectors have co-evolved with different hosts in different niches [61]. For instance, type I strains of T. gondii are super virulent in laboratory mice, which is attributed in part to the evasion from IRG-mediated killing mechanisms by Toxoplasma type I ROP18. However, IRG genes have high sequence diversity both within and between species, and some genes were lost during evolution [31]. In nature, it is possible that type I and other super-virulent parasites co-evolved in hosts which are naturally resistant to Toxoplasma or less dependent on IRG mediated parasite-killing, such that the super-virulence observed in laboratory mice might be an ‘artifact’ of its selection in another host. Alternatively, super-virulence might be a trait that was selected to allow superinfection of chronically infected animals. This would increase the chance of a feline becoming infected simultaneously with cysts from two or more strains by eating a single super-infected animal resulting in recombinant F1 progeny, possibly with greater fitness than either parent.
Complimenting this hypothesis, we recently argued that the host’s macrophage response is a niche that selects for strain-specific combinations of different Toxoplasma effectors. For example, there is considerable mouse strain variation in the ability to generate classically (M1) or alternatively (M2) activated macrophages [62]. M1 macrophages are driven by Th1 type cytokines (e.g. IFNγ) and are implicated in cytotoxic and antimicrobial functions against intracellular pathogens, including T. gondii [63, 64]. In contrast, M2 macrophages develop in a Th2 cytokine environment (IL-4, IL-13) and secrete anti-inflammatory molecules that can down-regulate Th1 immune responses [62]. Since GRA15 and ROP16 induce M1 and M2 activation, respectively [43], strain-specific expression of these effectors may have evolved to counteract a host’s predisposition to certain types of macrophage responses and toxoplasmacidal activities.
In the same line of reasoning, it was recently shown that human NLRP1 single nucleotide polymorphisms (SNPs) are associated with susceptibility to congenital toxoplasmosis [17]. Downregulation of Nlrp1 expression in human cells leads to increased parasite numbers and cell death after in vitro infection with T. gondii [17]. Similarly, the innate resistance of the Lewis rat strain to toxoplasmosis is determined by a genomic locus that includes the Nlrp1 gene [65, 66]. Another possible receptor involved in activation of the inflammasome following T. gondii infection is the P2X7 receptor, as polymorphisms in the human P2XR7 gene are associated with susceptibility to congenital toxoplasmosis [67]. Activation of the inflammasome may have different consequences depending on the host, as it seems to be deleterious in the mouse model [68], but may help to eliminate the parasite in rats and humans [18, 54, 66]. Besides host differences, it remains to be established if there are also strain specific Toxoplasma virulence factors that regulate inflammasome activation.
In conclusion, ending up in the wrong host could result in failure to establish chronic infection due to death of the parasite, as observed in resistant animal models, including Lewis rats [65], or death to the host, as observed in C57BL/6 following type II infection, but not in resistant BALB/c mice [69]. Under-activation of the immune response could also result in death of the host by excessive parasite burden, as observed in mice challenged with type I strains (Figure 2). Furthermore, relatively little is known regarding Toxoplasma effectors of the South American strain types IV through XII, which should be an active avenue of future research. Even less is known regarding how any of these effectors interact with the parasite’s definitive host, the cat. For example, do Toxoplasma effectors promote parasite sexual reproduction or feline survival following infection? From a clinical point of view it will also be important to establish if any of the aforementioned polymorphic effectors play a role in determining severity of toxoplasmosis in humans. This might be achieved if polymorphic peptides from these effectors contain B-cell epitopes which can be used to serotype strain-specific infections in human patients [70]. The future of Toxoplasma research should reveal more interesting parasite effectors that modulate the host’s inflammatory responses and disease.
Acknowledgements
J. Saeij is supported by the American Heart Association (0835099N), by a Massachusetts Life Sciences Center New Investigator Award, by the Singapore-MIT Alliance for Research and Technology (SMART), by a NERCE Developmental Grant and by NIH RO1-AI080621. K. Jensen is supported by the Irvington Postdoctoral Fellowship Program of the Cancer Research Institute (CRI). M. Melo was supported by the Knights Templar Eye Foundation.
Glossary
- Autophagy
A cellular process by which the cell removes large damaged organelles, particulates and possibly Toxoplasma containing vacuoles for degradation via the lysosome.
- CD4+ T cell
T cells that recognize peptides presented on MHCII, a complex which specializes in presenting peptides derived from extracellular antigens targeted to the lysosome via the phagocytic pathway.
- CD8+ T cell
T cells that recognize peptides presented on MHCI, a complex which specializes in presenting peptides derived from intercellular compartments and pathogens.
- GAS
Interferon gamma-activated sequence: promoter element.
- GTPase
family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP).
- IKK
IκB kinase, an enzyme complex that is part of the upstream NF-κB signaling cascade.
- Inflammasome
a multiprotein oligomer responsible for the activation of pro-inflammatory caspases, including caspase-1.
- IRG
Interferon-regulated guanine triphosphatases, a family of proteins that has been implicated in resistance to intracellular pathogens; sometimes called p47 GTPases to reflect their molecular weight.
- ISRE
Interferon-stimulated response element: promoter element.
- JAK
Janus kinases, a family of intracellular non-receptor tyrosine kinases that transduce cytokine-mediated signal.
- LD100
dose of parasites necessary to kill 100% of infected animals.
- LD50
dose of parasites necessary to kill 50% of infected animals.
- MAPK
Mitogen-activated protein kinases, serine-threonine kinases that respond to extracellular stimuli and regulate various cellular activities, such as gene expression, mitosis, differentiation, proliferation, and cell survival/apoptosis.
- MyD88
Myeloid differentiation primary response gene 88, a universal adapter protein as that is used by all TLRs, except TLR3, to activate the transcription factor NF-κB.
- Nanotubular network
a network of interconnecting nanotubules derived from the membrane of the PVM and is maintained by the dense granule proteins GRA6 and GRA2.
- NF-κB
Nuclear factor kappa B, a family of transcription factors that includes the proteins RelA (p65), RelB, c-Rel, p50 and p52. These factors play a key role in regulating immune responses.
- PAMP/DAMP
Pathogen or danger associated molecular patterns, molecular motifs associated with groups of pathogens or non-infectious stimuli, for example cellular debris from dying cells, that are recognized by cells of the innate immune system.
- Pyroptosome
a large supramolecular complex composed of Pycard dimers that mediates inflammatory programmed cell death (pyroptosis) through caspase-1 activation.
- QTL
Quantitative trait locus, stretches of DNA containing or linked to the genes that underlie a quantitative trait.
- STAT
Signal transducers and activators of transcription, transcription factors activated by Janus kinases that regulate several cellular processes, including growth, differentiation and immune activation.
- Th1
CD4+ T cells that produce IFNγ, IL-12 promotes their development in vivo.
- Th17
CD4+ T cells that produce IL-17; combinations of IL-1β, IL-6, and TGFβ or IL-23 induces their development; IL-17 promotes neutrophil homeostasis.
- Th2
CD4+ T cells that produce IL-4; IL-4 promotes allergic responses and the host response to worm infections
- TRAF
TNF receptor-associated factors, a family of proteins primarily involved in the regulation of inflammation, antiviral responses and apoptosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dubremetz JF. Host cell invasion by Toxoplasma gondii. Trends Microbiol. 1998;6:27–30. doi: 10.1016/S0966-842X(97)01165-7. [DOI] [PubMed] [Google Scholar]
- 2.Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 3.Morisaki JH, et al. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. Journal of Cell Science. 1995;108(Pt 6):2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- 4.Plattner F, Soldati-Favre D. Hijacking of host cellular functions by the Apicomplexa. Annu Rev Microbiol. 2008;62:471–487. doi: 10.1146/annurev.micro.62.081307.162802. [DOI] [PubMed] [Google Scholar]
- 5.Dubey JP, et al. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blader IJ, Saeij JP. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS. 2009;117:458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peixoto L, et al. Integrative Genomic Approaches Highlight a Family of Parasite-Specific Kinases that Regulate Host Responses. Cell host & microbe. 2010;8:208–218. doi: 10.1016/j.chom.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazzinelli RT, et al. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 9.Pifer R, Yarovinsky F. Innate responses to Toxoplasma gondii in mice and humans. Trends in parasitology. 2011 doi: 10.1016/j.pt.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, et al. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YO, et al. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, et al. Virulent Toxoplasma gondii evade immunity-related GTPase-mediated parasite vacuole disruption within primed macrophages. J Immunol. 2009;182:3775–3781. doi: 10.4049/jimmunol.0804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharton-Kersten TM, et al. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade RM, et al. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling YM, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witola WH, et al. NALP1 Influences Susceptibility to Human Congenital Toxoplasmosis, Pro-Inflammatory Cytokine Response and Fate of T. gondii-Infected Monocytic Cells. Infection and Immunity. 2010 doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lees MP, et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010;184:7040–7046. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazzinelli RT, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 20.Suzuki Y, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 21.Vossenkämper A, et al. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur. J. Immunol. 2004;34:3197–3207. doi: 10.1002/eji.200424993. [DOI] [PubMed] [Google Scholar]
- 22.Sibley LD, Ajioka JW. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol. 2008;62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- 23.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 24.Taylor S, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 25.Saeij JPJ, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behnke MS, et al. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9631–9636. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Hajj H, et al. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007;3:e14. doi: 10.1371/journal.ppat.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fentress SJ, et al. Phosphorylation of Immunity-Related GTPases by a Toxoplasma gondii-Secreted Kinase Promotes Macrophage Survival and Virulence. Cell host & microbe. 2010;8:484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinfeldt T, et al. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS biology. 2010;8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlowski N, et al. The activation mechanism of Irga6, an interferon-inducible GTPase contributing to mouse resistance against Toxoplasma gondii. BMC Biol. 2011;9:7. doi: 10.1186/1741-7007-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunn JP, et al. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mammalian genome : official journal of the International Mammalian Genome Society. 2010 doi: 10.1007/s00335-010-9293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh SB, et al. Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria. Nature cell biology. 2010;12:1154–1165. doi: 10.1038/ncb2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh SB, et al. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto M, et al. ATF6{beta} is a host cellular target of the Toxoplasma gondii virulence factor ROP18. The Journal of experimental medicine. 2011;208:1533–1546. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldszmid RS, et al. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med - Suppl. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reese ML, Boothroyd JC. A helical membrane-binding domain targets the Toxoplasma ROP2 family to the parasitophorous vacuole. Traffic. 2009;10:1458–1470. doi: 10.1111/j.1600-0854.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reese ML, et al. A polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1015980108. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Hajj H, et al. The ROP2 family of Toxoplasma gondii rhoptry proteins: proteomic and genomic characterization and molecular modeling. Proteomics. 2006;6:5773–5784. doi: 10.1002/pmic.200600187. [DOI] [PubMed] [Google Scholar]
- 39.Reese ML, Boothroyd JC. A conserved noncanonical motif in the pseudoactive site of the ROP5 pseudokinase domain mediates its effect on Toxoplasma virulence. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Hajj H, et al. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cellular microbiology. 2007;9:54–64. doi: 10.1111/j.1462-5822.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 41.Saeij JPJ, et al. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosowski EE, et al. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. The Journal of experimental medicine. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen KD, et al. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell host & microbe. 2011;9:472–483. doi: 10.1016/j.chom.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ong Y-C, et al. Toxoplasma Rhoptry Protein 16 (ROP16) Subverts Host Function by Direct Tyrosine Phosphorylation of STAT6. Journal of Biological Chemistry. 2010;285:28731–28740. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto M, et al. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med. 2009 doi: 10.1084/jem.20091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature reviews. Immunology. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 47.Leng J, et al. Dysregulation of macrophage signal transduction by Toxoplasma gondii: past progress and recent advances. Parasite Immunol. 2009;31:717–728. doi: 10.1111/j.1365-3024.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robben PM, et al. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 49.Asai T, et al. Detection of nucleoside triphosphate hydrolase as a circulating antigen in sera of mice infected with Toxoplasma gondii. Infection and Immunity. 1987;55:1332–1335. doi: 10.1128/iai.55.5.1332-1335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asai T, et al. High correlation in antibody titers between the Sabin-Feldman dye test and an enzyme-linked immunosorbent assay detecting immunoglobulin G antibodies to the nucleoside triphosphate hydrolase of Toxoplasma gondii. Journal of clinical microbiology. 1992;30:1291–1293. doi: 10.1128/jcm.30.5.1291-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asai T, et al. Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. J Biol Chem. 1995;270:11391–11397. doi: 10.1074/jbc.270.19.11391. [DOI] [PubMed] [Google Scholar]
- 52.Stutz A, et al. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 54.Corrêa G, et al. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mordue DG, et al. A patatin-like protein protects Toxoplasma gondii from degradation in activated macrophages. Molecular microbiology. 2007;63:482–496. doi: 10.1111/j.1365-2958.2006.05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollard AM, et al. A transmembrane domain-containing surface protein from Toxoplasma gondii augments replication in activated immune cells and establishment of a chronic infection. Infection and Immunity. 2009;77:3731–3739. doi: 10.1128/IAI.00450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox BA, et al. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryotic Cell. 2009;8:520–529. doi: 10.1128/EC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yarovinsky F, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann S, et al. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. Journal of immunology. 2006;176:1840–1847. doi: 10.4049/jimmunol.176.3.1840. [DOI] [PubMed] [Google Scholar]
- 61.Boothroyd JC. Expansion of host range as a driving force in the evolution of Toxoplasma. Memorias do Instituto Oswaldo Cruz. 2009;104:179–184. doi: 10.1590/s0074-02762009000200009. [DOI] [PubMed] [Google Scholar]
- 62.Mills CD, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. Journal of immunology. 2000;164:6166–6173. [Google Scholar]
- 63.Dunay IR, et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunay IR, et al. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infection and Immunity. 2010;78:1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sergent V, et al. Innate refractoriness of the Lewis rat to toxoplasmosis is a dominant trait that is intrinsic to bone marrow-derived cells. Infection and Immunity. 2005;73:6990–6997. doi: 10.1128/IAI.73.10.6990-6997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cavaillès P, et al. The rat Toxo1 locus directs toxoplasmosis outcome and controls parasite proliferation and spreading by macrophage-dependent mechanisms. Proc Natl Acad Sci USA. 2006;103:744–749. doi: 10.1073/pnas.0506643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jamieson SE, et al. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010;11:374–383. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hitziger N, et al. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell Microbiol. 2005;7:837–848. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 69.Liesenfeld O, et al. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kong JT, et al. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis. 2003;187:1484–1495. doi: 10.1086/374647. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 72.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 73.Sukhumavasi W, et al. TLR adaptor MyD88 is essential for pathogen control during oral Toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol. 2008;181:3464–3473. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scanga CA, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 75.Liu C-H, et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 76.LaRosa DF, et al. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Natl Acad Sci USA. 2008;105:3855–3860. doi: 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melo MB, et al. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 79.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 80.Hunter CA, et al. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 81.Cai G, et al. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infection and Immunity. 2000;68:6932–6938. doi: 10.1128/iai.68.12.6932-6938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones LA, et al. IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur. J. Immunol. 2010;40:426–436. doi: 10.1002/eji.200939705. [DOI] [PubMed] [Google Scholar]
- 83.Dardé M-L. Genetic analysis of the diversity in Toxoplasma gondii. Ann Ist Super Sanita. 2004;40:57–63. [PubMed] [Google Scholar]
- 84.Khan A, et al. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerging Infect Dis. 2006;12:942–949. doi: 10.3201/eid1206.060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pena HFJ, et al. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int J Parasitol. 2008;38:561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Miller MA, et al. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. International journal for parasitology. 2004;34:275–284. doi: 10.1016/j.ijpara.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 87.Khan A, et al. Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Natl Acad Sci USA. 2007;104:14872–14877. doi: 10.1073/pnas.0702356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–442. doi: 10.1016/s1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 89.Holland GN. Reconsidering the pathogenesis of ocular toxoplasmosis. Am J Ophthalmol. 1999;128:502–505. doi: 10.1016/s0002-9394(99)00263-9. [DOI] [PubMed] [Google Scholar]
- 90.Roberts F, McLeod R. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol Today. 1999;15:51–57. doi: 10.1016/s0169-4758(98)01377-5. [DOI] [PubMed] [Google Scholar]
- 91.Grigg ME, et al. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. Journal of Infectious Diseases, The. 2001;184:633–639. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- 92.Plattner F, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Debierre-Grockiego F, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 94.Kim L, et al. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J Immunol. 2006;177:2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- 95.Aliberti J, et al. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat Immunol. 2000;1:83–87. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 96.Tait ED, et al. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J Immunol. 2010;185:1502–1512. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muñoz M, et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelly MN, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]