Abstract
Background & Aims
Hepatocellular carcinoma (HCC) can result from hepatitis C (HCV)-related liver disease and is the fastest-growing cause of cancer-related death in the United States. Alpha-fetoprotein (AFP) has been used as a prognostic factor for HCC, but the value of AFP as a prognostic factor for HCV-related HCC in the United States is unknown. We investigated whether higher levels of AFP at the time of diagnosis are associated with increased mortality of patients with HCV-related HCC.
Methods
In a retrospective study, we collected data from a cohort of HCV-infected veterans, identifying incident HCC cases from October 1, 1998 to January 1, 2007 (n=1480 patients). Mean serum levels of AFP, obtained within 60 days before to 30 days after HCC diagnosis, were determined for 1064 patients and categorized as <10 ng/ml (18%), 10–<100 ng/ml (30%), 100–<1000 ng/ml (22%), or ≥1000 ng/ml (29%). Cox proportional hazard models were used to associate serum levels of AFP with mortality, adjusting for demographic features, clinical factors, and treatment.
Results
The median survival times were significantly lower among patients with higher levels of AFP: 709 d for patients with <10 ng/ml, 422 d for 10–<100 ng/ml, 208 d for 100–<1000 ng/ml, and 68 d for ≥1000 ng/ml. In the multivariate analysis, increased levels of AFP (10–<100, 100–<1000, ≥1000) were significantly associated with increased mortality, compared to a serum level of AFP <10; hazard ratios were 1.50, 2.23, and 4.35, respectively.
Conclusions
Serum level of AFP at the time of diagnosis with HCV-related HCC is an independent predictor of mortality.
Keywords: liver disease, risk, prognosis, epidemiology, blood test
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy 1. The incidence of HCC has been increasing recently in the United States, becoming the fastest growing cause of cancer-related death 1-4. The 5-year survival rates are poor ranging between 0 and 10% among patients detected at a symptomatic stage 2. The increased incidence of HCC can be mostly attributed to increases in hepatitis C virus (HCV)-related liver disease 3, 4. There are approximately 2.7 million people in the United States with chronic HCV 5. Individuals with HCV-related cirrhosis have a 2-8% annual incidence of HCC 2, 3. Therefore, a better understanding of the prognostic factors in HCV-related HCC is needed in the United States.
Prognosis of patients with HCC is related to the degree of liver dysfunction, tumor size and overall functional status 2, 6-9. Studies have also examined serum alpha-fetoprotein (AFP) as a modality for HCC surveillance as well as a prognostic factor for HCC 8, 9. These studies have shown that higher AFP levels at the time of HCC diagnosis are associated with worse survival 8-11 even after adjusting for tumor size and stage 3, 6, 7, 10-13. Therefore, AFP has been incorporated into at least three of the major staging and prognostic scoring systems of HCC: The Cancer of the Liver Italian Program (CLIP), the Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire (GRETCH), and the Chinese University Prognostic Index (CUPI) 6, 7, 14, 15. There are several other prognostic systems that have not incorporated AFP 16.
The value of AFP as a prognostic factor for HCC among patients with HCV-related HCC in the United States is unknown. The studies that examined AFP levels as a part of CLIP, GRETCH or CUPI were not conducted in the United States and evaluated patients with liver disease of various etiologies 6, 14, 15. The CLIP validation study contained the largest proportion of patients with HCV-related liver disease (85.5%), while these proportions were considerably smaller in the GRETCH (18%) and CUPI (3.3%) studies 6, 14, 15. These findings may not be generalizable to HCV-related HCC. For example, it has been reported that AFP levels are higher in hepatitis B virus (HBV)-positive patients than HBV-negative patients irrespective of HCC 9, 10. In addition, several established clinical and pathogenic differences exist between HBV- and HCV-related HCC 3, 13. Given these differences and the fact that AFP is an inexpensive, widely available and easily interpretable test it is important to examine the prognostic properties of AFP in a HCV-specific HCC population in the United States.
The Veterans Administration (VA) has the largest integrated healthcare system in the United States. The VA provides care to a large number of patients with HCV and collects a substantial amount of clinical data on these individuals. Using information obtained from the VA HCV Clinical Case Registry we conducted a retrospective cohort study of HCV-infected patients who developed HCC to determine whether AFP levels at the time of HCC diagnosis are predictive of mortality.
METHODS
Data Source
Data were obtained from the national VA HCV Clinical Case Registry. Patients were identified based on positive HCV antibody test or International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) code for HCV (V02.62, V070.41, V070.51, V070.44, and V070.54) at any of the 128 VA healthcare facilities nationwide. Data elements include demographics, laboratory tests, along with inpatient and outpatient diagnosis and procedure (CPT) codes. Details on the Clinical Case Registry data are published elsewhere 17. The date of death was obtained from the VA vital status file. Follow-up information was available through 9/30/2009.
Study Population
The details of this study cohort have been previously described 18. All HCV-infected patients 18 years of age or older with incident HCC were identified between October 1, 1998 and January 1, 2007. Patients with HCV were defined by one positive HCV antibody test combined with at least one HCV ICD-9-CM code. The date of first occurrence of a positive HCV antibody test or HCV ICD-9-CM code served as the HCV index date. HCC was defined using a previously validated algorithm19. To ensure that patients were using the VA for their healthcare, inclusion required at least one inpatient or outpatient encounter at any VA facility within the two-years prior to and one-year following HCC diagnosis date. Patients who developed HCC or died within 12 months following HCV or cirrhosis index date were excluded.
Study Variables and Definitions
The main exposure variable of interest was serum AFP level at HCC diagnosis. The main outcome variable was time to death after HCC diagnosis. Serum AFP level at time of HCC diagnosis was defined as the serum AFP level within 60 days before to 30 days after HCC diagnosis. Those without AFP levels during this time period were recorded as not having serum AFP tested. If patients had more than one AFP level during this time period the mean value was used, resulting in each patient having only one AFP level analyzed.
Cirrhosis was identified by one of several previously validated ICD-9-CM codes (571.2, 571.5, or 571.6) and a cirrhosis index date was based on the first appearance of a cirrhosis code 20. Liver disease severity was assessed with the Model for End-stage Liver Disease (MELD) score, which was calculated using laboratory values for serum creatinine, bilirubin, and INR within 6 months prior to or following the HCV index date 21. The Child-Pugh class would have been more informative for this cohort, but based on the lack of detailed information on encephalopathy and ascites with its treatment in the Clinical Case Registry we were unable to determine an accurate class. Therefore, MELD was the next best choice for determining liver disease severity. Laboratory data were used to determine hepatitis B virus surface antigen status. Alcohol, cocaine, and cannabis use were identified by positive lab tests or ICD-9-CM codes. We also assessed the presence of ICD-9-CM codes for ascites, varices, encephalopathy, medical co-morbidities (diabetes, coronary artery disease, chronic obstructive pulmonary disease, respiratory failure, congestive heart failure [CHF], hypertension, HIV and end stage renal disease), and mental health disorders (anxiety, post-traumatic stress disorder, depression, bipolar disorder, psychosis), as well as to calculate the Deyo co-morbidity index22. HCC treatment was ascertained by ICD-9 procedure and CPT codes for liver transplant, surgical resection, local ablation with alcohol or radiofrequency (RFA), as well as transarterial chemoembolization (TACE) within 90 days before and 24 months after the HCC diagnosis date.
The study protocol was approved by the Baylor College of Medicine Institutional Review Board and the Office of Human Subjects Research of the National Institutes of Health.
Data Analysis
The primary outcome measure was time to death after HCC diagnosis. Follow-up started at HCC diagnosis and ended September 30, 2009. Any patients alive at the end of follow-up were censored. In preliminary analyses, AFP levels were examined as a continuous variable and logarithmic transformation was used for normalization. The main analysis examined the serum AFP level for each patient within the specified 60 days before to 30 days after HCC diagnosis or the mean AFP value in the presence of multiple tests. Based on the results from preliminary analyses and clinical knowledge, the AFP levels (ng/ml) at the time of HCC diagnosis were converted to a categorical variable with four groups: <10, 10-<100, 100-<1000, and ≥1000. For each group, survival rates were estimated using a Kaplan-Meier approach. In a multivariate Cox proportional hazards regression model, the demographic and clinical variables listed above were tested as potential determinants of survival. Variables with p<0.10 in univariable Cox regression model were further evaluated using a stepwise regression analysis to identify independent prognostic factors. Only variables with a p-value less than 0.10 were retained in the final model. Hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated for each predictor variable with the final level of significance set at 0.05. The graphical and numerical methods of Lin, Wei, and Ying (1993) were performed to check the functional form of AFP and the proportional hazards assumption for the Cox model 23.
We also conducted sensitivity analyses in which we examined only the first AFP level among patients with more than AFP level within 60 days before to 30 days after HCC diagnosis date. We also analyzed the change in serum AFP level in patients who had more than one AFP test during the period that spanned 2 years before to 30 days after HCC diagnosis. The change in AFP was based on a slope estimated from a linear regression and categorized as no increase, <2-fold increase, 2-<10-fold increase, ≥10-fold increase. The relationship between serum AFP level and HCC treatments was determined by comparing the median AFP for each treatment group to the median AFP level in patients who did not receive HCC treatment. The Wilcoxon rank sum test was used to determine statistical significance between groups. All statistical analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
The study cohort was comprised of 1,480 patients who were diagnosed with HCC from 10/1/1998 - 1/1/2007 and fulfilled the inclusion and exclusion criteria. The characteristics of this cohort (Table 1) were previously published 18. The mean age of patients was 58.1 years (SD ± 8.6) at the HCC diagnosis date. Most patients were men (99.3%) and Caucasians (55.6%). The diagnosis of cirrhosis was recorded in 599 (40.5%) patients. The mean duration of time between the HCV index date and the HCC diagnosis date was 3.86 years (SD ± 1.96), and time between the cirrhosis index date (if present) and the HCC diagnosis date was 3.54 years (SD ± 2.44). Following HCC diagnosis, most patients (87.3%) died during a total follow-up of 2,711.3 patient-years (1.8-year mean follow-up duration). The overall median survival after HCC diagnosis was 262 days and the 1-year, 3-year, and 5-year survival rates were 43.4%, 19.4%, and 12.2%, respectively.
Table 1.
Characteristics of the study cohort of 1,480 patients with HCV-related HCC
| HCC diagnosis year | Number (%) |
| 1998 | 2(0.1) |
| 1999 | 14 (1.0) |
| 2000 | 56(3.8) |
| 2001 | 98(6.6) |
| 2002 | 145(9.8) |
| 2003 | 208(14.1) |
| 2004 | 280(18.9) |
| 2005 | 317(21.4) |
| 2006 | 360(24.3) |
| Age | |
| <50 | 194(13.1) |
| 50-54 | 434(29.3) |
| 55-60 | 436(29.5) |
| ≥60 | 416(28.1) |
| Gender | |
| Female | 10(0.7) |
| Male | 1470(99.3) |
| Race | |
| White | 823(55.6) |
| Black | 401(27.1) |
| Other | 172(11.6) |
| Missing | 84(5.7) |
| MELD score | |
| <15 | 890(60.1) |
| 15-20 | 162(11.0) |
| >20 | 37(2.5) |
| Missing | 391(26.4) |
| Treatment | |
| Liver Transplant | 48(3.2) |
| Resection | 65(4.4) |
| Ablation | 113(7.6) |
| TACE | 310(21) |
In 1,064 (72%) patients, at least one AFP level was tested within the 60 days before and 30 days after HCC diagnosis. Within the specified time period 86 patients had 3 or more AFP levels tested, 300 patients had only 2 tests, and 678 patients had only 1 test. Of the 386 patients with more than one AFP level, 315 (82%) had their subsequent AFP levels fall within the same AFP category as their initial AFP level. The distribution of AFP levels (ng/ml) by category (<10, 10-<100, 100-<1000, ≥1000, not tested) is shown in Figure 1. Median AFP levels were significantly lower in patients receiving liver transplantation with a median of 28.5 (interquartile range: 10.0-135.1), resection 44.1 (18.0-355.9), TACE 47.2 (12.6-451.1), or RFA 41.0 (9.9-120.1). In general, patients who received any of these treatments had lower AFP levels compared with non-treated patients, 50.3 ng/ml versus 182.9 ng/ml, respectively (p-value <0.0001).
Figure 1.
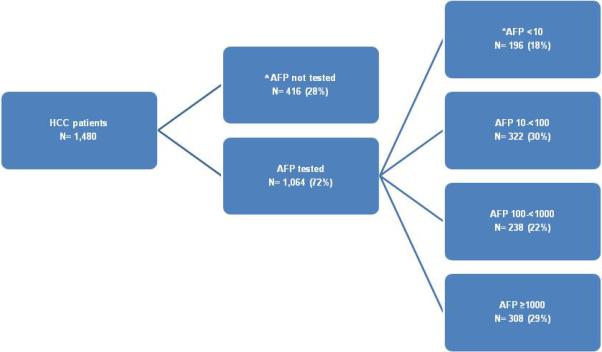
HCV cohort by serum AFP level at the time of HCC diagnosis
^No serum AFP level tested between 60 days before to 30 days after HCC diagnosis
*AFP in ng/ml
The differences in survival overtime based on AFP level are displayed in Kaplan Meier curves (Figure 2). The median survival was significantly lower in patients with higher AFP levels (Table 2). The median survival (25th-75th percentiles) for patients with AFP <10 was 709 days (237-1,721 days), for AFP 10-<100 was 422 days (143-1,111 days), for AFP 100-<1000 was 208 days (61-592 days), and for AFP ≥1000 was 68 days (28-182 days). The median survival in patients without an AFP test was near the middle of the cohort at 343 days (90-982 days). Similarly, the 1-, 3- and 5-year survival rates were progressively lower with higher AFP levels (Table 2). The 5-year survival rate for HCC was low in general (12.2%), but was very low when comparing those with serum AFP levels <10 to those with serum AFP levels ≥1000 (24% vs. 1%).
Figure 2.
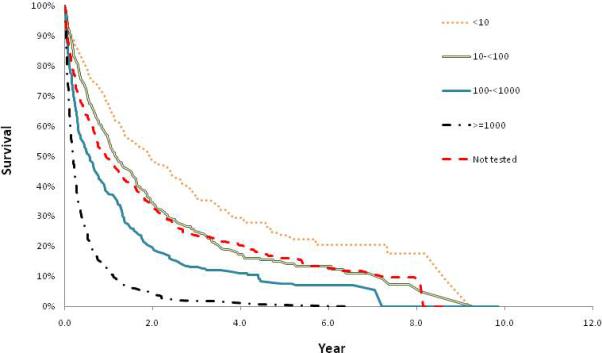
Kaplan Meier curves of survival based on AFP category in 1,480 patients with HCV-related HCC; AFP levels are in ng/ml
Table 2.
Median survival following HCC diagnosis among 1,480 patients with HCV-related HCC based on serum AFP level at time of diagnosis
| AFP Levels (ng/ml) | Number of Patients (%) | Median survival (days) | 1-Year survival rate | 3-Year survival rate | 5-Year survival rate | p-value |
|---|---|---|---|---|---|---|
| <10 | 196(13) | 709 | 67% | 37% | 24% | <0.0001 |
| 10-<100 | 322(22) | 422 | 56% | 25% | 15% | |
| 100-<1000 | 238(16) | 208 | 37% | 13% | 8% | |
| ≥1000 | 308(21) | 68 | 12% | 2% | 1% | |
| Not tested | 416(28) | 343 | 49% | 24% | 16% |
In the unadjusted Cox proportional hazard models, each level of AFP (10-<100, 100-<1000, ≥1000) compared to serum AFP levels <10 was significantly (p-value <0.01) associated with increased mortality with HRs of 1.39, 2.06, and 4.50, respectively. The association between AFP levels and mortality persisted in multivariate Cox proportional hazard analysis. The risk of death progressively increased by AFP level (10-<100, 100-<1000, ≥1000) independent of age, sex, race/ethnicity, year of HCC diagnosis, ascites, encephalopathy, CHF, MELD, transplantation, resection, TACE, or RFA (Table 3). The HR and 95% CI for patients with no AFP tested was 1.53 (1.26-1.86). Age ≥60, MELD ≥15, ascites and encephalopathy were also significant independent risk factors for death. All treatment variables (transplantation, resection, TACE, RFA) were associated with a significantly decreased risk of death. No significant association could be identified between sex, race/ethnicity or year of HCC diagnosis and risk of death. Of note, cirrhosis did not meet criteria for inclusion into the final Cox proportional hazard model.
Table 3.
Multivariate Cox proportional hazard analysis of prognostic factors for mortality in 1,480 patients with HCV-related HCC, including serum AFP level at time of HCC diagnosis
| Adjusted Hazard Ratio (95% CI) | P-value | |
|---|---|---|
| AFP (ng/ml) at HCC diagnosis | ||
| <10 | Reference | |
| 10-<100 | 1.50 (1.22-1.83) | <0.0001 |
| 100-<1000 | 2.23 (1.80-2.76) | <0.0001 |
| ≥1000 | 4.35 (3.54-5.36) | <0.0001 |
| Not tested | 1.53 (1.26-1.86) | <0.0001 |
| Age | ||
| <50 | Reference | |
| 50-54 | 1.08 (0.90-1.29) | 0.45 |
| 55-59 | 1.03 (0.85-1.25) | 0.75 |
| ≥60 | 1.22 (1.01-1.47) | 0.04 |
| Sex | ||
| Female | Reference | |
| Male | 1.25 (0.65-2.42) | 0.51 |
| Race/Ethnicity | ||
| White | Reference | |
| Black | 1.05 (0.92-1.20) | 0.49 |
| Other | 0.95 (0.79-1.14) | 0.59 |
| Missing | 1.01 (0.79-1.29) | 0.93 |
| HCC Diagnosis | ||
| 1998-2002 | Reference | |
| 2003-2006 | 1.03 (0.89-1.18) | 0.71 |
| *Ascites | 1.74 (1.52-1.99) | <0.0001 |
| *Encephalopathy | 1.31 (1.11-1.54) | 0.001 |
| *CHF | 1.25 (0.96-1.61) | 0.09 |
| MELD | ||
| <15 | Reference | |
| 15-20 | 1.53 (1.27-1.84) | <0.0001 |
| >20 | 2.80 (1.98-3.94) | <0.0001 |
| Missing | 0.99 (0.86-1.13) | 0.83 |
| HCC treatment | ||
| Transplantation | 0.16 (0.10-0.26) | <0.0001 |
| Resection | 0.56 (0.42-0.74) | <0.0001 |
| TACE | 0.74 (0.64-0.85) | <0.0001 |
| RFA | 0.61 (0.49-0.77) | <0.0001 |
Within 1 year before HCC diagnosis
Sensitivity analyses
Among those with more than one AFP level within 60 days before to 30 days after HCC diagnosis, we examined the first recorded AFP level in the multivariate Cox proportional hazard model (adjusting for covariates) and found significantly increased mortality based on serum AFP level (10-<100, 100-<1000, ≥1000) with HRs (95%CI) of 1.51 (1.24-1.85), 2.29 (1.85-2.83), and 4.22 (3.43-5.20), respectively and p-values <0.0001. We also examined the change in serum AFP level within 2 years before to 30 days after HCC diagnosis (<2-fold increase, 2-<10-fold increase, ≥10-fold increase) compared to no increase and found significantly increased mortality with HRs (95% CI) of 1.38 (1.09-1.75), 1.61 (1.29-2.02), 2.64 (2.10-3.33), respectively and p-values <0.01 (See supplement A for details of serum AFP change in Figure 3 and Table 4).
DISCUSSION
This is the largest study in a United States HCV-infected cohort to report serum AFP levels are predictive of mortality after HCC diagnosis. It was also able to show that incremental changes in AFP levels (10-<100, 100-<1000, ≥1000) at the time of HCC diagnosis are significantly associated with increased mortality independent of several demographic factors (age, sex, and race/ethnicity), clinical factors (ascites, encephalopathy, CHF, and MELD) or treatment (transplantation, resection, TACE, and RFA).
The finding that AFP level is an independent predictor of mortality after HCV-related HCC diagnosis is consistent with other international studies. Between 1998-2002 AFP levels were incorporated into the CLIP, GRETCH, and CUPI scoring systems. The CLIP system has been validated in different countries and was recommended by the American Joint Committee on Cancer as the clinical staging system of choice 24-27. More recently, in 2010 another group from Taiwan proposed a scoring system to predict HCC survival based on total tumor volume. The predictive accuracy of total tumor volume was enhanced with the addition of AFP level >400 resulting in decreased survival 28.
The training sets used to establish AFP as a prognostic factor in the three major scoring systems found similar risk estimates to our study, further supporting our results. In the CLIP study, AFP ≥400 had a median relative risk of 1.79 7. In the GRETCH study, AFP ≥35 had a relative risk of 1.68 with a 95%CI of 1.36-2.07 6. In the CUPI study, AFP ≥500 had a HR of 1.41 (p-value 0.0006) 15. The range of risk estimates from these studies is 1.41-1.79. The elevated mortality estimates based on AFP levels at the time of HCC diagnosis in this study ranged from 1.50-2.23 and varied based on the cutoffs used to define AFP categories (10-<1000).
The main limitation to concluding that AFP levels are independently associated with increased mortality after HCV-related HCC diagnosis in this study is the absence of detailed staging data including size, number or spread of tumor. However, numerous studies, including those used for the CLIP, GRETCH, and CUPI scoring systems have shown AFP to be an independent prognostic factor after adjusting for tumor size and stage 3, 6, 7, 10-13, 15. Although a focus of this study is on HCV-related HCC it should be acknowledged that some patients may also have alcohol use or co-infection with HBV or HIV. However, unlike previous AFP-HCC prognosis studies, 100% of this cohort is infected with HCV. Another potential limitation is that 28% of the cohort did not have an AFP level tested within the time period around HCC diagnosis. However, given that the median survival for this group is close to the middle of the cohort, selection bias related to receipt of AFP testing is less likely. Therefore, they are unlikely to have altered the conclusions of this study. The cohort consists of only veterans who are majority men using the VA for healthcare, which limits the generalizability of the results. The ability to identify sex and date of HCC diagnosis as independent predictors of mortality may have been limited by the small number of females and lower number of patients with HCC diagnosed from 1998-2002. Additionally, the identification of HCC treatments as independent predictors of decreased mortality may be, at least in part, due to the better baseline prognosis in the treated patients as suggested by their significantly lower AFP levels at the time of HCC diagnosis.
There are several strengths of this study. The large sample size (1,480 patients) of well-defined HCV-infected patients with HCC enabled adequate evaluation of significant survival differences among groups based on AFP levels. Several categories of AFP levels (<10, 10-<100, 100-<1000, ≥1000) could be analyzed. The dose-response relationship between increases in AFP levels and increased mortality strengthens the role of AFP as a predictor. The findings of this study are strengthened by the sensitivity analyses. Using the first AFP level at HCC diagnosis lead to the same conclusion, AFP is a significant predictor of mortality. In addition, greater incremental changes in AFP were associated with a corresponding increase in mortality risk.
In conclusion, our study is important because it focused on a United States cohort with HCC that was specifically related to HCV, which has not been previously addressed. We found serum AFP levels at HCC diagnosis are predictive of subsequent mortality. Testing for AFP at HCC diagnosis is inexpensive, widely available, easily interpretable and may guide prognosis. We believe that there could be clinical utility in staging systems that incorporate AFP levels at the time of HCC diagnosis, and such systems would be relevant to a Unites States population with an increasing incidence of HCV-related HCC. Future studies should examine refining HCC staging systems based on serum AFP gradation as there appears to be a dose-response relationship between AFP and mortality.
Supplementary Material
Supplement A
Table 4. Multivariate Cox proportional hazard analysis of prognostic factors for mortality in 1,480 patients with HCV-related HCC, including change in serum AFP level determined for patients with multiple tests during the period 2 years before to 30 days after HCC diagnosis
Supplement A
Figure 3.
The change in serum AFP level during 2 years before to 30 days after HCC diagnosis among 1,480 HCV-infected patients with HCC.
^Less than 2 recorded serum AFP levels
Acknowledgments
Funding: This work is partly funded by NIH grant T32 DK083266-01A1, NIH grant R01-CA-125487, NIH/National Institute of Diabetes and Digestive and Kidney Disease, Center Grant P30 DK56338 and the Houston Veterans Affairs Health Services Research and Development Center of Excellence HFP90-020 and MRP05-305.
Abbreviations
- AFP
alpha-fetoprotein
- CLIP
Cancer of the Liver Italian Program
- CUPI
Chinese University Prognostic Index
- GRETCH
Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- MELD
Model for End-stage Liver Disease
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest exist for Drs. Tyson, Duan, Kramer, Davila, Richardson or El-Serag.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Writing Assistance: This manuscript was written by Dr. Tyson and edited by the co-authors.
Reference List
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999 March 11;340(10):745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: An Update. Hepatology. July;000(000):1–35. doi: 10.1002/hep.24199. 10 A.D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008 March 21;14(11):1652–6. doi: 10.3748/wjg.14.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011 January 18;154(2):85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006 May 16;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 6.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999 July;31(1):133–41. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 7.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998 September;28(3):751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 8.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989 October 15;64(8):1700–7. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004 October 20;112(1):44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 10.Chen TH, Chen CJ, Yen MF, et al. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int J Cancer. 2002 March 10;98(2):257–61. doi: 10.1002/ijc.10122. [DOI] [PubMed] [Google Scholar]
- 11.Sakar B, Ustuner Z, Karagol H, Aksu G, Camlica H, Aykan NF. Prognostic features and survival of inoperable hepatocellular carcinoma in Turkish patients with cirrhosis. Am J Clin Oncol. 2004 October;27(5):489–93. doi: 10.1097/01.coc.0000136019.94333.09. [DOI] [PubMed] [Google Scholar]
- 12.Izumi R, Shimizu K, Kiriyama M, et al. Alpha-fetoprotein production by hepatocellular carcinoma is prognostic of poor patient survival. J Surg Oncol. 1992 March;49(3):151–5. doi: 10.1002/jso.2930490305. [DOI] [PubMed] [Google Scholar]
- 13.Tanizaki H, Ryu M, Kinoshita T, et al. Comparison of clinical features and survival in patients with hepatitis B and C virus-related hepatocellular carcinoma. Jpn J Clin Oncol. 1997 April;27(2):67–70. doi: 10.1093/jjco/27.2.67. [DOI] [PubMed] [Google Scholar]
- 14.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000 April;31(4):840–5. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 15.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002 March 15;94(6):1760–9. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 16.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005 April;41(4):707–16. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 17.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009 November;16(6):775–83. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Serag HB, Kramer Jennifer R, Chen G John, Duan Zhigang, Richardson Peter A, Davila Jessica A. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011 doi: 10.1136/gut.2010.230508. Epublished. [DOI] [PubMed] [Google Scholar]
- 19.Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007 September;41(8):777–82. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 20.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008 February 1;27(3):274–82. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 21.2010 http://www.unos.org/resources/meldpeldcalculator.asp.
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 June;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Lin D, Wei L, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3) 557-572. [Google Scholar]
- 24.Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002 June;50(6):881–5. doi: 10.1136/gut.50.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000 December 1;89(11):2266–73. [PubMed] [Google Scholar]
- 26.Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001 September;34(3):529–34. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 27.Henderson JM, Sherman M, Tavill A, Abecassis M, Chejfec G, Gramlich T. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford) 2003;5(4):243–50. doi: 10.1080/13651820310015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu CY, Huang YH, Hsia CY, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010 July;53(1):108–17. doi: 10.1016/j.jhep.2010.01.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement A
Table 4. Multivariate Cox proportional hazard analysis of prognostic factors for mortality in 1,480 patients with HCV-related HCC, including change in serum AFP level determined for patients with multiple tests during the period 2 years before to 30 days after HCC diagnosis
Supplement A
Figure 3.
The change in serum AFP level during 2 years before to 30 days after HCC diagnosis among 1,480 HCV-infected patients with HCC.
^Less than 2 recorded serum AFP levels


