Abstract
Resolvins, including D and E series resolvins, are endogenous lipid mediators generated during the resolution phase of acute inflammation from the omega-3 polyunsaturated fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Resolvins are known to have potent anti-inflammatory and pro-resolution actions in several animal models of inflammation. Recent findings also demonstrate that resolvin E1 and resolvin D1 can each potently dampen inflammatory and postoperative pain. This review focuses on the mechanisms by which resolvins act on their receptors in immune cells and neurons to normalize exaggerated pain, via regulating inflammatory mediators, transient receptor potential (TRP) ion channels, and spinal cord synaptic transmission. Resolvins may offer novel therapeutic approaches for preventing and treating pain conditions associated with inflammation.
Introduction
Tissue injury results in inflammation and inflammatory pain, such as pain associated with arthritis, temporomandibular (TMJ) joint disorder, lower back injury, and surgery. Inflammatory and postoperative pains are typically characterized by heat and mechanical hyperalgesia, increased response to noxious heat and mechanical stimuli, and mechanical allodynia in which a nociceptive response occurs to normally innocuous mechanical stimulus such as light touch1–4. It is generally believed that inflammatory and postoperative pain can manifest as an expression of neuronal plasticity characterized by peripheral sensitization of primary sensory neurons in the dorsal root ganglion (DRG) and trigeminal ganglion 4–6 and central sensitization of spinal dorsal horn and cortical neurons 1,7,8. Although neuropathic pain after nerve injury and certain surgical procedures, such as amputation and thoracotomy 9, also engages the mechanisms of peripheral and central sensitization, this review focuses on the mechanisms and potential treatments of tissue-injury-induced inflammatory pain and postoperative pain.
Peripheral sensitization is induced by inflammatory mediators released after tissue insults, such as bradykinin, prostaglandins, H+, nerve growth factors (NGF), pro-inflammatory cytokines [such as tumor necrosis factor-alpha (TNF-α), interleukin-1β(IL-1β), and interleukin-6 (IL-6)] and proinflammatory chemokines [such as chemokine CC-motif ligand (CCL2)]10–13. Notably, the peripheral terminals of nociceptor express the receptors for all these inflammatory mediators14. Activation of these receptors causes hyperactivity of key transduction molecules, such as transient receptor potential subtype V1 (TRPV1) and A1 (TRPA1) and conduction molecules such as sodium channels Nav1.7/1.8/1.9. As a result, the sensitivity and excitability of nociceptors are increased, via activation of protein kinases, such as protein kinase A (PKA), protein kinase C (PKC), and mitogen-activated protein kinases (MAPKs)2,6,13–17.
Tissue injury-triggered hyperactivity of nociceptors will lead to increased release of neurotransmitters (e.g., glutamate) and neuromodulators [eg. substance P and brain-derived neurotrophic factor (BDNF)] from nociceptor central terminals in the spinal cord, causing hyperactivity of postsynaptic dorsal horn neurons (i.e. central sensitization)8. Activation of NMDA receptors plays an essential role in the induction and maintenance of central sensitization 18–20. Central sensitization is important for maintaining persistent pain and generating secondary pain outside the initial injury site 7,8,20. Phosphorylation of extracellular signal-regulated kinase (pERK), a MAPK family member, in dorsal horn neurons is nociceptive-specific and serves as a marker for central sensitization 21,22. Accordingly, pERK plays a critical role in the induction and maintenance of central sensitization by increasing NMDA receptor activity, inhibiting Kv4.2 potassium channel activity, and inducing AMPA receptor trafficking and transcription of pronociceptive genes 19,21,23. Recent progress also points to an important role of glial cells, such as microglia and astrocytes, in the spinal cord and brain for the genesis of central sensitization linked to the development of inflammatory and postoperative pain 24–32. Tissue injury produces TNF-α and IL-1β in spinal microglia and astrocytes to drive central sensitization by increasing excitation and decreasing inhibition in dorsal horn neurons 33–35. In addition, descending facilitation from the cortex and brain stem also facilitates persistent pain states after tissue injury 36,37. In this review, we discuss how novel pro-resolving lipid mediators, namely resolvins, can reduce tissue injury-induced inflammatory and postoperative pain, as well as tissue injury-induced peripheral and central sensitization.
Resolution of inflammation and pain
Although we know a lot about how inflammatory pain is initiated by pro-inflammatory mediators, we know little as to how inflammatory pain is resolved. What is often overlooked is that tissue injury not only produces pro-inflammatory mediators but also generates novel local mediators that are both anti-inflammatory and pro-resolving, resulting in spontaneous or self-limited recovery of acute inflammation and acute pain. Resolution of acute inflammation, once thought to be a passive process, has now been shown to involve a homeostatic recruitment of active biochemical programs that enable inflamed tissues to return to the pre-inflammatory states 38. Accumulating evidence indicates that anti-inflammation and pro-resolution are distinct mechanisms for the control of inflammation39. The actions of pro-resolution mediators are in sharp contrast to those of the current and widely used anti-inflammatory therapeutics such as inhibitors of cyclooxygenase-2 (COX-2), which, ironically, disrupt endogenous resolution mechanisms38,40. Phagocytosis of macrophages is a cardinal step toward the resolution of inflammation, since such a process allows for the removal of apoptotic cells, cell debris, and pathogens.
COX-2 inhibitors reduce the production of key local lipid mediators, leading to deficits in inflammation resolution 38,40–44. It was found in an early study using a rat inflammation model (pleurisy) that the COX-2 inhibitor NS-398 reduced inflammation at 2 hours but enhanced inflammation at 48 hours 40. Therefore, it was proposed that COX-2 is proinflammatory during the early phase of acute inflammation, dominated by polymorphonuclear leucocytes, but promoted inflammatory resolution in the later phase, dominated by mononuclear cells 40,42. Indeed, COX-2 is required for the biosynthesis of pro-resolving mediators such as lipoxins and resolvins in the resolution phase of inflammation 38. Thus, the net effect of a COX-2 inhibitor on pain is a balance between its anti-inflammatory/antinociceptive actions and anti-resolving/pronociceptive actions. Although COX-2 inhibitors may still produce transient anti-hyperalgesic effects in some chronic inflammation/pain conditions, they may also prolong the duration of pain after treatment by disrupting endogenous resolution circuits.
While reduction of cell recruitment (influx) to the inflammation site reflects an anti-inflammation action, increase in cell exit (efflux) could demonstrate a pro-resolution action. Notably, resolvins at very low concentrations (pico- to nano-molar range) can markedly promote phagocytic activity of macrophages 38,44,45. It is believed that disruption of acute resolution processing will lead to uncontrolled inflammation that has been implicated in the pathogenesis of many chronic diseases 38. Similarly, a failure in resolution of acute inflammation may also lead to transition from acute pain to chronic pain. Unresolved inflammation will lead to chronic inflammation, which has been largely ignored in the pain research field, in part, due to its low intensity and local nature and lack of signs in circulating blood. This chronic inflammation should also include neuroinflammation in the peripheral nervous system (PNS) and central nervous system (CNS) involving activation of glial cells (e.g., microglia, astrocytes, and satellite glia). Chronic ongoing inflammation should play an important role in maintaining neural plasticity and chronic pain.
It is also important to point out that chronic pain is not just an extension of acute pain, but rather is the result of ongoing changes in pain processing, namely neuroplasticity. Targeting the transition phase from acute to chronic pain – for instance, by dampening acute inflammation - is likely to be important in preventing the development of chronic pain. However, reducing inflammation in the chronic phase per se may not be sufficient to treat chronic pain. Novel therapies for chronic pain should also directly target neural plasticity in both the PNS and CNS.
Resolvins and resolution of inflammation
Resolvins, such as resolvin D1 (RvD1), aspirin-triggered resolvin D1 (AT-RvD1), and resolving E1 (RvE1), represent a new family of pro-resolution mediators that are biosynthesized from the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) 46 (Fig. 1). Resolvins were originally isolated in exudates formed in the resolution phase of acute inflammation in both rodents and humans 43,46,47. Protectins, another class of endogenous molecules that include neuroprotectin D1, were also isolated from resolving inflammatory exudes [46]. These compounds will not be discussed further here, but they also exhibit many beneficial actions in the retina and brain (reviewed in [58]).
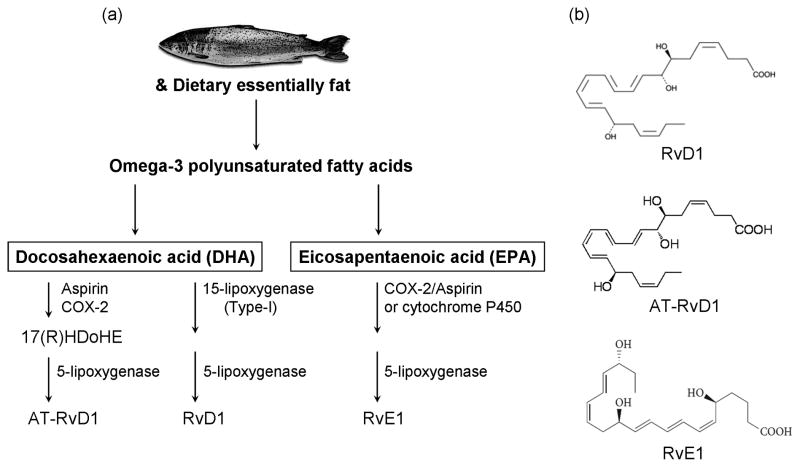
Figure 1.
Resolvins and their routes of formation. (a) Omega-3 polyunsaturated fatty acids include DHA and EPA, and are derived from dietary essentially fat (especially enriched in fish). RvD1 and AT-RvD1 are derived from DHA, whereas RvE1 is derived from EPA. Distinct synthetic enzymes, including COX-2, cytochrome P450, and 5- and 15-lipoxygenase are involved in these processes. See Ref [49] for detailed review on the biosynthesis of resolvins. (b) Chemical structures of the resolvins that are discussed in this review.
EPA and DHA are enzymatically converted to RvE1 and RvD1/AT-RvD1 in a multi-step reaction that proceeds through a transcellular biosynthetic route during endothelial/neutrophil cell-cell interactions 43,48. The biosynthesis of the resolvins has recently been reviewed in detail49. Briefly, RvE1 (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid) requires several enzymes such as COX-2 or cytochrome P450, and 5-lipoxygenase (5-LOX) for its biosynthesis from EPA50 (Fig. 1). Notably, aspirin initiates the biosynthetic pathway via acetylating COX-2, which may partly explain the mechanisms underlying the beneficial effects of low-dose aspirin in inflammation-associated chronic diseases 51,52. By comparison, RvD1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) utilizes 15-LOX and 5-LOX for its biosynthesis from DHA 53 (Fig. 1). It is important to point out that consumed (digested) fish oil (DHA and EPA) will not automatically be metabolized to resolvins under normal conditions, partly because the biosynthetic enzymes have low expression levels in most tissues in the non-injured states (Box-1). These enzymes are strongly induced by acute inflammation. For example, COX-2 and LOX-15 are induced not only at the site of peripheral inflammation, but also at the levels of the DRG and spinal cord 54–56. Of note, COX-2 is also constitutively expressed in the nervous system (including spinal cord and retina), as in the vasculature 57,58.
Box 1. Outstanding questions.
What is the relationship of dietary EPA and DHA to local resolvin production in humans and in regulating pain? Resolvins of the D- and E-series are biosynthesized from essential n-3 polyunsaturated acids during the resolution of acute inflammatory responses by inflammatory exudates (ref [38]), thus, tissue levels of EPA and DHA are key determinates in the timely resolution and operation of the local effector immune response. What are the minimal dietary requirements for n-3 in humans that are needed to evoke endogenous resolvin biosynthesis and the timely resolution of inflammation and pain?
Can resolvins be used to treat other persistent pain conditions, such as neuropathic pain and cancer pain, in addition to inflammatory and postoperative pain? An initial study has demonstrated anti-hyperalgesic effects of RvE1 in early-phase neuropathic pain ([71], Fig. 2d), suggesting that resolvins may be of therapeutic potential for a broader range of pain conditions than currently considered.
Do resolvins also differentially regulate ion channels other than TRP channels, such as acid-sensitive ion channels, calcium channels, and sodium channels?
Can resolvins also act on currently unknown receptors? Can small molecule agonists of resolvin receptors be developed for pain management?
What are the downstream signaling pathways that are activated or inactivated by resolvins with respect to pain? Can we harness these for a new approach to more effectively treat pain in humans?
RvE1 has been demonstrated to exhibit high potency in treating inflammation-related diseases in various animal models, some of which are listed in Table-1. In a murine peritonitis model, RvE1 (at nanomolar levels), was shown to significantly reduce dermal inflammation, peritonitis, neutrophil recruitment, dendritic cell migration, and modulate the expression of cytokines and chemokines 48,59. Topical administration of RvE1 was also found to reduce inflammation-induced bone loss in a rabbit model of periodontal disease, a painless yet important public health concern60. Vessel loss and subsequent neovascularization are two critical phases in many sight-threatening diseases. RvE1 potently protected against neovascularization in a mouse model of oxygen-induced retinopathy, in part by reducing TNF-α production in microglia associated with retinal vessels 61. RvE1 also protected against intestinal inflammation and colitis in mice by increasing the survival rates and decreasing leukocyte infiltration 62. RvE1 also mediates resolution of allergic inflammation, via regulating natural killer (NK) cell migration in vivo and NK cell cytotoxicity in vitro63,64. Stromal keratitis, a chronic immunopathological disease after herpes simplex virus (HSV) infection often causes blindness in humans. RvE1 treatment significantly reduced the extent of angiogenesis and stromal keratitis lesions, by reducing the numbers of inflammatory cells including T helper (Th1/Th17) cells and neutrophils in the cornea, increasing production of the anti-inflammatory cytokine IL-10, and inhibiting the production of pro-inflammatory mediators in mice 65. Most cases of pneumonia can spontaneously resolve, which also engages resolvins. In a murine model of aspiration pneumonia, RvE1 treatment decreased neutrophil accumulation and enhanced clearance of E. coli in the lung 66.
Table 1.
Anti-inflammatory actions of resolvins in animal models of inflammation
| Resolvins | Animals | Inflammatory conditions | Anti-inflammatory effects | Refs |
|---|---|---|---|---|
| RvE1 | Mouse | Dorsal air pouch | Stop neutrophil recruitment | [42] |
| Peritonitis | Reduce neutrophil recruitment | [48,59] | ||
| Regulate cytokine and chemokine expression | [48,59] | |||
| Retinopathy | Alleviate neovascularization and reduce TNF-a expression | [61] | ||
| Colitis | Decrease neutrophil recruitment and improve survival | [62] | ||
| Allergy | Resolve allergic airway inflammation | [63, 64] | ||
| Regulate NK cell migration | [63, 64] | |||
| Ocular Lesion | Control HSV-induced ocular inflammatory lesions | [65] | ||
| Increase IL-10 and decrease IL-6 expression | [65] | |||
| Lung injury | Protect bacterial pneumonia and acute lung injury | [66] | ||
| Enhance clearance of bacteria | [66] | |||
| RvD1 | Mouse | Kidney ischemia | Protect from ischemia-reperfusion-induced kidney damage | [67] |
| Regulate macrophage function | [67] | |||
| Peritonitis | Stop neutrophil recruitment and modulate miRNAs | [68] | ||
| Dorsal skin air pouch | Stop neutrophil recruitment | [46] | ||
| Oxidative stress | Control inflammation during oxidative stress | [69] | ||
| AT-RvD1 | Mouse | TMJ inflammation | Protect inflammation in the TMJ | [70] |
RvD1 and AT-RvD1 (the aspirin triggered form) also exhibit potent anti-inflammatory and pro-resolution actions in rodent models of inflammation (Table-1). Mouse kidney produces RvD1 in response to bilateral ischemia/reperfusion injury, and RvD1 administration before the ischemia alleviated functional and morphological kidney injury, reduced interstitial fibrosis and leukocytes infiltration, and blocked TLR-mediated activation of macrophages 67. Notably, RvD1 also regulates microRNA (miRNA) expression in self-limited murine peritonitis68. In a murine dorsal skin air pouch model, in which resolvins were originally identified, RvD1 blocked neutrophil recruitment 46. RvD1 also controls inflammation after oxidative stress 69. Recently, AT-RvD1 has been shown to protect against inflammation in a murine model of TMJ disease 70.
Resolvins and resolution of inflammatory and postoperative pain
Resolvins also reduce inflammation and pain in well-established rodent models of inflammatory pain. Intraplantar pretreatment of mice with RvE1, at a very low dose (20 ng), reduced carrageenan (CRG)-induced neutrophil infiltration and paw edema, as well as expression of proinflammatory cytokines and chemokines (e.g., TNF-α, IL-1β, IL-6, and CCL2) in inflamed paw tissues 71. Furthermore, intraplantar pretreatment of either RvD1 or RvE1 was found to reduce formalin-induced spontaneous pain and prevent CRG-induced heat hyperalgesia (Table 2 and Fig. 2a,b)71,72. Interestingly, RvE1 is more effective in reducing inflammatory pain than reducing tissue trafficking of inflammatory leukocytes, because the same dose of RvE1 largely inhibited heat hyperalgesia but only partially inhibited edema and neutrophil infiltration in the CRG model 71. The analgesic potency of resolvins is attributed in part to their capability of inhibiting the actions of TRP channels, such as TRPV1 and TRPA1, that have been strongly implicated in the genesis of inflammatory pain 14,73–75. Thus, intraplantar or intrathecal administration of RvE1 in mice abolished the spontaneous pain induced by capsaicin (TRPV1 agonist) but not by mustard oil (TRPA1 agonist) 71. In contrast, intraplantar RvD1 administration blocked the TRPA1 but not TRPV1 agonist-elicited pain 72. Intraplantar post-treatment of RvD1 also reduced CFA-induced mechanical hyperalgesia and allodynia via TRPA1 inhibition 72. Further, repeated systemic administration of AT-RvD1 effectively attenuated mechanical hyperalgesia in the complete Freund’s adjuvant (CFA) inflammatory pain model in rats 55 (Table 2). Such an effect was likely due to the observed reduction in TNF-α and IL-1β expression levels in the CFA-inflamed paw tissue, as well as reductions in NF-κB expression in the DRG and spinal cord 55. It is also of interest to note that 17(R)-HDoHE [17(R)-hydroxy-4Z,7Z,10Z,13Z,15E,17R,19Z-docosahexaenoic acid], the precursor of AT-RvD1, attenuated CFA-induced arthritic pain (stiffness) but not edema in rats 55. 17(R)-HDoHE can also be converted to other aspirin-triggered D series resolvins, including AT-RvD2, AT-RvD3, AT-RvD4 and AT-RvD5 46,55,76, but their roles in resolving pain have yet to be established.
Table 2.
Antinociceptive and anti-inflammatory actions of resolvins in rodent models of inflammatory and postoperative pain.
| Resolvins | Animals | Pain models | Antinociceptive/anti-inflammatory actions | Refs |
|---|---|---|---|---|
| RvE1 | Mouse | Formalin | Reduce inflammatory pain | [71] |
| CRG | Decrease inflammatory pain Reduce IL-1β, IL-6, TNF-α, and CCL2 expression Reduce edema and neutrophil infiltration |
[71] | ||
| CFA | Reduce inflammatory pain | [71] | ||
| Capsaicin | Reduce spontaneous pain | [71] | ||
| TNF-α | Abolishes heat hyperalgesia and mechanical allodynia | [71] | ||
| Incision | Reduce postoperative pain | [71] | ||
| RvD1 | Mouse | CRG | Reduce inflammatory pain | [71] |
| CFA | Reduce inflammatory pain | [71, 75] | ||
| Formalin | Reduce inflammatory pain | [75] | ||
| Mustard oil | Reduce spontaneous pain | [75] | ||
| PGE2 | Reduce inflammatory pain | [75] | ||
| Rat | Incision | Reduce postoperative pain | [82] | |
| SMIR | Reduce postoperative pain | [82] | ||
| AT-RvD1 | Rat | CFA | Reduce inflammatory pain Reduce TNF-α, IL-1β, COX-2, and NF-κB expression |
[55] |
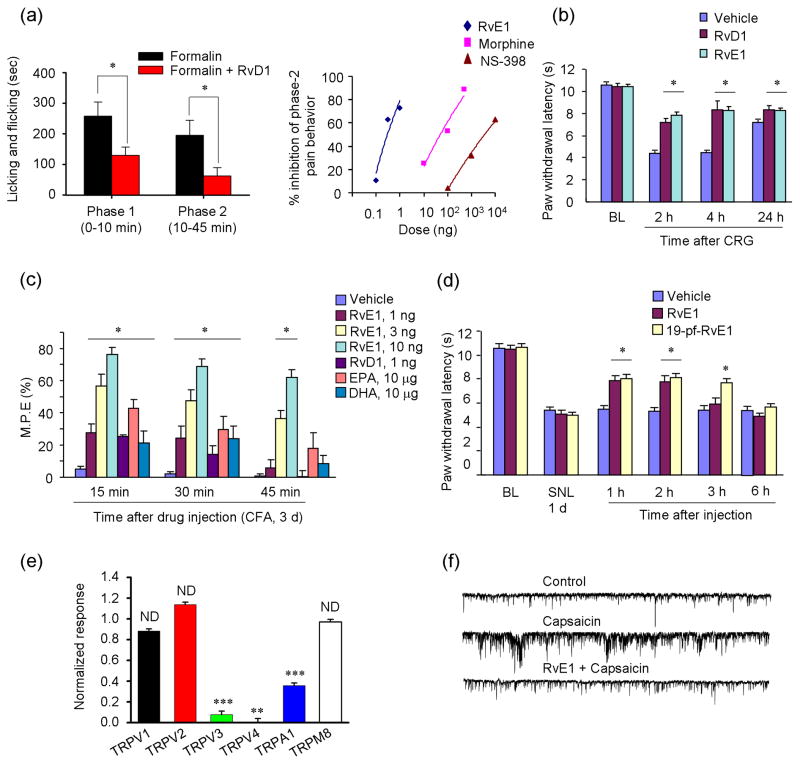
Figure 2.
Resolvins (RvE1 and RvD1) inhibit inflammatory and neuropathic pain in mice and suppress TRP channel activity. (a) Mouse model of formalin-induced inflammatory pain. Left panel, summary of the accumulated licking/flicking time of the 1st and 2nd phases. *P < 0.05, vs. vehicle. Right panel, dose-response curves showing the inhibition of formalin-induced 2nd phase pain by intrathecal RvE1, morphine, and NS-398 (a selective COX-2 inhibitor). Note that RvE1, NS-398, and morphine have similar molecular weights. (b) Carrageenan (CRG)-induced heat hyperalgesia is reduced by intraplantar pretreatment of RvE1 and RvD1. *P<0.05, vs. vehicle. (c) CFA-induced heat hyperalgesia on day 3 is differentially reduced by intrathecal administration of RvE1, RvD1, DHA, and EPA. M.P.E, maximum possible effect of anti-hyperalgesia. *P<0.05, vs vehicle. (d) Spinal nerve ligation (SNL)-induced neuropathic pain. SNL-induced heat hyperalgesia on day 1 is reduced by RvE1 and 19-pf-RvE1 (a metabolically stable analogue of RvE1). *P<0.05, vs vehicle. (e) Intracellular Ca2+ responses in HEK293T cells and differential effects of RvD1 (300 nM) on TRP agonist-induced intracellular Ca2+ increases. HEK293T cells were transiently transfected with individual thermoTRP channels and stimulated with appropriate agonists: 0.2 mM capsaicin for TRPV1; 100 mM probenecid for TRPV2; 4 mM camphor for TRPV3; 10 mM 4a-PDD for TRPV4; 300 mM menthol for TRPM8; and 300 mM cinnamaldehyde for TRPA1. **P < 0.01, ***P < 0.001; compared with respective agonist alone. (f) Patch-clamp recordings of traces of spontaneous postsynaptic currents (sEPSCs) in spinal cord slices of mice. Note that the sEPSC frequency after capsaicin perfusion is blocked by RvE1 pretreatment. Reproduced/modified, with permission, from [71] [panels a (right), c–d, and f) and [72] (panels a (left) and e).
Resolvins also reduce inflammatory pain via central actions. Pretreatment of RvE1 intrathecally (ie. at the spinal cord level), at very low doses (0.3 and 1 ng), reduced formalin-induced 2nd phase pain (Fig. 2a), which is known to be mediated via a spinal cord mechanism (ie. central sensitization)71. Strikingly, the dose of RvE1 required to reduce the 2nd phase pain is 100 times lower than that of morphine and 10000 times lower than that of the COX-2 inhibitor NS-398 (Fig. 2a). Intrathecal post-treatment with either RvE1 and RvD1 also rapidly reduced CFA-induced heat and mechanical hypersensitivity 71,72. Although dietary omega-3 fatty acids (e.g., DHA and EPA) were shown to alleviate inflammatory pain in patients 77, presumably via their own G-protein coupled receptors (GPCRs) such as GRP120 78, the efficacy of these precursors of resolvins for pain relief is much lower; the intrathecal dose required for either DHA and EPA to reduce CFA-induced heat hyperalgesia is >1000 times higher than that of RvE1 71. Thus, endogenous resolvins may provide a missing link between dietary omega-3 fatty acids and the reduction of inflammation 79 and inflammatory pain.
Postoperative pain caused by skin/muscle incision normally resolves within 1 week, and such pain is commonly recapitulated in rodent models of paw incision 3. Postoperative pain after prolonged muscle retraction lasts 3–4 weeks in humans and rodents 80. It is of great significance to effectively and aggressively manage acute postoperative pain and shorten the duration of patients’ hospital stay. Indeed, fast-track surgery has been strongly advocated in an effort to optimize surgical outcomes and control ever-increasing health care costs 81. Effective management of acute postoperative pain may also reduce the incidence of chronic postoperative pain, which can persist 3–6 months after surgery 82. Resolvins have also displayed potent analgesic actions in rodent models of postoperative pain (Table-2). RvE1 and RvD1 prevented paw incision-induced postoperative pain in mice and rats 71,82. In a recently developed skin-muscle retraction model (SMIR), surgery-induced mechanical hypersensitivity can last up to 4 weeks, yet a single treatment of RvD1 on postsurgical day 2 largely prevented this postoperative hyperalgesia in rats 82]. Of note, RvD1 treatment at later time points (e.g., postoperative day 9) only produced transient pain relief (< 1 day) 82, suggesting a time-dependent efficacy of resolvin treatment. Nevertheless, resolvin still displays efficacy in treating late-phase pain.
Importantly, resolvins do not interfere with normal pain perception. Thus, intraplantar, intrathecal, or systemic injection of resolvins was not observed to affect either thermal and mechanical pain sensitivity in rat and mice 55,71,82. In sharp contrast, the classic analgesics such as morphine dramatically decrease pain sensitivity 83. Physiological pain has evolved as a protective response. For people who can not feel pain, due to genetic mutation of the pain genes encoding neurotrophic tyrosine kinase receptor type A (TrkA) and sodium channel Nav1.7, life is often tragic, with self-mutilation and short life span 84. Thus, resolvins may serve as a new class of analgesics that act to block abnormal pain and restore normal sensitivity, rather than blocking pain transmission like classical analgesics.
Mechanisms underlying resolvin’s analgesic actions
Resolvin’s analgesic actions are thought to be mediated by specific GPCRs. The specific binding of RvE1 to the Gαi-associated ChemR23 receptor has been demonstrated using synthetic [3H]-labeled RvE1 48,85. Several lines of evidence indicate that ChemR23 mediates RvE1’s analgesic action in formalin-induced 2nd phase pain in mice 71. First, RvE1’s action in this pain model was abolished by pertussis toxin treatment, a specific inhibitor for Gi-coupled GPCRs, but not by naloxone, an opioid receptor antagonist71. Second, RvE1’s analgesic effect was abrogated by knockdown of ChemR23 with specific siRNA treatment71. Third, RvE1’s analgesic action could be recapitulated by chemerin, a natural peptide ligand for ChemR2371. Similarly to RvE1, chemerin reduces transepithelial migration of neutrophils and promotes apical clearance of neutrophils 45. Chemerin also inhibits the production of the pro-inflammatory mediators (e.g., TNF-α, IL-1β, IL-6 and IL-12) and induces the expression of anti-inflammatory cytokines such as transforming growth factor-beta (TGF-β) and IL-10 in macrophages, in a pertussis toxin–sensitive manner 86.
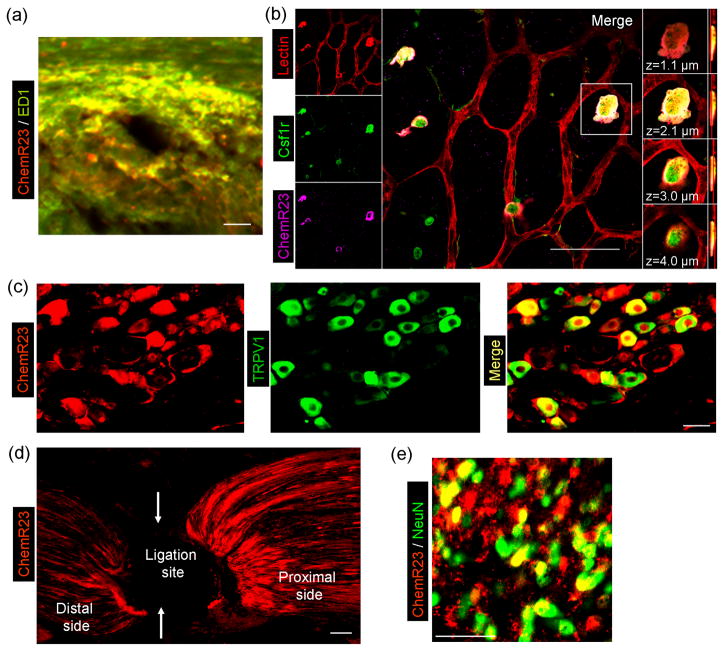
A broad expression of ChemR23 in various cell types may explain the versatile actions of RvE1 (Fig. 3). Earlier studies demonstrated ChemR23 expression in macrophages, microglia, and dendritic cells 48,61,86,87 (Fig. 3a, b). Recent findings have revealed that ChemR23 is also expressed by primary sensory DRG neurons. In particular, ChemR23 is heavily colocalized with TRPV1, a heat sensor in nociceptors (Fig. 3c). ChemR23 synthesized in DRG cell bodies is transported axonally (Fig. 3d) to central terminals in the spinal cord 71 and presumably, to peripheral terminals. ChemR23 is also expressed in spinal cord neurons (Fig. 3e). Furthermore, inflammation induces ChemR23 expression in skin macrophages (Fig. 3a). In addition, AT-RvD1 and RvD1 are known to activate the same GPCRs, GPR32 (human) as well as the LXA4 receptor ALX/FPR2 (murine) 88, and the latter is expressed in spinal astrocytes 89. However, the expression patterns of these receptors and their co-existence with ChemR23 awaits further investigation.
Figure 3.
The RvE1 receptor ChemR23 is widely expressed in immune cells, glial cells, and neurons in mouse tissues. (a) Double staining of ChmR23 and ED1 shows that ChemR23 is largely colocalized with the macrophage marker ED1 in the dermis of the CFA-inflamed skin. Scale bar: 50 μM. (b) Triple staining in retinal whole-mounts demonstrates that ChemR23 is localized to a subset of colony-stimulating factor-1-receptor–positive (Csf1r+) microglia. Left column, triple staining for lectin (endothelial cells and microglia, red), Csf1r (green) and ChemR23 (magenta). Central panel, merged image (scale bar: 50 μm); white indicates the colocalization of all three stains. Right column, four images of one Csf1r+ cell at indicated focal planes. (c) Double staining of ChemR23 and TRPV1 shows that ChemR23 is largely co-localized with TRPV1 in DRG neurons. Note that ChemR23 is also expressed in satellite glial cells surrounding neurons. Scale bar: 30 μM. (d) ChemR23 staining shows that after ligation of the sciatic nerve ChemR23 is accumulated near the ligation site (arrows), indicating axonal transport of ChemR23. Scale bar: 30 μM. (e) Double staining of ChemR23 and NeuN shows that ChemR23 co-colocalizes with the neuronal marker NeuN in the spinal cord dorsal horn. Scale bar: 50 μM. Note that ChemR23 is also expressed in NeuN-negative glial cells in the spinal cord. Reproduced/modified, with permission, from [61] (panel b), [71] (panels a, c–e).
Strikingly, different resolvins may differentially regulate TRP channels. RvE1 is known to block capsaicin-induced spontaneous pain, ERK activation in DRG neurons, and spinal cord synaptic plasticity (discussed below), without affecting TRPA1-induced pain71. In contrast, RvD1 inhibited TRPA1, TRPV3 and TRPV4, but not TRPV1 currents, in cultured human embryonic kidney 293 (HEK293) cells and DRG neuron cultures 72. Consistent with these findings, RvE1 is effective at low doses in reducing TRPV1-mediated heat hyperalgesia71, whereas RvD1 and AT-RvD1 are very effective in inhibiting mechanical hyperalgesia 55, whichis known to involve the activation of TRPA1/TRPV4 90,91. It should be noted that RvE1 can also reduce CFA-induced mechanical allodynia [71], but this occurs at much higher doses than those observed for the inhibition of heat hyperalgesia. Finally, a recent study demonstrated that 17(R)-RvD1, an analogue of RvD1, specifically inhibited TRPV3 in vitro 92. Thus, different resolvins may regulate different modalities of pain that are controlled through distinct TRP channels.
Tissue injury-induced spinal cord synaptic plasticity (i.e. central sensitization) has been strongly implicated in the genesis of persistent pain8. Such plasticity is measured in part as changes in spontaneous excitatory postsynaptic currents (sEPSCs), which could indicate both presynaptic mechanisms (frequency changes) and postsynaptic mechanisms (amplitude changes). Perfusion of spinal cord slices with RvE1 does not alter basal synaptic transmission, however, it does abolish capsaicin- and TNF-α-induced sEPSC frequency increases in lamina II neurons [71]. This indicates that RvE1 can normalize spinal cord synaptic plasticity, presumably by inhibiting ERK phosphorylation and glutamate release in presynaptic terminals (Figure 4f)71.
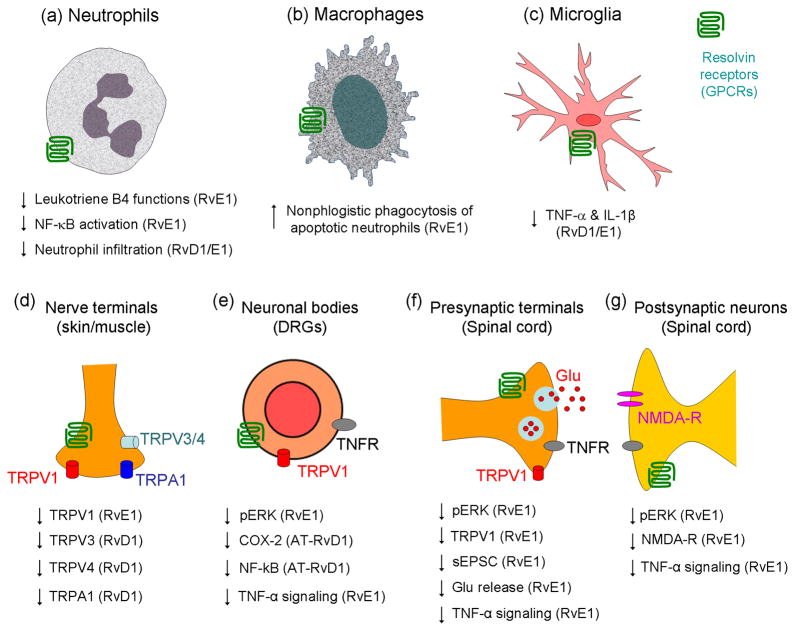
Figure 4.
Schematic of selective actions of resolvins in the resolution of abnormal pain. (a) RvE1 and RvD1 reduce leukotriene B4 functions, NF-κB activation, and infiltration in neutrophils [38]. (b) RvE1 acts on macrophages to induce nonphlogistic phagocytosis of apoptotic neutrophils (Ref [38]). (c) RvE1 and RvD1 inhibit TNF-α and IL-1β expression in microglia (Ref [38, 61]). (d) In peripheral nerve terminals in skin and muscle, RvE1 inhibits TRPV1 activity (Ref [71]), and RvD1 inhibits TRPA1, TRPV3, and TRPV4 activity (Ref [72]). (e) In DRG neuronal cell bodies, RvE1 inhibits capsaicin-induced ERK activation (Ref [71]), and AT-RvD1 inhibits CFA-induced COX-2 and NF-κB and expression (Ref [55]). RvE1 also inhibits TNF-α-induced ERK activation (Ref [71]). (f) At presynaptic sites of spinal cord primary afferent terminals, RvE1 inhibits TRPV1- and TNF-α-induced sEPSC frequency increase. This presumably occurs as a result of reduced glutamate release, via suppression of ERK activation [71]. (g) At postsynaptic sites of spinal cord dorsal horn neurons, RvE1 inhibits TNF-α-induced ERK activation and NMDA receptor hyperactivity (Ref [71]).
Activation of NMDA receptors in dorsal horn neurons is a key element for central sensitization and chronic pain development 7,18,93. TNF-α not only increases sEPSC frequency but also increases NMDA-induced currents in dorsal horn neurons in an ERK-dependent manner71. Notably, RvE1 blocks both the TNF-α-induced ERK phosphorylation and the correlated NMDA receptor activation in dorsal horn neurons 33,71 (Figure 4g). Thus, it is conceivable that RvE1 abrogates central sensitization via both presynaptic and post/extra-synaptic mechanisms. The underlying signaling mechanisms are largely unknown, although modulation of the ERK pathway - a critical pathway involved in central sensitization 8 - is likely to be involved 71.
Lastly, it should be mentioned that given the important role of spinal cord microglia in the genesis of arthritic and postoperative pain (see Introduction) and the known ChemR23 expression in microglia (Fig. 2a), it is reasonable to suggest that resolvins may also dampen pain via blocking microglia activation 46,61 (Fig. 4). Although this has yet to be tested in the spinal cord, RvE1 has been shown to inhibit TNF-α production in retina microglia in a mouse model of oxygen-induced retinopathy 61.
Conclusions and future perspectives
Mounting evidence indicates that resolvins, such as RvE1, RvD1, and AT-RvD1, are potent agonists that reduce inflammation by stimulating its resolution, as well as reducing inflammation-associated pain. The analgesic effects of resolvins are mediated by specific GPCRs (e.g., ChemR23, GPR-32), which are widely expressed by immune cells, glial cells, and neurons. Thus, resolvins can dampen abnormal pain via multiple mechanisms acting on various cell types, by reducing inflammation, glial activation, and spinal cord synaptic plasticity (Fig. 4), while at the same time leaving normal pain sensation intact. RvE1 blocks the ERK signaling pathway, a pathway that is known to regulate peripheral sensitization, central sensitization, and glia activation in the DRG and spinal cord21. Resolvins are also known to inhibit the NF-κB pathway to dampen the biosynthesis of pro-inflammatory mediators 38.
Inflammation is widely associated with various clinical pain conditions throughout the body and under a number of different injury conditions. A typical example is arthritis including rheumatoid arthritis (RA) and osteoarthritis (OA). Pain (and joint stiffness) is the initial and prevailing symptom of arthritis that demands immediate treatment. Postoperative pain, lower back pain, cancer pain, and temporomandibular joint (TMJ) pain also have components of inflammatory pain. Although fibromyalgia and inflammatory bowel syndrome (IBS) are classified as atypical pain or central pain, due to the lack of obvious signs of pathology and inflammation, the possibility that chronic ongoing and subthreshold/subclinical inflammation may exist in these diseases should not be excluded. Inflammatory pain is often treated with opioids and non-steroid anti-inflammatory drugs such as COX-2 inhibitors. However, these treatments are currently limited by well-known side effects. Acute opioid treatment produces respiratory depression, sedation, nausea, constipation, and vomiting94,95, and long-term treatment with opioids and COX-2 inhibitors is associated with the development of addiction and cardiovascular defects, respectively 96,97. Anti-TNF-α treatment has shown promise for inflammatory pain. For instance, arthritis TNF-α neutralization has been shown to inhibit pain in patients with RA much faster than it improves the signs of arthritis itself (ie. joint swelling), probably by rapid inhibition of central sensitization98. Moreover, the levels of TNF-α, IL-1β, and IL-6 are positively associated with pain severity in several painful musculoskeletal diseases, including fibromyalgia 99,100. However, anti-TNF-α and cytokine treatment may lead to infection due to immune suppression 71,94. Furthermore, due to the redundancy in the immune system, the approach of focusing on a single cytokine may be beneficial in only a fraction of patients.
Resolution of inflammation and inflammatory pain by endogenous pro-resolving mediators represents a novel therapeutic approach. By employing the multiple actions of resolvins (ie. anti-inflammatory, pro-resolving, and anti-hyperalgesic), this strategy may more effectively address the redundancy issue. Given the potent analgesic efficacy and safety profiles of endogenous lipid mediators, which includes dietary supplements which are taken in mg to gram amounts daily 77, resolvins and their analogues may offer new therapeutic approaches for the management of inflammation-associated pain. Finally, the urgent need to treat existing pain conditions is paralleled by the significant potential to prevent the development of chronic pain after certain surgical procedures (e.g., amputation and thoracotomy) with resolvin-derived therapies.
It is also important to point out the limitations of lipid mediators as potential new therapeutics. It is well known that endogenous local acting lipid mediators are, in general, metabolically unstable in that they are rapidly inactivated in vivo. For example, RvE1 reduces CFA-induced heat hyperalgesia for less than 2 hours71. Along these lines, a modified form of RvE1 (19-pf-RvE1), which is metabolically stable and resistant to local rapid metabolic inactivation has been developed 101. This more-stable form of RvE1 has been demonstrated to reduce heat hyperalgesia in mice for more than 6 hours71. Furthermore, the anti-inflammatory and pro-resolving actions of AT-RvD1 have been prolonged by constructing novel humanized nanoparticles that contain AT-RvD170. Hence, the development of more stable forms of resolvins, or more stable delivery methods, should prolong and enhance the analgesic effects of resolvins.
The detailed signaling mechanisms of how resolvins mediate pain relief remain illusive. A better understanding of the molecular means by which resolvins are so effective and potent for pain relief will be critical for future clinical development for targeting resolvin receptors and signaling pathways using small molecule agonists (Box-1). The analgesic efficacy of resolvins appear to be time-dependent, with more significant effects generally being observed when treatments were administrated at early stages, although resolvins are still effective in reducing late-phase pain in some animal models. Specific resolvins (i.e. RvD1, RvD2, RvE1) may differentially regulate distinct TRP channels (e.g., TRPA1, V1, V4) to control selective modalities of pain (e.g., heat vs mechanical hyperalgesia). It will be of interest to examine in the future whether a combination of several resolvins can achieve additive, and even synergistic analgesic effects.
Acknowledgments
The work was supported by National Institutes of Health grants R01-DE17794 and NS54932 (to R.R.J.), P01-GM 95467 (to C.N.S.), R01-NS67686 (to R.R.J. and C.N.S.), and R01-CA080153 (to G.R.S).
Footnotes
Statement of Conflicts of Interest
C.N.S. may have competing financial interests. Resolvins are biotemplates for stable analogs. Patents on these and their clinical indications are awarded and assigned to the Brigham and Women’s Hospital, and C.N.S. is the inventor. These patents are licensed for clinical development. C.N.S. retains scientific founder stock in Resolvyx Pharmaceutical Company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 2.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Brennan TJ, et al. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 4.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 6.Bhave G, Gereau RW. Posttranslational mechanisms of peripheral sensitization. J Neurobiol. 2004;61:88–106. doi: 10.1002/neu.20083. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 8.Ji RR, et al. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H, et al. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 10.Oh SB, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Stein C, et al. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv. 2007;7:26–41. doi: 10.1124/mi.7.1.6. [DOI] [PubMed] [Google Scholar]
- 14.Basbaum AI, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamboulian S, et al. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci. 2010;30:1637–1647. doi: 10.1523/JNEUROSCI.4872-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binshtok AM, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XJ, et al. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 21.Ji RR, et al. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji RR, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 23.Hu HJ, et al. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Guo W, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen YR, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–165. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 26.Raghavendra V, et al. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 27.Sun S, et al. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda M, et al. Pain and purinergic signaling. Brain Res Rev. 2010;63:222–232. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang ZY, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki Y, et al. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, et al. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci. 2010;30:12844–12855. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark AK, et al. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–582. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ossipov MH, et al. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Serhan CN, et al. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 41.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy BD, et al. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 43.Serhan CN, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwab JM, et al. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell EL, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 46.Serhan CN, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong S, et al. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 48.Arita M, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh SF, et al. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiang N, et al. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris T, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 53.Sun YP, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 54.Okubo M, et al. Expression of leukotriene receptors in the rat dorsal root ganglion and the effects on pain behaviors. Mol Pain. 2010;6:57. doi: 10.1186/1744-8069-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lima-Garcia J, et al. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samad TA, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 57.Ghilardi JR, et al. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. J Neurosci. 2004;24:2727–2732. doi: 10.1523/JNEUROSCI.5054-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bazan NG, et al. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bannenberg GL, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 60.Hasturk H, et al. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 61.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arita M, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haworth O, et al. NK cells are effectors for resolvin e1 in the timely resolution of allergic airway inflammation. J Immunol. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haworth O, et al. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajasagi NK, et al. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seki H, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duffield JS, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 68.Recchiuti A, et al. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spite M, et al. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br J Pharmacol. 2009;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Norling LV, et al. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu ZZ, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bang S, et al. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. 2010;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dai Y, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji RR, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 75.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 76.Xu ZZ, Ji RR. Resolvins are potent analgesics for arthritic pain. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01348.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. doi: 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 78.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- 80.Flatters SJ. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Pain. 2008;135:119–130. doi: 10.1016/j.pain.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 82.Huang L, et al. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain. 2011;152:557–565. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 84.Raouf R, et al. Pain as a channelopathy. J Clin Invest. 2010;120:3745–3752. doi: 10.1172/JCI43158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arita M, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 86.Cash JL, et al. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wittamer V, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnamoorthy S, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Svensson CI, et al. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alessandri-Haber N, et al. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei H, et al. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- 92.Bang S, et al. 17(R)-resolvin D1 specifically inhibits TRPV3 leading to peripheral antinociception. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01568.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren K, et al. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- 94.Sommer C, Birklein F. Fighting off pain with resolvins. Nat Med. 2010;16:518–520. doi: 10.1038/nm0510-518. [DOI] [PubMed] [Google Scholar]
- 95.Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med. 2009;10:654–662. doi: 10.1111/j.1526-4637.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 96.Schnitzer TJ. Update on guidelines for the treatment of chronic musculoskeletal pain. Clin Rheumatol 25 Suppl. 2006;1:S22–S29. doi: 10.1007/s10067-006-0203-8. [DOI] [PubMed] [Google Scholar]
- 97.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 98.Hess A, et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A. 2011;108:3731–3736. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukai E, et al. Comparative study of symptoms and neuroendocrine-immune network mediator levels between rheumatoid arthritis patients and healthy subjects. Clin Exp Rheumatol. 2000;18:585–590. [PubMed] [Google Scholar]
- 100.Wallace DJ. Is there a role for cytokine based therapies in fibromyalgia. Curr Pharm Des. 2006;12:17–22. [PubMed] [Google Scholar]
- 101.Arita M, et al. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]