Abstract
FOXP3 is critical for the development and function of CD4+CD25bright natural regulatory T cells (nTreg). Individuals harboring mutations in FOXP3 develop immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX). We describe a child diagnosed with IPEX who underwent a reduced intensity, T and B cell depleted, matched unrelated donor bone marrow transplant followed by clinical resolution. Using lineage-specific donor chimerism studies, we demonstrate that non-myeloablative HSCT resolves disease in the context of low level donor hematopoietic stem cell (HSC) engraftment. Despite low-levels of donor HSC, thymically-derived nTreg and to a lesser extent CD4+ and CD8+ T cells, exhibit a selective in vivo growth advantage for populations containing a functional FOXP3 gene. Moreover, nTreg from this patient show regulatory function directly ex vivo. These results have implications for improving clinical therapy for patients with IPEX and provide mechanistic insight into the in vivo development of human nTreg and unexpectedly, non-regulatory T cells.
Keywords: IPEX, FOXP3, regulatory T cell, non-myeloablative transplantation, human T cell development, in vivo selection
1. Introduction
Natural regulatory T cells (nTreg) are characterized by a general CD4+CD25brightCD127loFOXP3+ phenotype, with additional molecules potentially serving to delineate functional subgroups [3–5;9;10;13;15;21]. FOXP3 is a winged/helix forkhead transcription factor crucial for nTreg development and function. While FOXP3 has been a useful lineage specification marker in rodents, analogous immune monitoring studies in humans have been complicated by species-specific differences in gene regulation [20]. Two notable differences are that all human T cells transiently express FOXP3 without commensurate acquisition of suppressive function [1;17;19;25]. This trait raises the possibility of an additional role for FOXP3 in general T cell function, as well as for those with regulatory activity. Another distinct feature of human FOXP3 is the expression of two protein isoforms; a full length molecule and shorter protein lacking the amino acids encoded by exon 2 [27]. The unique biology of FOXP3 highlights the need for additional studies which provide insight into the in vivo development and function of human nTreg.
Most patients with IPEX do not live beyond three years of age. Immunosuppressive therapy has been generally ineffective and hematopoietic stem cell transplantation (HSCT) remains the only curative option. Conventional myeloablative conditioning prior to HSCT has resulted in substantial regimen-related mortality, whereas reduced intensity conditioning has been used with some success [8;14;18;26]. Here, we used lineage-specific donor chimerism studies to demonstrate that non-myeloablative HSCT can resolve clinical symptoms of IPEX in the context of low level donor hematopoietic stem cell (HSC) engraftment. We demonstrate a selective in vivo growth advantage of nTreg, as well as CD4+ and CD8+ T cells, from a minority population of donor HSC having a functional FOXP3 gene. These results have implications for improving clinical therapy for patients with IPEX and provide evidence for a previously unappreciated role for FOXP3 in the development of CD4+ and CD8+ T cells.
2. Methods
2.1 Patient
A six week old white male presented with a generalized, pruritic, desquamating rash, feeding intolerance, diarrhea and failure to thrive. He developed multiple infections including Pneumocystis pneumonia and bacteremia with enteric pathogens. Maternal family history revealed multiple male infant deaths. Pertinent laboratory findings included a peripheral blood eosinophilia of 27%, a hemoglobin level of 9.1 g/L, positive direct antiglobulin test (DAT), albumin level of 1.9 gm/dL, IgE initially elevated at 157 IU/mL (normal </ = 17 IU/mL) which later increased to greater than 1000 IU/mL and IgA low at <6.9 mg/dL. IgG and IgM were within normal limits for age at 244 and 31 mg/dL, respectively. He was dependent on red cell transfusion to maintain an adequate hemoglobin level. Four doses of rituximab were given to treat autoimmune hemolytic anemia. He had a normal number of lymphocytes and mitogen responsiveness in vitro.
A presumptive diagnosis of IPEX was given and the patient was treated with immunosuppression consisting of cyclosporine and prednisone. IPEX was confirmed by molecular studies identifying a missense mutation in the forkhead DNA binding region of the FOXP3 gene (A384T). After obtaining IRB and FDA approval for the proposed therapy, the patient, at 7 months of age, received a reduced intensity preparative regimen with alemtuzumab (33 mg total) on days −22 to −19, fludarabine (200 mg/m2 total) on days −8 to −4, thiotepa (10 mg/kg total) on day −3 and melphalan (140 mg/m2 total) on days −2 and −1. On day 0, the patient received a 10 of 10 HLA allele-matched, T and B cell depleted, unrelated bone marrow graft. The graft was CD3 and CD19 depleted on the CliniMACS Selection System® (Miltenyi Biotec, Bergisch Gladbach, Germany) by negative selection. Five million CD3+ cells/kg were added back to the marrow graft to enhance engraftment. The graft contained 2.1 × 108 total nucleated cells/kg, 4.7 × 106 total CD34+ cells/kg, and 1.02 × 106 CD19+ cells/kg. The number of CD4+CD25bright T cells infused with the graft is not known. Cyclosporine A, which had been used to treat IPEX symptoms, was continued for graft vs. host disease prophylaxis. Palifermin (60mcg/kg/dose) was administered daily for 3 days prior to the start of the preparative regimen (days −11 to −9) and 3 days after the stem cell infusion (days +1 to +3) for mucositis prevention and gastrointestinal protection. He tolerated the conditioning regimen and infusion of graft well without unexpected or severe complications. He achieved myeloid (ANC>500/mm3) and platelet (platelets>50,000/mm3) engraftment on days +13 and +37, respectively. Complications within 6 months after HSCT included coagulase negative Staphylococcus and Staphylococcus aureus bacteremia, an episode of dehydration, and adenovirus and Clostridium difficile diarrhea. Currently, he is greater than 3 years after HSCT, off immunosuppression, maintaining a stable mixed donor chimerism, and is growing; all clinical symptoms of disease have resolved. Donor lymphocyte infusions were not prescribed as he did not exhibit signs of IPEX, despite mixed donor chimerism.
2.2 VNTR
DNA was extracted from flow sorted populations and engraftment studies were performed using the D22S683 marker with methods previously described 20.
2.3 Flow cytometry
Antibodies used: anti-CD4 APC (clone SK3, BD Biosciences, San Jose, CA) anti-CD25 PE (clone 4E3, Miltenyi Biotec Inc.), anti-CD45RA (clone L48, Becton Dickinson), anti-CD31 (clone WM59; BD Biosciences), and anti-FOXP3 [anti-human FOXP3 flow kit, BD Biosciences; Alexa-Fluor 488 anti-FOXP3 (clone 259D), Alexa-Fluor 488 mouse IgG1 k isotype control and anti-human CD4-PE-Cy5/CD25-PE cocktail (clones RPA-T4/BC96)]. Samples were run on a FACS Calibur using Cell Quest software (BD Biosciences) and analyzed using FlowJo 8.82 software (Ashland, OR).
2.4 Suppression microassay
The suppression microassay was done as previously described [16]. In brief, flow cytometry sorted CD4+CD25− T cells were stimulated with anti-CD2/CD3/CD28 antibody-coated beads (MACS® T Cell Activation/Expansion Kit, Miltenyi Biotec Inc.) in the presence or absence of CD4+CD25bright T cells for 6–8 hours (the ratios are as indicated). Additional controls included each cell population cultured separately.
2.5 mRNA analysis
Human IL2 mRNA was tested using ABL1 as the endogenous control. mRNA extracted from nTreg cultures were used as a calibrator sample and mRNA from CD4+CD25− T cells as a positive control (Applied Biosystems; IL2: Hs00174114_m1, ABL1: Hs00245445_m1).
2.6 Analysis and statistics
Where possible, suppression assays were set up in triplicate and each respective cDNA was analyzed for IL2 in triplicate. Standard deviations (SD) were determined by paired t test using GraphPad Prism software (San Diego, CA).
2.7 Purification of CD4+CD25− and CD4+CD25bright T lymphocytes
Peripheral blood was obtained from the child with IPEX and his mother at St. Jude Children’s Research Hospital with permission from the Institutional Review Board (IRB) and parental consent. Peripheral blood mononuclear cells (PBMC) were magnetically labeled. The CD4+CD25− and CD4+CD25bright T cell fractions were isolated using an AutoMACS® cell sorter following manufacturer’s instructions (CD4+CD25+ Regulatory T Cell Isolation Kit, Miltenyi Biotec Inc., Auburn, CA). Purities were assessed by flow cytometry (greater than 95%).
3. Results and Discussion
We describe a six week male infant diagnosed with IPEX who harbored an A384T mutation in FOXP3 and examine the molecular dynamics of hematopoietic development and homeostasis following non-myeloablative HSCT (see Methods for case description). Prior to HSCT, the patient had a slightly higher percentage of CD4+CD25bright T cells compared to his mother (20% vs. 14%). Notably, a markedly reduced proportion of patient T cells (gated on the CD4+CD25bright subset as shown in Figure 2B) stained for FOXP3 (15% vs. 62%; Figure 1A), perhaps reflecting protein instability due to the A384T mutation in the forkhead domain. Unfortunately, the low number of CD4+CD25bright FOXP3+ cells in the patient’s peripheral blood (PB) precluded formal functional evaluation. However, these cells likely lack robust activity given that nTreg clones from a patient with an identical genetic mutation exhibited poor suppressive function in vitro [6].
Figure 2. CD45RA and CD31 expression on CD4+CD25-, CD4+CD25bright, and CD8+ T cell populations and the functional activity of sorted CD4+CD25bright T cells.
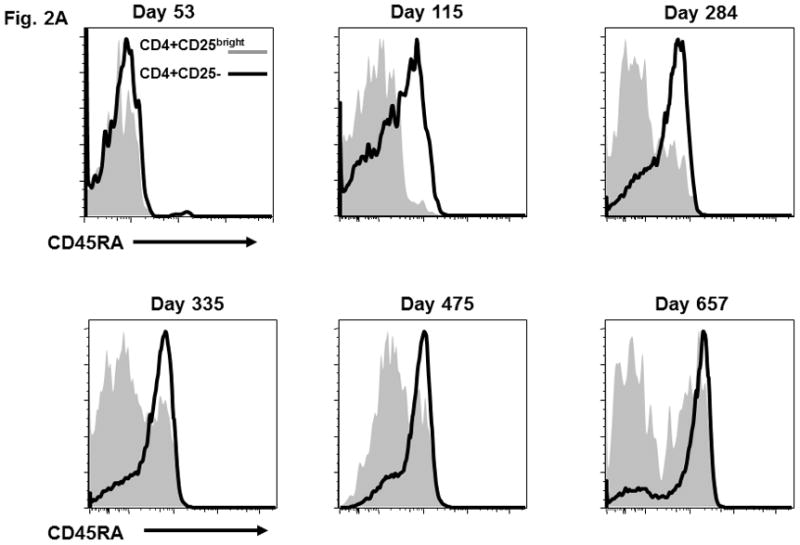
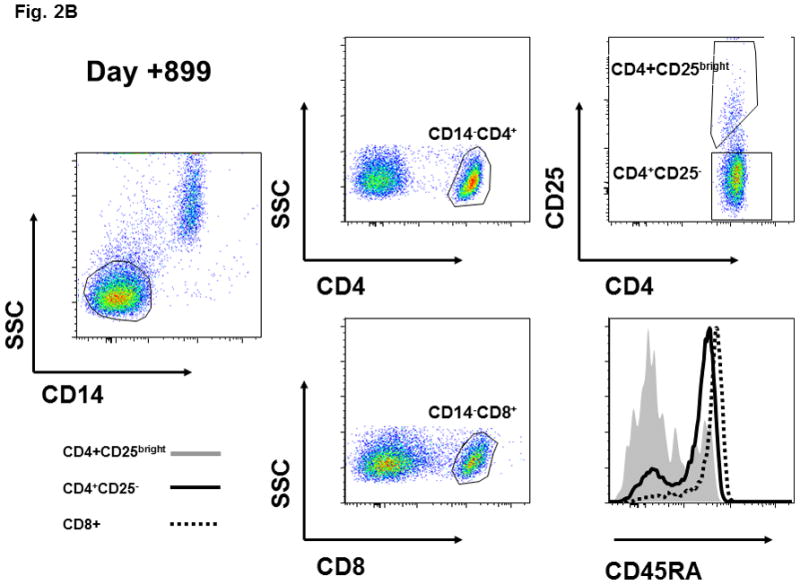
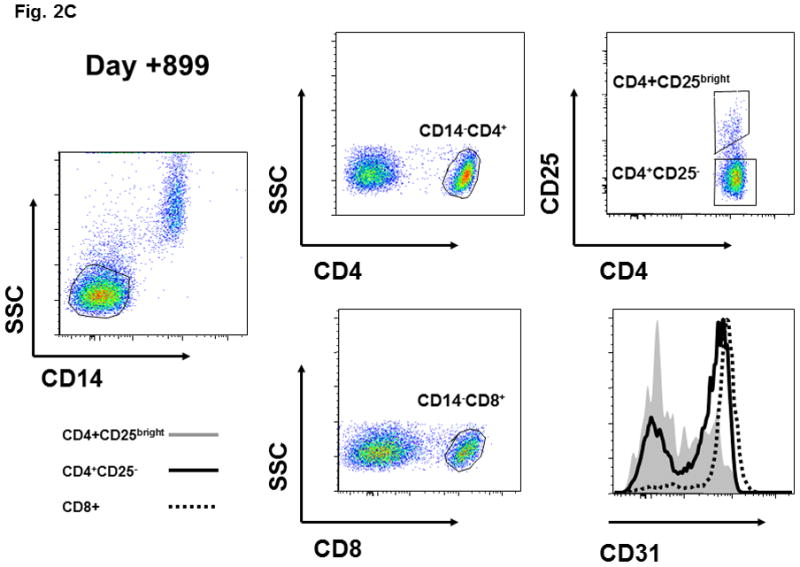
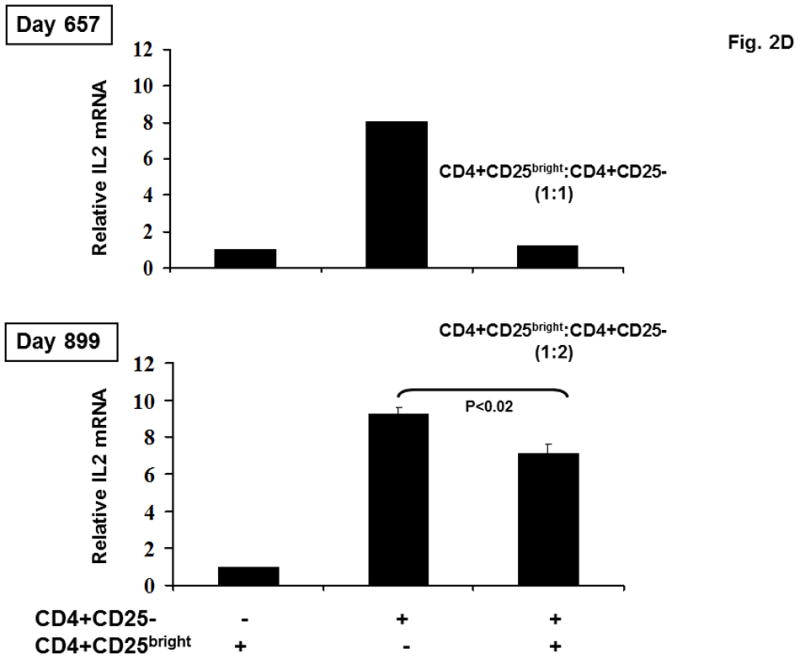
(A) CD45RA status of patient CD4+CD25bright (shaded area) and CD4+CD25- (solid line) T cells is shown at various times after HSCT. (B) CD45RA expression on day +899 is shown for the CD4+CD25bright (shaded area), CD4+CD25− (solid line), and CD8+ (dotted line) T cell populations. The gating strategy excluded CD14+ monocytes and positively gated either CD4+ or CD8+ T cells. The CD4+CD25− and CD4+CD25bright populations were gated as shown. (C) The gating strategy and CD31 expression is depicted for the CD4+CD25bright (shaded area), CD4+CD25− (solid line), and CD8+ (dotted line) T cell populations on day +899. (D) CD14−CD4+CD25− and CD14−CD4+CD25bright T cells were sorted as described and immediately assayed for functional capability. Relative IL2 mRNA levels were measured after a 6-hour in vitro stimulation with anti-CD3, anti-CD28, and anti-CD2 conjugated beads either alone or in co-culture at the indicated ratios. ABL1 was used as the endogenous control, IL2 from CD14−CD4+CD25bright cells as the calibrator, and CD14-CD4+CD25− as the positive control. Sufficient cells were available for stimulation cultures to be done in triplicate on day +899 and statistical significance by paired t-test analysis is shown.
Figure 1. Immune reconstitution in a patient with IPEX after non-myeloablative HSCT.
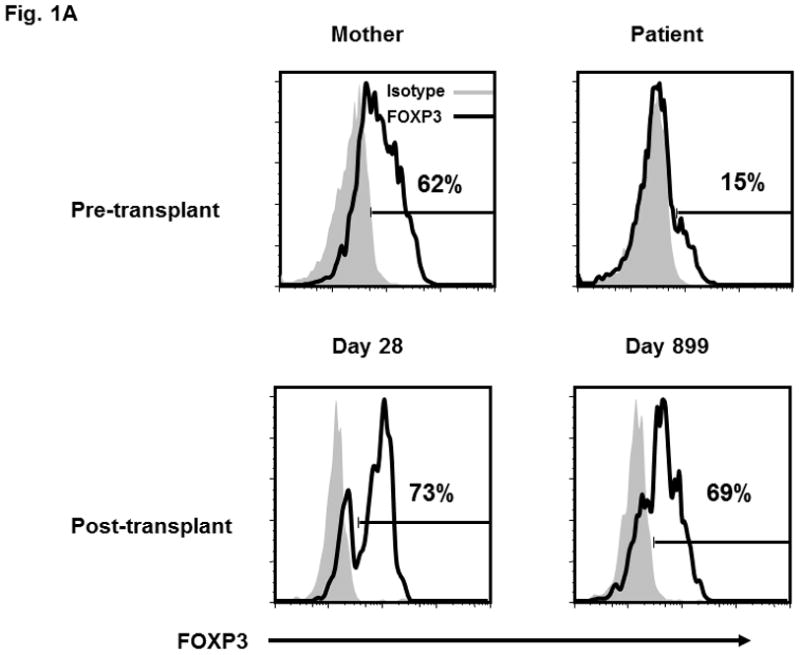
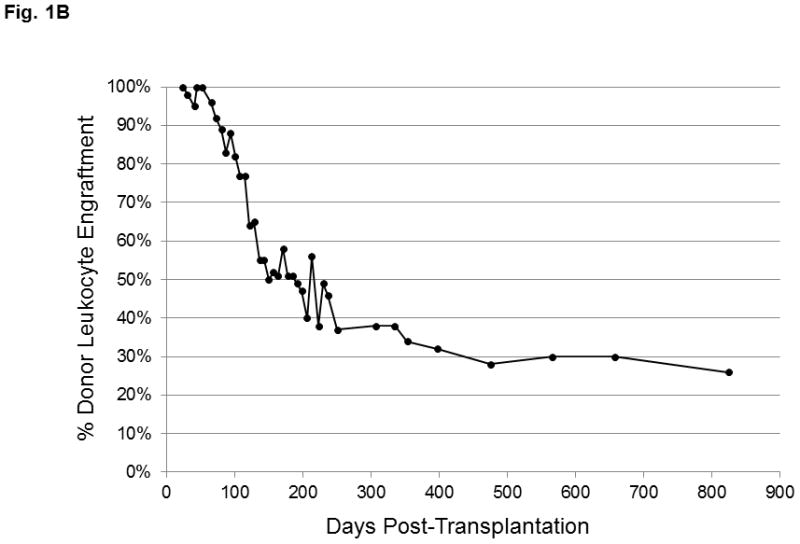
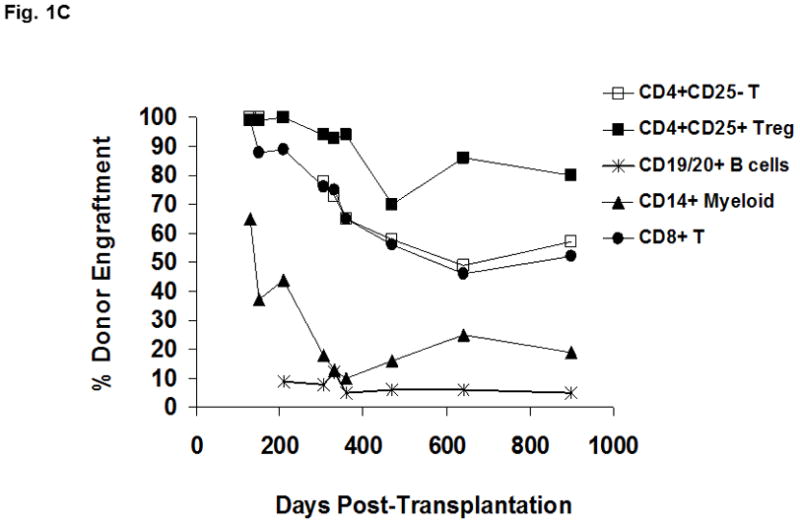
(A) Lymphocytes were selected based on exclusion of monocytes and granulocyes and the CD4+ T cell population was partitioned into the CD4+CD25− and CD4+CD25bright subsets (as shown in Figure 2B and 2C). FOXP3 expression within the CD4+CD25bright T cells (solid line) is compared to an isotype control antibody (shaded area). (B) Donor chimerism of total peripheral blood leukocytes at various times after HSCT, as assessed by VNTR. (C) Multi-color flow cytometry was used to sort lineage-specific cell populations prior to chimerism analysis. Donor chimerism of the purified subsets as determined by VNTR is indicated.
At 7 months of age, the patient received a reduced intensity preparative regimen (alemtuzumab, fludarabine, thiotepa, and melphalan) and a 10 of 10 HLA allele-matched, T and B cell depleted, unrelated bone marrow graft (see Methods for detailed treatment plan).The patient obtained neutrophil and platelet engraftment at days +15 and day +37, respectively. Initial peripheral blood chimerism studies showed complete donor engraftment, but continuing follow-up revealed a decrease in donor leukocyte chimerism during the first year, followed by long term stabilization in the 25–30% range (Figure 1B). Since peripheral blood CD14+ myeloid cells undergo constant turnover, they are reflective of the level of donor HSC engraftment in the bone marrow. To resolve whether the observed decline in peripheral blood donor chimerism was due to loss of the graft or the establishment of stable mixed chimerism, sequential VNTR chimerism analyses on the different sorted lineage populations were done. Analysis of the different leukocyte subsets demonstrated that the observed loss in donor chimerism was predominantly due to a decline in the myeloid (CD14+) compartment which eventually reached a plateau of 19% (Figure 1C). Importantly, these data verified stable low-level donor HSC engraftment almost three years after HSCT. Donor-derived B cells were present in very low numbers from the outset. In contrast, CD4+ and CD8+ T cells demonstrated donor chimerism at 57% and 52%, respectively, while donor-derived CD4+CD25bright T cells exhibited the greatest selective advantage (80%, day +899; Figure 1C). The majority of CD4+CD25bright T cells displayed FOXP3 expression (69%; Figure 1A). These latter data mirror the in vivo selection pattern described in healthy female carriers of mutant FOXP3 alleles. In that study X-chromosome inactivation in the CD4+CD25bright population was skewed towards the functional gene, while the CD4+ naïve and memory T cell populations in the carriers exhibit a random pattern [7]. The pattern of immune reconstitution in our patient is consistent with a selective in vivo growth advantage for nTreg. Additionally, the persistence of donor-derived CD4+ and CD8+ T cells at consistently higher proportions than CD14+ cells in our patient suggests the interesting possibility that functional FOXP3 in non-regulatory T cells may be important. The earlier observation that CD4+CD25− T cells from patients with IPEX exhibit diminished immune function is also consistent with a putative role for FOXP3 in effector T cell activity [2].
Next, we verified that the various T cell subsets were thymic-derived from donor hematopoietic precursors, and not simply transplanted T cells that underwent peripheral expansion in the context of lymphopenia. CD45RA expression, in association with other markers such as CD31, is a phenotypic hallmark of recent thymic emigrants (RTE) which have not undergone peripheral expansion [11;12]. Although T cells which have expanded in the context of IL7 therapy can retain a RTE phenotype, our patient did not receive this treatment and thus thymic-derivation of T cells is most consistent with the observed phenotype [23]. Serial analysis in our patient demonstrated the emergence of CD45RA+ T cells in the CD4+CD25− population (day +115), and subsequently in the CD4+CD25bright subset (day +284; Figure 2A). By day +899 the CD4+CD25− and CD8+ subsets were predominantly CD45RA+ (Figure 2B) and CD31+ (Figure 2C). At day +899 the mixed CD45RA/CD31 expression in CD4+CD25bright T cells likely reflects the elevated in vivo turnover rate of these cells and the establishment of homeostatic equilibrium between thymic output and turnover. Specific reconstitution of donor-derived CD4+CD25brightFOXP3+ T cells following non-myeloablative allogeneic transplant for IPEX has been previously reported on two occasions. However, in these studies the CD4+CD25brightFOXP3+ T cells observed after transplant very likely derived from the large quantities of T cells contained in the grafts [22;26]. Neither study reported evidence for de novo thymic output. Although loss of donor-derived nTreg has not been reported thus far, long-term self-renewal of functional CD4+CD25brightFOXP3+ T cells could be problematic if there is no renewable HSC source.
To analyze the function of the de novo nTreg directly, we developed a microassay to evaluate the ability of CD4+CD25bright cells to suppress production of IL2 mRNA production by CD4+CD25− T cells [16]. Adequate numbers of CD4+CD25bright cells were available at days +657 and +899 and showed significant regulatory function, consistent with the resolution of disease and the in vivo enrichment of CD4+CD25bright T cells containing a functional FOXP3 allele (Figure 2D). The yield of purified nTreg from this patient was consistently low and the functional assay could be performed at a 1:1 ratio (CD4+CD25bright T cells:CD4+CD25− T cells) in duplicate as shown on day +657, or at 1:2 ratio in triplicate as shown on day +899. Triplicate analysis and statistical evaluation was an important priority. Thus, a 1:2 ratio (CD4+CD25bright T cells:CD4+CD25− T cells) was used to accommodate the cell yield on day +899 and does not reflect an observed change in suppressive activity. This is the first direct ex vivo demonstration that nTreg are functionally active in a patient with IPEX following reduced intensity conditioning HSCT.
Until this report, evidence chronicling the development of nTreg in humans from HSC through a thymic intermediate has been lacking. In fact, recent data support the idea that in adults, peripheral nTreg are a rapidly proliferating population with a short lifespan and limited self-renewal [5;24]. Given the thymic involution and loss of function that occurs with age, a peripheral mechanism for the lifelong production of nTreg in adults is quite likely. Our data show that a significant proportion of nTreg are thymus-derived following HSCT in a child with IPEX syndrome. The later emergence of nTreg with reduced CD45RA and CD31 expression (as shown in Fig. 2B and 2C) supports the notion that in vivo turnover is accelerated in pediatric populations as well, and may contribute to the selective enrichment of donor-derived nTreg observed in our patient. A majority of donor-derived CD4+CD25brightFOXP3+ T cells (80%) from a minority population of donor HSC (reflected by the 19% CD14+ donor chimerism) suggests a strong in vivo growth selection for functional nTreg. A growth advantage for non-regulatory T cells, albeit to a lesser degree, is also evident from the persistence of donor-derived CD4+ and CD8+ T cells at higher proportions than CD14+ cells. A role for FOXP3 in the biology of non-regulatory T cells has been controversial. It was first noted that CD4+CD25− T cells from patients with IPEX exhibit diminished immune function, suggesting a possible role [2]. However, later studies examining X-chromosome inactivation did not identify skewing towards the functional allele [7]. Our data reconcile these seemingly contradictory observations and suggests a model where active FOXP3 may be important development or function for at least some de novo T cells. Taken together, these data further validate a non-myeloablative approach to HSCT for IPEX and provide mechanistic insight into the in vivo development of human nTreg and unexpectedly, non-regulatory T cells. Importantly, demonstration of de novo thymic output from a limited pool of donor HSC implies a long-term self-renewing potential for functional nTreg which is critical for sustained correction of disease.
Highlights.
Immune reconstitution after non-myeloablative HSCT for IPEX
In vivo growth advantage of regulatory T cells
Role for FOXP3 in non-regulatory T cell development
Establishment of stable mixed chimerism
De novo thymic development of regulatory and non-regulatory T cells expressing a functional allele of FOXP3
Acknowledgments
The authors would like to thank Dr. Mary Ellen Conley and members of the Division of Bone Marrow Transplantation and Cellular Therapy for care of the patient, Jim Houston and Drs. Anne Marie Hamilton-Easton and Richard Ashmun for invaluable assistance with flow cytometry, and members of the Molecular Pathology Lab at SJCRH for assistance with VNTR analysis. This work was supported by the Assisi Foundation of Memphis, Cancer Center Support Grant 2P30CA021765 and the American Lebanese and Syrian Associated Charities (ALSAC).
Abbreviations
- nTreg
natural regulatory T cells
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
- HSC
hematopoietic stem cell
- HSCT
hematopoietic stem cell transplantation
- DAT
direct antiglobulin test
- IRB
Institutional Review Board
- PBMC
Peripheral blood mononuclear cells
- PB
peripheral blood
- RTE
recent thymic emigrants
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Reference List
- 1.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 2.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, gna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 5.Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, Vukmanovic-Stejic M. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 6.d'Hennezel E, Ben-Shoshan M, Ochs HD, Torgerson TR, Russell LJ, Lejtenyi C, Noya FJ, Jabado N, Mazer B, Piccirillo CA. FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N Engl J Med. 2009;361:1710–1713. doi: 10.1056/NEJMc0907093. [DOI] [PubMed] [Google Scholar]
- 7.Di NS, Cecconi M, Passerini L, McMurchy AN, Baron U, Turbachova I, Vignola S, Valencic E, Tommasini A, Junker A, Cazzola G, Olek S, Levings MK, Perroni L, Roncarolo MG, Bacchetta R. Wild-type FOXP3 is selectively active in CD4+CD25(hi) regulatory T cells of healthy female carriers of different FOXP3 mutations. Blood. 2009;114:4138–4141. doi: 10.1182/blood-2009-04-214593. [DOI] [PubMed] [Google Scholar]
- 8.Dorsey MJ, Petrovic A, Morrow MR, Dishaw LJ, Sleasman JW. FOXP3 expression following bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Immunol Res. 2009;44:179–184. doi: 10.1007/s12026-009-8112-y. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, Scherer F, Shalaby T, Grotzer M, Siler U, Seger R, Gungor T. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37:3270–3280. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 12.Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, Thiel A. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St GB, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas KG, Ungar D, Comito M, Bayerl M, Groh B. Submyeloablative cord blood transplantation corrects clinical defects seen in IPEX syndrome. Bone Marrow Transplant. 2007;39:55–56. doi: 10.1038/sj.bmt.1705542. [DOI] [PubMed] [Google Scholar]
- 15.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Morales-Tirado V, Wichlan DG, Leimig TE, Street SE, Kasow KA, Riberdy JM. 1alpha,25-dihydroxyvitamin D3 (vitamin D3) catalyzes suppressive activity on human natural regulatory T cells, uniquely modulates cell cycle progression, and augments FOXP3. Clin Immunol. 2011;138:212–221. doi: 10.1016/j.clim.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der ZL, de Vries RR, Huizinga TW, Ottenhoff TH, Toes RE. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J, Shenoy S. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood. 2007;109:383–385. doi: 10.1182/blood-2006-05-025072. [DOI] [PubMed] [Google Scholar]
- 19.Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 21.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St GB. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidel MG, Fritsch G, Lion T, Jurgens B, Heitger A, Bacchetta R, Lawitschka A, Peters C, Gadner H, Matthes-Martin S. Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood. 2009;113:5689–5691. doi: 10.1182/blood-2009-02-206359. [DOI] [PubMed] [Google Scholar]
- 23.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van LM, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan H, Sinclair J, Adams S, Cale CM, Murch S, Perroni L, Davies G, Amrolia P, Qasim W. Immune reconstitution and recovery of FOXP3 (forkhead box P3)-expressing T cells after transplantation for IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome. Pediatrics. 2008;121:e998–1002. doi: 10.1542/peds.2007-1863. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]


