Abstract
BMPs (Bone morphogenetic proteins) such as BMP2 and BMP7 have been used about one decade as bone anabolic agents in orthopaedics. The BMP receptor ACVR1, which is a key receptor of BMP7, is expressed in bone. The pathological role of ACVR1 in humans has been reported: a point mutation in ACVR1 can cause fibrodysplasia ossificans progressiva (FOP) in which ectopic ossification occurs in skeletal muscles and deep connective tissues. The physiological function of ACVR1 in bone, however, is totally unknown. The purpose of this study is to investigate the endogenous role of ACVR1 in osteoblasts, one of the most dominant cell-types in bone. We generated Acvr1-null mice in an osteoblast-specific manner using an inducible Cre-loxP system. Surprisingly, we found that bone mass was increased in the Acvr1-null mice. Interestingly, canonical Wnt signaling was increased and expression levels of Wnt inhibitors Sost and Dkk1 were both suppressed in the null bones during the developmental stages. In addition, we confirmed that expression levels of both Sost and Dkk1 were upregulated by BMP7 dose-dependently in vitro. These results suggest that the Acvr1-deficiency can increase bone mass by activating Wnt signaling in which both Sost and Dkk1 expression levels are diminished. This study leads to a new concept of the BMP7-ACVR1-SOST/DKK1 axis in osteoblasts, in which BMP7 signaling through ACVR1 can reduce Wnt signaling via SOST/DKK1 and then inhibits osteogenesis. Although this concept is beyond the current known function of BMP7, it can explain the varied outcomes of BMP7 treatment. We believe BMP signaling can exhibit multifaceted effects by context and cell type.
Keywords: BMP, ACVR1, Wnt, SOST, DKK1, Osteoblast
Introduction
ACVR1 is a key receptor of BMP (bone morphogenetic protein) 7 [1], which is also called OP1 (osteogenic protein 1). BMP7, approved by the FDA (Food and Drug Administration) in 2001, has been clinically applied as a bone anabolic agent for lumbar spinal fusion and treatment of long bone non-union fracture [2] because of its ability to induce osteoblast differentiation in vitro and ectopic bone formation in vivo [3]. The advantage of BMP7 therapy has been highlighted commercially, but its effectiveness reviewed during one decade is not robust because the outcomes are varied [4].
Marshall Urist made the key discovery that demineralized bone matrix induced bone formation in 1965 [5]. BMPs are members of the transforming growth factor-β (TGF-β) superfamily [6]. BMP signals, like those of other TGF-β family members, are mediated through a heteromeric receptor complex of type I and type II transmembrane Ser/Thr kinase receptors [7]. Upon ligand binding, type II receptors, which are constitutively active kinases, phosphorylate and activate type I receptors (also called ALKs). There are three BMP type I receptors, type IA (BMPR1A or ALK3), type IB (BMPR1B or ALK6), and ACVRI (or ALK2). ACVR1 is expressed in bone [8]; however, the physiological role of ACVR1 in osteoblasts has not been studied yet [9].
By contrast, a pathological role of ACVR1 in humans has been reported. A single point mutation of ACVR1 has been linked as the causative mutation in patients with fibrodysplasia ossificans progressiva (FOP; OMIM ID: 135100) [10] that display congenital malformations of the progressive heterotopic ossification in skeletal muscles and other connective tissues [11; 12]. It is recently reported that cells mediating heterotopic ossification in FOP may be of endothelial origin [13] because exceeded BMP signaling through ACVR1 can convert vascular endothelial cells into multipotent stem-like cells. Because endogenous bone in FOP patients is not affected in general, it is important to identify distinct molecular mechanisms of ACVR1 in endogenous ossification versus ectopic (i.e. heterotopic) ossification.
To elucidate the endogenous role of ACVR1 in bone development, it is necessary to study loss-of-function of ACVR1 using animal models. The conventional Acvr1-null mice are not suitable for this purpose because they die before bone development [14]. In this study, we generated conditional Acvr1-null mice using a tamoxifen-inducible Cre-loxP system under the control of a 3.2 kb type I collagen promoter. In the conditional null mice, we unexpectedly found increased bone mass. Intriguingly, canonical Wnt signaling was upregulated in the null bones in conjunction with a reduction of Wnt inhibitors Sost and Dkk1. Based on our findings, we conclude that ACVR1 negatively regulates bone mass by suppressing Wnt signaling through SOST and DKK1.
Material and Methods
Mice and tamoxifen administration
A transgenic mouse line expressing the tamoxifen (TM)-inducible Cre fusion protein CreERT under the control of a 3.2 kb mouse pro-collagen a1(I) promoter (Col1-CreERT) was generated by a pronuclear injection [15; 16; 17] and crossed with floxed Acvr1 mice [18], which become functional null after Cre recombination [19]. Tamoxifen (TM, Sigma) was dissolved in a small volume of ethanol, diluted with corn oil at a concentration of 10 mg/ml, and stored at −20°C until use. To generate cKO mice in embryonic stages, we set up a breeding pair (i.e. male; Col1-CreERT+:Acvr1fx/fx, female; Col1-CreERT−:Acvr1fx/fx), injected TM (75mg/kg) intraperitoneally into pregnant females from E13.5 to E17.5, and collected fetuses at E18.5 [16]. After the administration of TM, 50% of fetuses were expected to be cKO (Col1CreERT+:Acvr1fx/fx) and the rest were control (Col1CreERT−:Acvr1fx/fx). To generate cKO mice in weanling stages, we injected TM intraperitoneally into nursing females every three days from P2 (Postnatal day 2) until euthanasia at P21 [17]. Under this condition, TM was delivered to progeny through milk. For adult stages, TM was injected intraperitoneally twice a week for 10 weeks. Cre activity of Col1-CreERT mice was detected specifically in immature osteoblasts, mature osteoblasts, and osteocytes as we reported previously [16; 17]. TOPGAL [20] mice were obtained from the Jackson Laboratory. Wildtype tissues and osteoblasts were harvested from C57BL/6 mice [21]. The animal protocol was approved by the Institutional Animal Care and Use Committee.
X-ray and histological analyses
For X-ray analysis, rib bones from Acvr1 cKO and control mice at P21 were harvested. Images were taken using a Faxitron X-ray system (Faxitron). For H&E staining, bones (i.e. humerus, calvariae, and tibiae) were fixed in 4% paraformaldehyde, decalcified with 10% EDTA, and embedded in paraffin. Paraffin sections were cut at 8 µm and stained using a standard protocol. For β-galactosidase (β-gal) staining on sections, decalcified calvariae and tibiae from P21 mice were soaked in up to 30% sucrose before frozen sectioning. Sections were stained with X-gal for β-gal activity and counterstained with eosin. For β-gal staining on whole tissues, bones (i.e. tibiae and calvariae) were stained with X-gal as described previously [16; 17].
Quantitative real-time RT-PCR (qRT-PCR)
RNA was isolated from P21 calvariae and newborn tissues (i.e. heart, skeletal muscles, and skull bones) using Trizol (Invitrogen) and from primary osteoblasts using Picopure (Arcturus). cDNA was synthesized using the SuperScript™ Preamplification System (Invitrogen). PCR reactions, data quantification, and analysis were performed according to the manufacturer’s standard protocol for TaqMan gene expression assays (Applied Biosystems). Values were normalized to Gapdh using TaqMan Rodent GAPDH Control Reagents. All measurements were performed in triplicate and analyzed using the 2−ΔΔCt method [22].
Primary osteoblast culture
Newborn calvariae from C57BL/6 wild type mice were digested with type I collagenase and dispase II to isolate osteoblasts as previously described [16]. Primary osteoblasts were maintained in α-MEM containing 10% FBS and ascorbic acid (50 mg/ml, Sigma) and then treated with BMP7 (R & D) for 3 hr at increasing concentrations (10, 50, and 100 ng/ml).
Statistical analysis
All statistical analyses were performed using a two-tailed Student’s t-test. All results were expressed as mean ± SD and compared between control and Acvr1 cKO. A p value of <0.05 indicates statistical significance.
Results
Generation of Acvr1 cKO mice
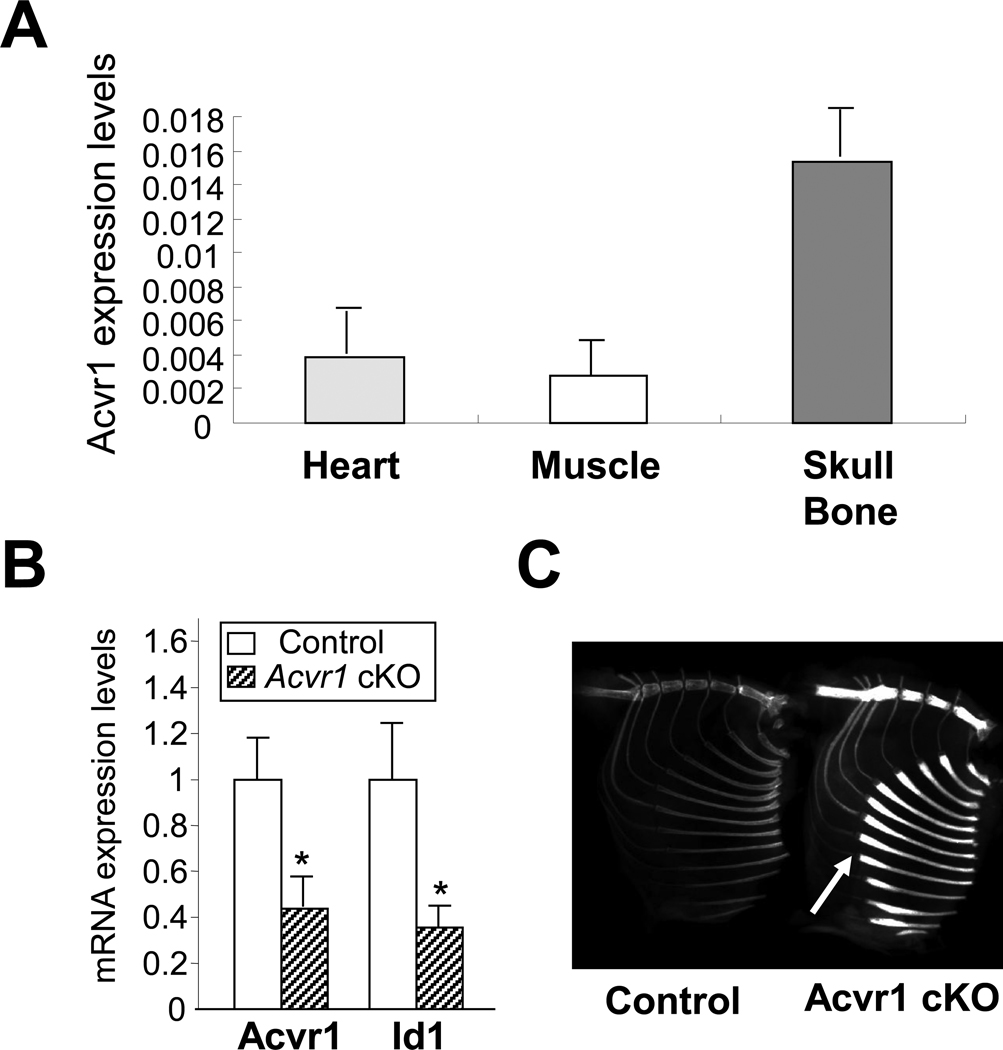
ACVR1 is known to be expressed in many tissues [8; 23], but its quantity by tissue is unknown. We quantitatively measured the expression levels of endogenous Acvr1 in the heart, skeletal muscles, and bones during postnatal development of wildtype mice. Interestingly, Acvr1 is highly expressed in skull bones compared with skeletal muscles at new bone stage (Fig. 1A). To investigate the physiological role of ACVR1 in bone, we next generated conditional knockout (cKO) mice for Acvr1 in an osteoblast-dependent manner using a tamoxifen-inducible Cre-loxP system. In cKO bones (i.e. skull bones), expression levels of Acvr1 was significantly reduced compared with control bones as assessed by qRT-PCR at P21 (Fig. 1B), suggesting a sucessful Cre recombination in the cKO mice. Consistent with this data, expression levels of Id1, a known downstream target of BMP signaling [24], was also significantly reduced. Although cKO mice appeared normal in skeletal size and length, X-ray analysis demonstrated a dramatic increase in radiodensity of adult cKO bones including the sternum and ribs (Fig. 1C). Bone mineral density assessed by DEXA was significantly increased in adult cKO bones (Control rib bones; 0.0193g/cm2, cKO rib bones; 0.0277g/cm2, p = 0.0006, n = 5). These results indicate that loss of ACVR1 in osteoblasts increases bone density.
Figure 1.
Acvr1 conditional knockout (cKO) mice. (A) Acvr1 expression levels in normal tissues. RNA was isolated from newborn wildtype mice. Endogenous expression levels of Acvr1 were assessed by qRT-PCR. Absolute values were expressed as mean ± SD (n = 3). (B) qRT-PCR analysis for Acvr1 and Id1 using P21 calvariae. Expression levels of Acvr1 and Id1 were significantly reduced in cKO bones. Values in cKO bones (striped bar) are expressed relative to controls (open bar). mean ± SD; *, p < 0.05. (C) X-ray image of Acvr1 cKO adult mice. The radiodensity of cKO rib bones was notably increased compared with controls. White arrows indicate the rib flaring in cKO mice.
Increased bone mass in Acvr1 cKO bones
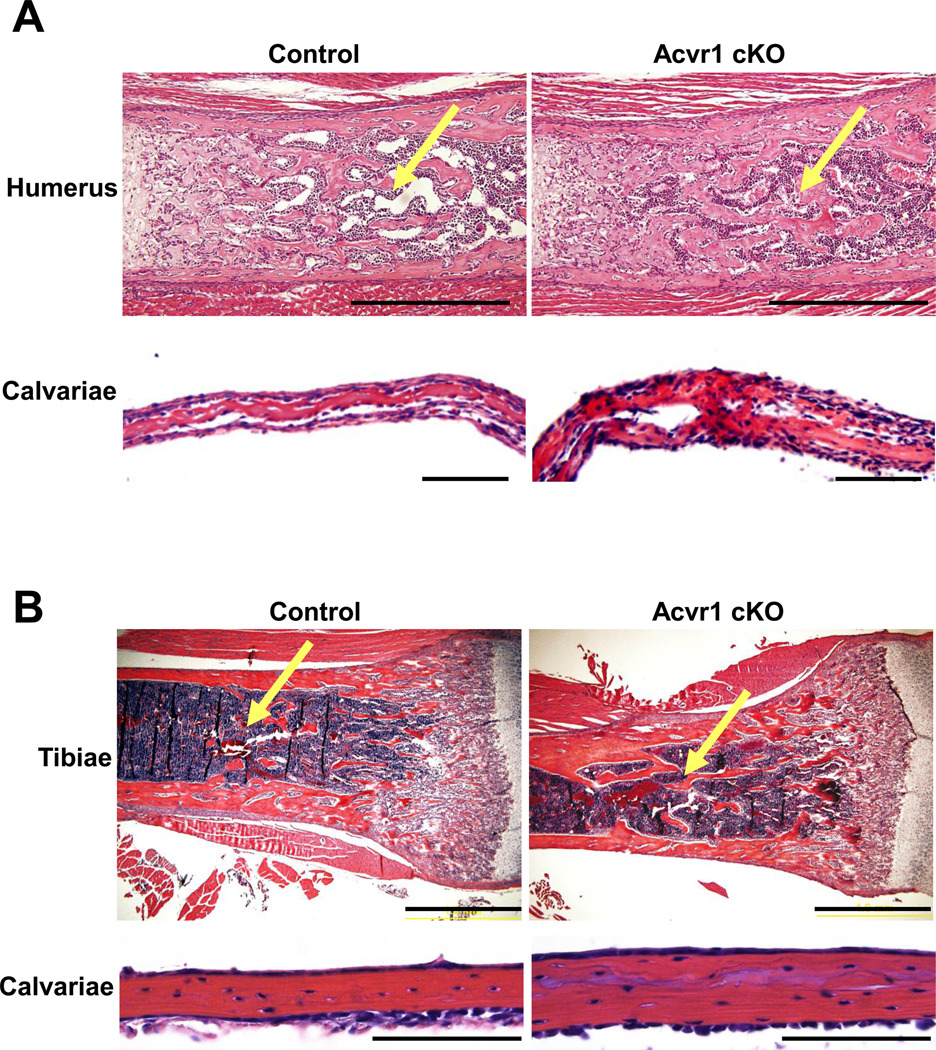
Although Acvr1 is highly expressed in bone during developmental stages (Fig. 1A), physiological roles of ACVR1 in bone development is totally unknown. To investigate the Acvr1-deficiency in bone development, we generated Acvr1 cKO during embryonic stages and postnatal stages. At E18.5 (i.e. embryonic day 18.5), trabecular bone mass in cKO humerus appeared increased compared with controls as assessed by H&E staining (Fig. 2A), which is consistent with the increase in radiodensity during adult stages (Fig. 1C). In addition, the thickness of skull bones (i.e. calvariae) was increased. Similarly, we observed increased trabecular bones in cKO tibiae as well as thickened lamellar bones in cKO calvariae compared with controls at P21 (i.e. postnatal day 21) (Fig. 2B). These results demonstrate that Acvr1 cKO mice exhibited an increase in bone mass during both embryonic and postnatal bone developmental stages. It is also suggested that Acvr1-deficiency affects regulation of bone mass both in the endochondral ossification process (i.e. humerus and tibiae) as well as the intramembranous ossification process (i.e. calvariae).
Figure 2.
Increased bone mass in Acvr1 cKO mice during embryonic and weanling stages. (A) H&E staining of humerus and calvariae was compared between control and cKO bones at E18.5. Bars: 500 µm (humerus), 100 µm (calvariae). (B) H&E staining of tibiae and calvariae was compared between control and cKO bones at P21. Bars: 1 mm (tibiae), and 500 µm (calvariae). Yellow arrows indicate the trabecular bone area where the bone mass is more increased in cKO bones compared with controls.
Upregulation of canonical Wnt signaling in Acvr1 cKO bones
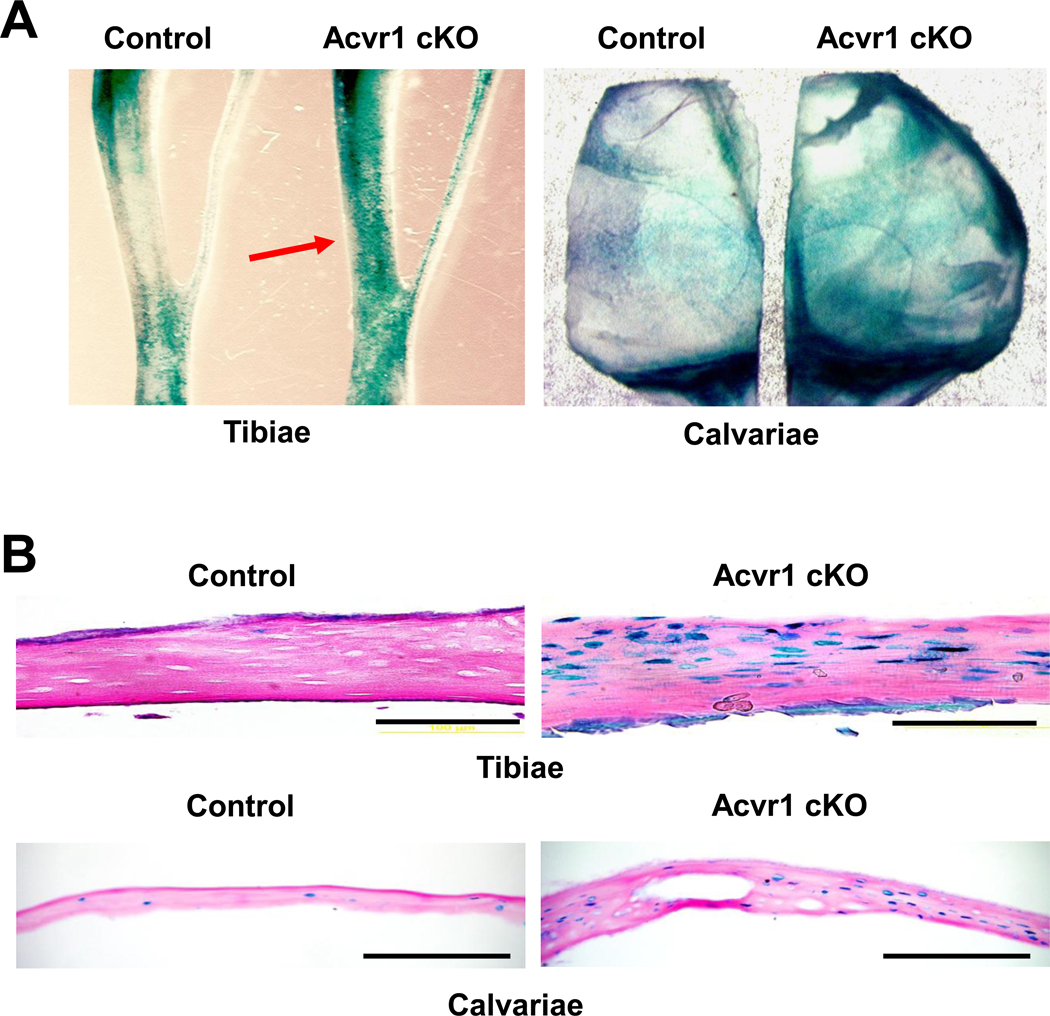
Activation of Wnt signaling in osteoblasts also increases bone mass [25; 26]. It is, however, largely unknown how BMP and Wnt signaling pathways affect each other in vivo. To assess canonical Wnt signaling in Acvr1 cKO mice, we used TOPGAL Wnt reporter mice that express a β-galactosidase transgene driven by a T cell factor (TCF) β-catenin responsive promoter [20]. When assessed by a whole mount via β-gal staining, Acvr1 cKO:TOPGAL mice demonstrated increased Wnt activity in the tibiae and calvariae at P21 compared to controls (Fig. 3A). Upregulation of canonical Wnt signaling was further confirmed by a sectioned specimen with β-gal staining (Fig. 3B). It is noted that the upregulation of Wnt signaling was observed in conjunction with increased bone thickness compared to controls. These facts suggest that enhanced canonical Wnt signaling resulting from a loss of BMP signaling through ACVR1 may be a cause of increased bone mass found in the cKO bones.
Figure 3.
Upregulation of canonical Wnt signaling in Acvr1 cKO bones. (A) Using TOPGAL mice, canonical Wnt signaling was assessed by whole mount β-gal staining at P21. Wnt activity was increased in both tibiae (red arrow) and calvariae. (B) Histological analysis of Wnt signaling by β-gal staining. Note that the number of β-gal positive osteoblasts was dramatically increased in both cKO tibiae and calvariae compared with controls at P21. Bars: 200 µm.
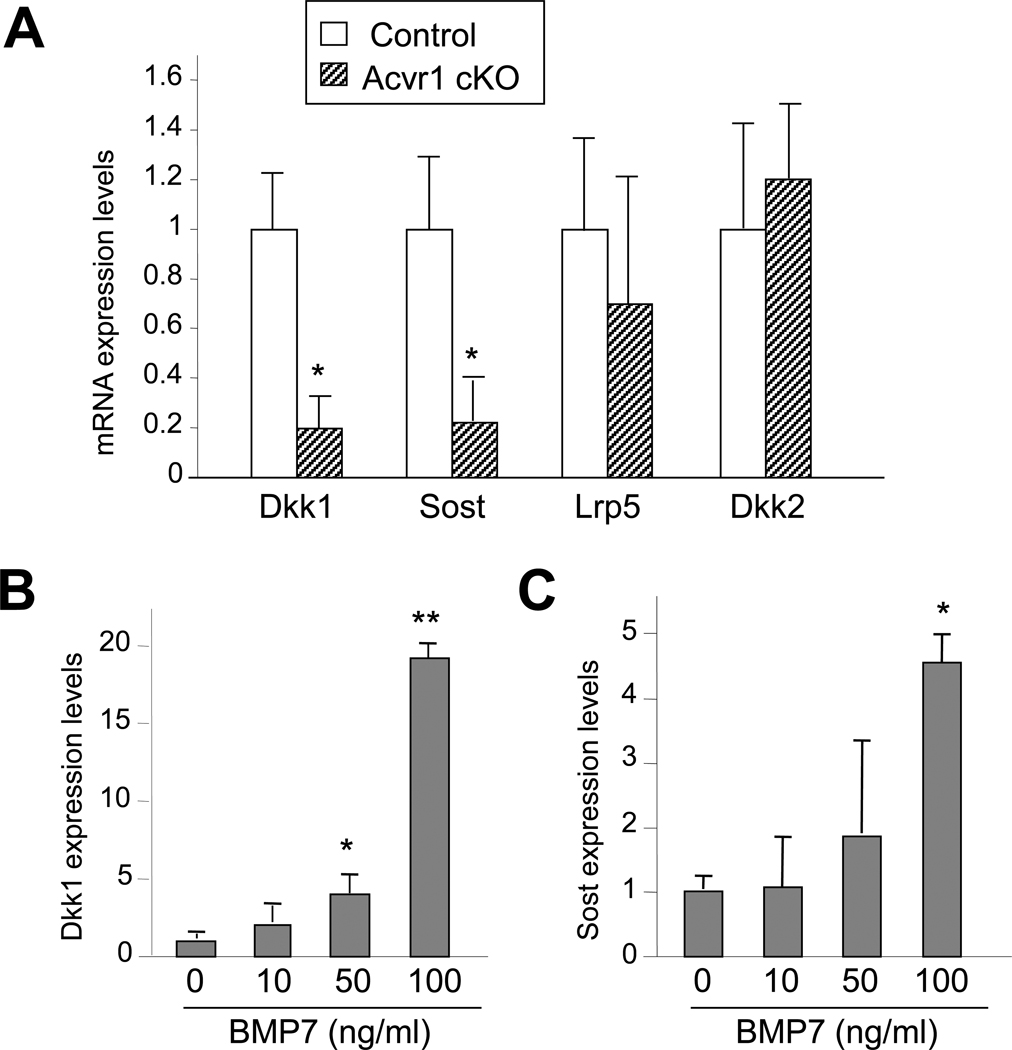
We further sought to determine the molecular mechanisms responsible for the increased Wnt signaling. In the P21 cKO calvariae, expression levels of Dkk1 and Sost mRNAs as assessed by qRT-PCR were significantly reduced while Dkk2 and Lrp5 were unchanged (Fig. 4A). We next investigated a potential link between the expression of Wnt inhibitors Sost and Dkk1 and BMP signaling using wildtype primary osteoblasts. In primary osteoblasts treated with BMP7, a potent ACVRI ligand [1], levels of Sost and Dkk1 increased up to 4.5- and 19-fold, respectively, after 3 hr as assessed by qRT-PCR (Fig. 4B, C). These results suggest that canonical Wnt signaling was upregulated in Acvr1 cKO bones in conjunction with downregulation of Wnt inhibitors Sost and Dkk1. In turn, ACVRI-mediated BMP signaling can negatively regulate canonical Wnt signaling in osteoblasts under physiological conditions.
Figure 4.
Reduction of Sost and Dkk1 expression in cKO bones. (A) qRT-PCR analysis for Dkk1, Dkk2, Lrp5, and Sost using P21 calvariae. Expression levels of Wnt inhibitors Dkk1 and Sost were significantly reduced in cKO calvariae, while expression of co-receptor Lrp5 was unchanged. Values in cKO bones (striped bar) are expressed relative to controls (open bar). mean ± SD; *, p < 0.05. (B, C) Positive regulation of Dkk1 and Sost expression by BMP7. Primary osteoblasts were isolated from wildtype newborn calvariae. mRNA was extracted from osteoblasts treated with BMP7 at indicated concentrations for 3 hr. Dose-dependent effects of BMPs on Dkk1 and Sost expression as assessed by qRT-PCR. Values are expressed relative to untreated osteoblasts. mean ± SD; t-test, *, p < 0.05; **, p < 0.01.
Discussion
The key finding of this study is an increase in endogenous bone mass in Acvr1 cKO mice. This observation is in striking contrast to the current understanding of BMPs as osteogenic growth factors, which has been extensively documented with numerous in vitro studies [27; 28]. Similar to our current results, negative regulation of bone mass by BMPs has been genetically demonstrated in mice: 1) overexpression of Bmp4 in osteoblasts reduced bone mass in embryos, 2) overexpression of Noggin, an antagonist of BMP2 and BMP4, in osteoblasts increased bone mass in embryos and weanlings [29], and 3) loss-of-function of BMPR1A, a potent receptor of BMP2 and BMP4, in osteoblasts demonstrated an increased bone phenotype in embryos, weanlings, and adults [16; 17; 21]. Collectively, these facts suggest that osteoblasts have ability to restrain bone mass by responding to BMPs. In fact, recent preclinical studies demonstrated that BMPs can reduce bone mass and density when applied to bone defect models [30; 31].
In addition to BMP signaling, Wnt signaling in osteoblasts has been examined for a decade because of its role in bone formation and bone mass [25; 26]. Both BMPs and Wnt are understood as bone mass inducers; however, the physiologic relationship between BMP and Wnt is largely unknown. This study, which focused on prenatal and postnatal developmental stages, demonstrates two key findings: 1) Wnt signaling was increased in the Acvr1 cKO bones where bone mass was also increased and 2) Wnt inhibitors SOST and DKK1 were both suppressed in the Acvr1 cKO bones. The physiological function of SOST and DKK1 is well documented in vivo: Conventional knockouts of Sost (Sost−/− mice) exhibit increased bone mass [32]. In humans, loss-of-function and hypomorphic mutations in SOST cause sclerosteosis [33] and Van Buchem disease [34], respectively, with a high bone mass (HBM) phenotype. Similarly, mice heterozygous for Dkk1 (Dkk1+/− mice) exhibit a HBM phenotype [35], while overexpression of Dkk1 in osteoblasts causes osteopenia [36]. These two Wnt inhibitors are upregulated by BMP7, a potent ligand of ACVRI (Fig. 4). We propose a working model of the BMP7-ACVR1-SOST/DKK1 axis, in which both SOST and DKK1 are downstream targets of ACVR1 and act as a Wnt inhibitor. As a new strategy of bone anabolic drugs, a clinical trial for treatment of osteoporosis using neutralizing antibodies for SOST (AMG785) and DKK1 (BHQ 880) has recently started [37]. Our study presented here may bring a new direction to develop ACVR1 inhibitors that can be used to increase bone mass as potential bone anabolic drugs for osteoporosis and bone fracture. For this direction, further characterization of bone anabolic parameters (i.e. bone formation rate, mineral apposition rate) in the cKO adult bones will be desired.
In conclusion, this study demonstrates that loss of BMP signaling via ACVR1 directs osteoblasts to increase endogenous bone mass. Signaling by BMPs via ACVR1 in osteoblasts negatively regulates endogenous bone mass and downregulates Wnt signaling possibly through Wnt inhibitor SOST and DKK1. This study, which proposes a new concept of BMP7-ACVR1-SOST/DKK1 functional axis, can help to understand complex roles of BMP signaling and varied outcomes of BMP treatments at molecular levels in vivo.
Highlights.
-
>
We investigate physiological roles of the BMP receptor ACVR1, a potent receptor for BMP7.
-
>
Loss of ACVR1 in osteoblasts increases bone mass and activates Wnt signaling in mice.
-
>
Wnt inhibitors SOST and DKK1 are both downregulated in the mutant bones.
-
>
ACVR1 can repress osteogenesis and Wnt signaling via SOST/DKK1.
-
>
This study proposes a new concept of the BMP7-ACVR1-SOST/DKK1 axis in osteoblasts.
Acknowledgements
We gratefully thank Drs. Tatsuya Kobayashi and Henry M. Kronenberg for providing us a 3.2 kb Col1-CreER™ transgenic mouse line. This work was supported by the Lilly Fellowship Foundation (N. K), R01DE013085 (V. K) and R01DE020843 (Y. M.)
Abbreviations
- ACVR1
Activin A Receptor Type I
- ALK
Activin Receptor-like Kinase
- BMP
Bone Morphogenetic Protein
- BMPR
BMP Receptor
- DEXA
Dual-emission X-ray Absorptiometry
- E
Embryonic day
- FOP
Fibrodysplasia Ossificans Progressiva
- HBM
High Bone Mass
- P
Postnatal day
- TGF-β
transforming growth factor-β
- qRT-PCR
quantitative Reverse Transcription Polymerase Chain Reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vesa M. Kaartinen, Email: vesak@umich.edu.
Yuji Mishina, Email: mishina@umich.edu.
References
- 1.Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- 2.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM, Pang RH, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–20362. [PubMed] [Google Scholar]
- 4.Garrison KR, Shemilt I, Donell S, Ryder JJ, Mugford M, Harvey I, Song F, Alt V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006950.pub2. CD006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 6.Massague J. Receptors for the TGF-beta family. Cell. 1992;69:1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- 7.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 8.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamiya N, Mishina Y. New insights on the roles of BMP signaling in bone-A review of recent mouse genetic studies. Biofactors. 2011;37:75–82. doi: 10.1002/biof.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, Pignolo RJ, Shore EM. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP) J Bone Miner Metab. 2008;26:521–530. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 15.Maes C, Kobayashi T, Kronenberg HM. A novel transgenic mouse model to study the osteoblast lineage in vivo. Ann N Y Acad Sci. 2007;1116:149–164. doi: 10.1196/annals.1402.060. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res. 2008;23:2007–2017. doi: 10.1359/JBMR.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaartinen V, Nagy A. Removal of the floxed neo gene from a conditional knockout allele by the adenoviral Cre recombinase in vivo. Genesis. 2001;31:126–129. doi: 10.1002/gene.10015. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu Y, Scott G, Nagy A, Kaartinen V, Mishina Y. BMP type I receptor ALK2 is essential for proper patterning at late gastrulation during mouse embryogenesis. Dev Dyn. 2007;236:512–517. doi: 10.1002/dvdy.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 21.Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the Type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res. 2010;25:200–210. doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 25.Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21:1022–1033. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- 30.McGee MA, Findlay DM, Howie DW, Carbone A, Ward P, Stamenkov R, Page TT, Bruce WJ, Wildenauer CI, Toth C. The use of OP-1 in femoral impaction grafting in a sheep model. J Orthop Res. 2004;22:1008–1015. doi: 10.1016/j.orthres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Seeherman HJ, Li XJ, Bouxsein ML, Wozney JM. rhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J Bone Joint Surg Am. 2010;92:411–426. doi: 10.2106/JBJS.H.01732. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 33.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 34.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]