Abstract
Background
Several occupations and occupational exposures have been investigated for associations with Parkinson’s disease. Common findings are increased risk associated with pesticide exposure and no association between Parkinson’s disease and welding.
Methods
We explored the association between a broad range of possible occupational risk factors and Parkinson’s disease as well as Parkinson’s disease plus other forms of parkinsonism (referred to as Parkinsonian disorders), using prospectively collected data in the population-based Swedish Twin Registry. A cohort of 14,169 Swedish men was followed for up to 43 years. We identified 234 Parkinsonian disorders cases including 204 Parkinson’s disease cases with complete data. We assessed exposure to 14 chemical and biological compounds through a job exposure matrix. Hazard ratios (HR) with 95% confidence intervals (CI) adjusted for age, smoking, and education were used to estimate the relative risk of disease associated with exposure.
Results
Exposure to inorganic dust was associated with increased risk of Parkinson’s disease and Parkinsonian disorders, HR 1.6 (95% CI 1.1–2.4) and 1.5 (1.0–2.2) respectively. There was no association between Parkinson’s disease or Parkinsonian disorders and occupational exposure to pesticides, welding smoke, metal dust, wood dust, animal handling, stone and concrete dust, chrome and nickel dust, quartz dust, organic dust, oil, asbestos, organic solvents and irritating gas.
Conclusions
Inorganic dust should be explored further as a potential risk factor for Parkinson’s disease. Occupational exposure to pesticides and twelve other compounds explored in this study may not be associated with risk of Parkinson’s disease in Swedish men.
Keywords: Parkinson disease, Occupational exposure, Prospective studies
1. Introduction
Specific genes are implicated in a small proportion of Parkinson’s disease (PD) cases, but heritability is low and environmental factors are important in the etiology. Risk factors for the disease are largely unknown [1].
Several occupations and occupational exposures have been investigated for associations with PD. Increased risk of PD has been reported for physicians [2], teachers [3, 4], cleaners [5, 6], agricultural workers/farmers [4, 6, 7], and for exposure to pesticides [8–13]. Pesticides and factors related to farming (working in agriculture, living in the country, drinking well water) were originally investigated for an association with PD because the neurological toxin MPTP, which is structurally similar to the pesticide Paraquat, causes rapid degeneration of dopaminergic neurons and subsequent parkinsonism [14]. Although several studies have shown increased PD risk associated with exposure to pesticides, epidemiological evidence is inconsistent and a causal relationship has not been established [8–13, 15–18]. Welding has been studied as a risk factor for PD mainly because of case reports of parkinsonian-like neurological symptoms attributed to manganese exposure in welders. Several studies have investigated welding in relation to PD without finding an association [19–21].
We wished to explore the relation between occupational exposure to a broad range of environmental factors in the time-period 1960–1980 and risk of PD as well as PD plus other forms of parkinsonism.
2. Methods
2.1. Study population
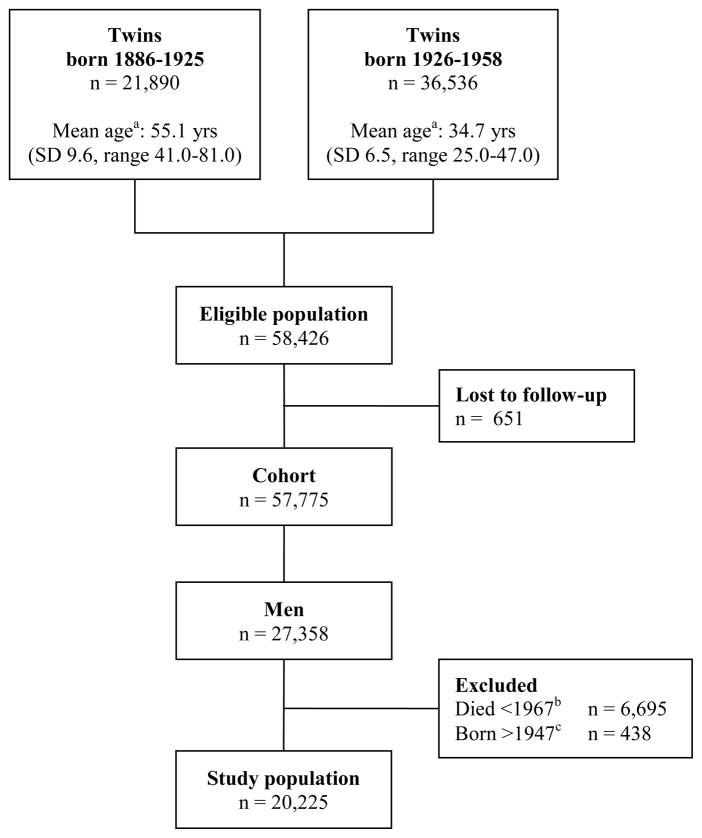
The Swedish Twin Registry (STR) [22] contains more than 170,000 twins born in Sweden from 1886 and onwards. For the present study we considered a cohort of like sexed male twins born 1886–1947. We excluded women because 48.4% of women in the cohort had missing information on occupation.
Twins born 1886–1925 were contacted with several questionnaires in the 1960’s. The present study included all men who completed the first questionnaire in 1961 and were still alive when a follow up questionnaire was sent in 1967. Twins born 1926–1958 were contacted with a questionnaire in 1973. All men who completed this questionnaire and were at least 25 years old at that time were included in the present study, effectively truncating the birth cohort in 1947 (figure 1).
Figure 1.
Flow chart describing the study population. aMean age at baseline. bMen born 1886–1925 who died between first contact in 1961 and start of follow-up for this study in 1967. cSince occupation in 1960–1980 was used as a proxy for exposure in this study population, all men born after 1947 were excluded as they were less than 25 years old at baseline in 1973. Yrs = years, SD = Standard deviation.
2.2. Occupational exposure assessment
For the twins born 1886–1925, questions about main occupation in adulthood were included in the 1967 questionnaire when the mean age of the twins was 55.1 (Standard Deviation, SD 9.6, range 41.0–81.0) years. For the twins born 1926–1947, questions about present occupation were included in the 1973 questionnaire when the mean age of the twins was 34.7 (SD 6.5, range 25.0–47.0) years. Occupations were coded according to the 3-digit level of occupational codes used in the 1970 census, based on the Nordic Standard Occupational Classification of 1965 (Nordisk yrkesklassificering, NYK), adapted from the International Standard Classification of Occupations (ISCO).
A job exposure matrix (JEM) was used to assess exposures for each person based on occupation at baseline. The JEM was developed by industrial hygienists at Karolinska Institutet for population based studies of occupational exposures in the years 1960–1980 [23, 24], corresponding to the average working age of the men in the study population. The JEM assesses probability of occupational exposure to chemical and biological compounds in four classes. Classes 0 to 3 represent increasing probability of specific exposures within families of occupations, from very low probability (class 0: less than 1/10 of persons within the occupational family exposed), to high probability of exposure (class 3: more than 2/3 exposed). The complete JEM contains information for 29 exposures in 248 occupations. Based on a cut-off level of at least 5% exposed in the study population, 14 of the 29 occupational exposures were selected for analysis.
2.3. Education and smoking assessment
Education and smoking were included to control for possible confounding. Information was taken from questionnaires in 1961 and 1963 for twins born 1886–1925 and in 1973 for twins born 1926–1947. Educational level was categorized as having completed compulsory school only or any higher education. In this study population, compulsory school was 6, 7 or 8 years of education depending on birth year. Smoking status was categorized as never, current, or past smoker at the time of the questionnaire.
2.4. Case identification
Incident cases of PD and other forms of parkinsonism were ascertained by record linkage of the STR with the National Patient Register (NPR) and the Cause of Death Register (CDR). Compilation of the NPR started in 1964 and since 1987 the register covers all hospital discharges in Sweden. In the middle of the 1970’s more than half of Sweden’s 26 counties reported to the register. The CDR covers all Swedish counties since 1961. Based on clinical work-ups [25], the positive predictive value (PPV) of a PD diagnosis in the NPR is 71% and the PPV of a diagnosis of any Parkinsonian disorder in the NPR is 88%. In the CDR, PPV is at least 70% for PD and at least 56% for Parkinsonian disorders. In the present study NPR data was available for 1967–2009 and CDR data was available for 1967–2008 (dates of death were available for the last year of follow-up in the NPR not covered by the CDR). Cases were defined as PD if there was a PD diagnosis and no parkinsonism diagnosis in the NPR or CDR.
Cases were defined as parkinsonism if there was a parkinsonism diagnosis in the NPR or CDR, regardless whether there was also a PD diagnosis. We considered two outcomes in the analyses: 1) PD and 2) Parkinsonian disorders that included cases of parkinsonism in addition to PD cases. The outcome Parkinsonian disorders was used to ensure capture of as many PD cases as possible in the registers. Date of ascertainment was defined as date of first hospital record of PD or parkinsonism, or, for cases only identified through the CDR, date of death.
International Classification of Diseases (ICD) codes used for PD were 350 (ICD-7); 342.00 (ICD-8); 332.0 (ICD-9); G20 (ICD-10). ICD codes used for parkinsonism were 342.08 and 342.09 (ICD-8); 332.1 and 333.0 (ICD-9); G21, G23.1, G23.2, G23.9, G25.9 (ICD-10).
2.5. Statistical methods
The study population was followed for PD and parkinsonism from baseline collection of occupational data until date of ascertainment of case status, December 31st 2009, or date of death, whichever came first. As the exact date of data collection was unknown, the standard date January 1st 1967 was chosen as baseline date for men born 1886–1925, and January 1st 1973 was chosen for men born 1926–1947.
Incidence rates (IR) of PD were calculated as events divided by time-at-risk, reported per 100,000 person-years (PYR) with 95% confidence intervals (CI) assuming a Poisson distribution.
The association between occupational exposure and PD was modeled using Cox proportional hazard regression yielding hazard ratios (HR) with 95% CI. Age was used as the underlying time scale. Educational level and smoking status were included as co-variates in the statistical model. The power to detect an HR of 1.75 for an exposure with 5% prevalence in a population of more than 20,000 with on average more than 29 years of follow-up is 77%.
The proportional hazards assumption was examined by including time by covariate interaction terms in the models. We found no evidence of nonproportionality. We did not account for correlation due to relatedness in the analysis, since such a correlation would be too weak to influence the standard errors in this population of a large number of small clusters (twin pairs). Presumably, occupational exposure is correlated to some extent within the twin pairs. However, our own data and other studies show that the correlation for PD within twin pairs is low [26].
Observations with missing information on any of the covariates were excluded in the regression analyses (table 1). Data analyses were performed using SAS software, Version 9.2 of the SAS System for Windows.
Table 1.
Available data in the study population
| N total | Occupation
|
Smoking
|
Education
|
Complete dataa |
|||||
|---|---|---|---|---|---|---|---|---|---|
| N with data | % | N with data | % | N with data | % | N with data | % | ||
| Study population | 20,225 | 15,174 | 75.0 | 17,583 | 86.9 | 16,450 | 81.3 | 14,169 | 70.1 |
| Men born 1886–1925 | 9,202 | 6,775 | 73.6 | 9,182 | 99.8 | 7,726 | 84.0 | 6,159 | 66.9 |
| Men born 1926–1947 | 11,023 | 8,399 | 76.2 | 8,401 | 76.2 | 8,724 | 79.1 | 8,010 | 72.7 |
Percentages calculated by available data divided by total N.
All men with complete data on occupation, smoking status and education available for multivariate proportional hazard regression analysis.
3. Results
The study population consisted of 20,225 men with a mean age of 44.0 years at baseline (SD 13.0) and a mean follow-up time of 29.1 years (SD 11.1). In total, the cohort accumulated 588,577 PYR over 43 years of follow-up.
We identified 336 incident cases of Parkinsonian disorders, whereof 293 (87.2%) were PD cases. Of 294 cases identified in the NPR, 119 cases (40.5%) were also identified in the CDR. Forty-two cases (12.5%) were identified in death records only. Of 43 Parkinsonian disorders cases, 29 (67.4%) had a PD diagnosis, but were classified as parkinsonism due to an additional parkinsonism diagnosis. Mean age of ascertainment of outcome for all cases was 75.5 (SD 8.6) years (range 43.4–94.6 years).
Of 336 cases, 254 (75.6%) had occupational data and 234 (69.6%) had complete data on occupation, education and smoking status (table 1).
The overall crude incidence rates for PD and Parkinsonian disorders are shown in table 2. Among men with occupational data, the crude incidence rate was 49.1 per 100,000 PYR (95% CI 43.0–56.0) for PD, and 56.1 per 100,000 PYR (95% CI 49.6–63.5) for Parkinsonian disorders. The rate among men without occupational data was 52.2 per 100,000 PYR (95% CI 41.3–65.8) for PD and 60.3 per 100,000 PYR (95% CI 48.5–74.8) for Parkinsonian disorders.
Table 2.
Descriptive statistics; numbers and crude incidence rates stratified by attained age, time period of follow-up, smoking status and educational level at baseline. Age-adjusted hazard ratios given for smoking status and educational level.
| Outcome: Parkinson’s disease
|
Outcome: Parkinsonian disorders
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IR/100,000 | IR/100,000 | ||||||||
| N | PYR | N | PYR | ||||||
| Study population | |||||||||
| (n=20 226) | 293 | 49.8 | 336 | 57.1 | |||||
| Attained age | <49 | 2 | 1.1 | 4 | 2.2 | ||||
| 50–64 | 30 | 13.0 | 36 | 15.6 | |||||
| 65–79 | 167 | 113.0 | 193 | 131.0 | |||||
| >80 | 94 | 315.0 | 103 | 346.0 | |||||
| Time period | 1967–1979 | 48 | 26.3 | 56 | 30.7 | ||||
| 1980–1989 | 87 | 53.1 | 94 | 57.3 | |||||
| 1990–1999 | 82 | 61.1 | 95 | 70.7 | |||||
| 2000–2009 | 74 | 79.1 | 89 | 95.1 | |||||
| HR | 95% CI | HR | 95% CI | ||||||
|
|
|
||||||||
| Smoking statusa | Never smoker | 149 | 84.1 | 1 | ref | 170 | 96.0 | 1 | ref |
| Current smoker | 78 | 37.6 | 0.51 | 0.39 – 0.68 | 90 | 43.4 | 0.52 | 0.40 – 0.67 | |
| Past smoker | 50 | 42.8 | 0.74 | 0.54 – 1.02 | 56 | 47.9 | 0.72 | 0.53 – 0.97 | |
| Educational level | Only compulsory | 135 | 54.2 | 1 | ref | 155 | 62.3 | 1 | Ref |
| Above compulsory | 101 | 43.6 | 1.12 | 0.86 – 1.44 | 117 | 50.5 | 1.12 | 0.88 – 1.42 | |
Smoking status in 1961 or 1963 for the men born 1886–1925 and in 1973 for the men born 1926–1947 (baseline).
IR = Incidence rate, PYR = Person-years, HR = Hazard ratio, CI = confidence interval.
As previously reported for this population [27], there was decreased risk of PD and Parkinsonian disorders for current smokers vs. never smokers. For past smokers vs. never smokers, risk of PD and Parkinsonian disorders was decreased, although statistically non-significant (table 2). There was no association between education and PD or Parkinsonian disorders. Previously we reported an association between higher education and PD [27], but this association was less pronounced in men than in women.
There was an increased risk of PD and Parkinsonian disorders for occupational inorganic dust exposure class 1 and all exposed vs. unexposed (table 3). The associations were still significant when adjusting for 2 comparisons (number of exposure levels for inorganic dust), but were not significant after correcting for 14 comparisons (number of exposures). There were no associations between PD or Parkinsonian disorders and any of the other 13 occupational exposures analyzed. Overall, results were similar for PD and Parkinsonian disorders (table 3).
Table 3.
Crude incidence rates and age-adjusted hazard ratios by occupational exposure for Parkinson’s disease and parkinsonian disorders.
| Occupational exposures | Exposure probability level | Outcome: Parkinson’s disease
|
Outcome: Parkinsonian disorders
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| IR/100,000 | IR/100,000 | ||||||||
| N | PYR | HR | (95% CI) | N | PYR | HR | (95% CI) | ||
| Metal dust | Unexposed | 212 | 49.8 | 1 | ref | 243 | 57.0 | 1 | ref |
| 1 | 10 | 43.1 | 0.9 | 0.4 – 1.8 | 11 | 47.4 | 0.8 | 0.4 – 1.6 | |
| 2 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| 3 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| All exposed | 10 | 37.7 | 0.7 | 0.4 – 1.5 | 11 | 41.4 | 0.7 | 0.4 – 1.4 | |
| Stone and concrete dust | Unexposed | 206 | 50.3 | 1 | ref | 235 | 57.3 | 1 | ref |
| 1 | 10 | 32.7 | 0.9 | 0.5 – 1.7 | 12 | 39.3 | 1.0 | 0.5 – 1.7 | |
| 2 | 0 | 0 | n/a | 1 | 28.8 | 0.9 | 0.1 – 6.3 | ||
| 3 | 6 | 69.6 | 1.9 | 0.8 – 4.2 | 6 | 69.6 | 1.6 | 0.7 – 3.7 | |
| All exposed | 16 | 37.5 | 1.1 | 0.6 – 1.8 | 19 | 44.6 | 1.1 | 0.7 – 1.7 | |
| Chrome and nickel dust | Unexposed | 203 | 49.5 | 1 | ref | 234 | 57.1 | 1 | ref |
| 1 | 18 | 48.0 | 0.9 | 0.5 – 1.6 | 19 | 50.7 | 0.8 | 0.5 – 1.4 | |
| 2 | 1 | 24.5 | 0.7 | 0.1 – 4.8 | 1 | 24.5 | 0.6 | 0.1 – 4.2 | |
| 3 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| All exposed | 19 | 44.9 | 0.9 | 0.5 – 1.5 | 20 | 47.2 | 0.8 | 0.5 – 1.3 | |
| Quartz dust | Unexposed | 200 | 48.9 | 1 | ref | 230 | 56.3 | 1 | ref |
| 1 | 2 | 31.8 | 0.7 | 0.2 – 3.0 | 2 | 31.8 | 0.7 | 0.2 – 2.6 | |
| 2 | 18 | 54.8 | 1.2 | 0.7 – 2.0 | 20 | 60.9 | 1.2 | 0.7 – 1.8 | |
| 3 | 2 | 44.9 | 1.0 | 0.3 – 4.1 | 2 | 44.9 | 0.9 | 0.2 – 3.5 | |
| All exposed | 22 | 50.5 | 1.1 | 0.7 – 1.8 | 24 | 55.0 | 1.1 | 0.7 – 1.6 | |
| Wood dust | Unexposed | 208 | 49.2 | 1 | ref | 239 | 56.6 | 1 | ref |
| 1 | 1 | 22.2 | 0.7 | 0.1 – 5.0 | 1 | 22.2 | 0.6 | 0.1 – 4.3 | |
| 2 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| 3 | 13 | 53.2 | 0.9 | 0.5 – 1.6 | 14 | 57.3 | 0.8 | 0.5 – 1.5 | |
| All exposed | 14 | 46.6 | 0.8 | 0.5 – 1.5 | 15 | 49.9 | 0.8 | 0.5 – 1.4 | |
| Organic dust | Unexposed | 181 | 44.8 | 1 | ref | 208 | 51.4 | 1 | ref |
| 1 | 11 | 74.0 | 1.6 | 0.9 – 3.1 | 11 | 74.0 | 1.4 | 0.7 – 2.7 | |
| 2 | 30 | 92.8 | 1.1 | 0.7 – 1.6 | 35 | 108.0 | 1.1 | 0.8 – 1.6 | |
| 3 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| All exposed | 41 | 85.1 | 1.2 | 0.8 – 1.7 | 46 | 95.5 | 1.2 | 0.8 – 1.6 | |
| Inorganic dust | Unexposed | 190 | 45.9 | 1 | ref | 220 | 53.2 | 1 | ref |
| 1 | 19 | 121.0 | 2.1 | 1.3 – 3.5 | 20 | 128.0 | 1.9 | 1.2 – 3.2 | |
| 2 | 13 | 56.1 | 1.3 | 0.7 – 2.3 | 14 | 60.4 | 1.2 | 0.7 – 2.1 | |
| 3 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| All exposed | 32 | 82.5 | 1.6 | 1.1 – 2.4 | 34 | 87.6 | 1.5 | 1.0 – 2.2 | |
| Pesticides | Unexposed | 196 | 46.6 | 1 | ref | 222 | 52.8 | 1 | ref |
| 1 | 5 | 67.3 | 0.8 | 0.3 – 2.2 | 7 | 94.2 | 1.1 | 0.5 – 2.5 | |
| 2 | 21 | 85.3 | 0.9 | 0.5 – 1.4 | 25 | 102.0 | 0.9 | 0.6 – 1.5 | |
| 3 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| All exposed | 26 | 81.1 | 0.9 | 0.5 – 1.3 | 32 | 99.8 | 1.0 | 0.6 – 1.4 | |
| Animal handling | Unexposed | 200 | 47.0 | 1 | ref | 228 | 53.5 | 1 | ref |
| 1 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| 2 | 21 | 85.3 | 0.9 | 0.5 – 1.4 | 25 | 102.0 | 0.9 | 0.6 – 1.5 | |
| 3 | 1 | 95.8 | 3.4 | 0.5 – 24.3 | 1 | 95.8 | 2.9 | 0.4 – 20.9 | |
| All exposed | 22 | 82.4 | 0.9 | 0.6 – 1.4 | 26 | 97.4 | 1.0 | 0.6 – 1.5 | |
| Welding smoke | Unexposed | 200 | 51.2 | 1 | ref | 232 | 59.4 | 1 | ref |
| 1 | 4 | 21.4 | 0.6 | 0.2 – 1.5 | 4 | 21.4 | 0.5 | 0.2 – 1.3 | |
| 2 | 15 | 46.0 | 1.3 | 0.7 – 2.2 | 15 | 46.0 | 1.1 | 0.6 – 1.9 | |
| 3 | 3 | 27.7 | 0.7 | 0.2 – 2.6 | 3 | 27.7 | 0.6 | 0.1 – 2.2 | |
| All exposed | 22 | 35.4 | 0.9 | 0.6 – 1.5 | 22 | 35.4 | 0.8 | 0.5 – 1.3 | |
| Oil | Unexposed | 190 | 49.3 | 1 | ref | 220 | 57.1 | 1 | ref |
| 1 | 31 | 55.3 | 1.1 | 0.8 – 1.7 | 33 | 58.9 | 1.0 | 0.7 – 1.5 | |
| 2 | 1 | 9.3 | 0.5 | 0.1 – 3.7 | 1 | 9.3 | 0.4 | 0.1 – 3.1 | |
| 3 | 0 | 0 | n/a | 0 | 0 | n/a | |||
| All exposed | 32 | 47.7 | 1.1 | 0.7 – 1.6 | 34 | 50.7 | 1.0 | 0.7 – 1.4 | |
| Asbestos | Unexposed | 187 | 50.4 | 1 | ref | 218 | 58.8 | 1 | ref |
| 1 | 25 | 53.3 | 1.1 | 0.7 – 1.8 | 26 | 55.5 | 1.0 | 0.6 – 1.6 | |
| 2 | 9 | 30.6 | 0.7 | 0.4 – 1.5 | 9 | 30.6 | 0.6 | 0.3 – 1.2 | |
| 3 | 1 | 18.0 | 0.5 | 0.1 – 3.4 | 1 | 18.0 | 0.4 | 0.1 – 2.9 | |
| All exposed | 35 | 42.8 | 0.9 | 0.6 – 1.4 | 36 | 44.0 | 0.8 | 0.6 – 1.2 | |
| Organic solvents | Unexposed | 160 | 47.2 | 1 | ref | 186 | 54.8 | 1 | ref |
| 1 | 39 | 53.3 | 0.9 | 0.6 – 1.3 | 44 | 60.2 | 0.9 | 0.6 – 1.2 | |
| 2 | 15 | 51.3 | 0.9 | 0.5 – 1.6 | 16 | 54.7 | 0.9 | 0.5 – 1.4 | |
| 3 | 8 | 73.8 | 1.4 | 0.6 – 2.9 | 8 | 73.8 | 1.2 | 0.6 – 2.5 | |
| All exposed | 62 | 54.8 | 0.9 | 0.7 – 1.3 | 68 | 60.1 | 0.9 | 0.7 – 1.2 | |
| Irritating gas | Unexposed | 159 | 48.7 | 1 | ref | 180 | 55.1 | 1 | ref |
| 1 | 19 | 55.0 | 0.9 | 0.5 – 1.5 | 22 | 63.7 | 0.9 | 0.6 – 1.5 | |
| 2 | 27 | 45.4 | 0.7 | 0.4 – 1.0 | 33 | 55.5 | 0.7 | 0.5 – 1.1 | |
| 3 | 17 | 53.1 | 1.3 | 0.8 – 2.2 | 19 | 59.3 | 1.2 | 0.7 – 2.0 | |
| All exposed | 63 | 50.0 | 0.9 | 0.6 – 1.2 | 74 | 58.7 | 0.9 | 0.6 – 1.2 | |
Analyses performed with Cox proportional hazard regression analysis adjusting for age in the time scale and education and smoking as co-variates.
IR = Incidence rate, PYR = Person-years, HR = Hazard ratio,
CI = confidence interval.
For pesticide exposure, the crude IR of PD and Parkinsonian disorders was almost doubled for class 2 probability of exposure vs. unexposed, but the multivariate adjusted HR did not show an association (table 3). Similar results were observed when adjusting for age only; HR 1.0 (95% CI 0.6–1.5) for PD and HR 1.0 (95% CI 0.7–1.6) for Parkinsonian disorders. Thus, the increased crude IR seen for class 2 probability of pesticide exposure was an effect of the higher age of those exposed; baseline mean age was 52.3 (SD 13.0) years for exposed vs. 42.8 (SD 12.1) years for unexposed. Similar differences in crude IRs between exposed and unexposed that were not reflected by the HRs were found for the exposures wood dust, animal handling, stone and concrete dust, quartz dust and organic dust.
4. Discussion
In this prospective population based cohort study of Swedish male twins, we found an association between occupational exposure to inorganic dust and PD and Parkinsonian disorders. To our knowledge, this is a novel finding, which warrants further research.
Inorganic dust is a constituent of the particulate matter (PM) in air pollution. The exposure is found in occupations such as farm workers, dental technicians, painters, construction workers, glass workers, dock laborers and cleaners. For example, cleaners have been associated with increased risk of PD [5, 6]. Inhaled PM may reach not only the systemic circulation but also the central nervous system, where it can cause neuroinflammation through the activation of microglia in particular [28, 29]. Activated microglia are found in the substantia nigra of PD patients. One neuropathological study [29] showed that young adult residents in cities with high air pollution had neuroinflammation, blood-brain barrier disruption and accumulation of alpha-synuclein. Thus, this is a possible mechanism by which inorganic dust exposure affects PD risk.
Few studies have investigated the association between occupational exposure and risk of PD in Scandinavian populations. A Danish study reported increased risk of PD for men working in agriculture and horticulture [6]. Two other studies in Sweden and Denmark investigated occupational welding as a risk for PD, and neither found an association [19, 20]. A register based Swedish study reported increased risk of PD in men associated with several occupations, including farmers and teachers [4]. Two other retrospective case-control studies at least partially conducted in Sweden (one was a multi-centre European study [11]) reported slightly increased risks of PD associated with occupational exposure to pesticides [5, 11].
We found no association between PD or Parkinsonian disorders and occupational exposure to pesticides. Although a metaanalysis [8] and some recent studies [9, 10, 12] found an increased risk, several studies were negative [15, 18] and more than half of the studies included in a recent review did not find significant associations [16]. Differences across time and between countries in occupational pesticide exposure may explain some of the inconsistencies between studies. In Sweden, herbicides are the most commonly used type of pesticide. Exposure has generally been reduced in affected occupations over time. In Sweden, substantial improvements in work environment were introduced in the 1980’s for farmers in particular, as many small farms were merged into larger units and use of pesticides became more regulated. If a long duration of exposure is needed to affect risk of PD, our chances to detect an association would be smaller than in countries that did not prohibit the use of specific pesticides. Another explanation for inconsistencies between studies conducted in different populations is gene by environment interactions [30, 31].
Using a JEM to assess occupational exposure may increase misclassification compared to expert assessments [32], but may increase sensitivity compared to using job codes alone. In the European multi-centre study mentioned above, the association between pesticides and PD was reported using a JEM, but when only job codes were used, no association was detected [17]. Sensitivity of JEMs is, however, not perfect, which is a limitation of our study leading to possible bias towards the null. Further, the JEM we used does not assess quantitative levels of exposure but rather probability of exposure for each occupation, and consequently we cannot assess whether there may be a dose-response relationship. The JEM does not provide any details about exposure to specific environmental compounds. Generally, few studies have detailed information on frequency and duration of occupational exposures (cumulative exposure) as well as data on specific compounds.
Strengths of this study include the long follow-up and prospective design, eliminating risk of recall bias and reverse causation, and minimizing possible bias due to missingness of occupational data. Limitations, other than lack of data on specific chemical compounds and quantitative levels of exposure, include lack of data on recreational exposures and method of case identification. As PD and parkinsonism are primarily treated in out-patient care, severe cases or cases with substantial co-morbidity may be over represented in the hospital and cause of death records. However, the proportion of exposed did not differ between cases identified in a separate study by telephone screening and clinical work-up [25] and cases identified through the registers, indicating that this potential bias is non-differential with regards to exposure and should thus not affect the estimated associations. Although these limitations were present, the direction of the effect would not be influenced (bias towards the null). However, the limitations may have introduced false negative findings. For example, we had limited power to detect an association between PD and occupational exposure to pesticides.
Unfortunately, we did not have information on occupational histories for the study persons, which would have increased the precision of exposure assessment. The older twins were asked about main occupation in life and these men were unlikely to change occupation after data collection. The younger twins were asked about present occupation and a portion of them might most likely have changed occupation. However, exposures for occupations held at a later time would most likely be different, for example due to advances in technology and work safety. In this prospective population based cohort study of Swedish men with up to 43 years of follow-up, we found an association between occupational inorganic dust exposure and increased risk of PD. Inorganic dust should be investigated further as a potential risk factor for PD, in particular since inorganic dust is a common exposure, being a component of air pollution. Occupational exposure to pesticides, welding smoke, metal dust, wood dust, animal handling, stone and concrete dust, chrome and nickel dust, quartz dust, organic dust, oil, asbestos, organic solvents and irritating gas was not associated with PD or Parkinsonian disorders. These exposures may not be associated with risk of PD and Parkinsonian disorders in Swedish men.
Supplementary Material
Acknowledgments
We would like to thank Dr. Nils Plato for contributing expertise on the Job Exposure Matrix. The study was supported by grant ES10758 from the National Institutes of Health, the Swedish Research Council, the Swedish Society for Medical Research, the Swedish Society of Medicine, the Parkinson Foundation in Sweden, and funds from Karolinska Institutet. Dr. Wirdefeldt received a research grant from Syngenta, Inc. All other authors have nothing to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Additional references included at the request of reviewers.
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Frigerio R, Elbaz A, Sanft KR, Peterson BJ, Bower JH, Ahlskog JE, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology. 2005;65:1575–83. doi: 10.1212/01.wnl.0000184520.21744.a2. [DOI] [PubMed] [Google Scholar]
- 3.Goldman SM, Tanner CM, Olanow CW, Watts RL, Field RD, Langston JW. Occupation and parkinsonism in three movement disorders clinics. Neurology. 2005;65:1430–5. doi: 10.1212/01.wnl.0000180361.74060.70. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Sundquist J, Sundquist K. Socioeconomic and occupational groups and Parkinson’s disease: a nationwide study based on hospitalizations in Sweden. Int Arch Occup Environ Health. 2009;82:235–41. doi: 10.1007/s00420-008-0327-z. [DOI] [PubMed] [Google Scholar]
- 5.Fall PA, Fredrikson M, Axelson O, Granerus AK. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Tuchsen F, Jensen AA. Agricultural work and the risk of Parkinson’s disease in Denmark, 1981–1993. Scand J Work Environ Health. 2000;26:359–62. doi: 10.5271/sjweh.554. [DOI] [PubMed] [Google Scholar]
- 7.Kirkey KL, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Gorell JM. Occupational categories at risk for Parkinson’s disease. Am J Ind Med. 2001;39:564–71. doi: 10.1002/ajim.1055. [DOI] [PubMed] [Google Scholar]
- 8.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res. 2001;86:122–7. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 9.Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 10.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–26. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64:666–72. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- 13.Petrovitch H, Ross GW, Abbott RD, Sanderson WT, Sharp DS, Tanner CM, et al. Plantation work and risk of Parkinson disease in a population-based longitudinal study. Arch Neurol. 2002;59:1787–92. doi: 10.1001/archneur.59.11.1787. [DOI] [PubMed] [Google Scholar]
- 14.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 15.Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165:364–74. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 16.Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease--is there a link? Environ Health Perspect. 2006;114:156–64. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dick S, Semple S, Dick F, Seaton A. Occupational titles as risk factors for Parkinson’s disease. Occup Med (Lond) 2007;57:50–6. doi: 10.1093/occmed/kql109. [DOI] [PubMed] [Google Scholar]
- 18.Firestone JA, Lundin JI, Powers KM, Smith-Weller T, Franklin GM, Swanson PD, et al. Occupational factors and risk of Parkinson’s disease: A population-based case-control study. Am J Ind Med. 2010;53:217–23. doi: 10.1002/ajim.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fored CM, Fryzek JP, Brandt L, Nise G, Sjogren B, McLaughlin JK, et al. Parkinson’s disease and other basal ganglia or movement disorders in a large nationwide cohort of Swedish welders. Occup Environ Med. 2006;63:135–40. doi: 10.1136/oem.2005.022921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fryzek JP, Hansen J, Cohen S, Bonde JP, Llambias MT, Kolstad HA, et al. A cohort study of Parkinson’s disease and other neurodegenerative disorders in Danish welders. J Occup Environ Med. 2005;47:466–72. doi: 10.1097/01.jom.0000161730.25913.bf. [DOI] [PubMed] [Google Scholar]
- 21.Stampfer MJ. Welding occupations and mortality from Parkinson’s disease and other neurodegenerative diseases among United States men, 1985–1999. J Occup Environ Hyg. 2009;6:267–72. doi: 10.1080/15459620902754703. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 23.Plato N, Steineck G. Methodology and utility of a job-exposure matrix. Am J Ind Med. 1993;23:491–502. doi: 10.1002/ajim.4700230312. [DOI] [PubMed] [Google Scholar]
- 24.Lope V, Perez-Gomez B, Aragones N, Lopez-Abente G, Gustavsson P, Plato N, et al. Occupational exposure to chemicals and risk of thyroid cancer in Sweden. Int Arch Occup Environ Health. 2009;82:267–74. doi: 10.1007/s00420-008-0314-4. [DOI] [PubMed] [Google Scholar]
- 25.Wirdefeldt K, Gatz M, Bakaysa SL, Fiske A, Flensburg M, Petzinger GM, et al. Complete ascertainment of Parkinson disease in the Swedish Twin Registry. Neurobiol Aging. 2008;29:1765–73. doi: 10.1016/j.neurobiolaging.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63:305–11. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- 27.Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and protective factors for Parkinson’s disease: a study in Swedish twins. Ann Neurol. 2005;57:27–33. doi: 10.1002/ana.20307. [DOI] [PubMed] [Google Scholar]
- 28.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–16. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 30.Elbaz A, Levecque C, Clavel J, Vidal JS, Richard F, Amouyel P, et al. CYP2D6 polymorphism, pesticide exposure, and Parkinson’s disease. Ann Neurol. 2004;55:430–4. doi: 10.1002/ana.20051. [DOI] [PubMed] [Google Scholar]
- 31.Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, et al. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117:964–9. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire V, Nelson LM, Koepsell TD, Checkoway H, Longstreth WT., Jr Assessment of occupational exposures in community-based case-control studies. Annu Rev Public Health. 1998;19:35–53. doi: 10.1146/annurev.publhealth.19.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.