Abstract
Effects of vitamin D on the immune system have been recognized for over thirty years and stemmed in part from analysis of the dysregulated vitamin D metabolism associated with granulomatous diseases. However, it is only in more recent years that a role for interaction between vitamin D and normal immune function has been proposed. As with the original studies, the basis for this new perspective on immunmodulation by vitamin D stems from studies of vitamin D metabolism by immune cells. In particular, induction of the vitamin D-activating enzyme CYP27B1 in monocytes via pathogen recognizing receptors has highlighted an entirely new function for vitamin D as a potent inducer of antibacterial innate immune responses. This has prompted a new potential role for vitamin D in protecting against infection in a wide range of tissues but has also prompted revision of the parameters for adequate vitamin D status. The following review describes some of the key developments in innate immune responses to vitamin D with particular emphasis on the role of key metabolic enzyme as determinants of localized immune activity of vitamin D.
Keywords: vitamin D, CYP27B1, CYP24A1, vitamin D receptor, toll-like receptor, monocyte, neutrophil, cathelicidin, defensins, tuberculosis
1. Introduction
Non-classical responses to vitamin D have been recognized for more than a quarter of a century ago since various neoplastic cell lines were shown to exhibit specific binding for the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) [1,2]. Subsequent studies showed that this interaction between 1,25(OH)2D and its cognate nuclear receptor, the vitamin D receptor (VDR), promoted antiproliferative and prodifferentiation responses in cancer cells [3,4]. These data highlighted an entirely new facet of vitamin D action distinct from its effects on calcium homeostasis and bone metabolism. The range of non-classical responses to vitamin D was then extended to include actions on cells from the immune system [5,6]. This stemmed initially from the observation that some patients with the granulomatous disease sarcoidosis present with elevated circulating levels of 1,25(OH)2D and associated hypercalcemia [7,8]. In these patients the high serum 1,25(OH)2D arises from elevated activity of the enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). However, in contrast to normal subjects where 1α-hydroxylase activity is classically localized in the kidney, the increased production of 1,25(OH)2D in granulomatous disease patients involves expression of CYP27B1 in disease-associated macrophages [9–11]. Studies carried out at about the same time showed that VDR expression is common to macrophages, T-lymphocytes (T-cells), B-lymphocytes (B-cells) and other cell types from the immune system [12,13]. Based on these observations it was concluded that the immune system has the potential to synthesize 1,25(OH)2D and elicit intracrine or paracrine responses from immune cells expressing the VDR [14].
Subsequent studies showed that dysregulation of 1,25(OH)2D synthesis was not restricted to sarcoidosis but was a common feature of many granulomatous disorders and some forms of cancer [15]. In a similar fashion, studies in vitro showed that it was possible to potently regulate a range of immune cell functions using 1,25(OH)2D or its synthetic analogs [16,17]. Despite these advances, the extent to which vitamin D could act as a physiological regulator of normal immune responses remained elusive, even though expression of CYP27B1 was reported in a diverse array of non-classical target tissues [18]. A breakthrough in the link between vitamin D physiology and normal immune function occurred five years ago with the first studies linking vitamin D and antibacterial activity in monocytes. The crucial feature of this new perspective on vitamin D and immunology arose from the ability of monocyte pathogen recognition receptors (PRR) to trigger localized metabolism of the precursor form of vitamin D, 25-hydroxyvitamin D (25OHD). The resulting synthesis of 1,25(OH)2D was sufficient to promote intracrine activation of VDR responses, and concomitant induction of innate immune responses. In view of the fact that 25OHD is the major circulating form of vitamin D, these observations provided a clear potential link between vitamin D status (serum 25OHD) and the efficacy of immune response. The aim of the current review will be to detail these developments with specific emphasis on the role of vitamin D metabolism as the central component of the interface between vitamin D and the immune system.
2. Vitamin D metabolism and innate immunity
The innate immune system is the body’s first line of the defence against pathogenic challenge, and occurs in an immediate and non-specific manner. Innate immune response to infection involves the complement system, antibacterial responses by neutrophils and macrophages, but also incorporates antigen presentation to lymphocytic cells from the adaptive or acquired immune system. Accumulating evidence indicates that vitamin D is involved in regulating various components of the innate immune system, and may therefore be a key environmental determinant of human responses to infection [19]. The initial data linking vitamin D and innate immunity arose from studies of vitamin D metabolism, and in particular the vitamin D-activating enzyme CYP27B1. In a classical renal setting, expression and activity of CYP27B1 is regulated in a sensitive fashion by endocrine factors associated with calcium and phosphate homeostasis such as parathyroid hormone and fibroblast growth factor 23 [20]. However, similar factors do not appear to play a role in regulating extra-renal activity of CYP27B1. Instead, elucidation of a distinct mechanism linking monocyte CYP27B1 and pathogen-sensing provided a major breakthrough in our understanding of the link between vitamin D metabolism and innate immunity.
Monocytes and macrophages are crucial members of the innate immune compartment, being able to both phagocytose pathogens, and sense the pathogen-associated molecular patterns (PAMPs) expressed by these pathogens. The latter is achieved by means of pathogen-recognition receptors (PRR), such as toll-like receptors (TLRs), that are expressed by many cells types including monocytes [21,22]. To date ten functional TLRs have been identified in humans and twelve in mice, with each receptor responding to specific PAMPs from a wide range of microbes that include bacteria, viruses, parasites and fungi [22]. Commonly studied TLRs include TLR2 which responds to Gram-positive bacteria and mycobacteria, TLR4 which responds to Gram-negative bacteria and TLR3 which responds to the double-stranded RNA associated with viral infections [21]. Although TLRs are intimately associated with innate monocyte responses to infection they have been detected on a wide range of cell types, including other immune cells such as dendritic cells and lymphocytes. Consequently it is now clear that pathogen recognition and associated immune responses are a common feature of many tissues notably those at so-called barrier sites in the body [22].
Cells such as monocytes are able to utilize TLRs to promote appropriate innate immune responses to pathogens internalized by phagocytosis, thereby limiting the potential for damage to the host cell by the pathogen. In 2006 studies to identify the spectrum of genes regulated in response to sensing of Mycobacterium tuberculosis (M. tb) by monocytic TLR2/1 showed specific induction of CYP27B1 and VDR [23]. These data suggested an intracrine system by which locally synthesized 1,25(OH)2D can bind to endogenous VDR and regulate monocyte gene expression. Potential targets for this intracrine response include the antibiotic protein cathelicidin (LL37) which is a direct transcriptional target for the 1,25(OH)2D-VDR complex [24,25]. Functional analyses showed that following M. tb-TLR 2/1 activation both 1,25(OH)2D and 25OHD induced expression of LL37 in macrophages, and that this was coincident with enhanced killing of M. tb [23]. These observations indicated that induction of monocyte LL37 involves TLR2/1 activation of CYP27B1 and VDR, but nevertheless may ultimately depend on the concentration of available 25OHD to support the intracrine induction of bacterial killing. Naturally-occurring variations in serum 25OHD levels have been shown to correlate with monocyte LL37 [26]. As a consequence, individuals with vitamin D-insufficiency (low serum 25OHD) will be less able to support monocyte induction of LL37 [23,26], and may therefore be at greater risk of infection. Conversely, supplementation of vitamin D-insufficient individuals in vivo has been shown to improve TLR-mediated induction of monocyte LL37 [26], and may therefore help to protect against infection.
The description of a TLR-mediated mechanism for induction of CYP27B1 and VDR provided a completely new perspective on the relationship between vitamin D and the immune system. However, it is important to recognize that vitamin D-mediated monocyte killing of M. tb was initially described many years ago in studies that used 1,25(OH)2D rather than 25OHD to enhance bacterial killing [27]. In this instance the effect of vitamin D was enhanced by addition of the cytokine interferon γ (IFNγ) indicating that other immunity pathways may be involved in regulating the monocyte vitamin D system. For example, expression of CYP27B1 is known to be induced by other TLR ligands such as lipopolysaccharide (LPS), which binds to TLR4 [26]. The precise mechanism by which TLR2/1 and TLR4 enhance transcription of VDR and CYP27B1 has yet to be fully defined. Studies using monocytic cell lines have shown that JAK-STAT, p38 MAP kinase, and NF-κB pathways are involved in stimulating CYP27B1 expression in the presence of either TLR4 ligand LPS, or IFNγ [28]. The pathways associated with CYP27B1 induction by TLR ligands alone remain unclear, although recent studies using monocytes treated with a TLR2 ligand have shown that the cytokine interleukin-15 (IL-15) can act as a potential intermediary in promoting localized activity of CYP27B1 [29]. Elevated expression of IL-15 is frequently associated with inflammatory diseases, notably the granulomatous disease sarcoidosis [30]. In view of the fact that over-production of 1,25(OH)2D is frequently observed in patients with granulomatous diseases [31], it is possible that IL-15-mediated induction of CYP27B1 provides a link between vitamin D as a regulator of normal innate immune responses, and the pathological dysregulation of 1,25(OH)2D production associated with some inflammatory diseases.
The vitamin D steroidogenic system is unique in that it includes a dedicate feedback control enzyme 24-hydroxylase (CYP24A1), which generates less active 24-hydroxylated metabolites from 25OHD or 1,25(OH)2D (see Figure 1). Expression of CYP24A1 is induced primarily by its main substrate, 1,25(OH)2D, and in monocytes locally synthesized 1,25(OH)2D stimulate CYP24A1 in concert with the intracrine induction of LL37 [23]. Thus CYP24A1 may be a key determinant of monocyte responses to 25OHD, by converting 1,25(OH)2D to less active 1,24,25-trihydroxyvitamin D (1,24,25(OH)3D) [32]. The importance of this is underlined by recent studies showing that the cytokine IL-4 promotes 24-hydroxylase activity in monocytes and in doing so attenuates TLR2/1-mediated intracrine induction of LL37 expression by 25OHD [33]. This effect contrasted with IFNγ which enhanced the intracrine vitamin D responses. Given that IFNγ is a marker of the T-helper (Th)1 subset of T-cell immune responses, whilst IL-4 is produced by Th2 T-cells, these data suggest that the two different types of T-cell adaptive immune activity have opposing effects on vitamin D metabolism [33]. By utilizing these cytokine-specific mechanisms for regulation of vitamin D activation and catabolism, vitamin D may therefore be an as yet unrecognized coordinator of the interface between the innate and adaptive immune function. The link between these two arms of the immune system is an important determinant of immune responses to pathogens such as M. tb, which cannot be eradicated by the innate immune system alone. As expected, the monocyte 24-hydroxylase activity induced by IL-4 was shown to be dependent on the CYP24A1 enzyme. However, treatment with the cytokine did not increase monocyte expression of mRNA for CYP24A1. Instead the cytokine suppressed expression of CYP24A1 and several other cytokines with putative capacity for 24-hydroxylation [33]. Thus, it is possible that IL-4 enhanced 24-hydroxylase activity in monocytes is due to indirect suppression of a competitor enzyme to CYP24A1.
Figure 1. Vitamin D metabolism and innate immune responses.
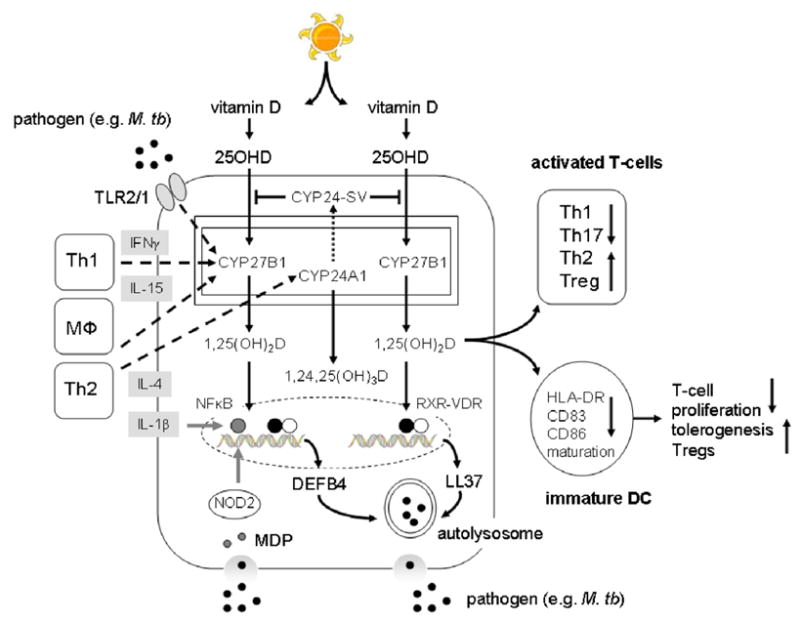
Metabolism of 25-hydroxyvitamin D (25OHD) to 1,25-dihydroxyvitamin D (1,25(OH)2D) by monocytes and the intracrine regulation of antibacterial activity (DEFB4/LL37 production and autophagy), antigen presentation (decreased HLA-DR, CD83 and CD86) and T-cell (Th1, Th2, Th17 and Treg) function (solid arrows). Effects of toll-like receptor (TLR)-mediated response to pathogens such as Mycobacterium tuberculosis (M. tb), and associated cytokine effects on monocyte vitamin D metabolism are show by dashed lines. Effects of cytokines and NOD2 intracellular pathogen recognition on nuclear factor-κB (NF-κB) enhancement of vitamin D-receptor (VDR)-retinoid X receptor (RXR)-mediated transcription of antibacterial factors are shown as solid gray lines.
Although induction of 24-hydroxylase activity is crucial to the efficacy of intracrine activity of vitamin D, monocytes and macrophages also express a truncated form of CYP24A1 in which the N-terminal mitochondrial targeting sequence of the protein is spliced out [34]. Despite being metabolically inactive, the CYP24A1 splice variant (CYP24-SV) retains its steroid binding domain and can therefore bind substrates such as 1,25(OH)2D or 25OHD, without being metabolically active. The abundant expression of CYP24-SV in monocytes/macrophages provides an explanation for the relatively low levels of 24-hydroxylase activity observed in these cells. Thus the conventional role of CYP24A1 in catalyzing catabolism of 1,25(OH)2D to 1,24,25(OH)3D may be less important in macrophages relative to other 1,25(OH)2D target cells, with CYP24A simply acting as an alternative to VDR as a binding site for the active form of vitamin D (see Figure 1). Binding to not catabolic CYP24-SV rather than conventional CYP24A1 may also explain the accumulation of 1,25(OH)2D levels, and concomitant lack of catabolism that is characteristic of macrophages associated with granulomatous disease (see Introduction). However, molecular modeling suggests that the substrate preference in CYP24-SV is switched from 1,25(OH)2D to 25OHD [35]. In this way CYP24-SV may be more important as a modulator of 25OHD, rather than 1,25(OH)2D, availability, thereby limiting substrate binding to CYP27B21. This has the advantage of being metabolically economical, particularly in cells such as monocytes where intracrine conversion of 25OHD to 1,25(OH)2D is the pivotal mechanism for vitamin D action. The precise manner by which CYP24-SV contributes to the effects of vitamin D on innate immunity remains to be determined, and it is interesting to note that the upregulation of 24-hydroxylase activity reported for IL-4-treated monocytes did not involve any differential regulation of CYP24-SV [33].
3. Alternative target cells for antibacterial activities of vitamin D
A wide range of cell types express PRRs and have the potential to initiate innate immune responses to infection. In addition to monocytes and macrophages, granulocytic cells such as neutrophils are the most abundant of all the leukocytes and therefore form the first line of response to infection. Initial reports of 1,25(OH)2D-induced LL37 expression in vitro indicated that this response occurred in neutrophils as well as monocytes [24]. Neutrophils express VDR but, unlike monocytes, there is no clear evidence that they express a functional CYP27B1 enzyme. Consequently, these cells may only exhibit systemic responses to 1,25(OH)2D. Nevertheless, the relative abundance of neutrophils suggests that they are likely to be the major source of circulating LL37 [36]. This is supported by the observation of an association between serum LL37 and levels of 1,25(OH)2D (but not 25OHD) in patients with chronic kidney disease [37]. Paradoxically, in patients with sepsis where there neutrophil numbers are increased, low circulating levels of LL37 have been shown to associated with low serum 25OHD [38].
Induction of LL37 by vitamin D metabolites has been reported for a variety of human cell types outside the classical immune system These include bronchial epithelial cells [39], myeloid cell lines [25], and decidual [40], and trophoblastic cells of the placenta [41]. Although the underlying molecular mechanism for 1,25(OH)2D-induced LL37 expression is similar in all of these cell types, the mechanism for localized metabolism of 25OHD may vary from one tissue to another. For example, normal human keratinocytes express relatively low levels of TLR2 and are therefore less sensitive to PAMPs than monocytes [42]. However, following epidermal wounding, transforming growth factor-beta is released from the keratinocytes and acts in a paracrine fashion to stimulate CYP27b1 expression in adjacent cells. The resulting localized accumulation of 1,25(OH)2D in turn enhances TLR expression, thereby increasing keratinocyte sensitivity to PAMPs and subsequent production of antimicrobial LL37 [42]. Another cell type, vascular endothelial cells, play a major role in the innate immune activation during infections and sepsis [43]. Human microvessel endothelial cells (HMEC) treated with 1,25(OH)2D showed inhibited LPS activation of NF-κB and expression of IL-6 and IL-8 [43]. This suggests that 1,25(OH)2D plays a role in LPS-induced immune activation of endothelial cells during Gram-negative bacterial infections, and supports a potential role for vitamin D as an adjuvant in the treatment of Gram-negative sepsis.
In other cell types antibacterial responses to vitamin D may occur in a non-infectious setting. Recent studies by our group using vitamin D-deficient mice show suppressed colonic expression of angiogenin-4, an antimicrobial protein produced primarily in Paneth cells which acts as a key regulator of tissue invasion by enteric bacteria [44]. Given that aberrant innate immune handling of the microbiota has been implicated as an initiator of the tissue inflammation associated with some types of inflammatory bowel disease [45], it is tempting to speculate that effects of vitamin D in protecting against this disease may involve the induction of innate antibacterial responses to enteric bacteria. This effect of vitamin D on a gastrointestinal antibacterial activity appears to be more specifically associated with tissue responses to the abundant commensal bacteria that make up the enteric microbiota. Responses to pathogenic enteric bacteria are more likely to involve conventional antibacterial proteins such as cathelicidin or DEFB4. The induction of these innate immune factors by colonic epithelial cells is variable and may require specific tissue microenvironments or TLR-mediated signaling to be effective [46,47].
4. Alternative antibacterial targets for vitamin D
The induction of LL37 transcription by 1,25(OH)2D occurs following interaction of the liganded VDR with a consensus vitamin D response element (VDRE) within the proximal promoter of the LL37 gene [24,25]. VDR interaction with the LL37 gene promoter is observed in humans and apes, as well as New World and Old World primates. However, other non-primate mammals such as mice lack the appropriate LL37 proximal promoter VDRE and do not appear to induce the antibacterial protein in response to 1,25(OH)2D [25]. The VDRE associated with vitamin D-induced hCAP expression in primates, arose through incorporation of an Alu short interspersed element (SINE) which placed this gene under the control of the VDR [48]. Over the subsequent 50–60 million years this genetic modification has presumably provided an innate immune advantage. Primates, such as Homo sapiens, would have originally lived in a vitamin D-enriched state due to relatively high levels of exposure to ultra violet (UV) light, which stimulates the production of vitamin D from 7-dehydrocholesterol in the skin. Under these conditions, vitamin D-induced antimicrobial function may have conferred significant advantages in combating infectious disease. By contrast, other mammals, such as mice, with less routine exposure to UV light would have benefited less from SINE incorporation of a VDRE into genes encoding antimicrobial proteins.
A VDRE is also present in the proximal promoter of the human gene for beta-defensin 2 (DEFB4) [24]. However, induction of DEFB4 expression by 1,25(OH)2D alone is far less striking than observed with LL37 [24]. Instead studies using squamous cell carcinoma cells [24] and monocytes [49] indicate that cooperation with nuclear factor kappa B (NF-κB) is required for 1,25(OH)2D-VDR-induced transcription of DEFB4. This effect is mediated via NF-κB response elements adjacent to the DEFB4 gene promoter VDRE [50]. Promotion of NF-κB signaling in this fashion may involve factors such as inflammatory cytokines [24,49], but alternative pathogen recognition mechanisms may also be involved. Treatment of a variety of cell types with 1,25(OH)2D potently induces expression of nucleotide-binding oligomerization domain containing 2 (NOD2) [51], an intracellular PRR which binds the bacterial cell membrane product muramyl dipeptide (MDP) [52]. Acting via NOD2, MDP promotes NF-κB activity in a similar fashion to that observed for cytokines, and studies in vitro have shown that combined treatment with 1,25(OH)2D and MDP synergistically induces expression of DEFB4 [51]. Thus, the induction of antimicrobial activity by vitamin D is not restricted to TLR-mediated signaling and direct vitamin D-induced transcription of LL37. Instead other PRRs and antibacterial proteins, as well as cooperative immune signaling pathways may also be involved.
LL37 and DEFB4 appear to be crucial factors in mediating antibacterial response to pathogens such as M. tb in primates [29]. However, in non-primate mammals such as mice the picture is less clear. The lack of appropriate gene proximal promoter VDREs in equivalent genes for LL37 and DEFB4 suggests that these unlikely to be targets for vitamin D in non-primates. However, vitamin D may also regulate other innate immune responses to infection. Previous studies have shown that monocytes infected with M. tb in the presence of 1,25(OH)2D produce high levels of bacteriocidal superoxide anions [53]. Another reactive oxygen species nitric oxide (NO) is also produced by monocytes [54]. The latter is known to be a particularly important mechanism for bacterial killing in mice [55], and it is therefore possible that this alternative antibacterial pathway compensates for the lack of vitamin D-mediated induction of LL37/DEFB in mice. Indeed, it is interesting to note that 1,25(OH)2D-induced suppression of M. tb growth in monocytes has been linked to the production of NO in both mice [56] and humans [56,57].
Vitamin D-mediated innate immunity in primates and non-primates may also extend beyond the simple induction of antibacterial factors. Initiation of intracellular bacterial killing following permeabilization of microbial cell membranes requires the fusion of antimicrobial-enriched lyzosomes with phagocytic vacuoles [58]. Recent studies indicate that this process also involves autophagy, a eukaryotic mechanism that utilizes encapsulation of organelles or cell proteins in a double-membrane autophagosome prior to fusion with lysosomes. Degradation of the autolysosomal contents is a fundamental feature of cytosolic homeostasis [59], but autophagy may also be involved in cellular response to infection [60,61]. Importantly, monocyte autophagy appears to be a key component of 1,25(OH)2D-induced responses to infection with M. tb [62], with autophagy responses to TLR2/1 activation being associated with induction of an intracrine vitamin D system [63]. To date studies of vitamin D-mediated autophagy have focused on the role of this mechanism in human monocytes, but similar activity may also be a feature of innate immunity in mice.
5. Dendritic cells and antigen presentation
Innate immune responses to infection are not restricted to antibacterial activity in monocytes, neutrophils and other cells that encounter pathogens. As outlined above, adequate immune management of infectious agents such as M. tb requires cooperation with the adaptive immune system. In order to this, lymphocytes need to be exposed to antigen from the pathogen. The most potent antigen-presenting cells (APCs) are dendritic cells (DCs), which act as the primary initiators of T cell-mediated immunity. They are broadly divided into 2 groups based on their origin: Myeloid (mDCs) and plasmacytoid (pDCs) which express different types of cytokines and chemokines and seem to exert complementary effects on T-cell responses, with mDCs being the most effective APCs [64] and pDCs being more closely associated with immune tolerance [65]. DCs isolated from lymphatic tissue were shown to express VDR [66], and subsequent studies showed that 1,25(OH)2D acted to attenuate antigen presentation by these cells [67]. 1,25(OH)2D3 [68] and its synthetic analogs [69] have also been shown to inhibit the maturation of monocyte-derived DCs, thereby suppressing their capacity for antigen presentation. This provided a mechanism by which vitamin D could act to promote immune tolerance, and further studies showed that 1,25(OH)2D-suppression of DC maturation was associated with concomitant enhancement of suppressor or regulatory T-cells (Treg) [70].
The link between vitamin D, DC maturation and tolerogenesis was underlined by observation that DCs express CYP27B1 in a similar fashion to macrophages [71,72], with expression and activity of the enzyme increasing as DCs differentiate towards a mature phenotype [71]. The simultaneous expression of VDR by DCs provided an intracrine system similar to that observed in monocytes, with 25OHD suppressing DC maturation and associated antigen presentation [71]. The precise manner by which the 1,25(OH)2D synthesized by DCs is able to influence DC phenotype, antigen presentation and T-cell function has yet to be defined and may involve, intracrine (DC maturation) or paracrine (direct effects on VDR-expressing T-cells) mechanisms [19,73]. At the intracrine level 1,25(OH)2D appears to preferentially regulate mDCs, suggesting that the key effect of vitamin D in this instance is to suppress activation of naive T cells. APC secretion of cytokines that are crucial for recruitment and activation of T-cells is also influenced by 1,25(OH)2D. Immunostimulatory IL-12 is inhibited by 1,25(OH)2D in DCs and other APCs [74]. IL-12 stimulates the development of Th1 T-cells and inhibits the development of Th2 T-cells, supporting the role of vitamin D in promoting a shift from Th1 to Th2 [75]. Conversely, DCs treated with 1,25(OH)2D show enhanced expression of the immunosuppressive cytokine IL-10 which opposes the Th1-driving effects of IL-12 [74,76]. Other factors secreted by APCs that are also regulated by 1,25(OH)2D include prostaglandin E2 [77].
The ability of vitamin D to influence antigen presentation underlines its potential as a key intermediary between the innate and adaptive immune systems. As outlined in section 2, this may involve the modulation of vitamin D-mediated innate immune responses by cells from the adaptive immune system. However, it is important to recognize that the synthesis of 1,25(OH)2D by innate immune cells may conversely affect adaptive immune responses (see Figure 1). In vitro, 1,25(OH)2D has been shown to preferentially inhibit Th1 cells associated with cellular immunity [75], whilst simultaneously promoting Th2 cells, a subset of T-cells associated with humoral (antibody)-mediated immunity [78,79]. However, the in vivo significance of this is unclear as studies using immune cells from the VDR gene knockout mouse indicate that these animals have reduced (rather than the predicted elevated) levels of Th1 cells [80]. More recently, the T-cell repertoire has been expanded to include another T-cell lineage distinct from Th1 or Th2 cells. Termed Th17 cells because of their capacity to synthesize interleukin-17 (IL-17) [81,82], Th17 cells play an essential role in combating certain pathogens but may also cause tissue damage and inflammation [83,84]. Studies of animal models of inflammatory disease have shown that treatment with 1,25(OH)2D reduces expression of IL-17 [85], whilst CYP27B1 gene knockout has been linked to elevated levels of this cytokine [86]. Thus, it possible that vitamin D exerts some of its effects on inflammation and autoimmune disease through the regulation of Th17 cells. In contrast to Th1, Th2 and Th17 cells, Tregs act as suppressor T-cells and treatment with 1,25(OH)2D alone can promote their differentiation [87]. Preferential induction of Tregs is a pivotal mechanism linking vitamin D and adaptive immunity, with potential beneficial effects for autoimmune disease and host-graft rejection [88–90]. As outlined above, the effects of 1,25(OH)2D on Treg development may be mediated by the induction of tolerogenic DCs [70], but direct effects on T-cells may also be important [91,92].
6. Innate immunity related diseases
Historically, vitamin D-deficiency was defined primarily by presence of the bone disease rickets (osteomalacia in adults). However, more recent studies have suggested that sub-optimal vitamin D status may occur even in the absence of rachitic bone disease. A new term - vitamin D ‘insufficiency’ – has been proposed in which serum levels of 25OHD are sub-optimal (< 75 nM) without necessarily impacting on skeletal homeostasis [93]. Because circulating levels of 25OHD are a direct reflection of vitamin D status, this may vary significantly in populations depending on individual access to vitamin D either through exposure to sunlight or through dietary intake. As a result of these new parameters for vitamin D status, a consensus statement from the 13th Workshop on Vitamin D concluded that vitamin D insufficiency was a worldwide epidemic. The key question now being considered is what is the physiological and clinical impact of global vitamin D insufficiency, particularly for non-classical effects such as immunomodulation? Epidemiology has highlighted possible links between vitamin D insufficiency and a variety of human diseases [93]. The final section of the review will detail some of the diseases related to innate immune function that may be influenced by variations in vitamin D status.
Epidemiological studies in many countries have suggested an association between vitamin D deficiency or insufficiency and the incidence and progression of tuberculosis TB. Three prospective studies [94,95] and three case-control studies [96–98] have been carried out in different populations, including Whites and Indian in UK, African in Kenya, Thai in Thailand, Chinese in Hong Kong, Gujarati Hindus resident in London, Indian in India. In each case vitamin D levels were shown to be significantly lower in pulmonary and/or extra-pulmonary TB patients (16.0 nmol/L ~ 39.75 nmol/L) compared to matched healthy control populations (27.25 nmol/L ~ 95.5 nmol/L). After a systematic review and meta-analysis it was concluded that low serum vitamin D levels are associated with higher risk of active TB [99]. A cohort study to assess the association between vitamin D deficiency and TB progression in Pakistan found that low vitamin D levels were associated with a 5-fold increased risk for progression to active TB in healthy household contacts with TB patients [100].
Host genetic variation may also contribute to the individual susceptibility to TB. Single nucleotide polymorphisms (SNPs) of VDR gene have become a target for investigation, since these inherited variations have been linked to VDR activity and subsequent downstream vitamin D-mediated effects [101]. Meta-analysis of 23 case-control or cohort studies from Asia and Africa/South America indicates that among Asians, the FokI ff VDR genotype showed a pronounced positive association with TB (OR 2.0) [101]. By contrast, the BsmI bb genotype showed a significant inverse association with TB (OR 0.5), and marginally significant associations were observed for TaqI and dApaI SNPs [101]. None of the SNPs were significantly related to TB among Africans or South Americans.
Vitamin D supplementation has also been used as a potential treatment strategy for TB [102–105]. In one report, a single oral dose of 2.5 mg vitamin D prior to testing, suppressed the growth of M. tb in patient blood samples [106]. Other studies have shown that adjunct vitamin D supplementation (0.25 mg vitamin D/day) of TB patients receiving conventional therapy for the disease reduced the time for sputum smear conversion from acid fast bacteria (AFB) positive to AFB-negative status [104]. Inherited variations in the vitamin D system may also influence patient responses to supplemental vitamin D. Recent reports of a UK population with TB, showed that vitamin D supplementation had only a limited overall effect in promoting sputum conversion [107]. In this study 146 TB patients were initially assessed at baseline and shown to be predominantly vitamin D-insufficient (95–98% of patients with serum levels of 25OHD less than 75 nM), with almost half the patients being profoundly vitamin D-deficient (60% of patients with serum levels of 25OHD less than 20 nM). Each patient was randomized to either 2.5 mg vitamin D3 (100,000 IU) or placebo on day 0, 14, 28 and 42 of standard TB therapy. Patients receiving supplementary vitamin D showed a 5-fold increase in serum levels of 25OHD compared to placebo patients but this resulted in only a moderate improvement in the time to sputum conversion. However, a significant improvement in sputum conversion time was observed in vitamin D supplemented patients with the tt genotype of the Taq1 polymorphism of the VDR gene [107]. These studies underline the increased risk of low vitamin D status in TB patients but also suggest that therapeutic benefits of vitamin D supplementation may be influenced by patient genetic variation.
The impact of variations in other key vitamin D genes such as CYP27B1 on innate immune responses to vitamin D supplementation has still to be determined. However, a link between TB and the gene for serum vitamin D binding protein (DBP) gene, originally referred to as group-specific component (Gc), has been reported [108]. Specifically the Gc2 allele was shown to be associated with active TB, but only in a cohort with extremely low vitamin D status [109]. DBP SNPs have also been linked to risk of serum vitamin D-insufficiency, the overall conclusion being that these gene variations can act as an inherited determinant of serum vitamin D status by influencing the serum concentrations of DBP [110]. However, gene variants that influence the binding affinity of DBP for vitamin D metabolites may also play a role in modulating the bioavailability of 25OHD to target cells such as monocytes [111]. Data from our laboratory have shown that antibacterial responses to 25OHD are more pronounced in the presence of low affinity forms of DBP [112]. This suggests that the intracrine machinery required for induction of antibacterial responses in monocytes is dependent on the availability of ‘free’ rather than DBP-bound 25OHD. In this way inherited variations in DBP may influence both the circulating levels of DBP and 25OHD, as well as the bioavailability of 25OHD to target tissues.
As outlined above, serum levels of 25OHD have also been linked to sepsis [38], a whole-body inflammatory response caused by systemic immune response to microbial infection [113,114]. A serious medical condition, severe sepsis leads to multiple organ failure, circulatory collapse and disseminated intravascular coagulation (DIC). Treatment with 1,25(OH)2D has been reported to be effectively protecting against DIC in a rat model [115]. The anti-DIC effect of vitamin D was as effective as more conventional therapies suggesting a possible role for vitamin D in the treatment of DIC [115].
Epidemiology has also linked low serum 25OHD with increased incidence or poor control of asthma, respiratory infection and chronic obstructive pulmonary disease (COPD) [116–119]. Inflammation is crucial to the pulmonary dysfunction in COPD, with matrix metalloproteinases (MMPs) being key factors in this process [120]. Vitamin D supplementation has been shown to significantly reduce the MMP-9 levels [121]. SNPs in the vitamin D-binding protein (DBP) have also been linked to COPD [122–124], with low affinity forms of DBP protecting against COPD [122]. Vitamin D may also play a role in upper respiratory infection, particularly as seasonal variations have been reported for infections such as influenza [125]. At a therapeutic level, protective effects of vitamin D supplementation have been described for colds and influenza [126], and this is endorsed by the ability LL37 to exhibit antiviral as well as antibacterial properties [127].
Recent studies have demonstrated expression of CYP27B1 and VDR in the human urinary bladder, with 25OHD being converted to 1,25(OH)2D in bladder epithelial cells [128]. This study also showed that 25OHD and 1,25(OH)2D can significantly enhance production of LL37 from the urinary bladder epithelium during uropathogenic E. coli infection [128]. In this way vitamin D status may influence susceptibility to urinary tract infection [129]. Vitamin D deficiency is also associated with bacterial vaginosis [130,131]. This, and the presence of an intracrine antibacterial system in placental cells [41,132], suggests a role for vitamin D in protecting against infection during pregnancy [133].
7. Conclusions
The last five years have witnessed a sea-change in our perspective on how vitamin D interacts with the immune system. Prominent new data have shown that the expression and activity of vitamin D metabolizing enzymes is central to normal immune responses, providing a mechanism for localized metabolism of 25OHD to 1,25(OH)2D at sites of infection. Unlike its renal endocrine counterpart, the vitamin D metabolic machinery within the immune system is exquisitely dependent on the availability of substrate 25OHD – in other words the vitamin D status of any individual. Thus, impaired serum levels of 25OHD associated with vitamin D-insufficiency may lead to dysregulation of immune responses. Although this has consequences for both the innate and adaptive arms of the immune system, much recent attention has focused on antibacterial actions of vitamin D where intracrine coordination of monocyte CYP27B1 and VDR appears to be a central feature of innate immunity. Further characterization of vitamin D metabolism and innate immunity will be crucial in supporting a broader role for vitamin D in maintaining human health. At a basic science level, more information on the mechanisms that underpin immune regulation of enzymes such as CYP27B1 and CYP24A1 is required. Likewise, almost all of the current data on how vitamin D can influence innate immune function has stemmed from studies of human cells. A limited number of animal models have been utilized with varying results [42,44,134,135], and future studies will need to improve this significantly. Finally, at a patient level more clinical trials are needed to determine how vitamin D affects infection in vivo, and whether the levels of vitamin D required to do this are the same as those required for classical skeletal functions of vitamin D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979;2:1335–6. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- 2.Manolagas SC, Haussler MR, Deftos LJ. 1,25-dihydroxyvitamin D3 receptors in cancer. Lancet. 1980;1:828. doi: 10.1016/s0140-6736(80)91332-x. [DOI] [PubMed] [Google Scholar]
- 3.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–6. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 4.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe E, Miyaura C, Tanaka H, Shiina Y, Kuribayashi T, Suda S, Nishii Y, DeLuca HF, Suda T. 1 alpha,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc Natl Acad Sci U S A. 1983;80:5583–7. doi: 10.1073/pnas.80.18.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133:1748–54. [PubMed] [Google Scholar]
- 7.Bell NH, Stern PH, Pantzer E, Sinha TK, DeLuca HF. Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979;64:218–25. doi: 10.1172/JCI109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papapoulos SE, Clemens TL, Fraher LJ, Lewin IG, Sandler LM, O’Riordan JL. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1:627–30. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 9.Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981;305:440–3. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 10.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985;161:755–65. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 13.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 14.Hewison M. Vitamin D and the immune system. J Endocrinol. 1992;132:173–5. doi: 10.1677/joe.0.1320173. [DOI] [PubMed] [Google Scholar]
- 15.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 16.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 17.Van Etten E, Decallonne B, Verlinden L, Verstuyf A, Bouillon R, Mathieu C. Analogs of 1alpha,25-dihydroxyvitamin D3 as pluripotent immunomodulators. J Cell Biochem. 2003;88:223–6. doi: 10.1002/jcb.10329. [DOI] [PubMed] [Google Scholar]
- 18.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 19.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103–11. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–8. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 23.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 24.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 25.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 26.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–63. [PMC free article] [PubMed] [Google Scholar]
- 28.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 29.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agostini C, Semenzato G. Cytokines in sarcoidosis. Semin Respir Infect. 1998;13:184–96. [PubMed] [Google Scholar]
- 31.Kallas M, Green F, Hewison M, White C, Kline G. Rare causes of calcitriol-mediated hypercalcemia: a case report and literature review. J Clin Endocrinol Metab. 95:3111–7. doi: 10.1210/jc.2009-2673. [DOI] [PubMed] [Google Scholar]
- 32.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–34. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 33.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, Modlin RL. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 107:22593–8. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280:20604–11. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 35.Adams JS, Chen H, Chun R, Ren S, Wu S, Gacad M, Nguyen L, Ride J, Liu P, Modlin R, Hewison M. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J Bone Miner Res. 2007;22(Suppl 2):V20–4. doi: 10.1359/jbmr.07s214. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–9. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 37.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, Thadhani R. Low Plasma Level of Cathelicidin Antimicrobial Peptide (hCAP18) Predicts Increased Infectious Disease Mortality in Patients Undergoing Hemodialysis. Clin Infect Dis. 2009 doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007 doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. Effects of 25-Hydroxyvitamin D3 and 1,25-Dihydroxyvitamin D3 on Cytokine Production by Human Decidual Cells. Biol Reprod. 2006 doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 41.Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M. Vitamin D Induces Innate Antibacterial Responses in Human Trophoblasts via an Intracrine Pathway. Biol Reprod. 2009;80:398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Equils O, Naiki Y, Shapiro AM, Michelsen K, Lu D, Adams J, Jordan S. 1,25-Dihydroxyvitamin D inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin Exp Immunol. 2006;143:58–64. doi: 10.1111/j.1365-2249.2005.02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 151:2423–32. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagishetty V, Chun RF, Liu NQ, Lisse TS, Adams JS, Hewison M. 1alpha-hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. J Steroid Biochem Mol Biol. 121:228–33. doi: 10.1016/j.jsbmb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kao CY, Kim C, Huang F, Wu R. Requirements for two proximal NF-kappaB binding sites and IkappaB-zeta in IL-17A-induced human beta-defensin 2 expression by conducting airway epithelium. J Biol Chem. 2008;283:15309–18. doi: 10.1074/jbc.M708289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-beta defensin 2 innate immune pathway defective in Crohn’s disease. J Biol Chem. 2009 doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 53.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276:35482–93. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 54.Kohchi C, Inagawa H, Nishizawa T, Soma G. ROS and innate immunity. Anticancer Res. 2009;29:817–21. [PubMed] [Google Scholar]
- 55.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009;182:3696–705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 57.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66:5314–21. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 59.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 61.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–49. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial Lipoprotein Activates Autophagy via TLR2/1/CD14 and a Functional Vitamin D Receptor Signaling. Cell Microbiol. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 65.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 66.Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, O’Riordan JL. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 67.Dam TN, Moller B, Hindkjaer J, Kragballe K. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J Investig Dermatol Symp Proc. 1996;1:72–7. [PubMed] [Google Scholar]
- 68.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 69.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701–8. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 70.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 71.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 72.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 73.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215:31–8. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 74.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 76.van Halteren AG, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C. Redirection of human autoreactive T-cells Upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3) Diabetes. 2002;51:2119–25. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- 77.Koren R, Ravid A, Rotem C, Shohami E, Liberman UA, Novogrodsky A. 1,25-Dihydroxyvitamin D3 enhances prostaglandin E2 production by monocytes. A mechanism which partially accounts for the antiproliferative effect of 1,25(OH)2D3 on lymphocytes. FEBS Lett. 1986;205:113–6. doi: 10.1016/0014-5793(86)80876-6. [DOI] [PubMed] [Google Scholar]
- 78.Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, Laureys J, Bouillon R, Mathieu C. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543) Diabetes. 2000;49:1301–7. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 79.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 80.O’Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109:1091–9. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 82.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 83.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–71. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of TNBS colitis with calcitriol is associated with a change of a Th1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 86.Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–83. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 88.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–74. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 89.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261–6. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 90.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030–7. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 91.Urry Z, Xystrakis E, Richards DF, McDonald J, Sattar Z, Cousins DJ, Corrigan CJ, Hickman E, Brown Z, Hawrylowicz CM. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119:387–98. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D(3) and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 94.Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985;40:187–90. doi: 10.1136/thx.40.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davies PD, Church HA, Brown RC, Woodhead JS. Raised serum calcium in tuberculosis patients in Africa. Eur J Respir Dis. 1987;71:341–4. [PubMed] [Google Scholar]
- 96.Chan TY, Poon P, Pang J, Swaminathan R, Chan CH, Nisar M, Williams CS, Davies PD. A study of calcium and vitamin D metabolism in Chinese patients with pulmonary tuberculosis. J Trop Med Hyg. 1994;97:26–30. [PubMed] [Google Scholar]
- 97.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 98.Sasidharan PK, Rajeev E, Vijayakumari V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 2002;50:554–8. [PubMed] [Google Scholar]
- 99.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–9. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 100.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin d deficiency and tuberculosis progression. Emerg Infect Dis. 16:853–5. doi: 10.3201/eid1605.091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:15–23. [PubMed] [Google Scholar]
- 102.Dowling GB, Prosser Thomas EW. Treatment of lupus vulgaris with calciferol. Lancet. 1946;1:919–22. doi: 10.1016/s0140-6736(46)90616-2. [DOI] [PubMed] [Google Scholar]
- 103.Morcos MM, Gabr AA, Samuel S, Kamel M, el Baz M, el Beshry M, Michail RR. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137:157–64. [PubMed] [Google Scholar]
- 104.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 105.Chocano-Bedoya P, Ronnenberg AG. Vitamin D and tuberculosis. Nutr Rev. 2009;67:289–93. doi: 10.1111/j.1753-4887.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- 106.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. A single dose of vitamin d enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 107.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK, Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V, Drobniewski FA, Eldridge SM, Griffiths CJ. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 377:242–50. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986;72:281–93. doi: 10.1007/BF00290950. [DOI] [PubMed] [Google Scholar]
- 109.Martineau AR, Leandro AC, Anderson ST, Newton SM, Wilkinson KA, Nicol MP, Pienaar SM, Skolimowska KH, Rocha MA, Rolla VC, Levin M, Davidson RN, Bremner SA, Griffiths CJ, Eley BS, Bonecini-Almeida MG, Wilkinson RJ. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2009 doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 95:3368–76. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-Binding Protein Directs Monocyte Responses to 25-Hydroxy- and 1,25-Dihydroxyvitamin D. J Clin Endocrinol Metab. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 114.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 115.Asakura H, Aoshima K, Suga Y, Yamazaki M, Morishita E, Saito M, Miyamoto K, Nakao S. Beneficial effect of the active form of vitamin D3 against LPS-induced DIC but not against tissue-factor-induced DIC in rat models. Thromb Haemost. 2001;85:287–90. [PubMed] [Google Scholar]
- 116.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 117.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D Deficiency is Highly Prevalent in COPD and Correlates with Variants in the Vitamin D Binding Gene. Thorax. 2009 doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 118.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 119.Dimeloe S, Nanzer A, Ryanna K, Hawrylowicz C. Regulatory T cells, inflammation and the allergic response-The role of glucocorticoids and Vitamin D. J Steroid Biochem Mol Biol. 2010;120:86–95. doi: 10.1016/j.jsbmb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 120.Sampsonas F, Karkoulias K, Kaparianos A, Spiropoulos K. Genetics of chronic obstructive pulmonary disease, beyond a1-antitrypsin deficiency. Curr Med Chem. 2006;13:2857–73. doi: 10.2174/092986706778521922. [DOI] [PubMed] [Google Scholar]
- 121.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Qjm. 2002;95:787–96. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 122.Wood AM, Bassford C, Webster D, Newby P, Rajesh P, Stockley RA, Thickett DR. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 66:205–10. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 123.Horne SL, Cockcroft DW, Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered. 1990;40:173–6. doi: 10.1159/000153926. [DOI] [PubMed] [Google Scholar]
- 124.Ishii T, Keicho N, Teramoto S, Azuma A, Kudoh S, Fukuchi Y, Ouchi Y, Matsuse T. Association of Gc-globulin variation with susceptibility to COPD and diffuse panbronchiolitis. Eur Respir J. 2001;18:753–7. doi: 10.1183/09031936.01.00094401. [DOI] [PubMed] [Google Scholar]
- 125.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aloia JF, Li-Ng M. Correspondence. Epidemiol Infect. 2007:1–4. [Google Scholar]
- 127.Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–5. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 128.Hertting O, Holm A, Luthje P, Brauner H, Dyrdak R, Jonasson AF, Wiklund P, Chromek M, Brauner A. Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder. PLoS One. 2010;5:e15580. doi: 10.1371/journal.pone.0015580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007;18:2810–6. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 130.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157–61. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O’Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23:45–52. doi: 10.1016/j.jpag.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 132.Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75:816–22. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 133.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 31:1027–34. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mathieu C, Van Etten E, Gysemans C, Decallonne B, Kato S, Laureys J, Depovere J, Valckx D, Verstuyf A, Bouillon R. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J Bone Miner Res. 2001;16:2057–65. doi: 10.1359/jbmr.2001.16.11.2057. [DOI] [PubMed] [Google Scholar]
- 135.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


