Abstract
Clinical studies indicate an increased incidence of impaired glucose tolerance in individuals with Parkinson’s disease (PD). The mechanisms that underlie this co-morbidity are currently unknown. The purpose of this study was to analyze peripheral glucose tolerance following severe unilateral nigrostriatal dopamine (DA) depletion, and to determine whether central and peripheral insulin signaling was affected in the 6-hydroxydopamine (6-OHDA) middle-aged rat model of PD. Although serum insulin levels differed significantly between the 6-OHDA and sham groups over the course of a glucose tolerance test six weeks post-lesion, no significant effect on glucose tolerance or insulin signaling in skeletal muscle was observed. In contrast, markers of striatal insulin resistance were evident in the rats. These data suggest that while 6-OHDA may affect serum insulin levels and striatal insulin signaling, the unilateral 6-OHDA lesion model does not induce glucose intolerance or peripheral insulin resistance, at least at the six-week post-lesion timepoint.
Keywords: Parkinson’s disease, insulin, glucose, diabetes, 6-hydroxydopamine
Introduction
Parkinson’s disease (PD) has been associated with an increased incidence of glucose intolerance or diabetes (Barbeau et al., 1961, Boyd et al., 1971, Lipman et al., 1974). While a direct link between these conditions remains controversial (Simon et al., 2007), hyperglycemia can decrease the efficacy of dopamine replacement therapy (Sandyk, 1993) and diabetes has been shown to increase motor symptom severity (Papapetropoulos et al., 2004) and the cost of medical care (Pressley et al., 2003) for individuals with PD. The relationship between PD and insulin resistance clearly warrants further study in preclinical models.
We recently reported that unilateral nigrostriatal dopamine depletion impairs striatal insulin signaling in young adult rats (Morris et al., 2008). Peripheral glucose and insulin levels, however, were not affected. Aging is a risk factor for both PD and insulin resistance (Supiano et al., 1993, Vanitallie, 2008), so it is possible that our use of young animals accounted for the lack of peripheral metabolic deficits in our study. Therefore, the goal of the current study was to extend our previous findings to middle-aged rats. We sought to determine whether unilateral nigrostriatal DA depletion would, in addition to impairing striatal insulin signaling, affect peripheral glucose tolerance in this more age-appropriate model.
Material and Methods
Sixteen male 16-month-old Fisher 344 rats were obtained from National Institutes on Aging colonies (Harlan). Protocols for animal use were approved by the University of Kansas Medical Center IACUC and adhered to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Rats were divided into two groups of similar average body weight. The 6-OHDA lesion procedure was based on previously published studies (Morris et al., 2008). Rats were anesthetized with Nembutal (50mg/kg) and placed into a stereotaxic frame. Animals receiving a 6-OHDA lesion (n=8) were infused with 12µL of 6-OHDA in 0.9% NaCl with 0.02% ascorbate into the right medial forebrain bundle (stereotaxic coordinates with respect to bregma: M/L 1.3, A/P −4.4, and D/V −7.8) at a dose of 2.25mg/mL. The infusion rate was 0.75µL/min over a period of 16 min and the cannula was withdrawn 1 min following infusion. Sham (saline) infused rats (n=8) received the same surgical procedure but were infused with saline (0.9% NaCl with 0.02% ascorbate) instead of 6-OHDA.
Food intake and body weight were measured weekly during the 6-week post-lesion period. Two days prior to tissue harvest, blood glucose and serum insulin levels were measured during an intraperitoneal glucose tolerance test (IPGTT) as previously described (Morris et al., 2008). At 6 weeks post-lesion, animals were deeply anesthetized for tissue harvest. Muscles were then dissected and incubated with insulin in vitro to determine insulin sensitivity (Gupte et al., 2008). Specifically, soleus (slow-twitch) and extensor digitorum longus (EDL; fast-twitch) muscles were carefully dissected from anesthetized animals. Muscles from each side were split longitudinally and incubated in a recovery solution for 90 min at 39°C. One half of each muscle was removed into a solution containing 1 mU/mL insulin for 10 min at 39°C (insulin-stimulated) while the other half (basal) remained in the recovery solution. Muscles were then clamp-frozen and stored at −80°C for Western blot analysis. At tissue harvest, adiposity was measured by weighing epidydymal fat from animals in each group. Gastrocnemius muscles were dissected and weighed to determine whether muscle atrophy had occurred.
Following the removal of muscles, brains were removed and placed on an ice cold brain block. Brain sections containing striatum, SN and hypothalamus were dissected for HPLC-EC and for Western blotting. For HPLC-EC analysis, striatal tissue was processed and analyzed as previously described (Morris et al., 2008). DA and norepinephrine (NE) levels were also analyzed in the SN and the hypothalamus. These samples were prepared identically to striatal samples, except the volume of burnt citrate acetate mobile phase was reduced to 250µL and DHBA concentration was reduced to 10−7M due to the smaller tissue size. Striatal sections for Western blotting were dissected freehand and frozen on dry ice. Brain and muscle samples were prepared and analyzed for western blot as previously described (Gupte et al., 2008, Morris et al., 2010).
Data for food intake, body weight and glucose and insulin levels during a glucose tolerance test were analyzed using two-way analyses of variance (ANOVA) time as the repeated measure. DA depletion was analyzed using one-way ANOVA. Western blot data was analyzed using student’s t-test. No protein expression or activation differences between lesioned and non-lesioned hemispheres were observed for any protein measures, so data from the left and right striatal hemisphere were pooled for each animal. When necessary, post-hoc analyses were performed using student’s t-test. Data were considered statistically significant at p<0.05.
Results and Discussion
While both groups lost weight after surgery, the 6-OHDA group lost more weight overall (F=7.18, p<0.05; Table 1). The 6-OHDA group also experienced a greater decrease in food intake immediately following surgery (weeks 1–4, F= 19.76, p<0.01; Table 1). However, food intake normalized by the end of the experiment (weeks 5–6). The 6-OHDA group also had less fat mass at tissue harvest (F=7.09, p<0.05; Table 1). Gastrocnemius muscle weight did not differ between groups, indicating that weight differences were due primarily to loss of fat mass and not muscle atrophy following the lesion.
Table 1.
Peripheral measures
| Control | 6-OHDA | |
|---|---|---|
| Pre-lesion BW (g) | 466.1 ± 6.6 | 466.4 ± 13.3 |
| Final BW (g) | 457.7 ± 6.75 | 407.7 ± 19.4* |
| Pre-lesion food intake (Kcal/day) | 75.1 ± 2.2 | 89.5 ± 2.3* |
| Food intake (wk 1–4) (Kcal/day) | 69.2 ± 2.0 | 47.5 ± 5.1* |
| Food intake (wk 5–6) (Kcal/day) | 78.1 ± 5.2 | 77.3 ± 5.4 |
| Contralateral Gastroc wt (g) | 1.86 ± 0.05 | 1.77 ± 0.06 |
| Ipsilateral Gastroc wt (g) | 1.88 ± 0.05 | 1.76 ± 0.07 |
| Fat Mass (g) | 14.86 ± 0.68 | 10.96 ± 1.3* |
p<0.05 6-OHDA vs. Sham
As expected, administration of 6-OHDA into the MFB substantially reduced DA content in the infused striatum, where it was reduced by ~95% (Table 2). This led to significant effects for group (F=74.21, p<0.001), side (F=64.57, p<0.001) and a group by side interaction (F=13.103, p<0.01). Surprisingly, saline infusion also decreased striatal DA concentration (~25%) in the infused hemisphere of the sham-treated rats. This may have resulted from neuronal damage from a higher infusion rate and greater infusion volume than used in our previous study (Morris et al., 2008).
Table 2.
Neurotransmitter concentration in various regions (ng/g tissue weight)
| Sham | 6-OHDA | ||||
|---|---|---|---|---|---|
| Tissue | Analyte | Contralateral | Ipsilateral | Contralateral | Ipsilateral |
| Striatum | DA | 7376.5 ± 664 | 5490.71 ± 494# | 5243.5 ± 302 | 266.21 ± 111*#† |
| NE | 116.4 ± 17 | 88.0 ± 17 | 104.3 ± 26 | 123.9 ± 54 | |
| Substantia Nigra | DA | 1064.2 ± 166 | 1185.0 ± 146 | 801.6 ±99.0 | 441.7 ± 95.9*# |
| NE | 547.3 ± 67 | 692.5 ± 76 | 534.7 ± 109 | 609.2 ± 198 | |
| Hypothalamus | DA | 916.4 ± 323 | 1110.7 ± 392 | 751.2 ± 265 | 615.1 ± 232 |
| NE | 26503.1 ± 9370 | 28525.6 ± 10085 | 13888.9 ± 4910 | 6876.2 ± 2431*# | |
p< 0.05 6-OHDA vs. Sham
p<0.05 Ipsilateral vs. Contralateral
p<0.05 Group × Side interaction
As expected, the 6-OHDA group also had significant (~50%) DA depletion in the SN. (F=14.78, p<0.001; Table 2). Saline-infused animals did not exhibit DA depletion in this region. Hypothalamic DA was not affected by 6-OHDA. Because 6-OHDA can also damage noradrenergic neurons, we also quantified NE content in all three nuclei. While 6-OHDA did not affect NE levels in the striatum or in the SN, there was a robust depletion of hypothalamic NE in the 6-OHDA group (F=28.9, p<0.001; Table 2). Although this reduction was bilateral, as expected, it was more marked in the hemisphere ipsilateral to the lesioned side.
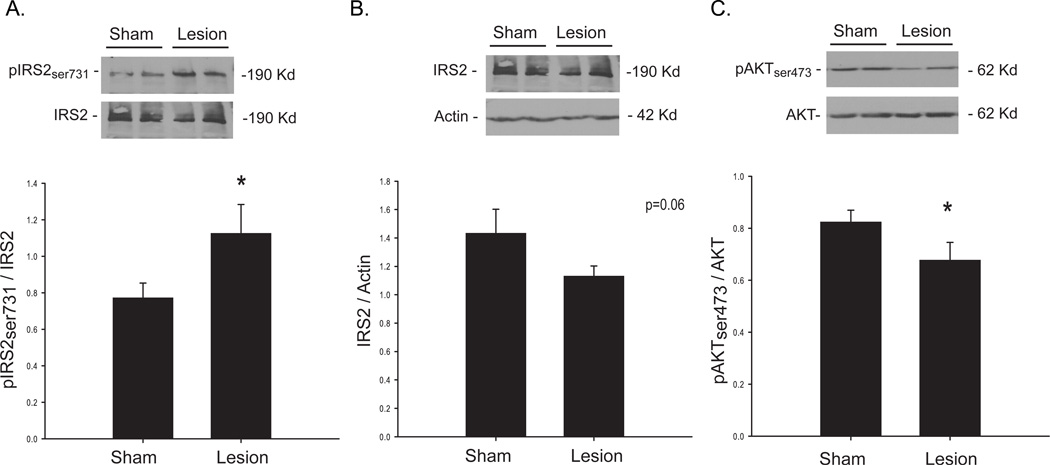
Consistent with our study in younger animals (Morris et al., 2008), insulin signaling was impaired in the striatum following 6-OHDA-induced DA depletion. Serine phosphorylation of insulin receptor substrate 2 (IRS2), which is inhibitory to insulin signaling (White, 2002), was increased in the lesion group (p<0.05; Figure 1A). Long term serine phosphorylation can result in IRS degredation (Pederson et al., 2001, Kim et al., 2005), and we observed a strong trend (p=0.06) for decreased protein content in lesioned animals (Figure 1B). The protein AKT is activated by phosphorylation, which occurs in response to normal insulin signaling to promote glucose uptake (Gonzalez and McGraw, 2006). As expected, we observed significantly decreased pAKT in the striatum, in line with increased serine phosphorylation of IRS2 (Figure 1C).
Figure 1. Striatal Insulin signaling in 6-OHDA treated middle aged rats.
Treatment with 6-OHDA significantly increased serine phosphorylation of IRS2 (A), indicating insulin resistance. A trend for decreased IRS2, which has been shown to occur with chronic serine phosphorylation, was observed in the severely depleted group (B). In line with these findings, serine phosphorylation of the downstream protein AKT, which is a positive indicator of insulin signaling, was significantly decreased in the lesion group (C). Values are means ± SE for 8 samples per group. *p<0.05 sham vs. lesion.
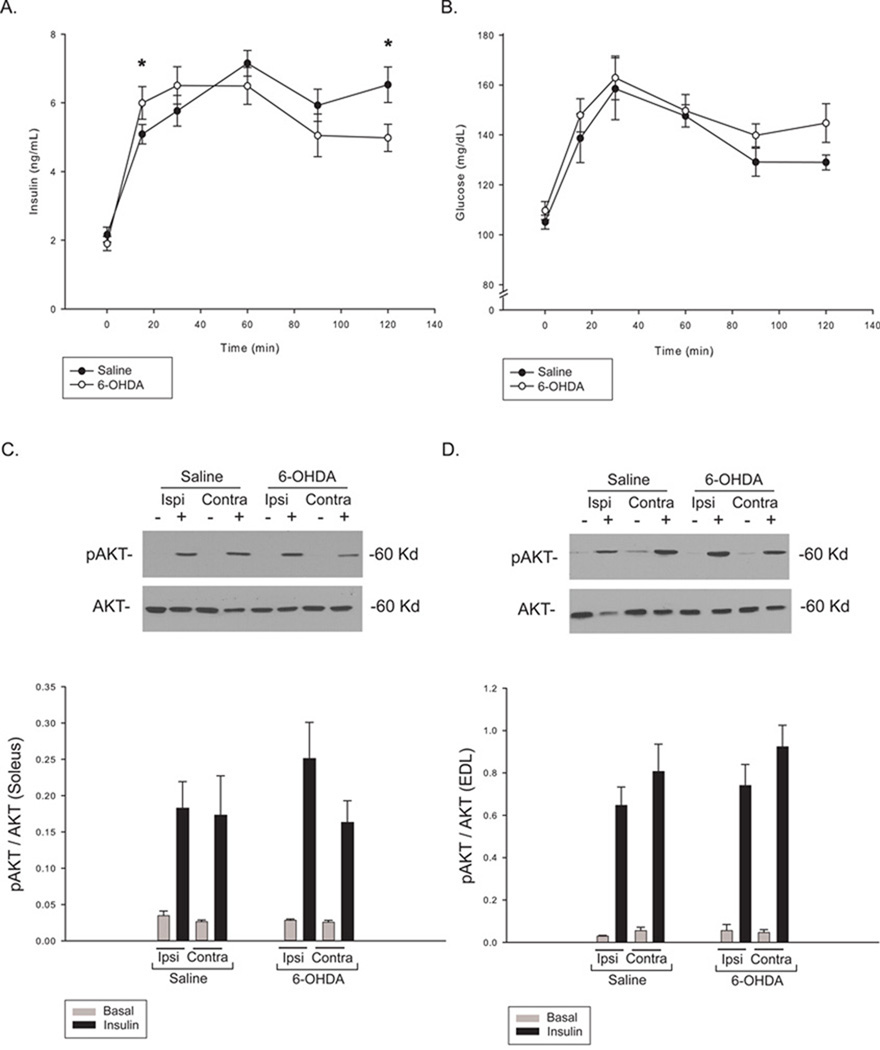
Although we observed effects on brain insulin signaling, peripheral glucose tolerance was not affected in the current study. However, the insulin response to a glucose bolus did differ between groups over the course of an IPGTT (F=3.93, p<0.01; Figure 2A). Post-hoc analysis showed that 6-OHDA lesioned animals exhibited a greater initial increase in insulin (15 min), but were not able to maintain this increased level, leaving insulin levels significantly lower at the final timepoint (120 min). Over the course of the test, blood glucose levels did not differ between groups (Figure 2B), but lesioned animals did exhibit higher glucose values at the final timepoint. Although this does not indicate an outright impairment in glucose tolerance, these subtle changes in insulin action may precede a larger-scale effect. To determine whether glucose uptake was affected, insulin signaling in skeletal muscle was analyzed. Insulin signaling, as measured by AKT phosphorylation, was not affected in either slow (Soleus) or fast (EDL) muscle (Figure 2C & 2D) at this post-lesion timepoint.
Figure 2. Peripheral glucose tolerance and insulin signaling following nigrostriatal 6-OHDA treatment.
Animals were fasted overnight for 12 hours prior to a glucose tolerance test. A glucose bolus (60% glucose, 2g glucose/kg body weight) was injected intraperitoneally at t=0. Insulin and glucose were measured in tail blood at six time points following the glucose bolus (injection at t=0). Serum insulin levels differed significantly between groups over the course of the test (A). Although there was no significant difference between glucose levels over the course of the test (B), glucose levels were higher at the final timepoint, after serum insulin levels had fallen. To assess insulin signaling, muscles were incubated with or without insulin in vitro and AKT phosphorylation analyzed by Western blot. Basal (gray bars) and insulin-stimulated (black bars) AKT phosphorylation was measured in both soleus (C) and EDL (D) muscles. No difference in insulin-stimulated pAKT was observed, indicating the absence of skeletal muscle insulin resistance. Values are means ± SE for 8 muscles per group. *p<0.05 sham vs. lesion.
It is possible that the increase in serum insulin levels early in the IPGTT may actually be compensatory and help maintain normal glycemia, preceding significant alterations in blood glucose. In support of this possibility, higher blood glucose levels were observed at 120 minutes in the 6-OHDA treated group, when insulin levels had decreased significantly. An alternative possibility is that insulin release was affected by decreased input of the sympathetic nervous system. The primary neurotransmitter used in the sympathetic nervous system is NE (Hall, 1990), and we did observe hypothalamic NE depletion. Sympathetic nerves inhibit insulin secretion (Ahren, 2000), and a decrease in the activity of these sympathetic nerves lessens their inhibitory effect (Teff, 2007). However, control of insulin release by the autonomic nervous system is complex and involves both sympathetic and parasympathetic inputs. We did not directly measure autonomic nervous system function, and thus cannot further speculate on the role of these neurons in the altered insulin response.
Additional factors that likely play a role in glucose tolerance are eating behavior and body weight. In our experiment, animals lost weight following 6-OHDA. Weight loss often occurs in PD patients. Although weight gain, not loss, is associated with impaired glucose tolerance, it has been suggested that PD patients ingest more fat than healthy individuals (Lorefat et al., 2006). Interestingly, in this study, PD patients who lost weight actually exhibited higher fat and energy intake than those who did not lose weight (Lorefat et al., 2006). However, eating behavior is difficult to measure in humans, as difficulties with recall and validation have led to equivocal results (reviewed in (Ishihara and Brayne, 2005)).
Conclusions
This study confirms previous work describing striatal insulin resistance in young rats following 6-OHDA and expands these findings to a middle-aged model and analysis of peripheral insulin signaling in skeletal muscle. Although serum insulin levels differed over the course of a glucose tolerance test, no effect on glucose tolerance or insulin signaling in skeletal muscles was observed. Although differences are not evident in middle-aged rats used in this study, it is possible that aged animals may exhibit more pronounced metabolic deficits. We cannot conclude from these findings that aging increases the risk of developing peripheral glucose tolerance following unilateral 6-OHDA treatment. However, the observed alterations in serum insulin levels are novel and interesting, warranting further study.
Research Highlights.
-
-
Severe unilateral dopamine depletion impairs insulin signaling in the striatum
-
-
Insulin signaling is not affected in muscle
-
-
Glucose tolerance is not affected in muscle
-
-
Serum insulin differs slightly between groups following dopamine depletion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Barbeau A, Giguere R, Hardy J. Clinical experience with tolbutamide in Parkinson's disease. L'union medicale du Canada. 1961;90:147–151. [PubMed] [Google Scholar]
- Boyd AE, 3rd, Lebovitz HE, Feldman JM. Endocrine function and glucose metabolism in patients with Parkinson's disease and their alternation by L-Dopa. The Journal of clinical endocrinology and metabolism. 1971;33:829–837. doi: 10.1210/jcem-33-5-829. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Molecular biology of the cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Geiger PC. Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol. 2008;105:839–848. doi: 10.1152/japplphysiol.00148.2008. [DOI] [PubMed] [Google Scholar]
- Hall WD. An Overview of the Autonomic Nervous System. 1990 [PubMed] [Google Scholar]
- Ishihara L, Brayne C. A systematic review of nutritional risk factors of Parkinson's disease. Nutr Res Rev. 2005;18:259–282. doi: 10.1079/NRR2005108. [DOI] [PubMed] [Google Scholar]
- Kim B, van Golen CM, Feldman EL. Insulin-like growth factor I induces preferential degradation of insulin receptor substrate-2 through the phosphatidylinositol 3-kinase pathway in human neuroblastoma cells. Endocrinology. 2005;146:5350–5357. doi: 10.1210/en.2005-0356. [DOI] [PubMed] [Google Scholar]
- Lipman IJ, Boykin ME, Flora RE. Glucose intolerance in Parkinson's disease. Journal of chronic diseases. 1974;27:573–579. doi: 10.1016/0021-9681(74)90031-9. [DOI] [PubMed] [Google Scholar]
- Lorefat B, Ganowiak W, Wissing U, Granerus AK, Unosson M. Food habits and intake of nutrients in elderly patients with Parkinson's disease. Gerontology. 2006;52:160–168. doi: 10.1159/000091825. [DOI] [PubMed] [Google Scholar]
- Morris JK, Bomhoff GL, Stanford JA, Geiger PC. Neurodegeneration in an animal model of Parkinson's disease is exacerbated by a high fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1082–R1090. doi: 10.1152/ajpregu.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Zhang H, Gupte AA, Bomhoff GL, Stanford JA, Geiger PC. Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson's disease. Brain Res. 2008;1240:185–195. doi: 10.1016/j.brainres.2008.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos S, Ellul J, Argyriou AA, Talelli P, Chroni E, Papapetropoulos T. The effect of vascular disease on late onset Parkinson's disease. Eur J Neurol. 2004;11:231–235. doi: 10.1046/j.1468-1331.2003.00748.x. [DOI] [PubMed] [Google Scholar]
- Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- Pressley JC, Louis ED, Tang MX, Cote L, Cohen PD, Glied S, Mayeux R. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology. 2003;60:87–93. doi: 10.1212/wnl.60.1.87. [DOI] [PubMed] [Google Scholar]
- Sandyk R. The relationship between diabetes mellitus and Parkinson's disease. The International journal of neuroscience. 1993;69:125–130. doi: 10.3109/00207459309003322. [DOI] [PubMed] [Google Scholar]
- Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology. 2007;69:1688–1695. doi: 10.1212/01.wnl.0000271883.45010.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supiano MA, Hogikyan RV, Morrow LA, Ortiz-Alonso FJ, Herman WH, Galecki AT, Halter JB. Aging and insulin sensitivity: role of blood pressure and sympathetic nervous system activity. J Gerontol. 1993;48:M237–M243. doi: 10.1093/geronj/48.6.m237. [DOI] [PubMed] [Google Scholar]
- Teff KL. Visceral Nerves: Vagal and Sympathetic Innervation. JPEN J Parenter Enteral Nutr. 2007;32:569–571. doi: 10.1177/0148607108321705. [DOI] [PubMed] [Google Scholar]
- Vanitallie TB. Parkinson disease: primacy of age as a risk factor for mitochondrial dysfunction. Metabolism. 2008;57 Suppl 2:S50–S55. doi: 10.1016/j.metabol.2008.07.015. [DOI] [PubMed] [Google Scholar]
- White MF. IRS proteins and the common path to diabetes. American journal of physiology. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]