Abstract
Objective
This study tested the hypothesis that minimally invasive microneedles cause less pain during injection of lidocaine but induce local anesthesia in human subjects with the same rapid onset and efficacy as intradermal lidocaine injection using hypodermic needles.
Methods
This study was a randomized, single-blinded, within subjects, controlled design. Hollow 500 μm-long microneedles were used to inject lidocaine to the forearm of 15 human subjects. The associated pain was recorded using a visual analog pain scale (VAS). The area and depth of numbness were determined at 0, 7.5, and 15 min after injection. Lidocaine was also injected to the dorsum of the hand near a vein, followed by placement of an intravenous catheter and measurement of associated pain. A 26-gauge intradermal bevel hypodermic needle similarly administered lidocaine on the opposite forearm/hand to serve as the positive control.
Results
VAS pain scores revealed that injection using microneedles was significantly less painful than hypodermic needles for both the forearm and dorsum of the hand injections. However, there was no significant difference in the area or depth of the resulting numbness between the two treatment methods at any time-point (0, 7.5, and 15 min) indicating that microneedles had immediate onset and were as effective as hypodermic needles in inducing dermal anesthesia. Moreover, insertion of an IV catheter immediately after lidocaine injection on the dorsum of the hand led to comparable pain scores for the microneedle and hypodermic needle treated sites, further confirming efficacy of microneedles in inducing rapid local anesthesia. Lastly, 77% of the subjects preferred microneedles and 80% indicated that they did not consider microneedles to be painful.
Discussion
This study demonstrates for the first time that microneedle-based lidocaine injection is as rapid and as effective as hypodermic injection in inducing local anesthesia while resulting in significantly less pain during injection.
Keywords: microneedle, intradermal injection, lidocaine, pain, topical anesthesia
Introduction
An ideal local anesthesia system for use in ambulatory clinical environments should be effective; have rapid onset; cause minimal pain; be fast and easy to load and administer with minimal user training; be portable without bulky equipment; and be cost effective (1). While several methods of inducing local anesthesia exist, each of them comes with limitations.
Hypodermic-needle injection is the most common means of administering local anesthesia and allows for rapid onset with good efficacy. Hypodermic-needles, however, can cause pain, anxiety and distress leading to poor patient compliance, especially among children (2,3) and require administration by experienced medical personnel. Topical anesthetic creams and gels provide a painless and simple means of administering anesthesia, however, these techniques rely upon passive diffusion across the skin's outermost layer (stratum corneum) which leads to slow onset times ranging from 30 min to 1.5 h (1,3). This delayed onset is the main barrier to widespread use of topical anesthetics for venous access procedures.
More recently, several needle-free, active drug delivery devices such as iontophoresis, ultrasound, jet injection, and laser/thermal ablation-based systems have been developed to administer local anesthetics. However, several such commercialized devices have had poor market acceptance and have been discontinued over the past few years (Anesiva Annual Report, 2008; Vyteris Annual Report, 2008).
We propose the use of micron-dimension needles called microneedles to administer local anesthetics in a simple and minimally invasive manner while allowing for rapid onset of analgesic action. Microneedles can be fabricated as solid or hollow structures that are long enough to deliver drug by breaching the stratum corneum, but are short enough to avoid stimulating nerve endings in the dermis (4).
Hollow microneedles administer drugs by means of active injection and can be used to deliver large volumes to the skin. Studies have demonstrated the local and systemic effects of hollow microneedle-based injection using methyl nicotinate (5) and insulin (6) in human subjects, respectively. Small (1.5 mm-long) hypodermic needles developed by Becton Dickinson have been used for influenza vaccination in elderly human subjects resulting in a stronger immune response than intramuscular injection (7). Further, arrays of hollow microneedles have also demonstrated influenza vaccine injection in human subjects (8). With respect to pain, previous studies have demonstrated that skin insertion of solid piercing structures with similar geometries as hollow microneedles used in this study is significantly less painful than 25 and 26-gauge hypodermic-needles (9–11). Further, short hypodermic- needles have also been found to be significantly less painful than 26-gauge needles inserted intradermally (12).
Guided by this previous literature, this paper reports for the first time (i) the pain associated with hollow microneedle injection, (ii) microneedle-based delivery for a local therapeutic drug response, and (iii) the efficacy of lidocaine administration using microneedles to cause local anesthesia in human subjects.
Materials and Methods
Participants
Fifteen healthy human subjects (11 male, 4 female; age: 22–56 years) were recruited. Subjects having diseased or abnormal skin, known diseases affecting nerve function or perception of pain, allergies to lidocaine, and veins on the dorsum of their hand without easy access were excluded. The study was approved by Emory University's Institutional Review Board and carried out in accordance with the Declaration of Helsinki protocol. All subjects provided informed consent prior to participation.
Endpoints
The following end points were measured: a) pain associated with microneedle and hypodermic needle-based lidocaine injection, b) area and c) depth of skin numbness at 0, 7.5 and 15 min after injection, d) efficacy based on pain associated with placement of an intravenous (IV) catheter immediately after lidocaine injection, and e) preferred treatment method among microneedle and hypodermic-needle injection.
Lidocaine
A preservative-free 2% lidocaine hydrochloride injectable solution (Hospira, Lake Forest, IL) was used for all injections
Microneedle fabrication and injection
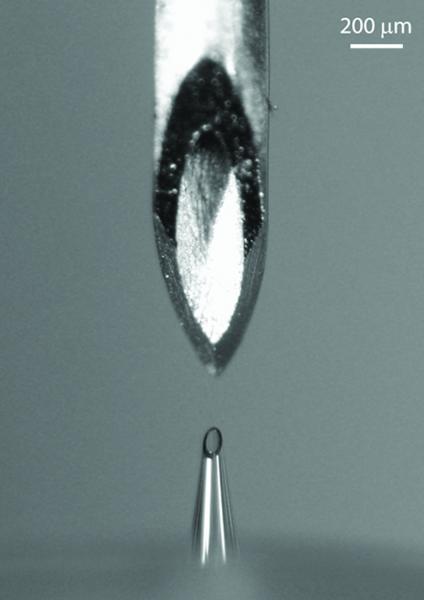
Hollow borosilicate-glass microneedles (Figure 1) with a 30° bevel angle and tip radii of 60–80 μm were fabricated as described previously (6). Microneedles were inserted at a 90° angle into skin to a depth of 500 μm using a custom rotary drilling device with ±10 μm precision, although the insertion depth into the skin was probably an order of magnitude less accurate because of the variable deformation of the skin during microneedle insertion (13). The microneedle was connected to a 3 mL syringe and a syringe pump (NE-1000, New Era Systems, Farmingdale, NY) which controlled lidocaine flow rate at 300 μl/min (6). Microneedles were removed from the skin approximately 5 s after injection.
Figure 1.
The bevel opening of a 26-gauge intradermal bevel hypodermic needle (top) compared to the entire length of a 500 μm-long microneedle (bottom).
Hypodermic-needle injection
A 26-gauge 3/8” intradermal bevel needle connected to a 1 mL syringe was used as the positive control. The needle was inserted at an angle into the intradermal space by experienced anesthesiology personnel and lidocaine was injected within 3–5 s.
Study design
This study was a randomized, within subjects, controlled, single-blinded design and was carried out in two phases. One phase was performed on the forearm while the other on the dorsum of the hand. The forearm phase assessed pain associated with microneedle-based injection and determined the efficacy of delivery by measuring area and depth of numbness. The dorsum of the hand phase further assessed pain associated with microneedle-based injection and determined efficacy by measuring pain during IV placement. All subjects received treatments with both devices on their forearms and the dorsum of their hands and each subject served as his/her own control. The sequence of these two phases (forearm or dorsum) was randomized among subjects. Within each phase, the treatment sequence (microneedle or hypodermic-needle) and the forearm/hand (left or right) were also randomized. Subjects were blinded throughout the study by placing their arms under a curtain barrier.
Forearm protocol
The volar forearm insertion site was identified and marked 7 cm from the midpoint of the antecubital fossa. The site was then wiped with isopropanol swabs followed by injection of 200 μl of lidocaine using a microneedle or hypodermic-needle as per the randomization schedule. The subject rated the pain associated with injection using a 100-mm visual analog scale (VAS) and the score was recorded by a blinded observer. The subject was then asked if he/she considered the procedure to be painful. The area of numbness was measured via a pin-prick test by gently tapping a 25-gauge 3.5” spinal needle (Becton Dickinson, Franklin Lakes, NJ) on the skin starting from the insertion point and extending radially outward in four directions until the subject felt a sensation. The depth of numbness was measured by inserting a 26-gauge 3/8” hypodermic-needle up to a maximum of 9.5 mm into the skin at the injection site until the subject indicated that he/she felt sensation.
The procedure took approximately 2 min to complete and was performed immediately after lidocaine injection (t=0) and again at 7.5 and 15 min after injection. This procedure was then repeated on the opposite forearm using the other injection method. At the end of both treatment procedures, subjects were asked to rank their preference between the microneedle and hypodermic-needle injection procedures.
Dorsum of hand protocol
A site near a vein accessible for venipuncture was identified and wiped with isopropanol swabs. Lidocaine (100 μl) was then injected using a microneedle or hypodermic-needle to generate a skin wheal. The subject rated the pain associated with injection using the VAS scale and indicated if the procedure was painful. A 22-gauge IV catheter (Optiva, Smiths Medical, Carlsbad, CA) was then inserted through the wheal into the vein until a small amount of blood appeared in the hub, upon which the IV catheter was immediately removed. The site was wiped with an isopropanol swab and covered with an adhesive bandage. The subject then rated the pain of inserting the IV catheter and indicated if the procedure was painful. The procedure was repeated on the opposite hand using the other injection method. At the end of both treatments, the subject was asked to indicate the preferred treatment method.
Local skin reaction
Immediately following lidocaine injection and 1 h post injection, treatment sites were visually examined for any evidence of skin irritation.
Statistical analysis
All data are expressed as mean ± standard deviation. VAS pain scores for hypodermic and microneedle-based lidocaine injection and venous cannulation pain scores were compared using paired, two-tailed Student's t-test. In all cases, p <0.05 was the minimum value considered acceptable for rejection of the null hypothesis. Differences in the radii of numbness (top, bottom, left, and right) for the two treatment methods over all three time points were compared using a repeated measures three-way ANOVA. The total area and depth measurements for the two devices were compared over the three time points using a repeated measures two-way ANOVA. All comparisons were performed using NCSS (Kaysville, UT) and Minitab (State College, PA) software. Data from incomplete injections due to leakage from either injection devices were excluded from the analyses.
Results
Study population
Fifteen healthy subjects gave informed consent and participated in this study. Of the enrolled subjects, data from 13 (87%) subjects were included in the forearm phase. The other two subjects experienced incomplete lidocaine delivery during the injection procedure. Data from 11 (73%) subjects were analyzed for the dorsum of the hand phase for the same reason.
Assessment of pain
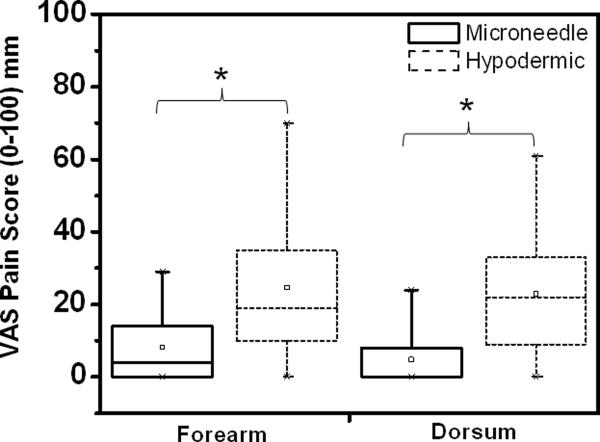
To test the hypothesis that microneedle-based injection is less painful than hypodermic needle-based injection, VAS pain scores were measured for the two injection methods (Table 1). Microneedles were significantly less painful than hypodermic needles for both forearm (Student's t-test; p=0.002) and dorsum of the hand (p=0.013) injection procedures. On average, the hypodermic needle received a VAS score much larger than the microneedle for both forearm injections and dorsum injections. Further, 92% of forearm protocol subjects reported the microneedle procedure to be painless as compared to only 25% for the hypodermic needle. For the dorsum of the hand injection, 82% of subjects reported the microneedle procedure to be painless, while only 27% thought the hypodermic needle caused no pain. Although the sample size in this study was small, post-hoc power analysis (G*Power3, Kiel, Germany) revealed that there was a ≥ 80% power of detecting a statistically significant difference, should it exist, for both phases using the chosen experimental design.
Table 1.
Comparison of Microneedle and Hypodermic Needle Results
| Microneedle | Hypodermic Needle | p-value | |
|---|---|---|---|
| FOREARM (n = 13) | |||
| Injection VAS pain score (mm) | 8.2 ± 10.4 | 24.9 ± 21.2 | 0.002 |
| Average numb radius at t = 0 min (mm)+ | 11.5 ± 2.7 | 10.6 ± 3.1 | 0.201 |
| Average numb radius at t = 7.5 min (mm)+ | 11.2 ± 2.3 | 11.0 ± 2.5 | 0.212 |
| Average numb radius at t = 15 min (mm)+ | 10.1 ± 2.8 | 11.9 ± 2.6 | 0.053 |
| Depth of numbness at t = 0 min (mm) | 9.1 ± 1.6 | 9.4 ± 1.5 | 0.656 |
| Depth of numbness at t = 7.5 min (mm) | 9.1 ± 1.2 | 9.5 ± 1.1 | 0.188 |
| Depth of numbness at t = 15 min (mm) | 8.7 ± 1.8 | 9.5 ± 0.8 | 0.084 |
| DORSUM OF HAND (n = 11) | |||
| Injection VAS pain score (mm) | 4.8 ± 7.9 | 22.0 ± 17.2 | 0.013 |
| IV catheter insertion pain score (mm) | 4.2 ± 4.8 | 1.6 ± 2.7 | 0.130 |
Data reported as mean ± standard deviation
the average numb radius at a given time point is determined by averaging the radii from the injection point in all four directions (top, left, right, and bottom),
Area of numbness
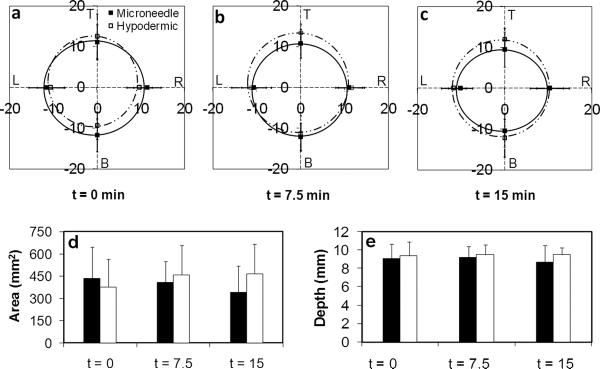
To determine if microneedle injection was as effective as hypodermic needles in inducing anesthesia over a clinically relevant area, the area of numbness was measured using the pin-prick protocol described above for the forearm at t = 0, 7.5, and 15 min after delivery. As seen in Figures 2a–c, uniform anesthesia was achieved in all directions, creating a circular area of numbness for both delivery methods. There were no significant differences in the anesthetized regions along any of the four axes of measurement or the total areas of numbness between the two treatment methods (repeated measures three-way ANOVA: p > 0.05) for all time points. The average areas over the 15 min measurement period were 4.0±1.8 cm2 and 4.3±1.9 cm2 for the microneedle and hypodermic-needle treated forearm sites, respectively (Figure 2d). There were no significant differences (repeated measures two-way ANOVA: p > 0.05) among the anesthetized areas for both treatment methods at any time point over the study period.
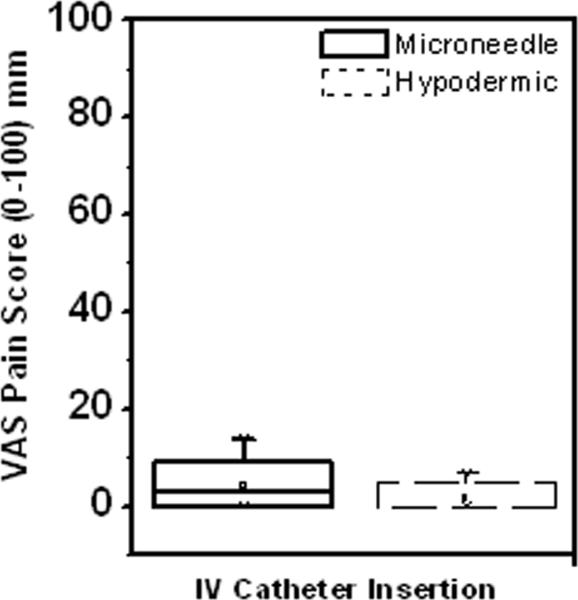
Figure 2.
Box plot representation of visual analog scale (VAS) pain scores associated with lidocaine injection for the forearm and dorsum of the hand. The rectangular box represents the interquartile range (25% to 75%) of the VAS pain scores for each treatment procedure (solid = microneedle; dashed = hypodermic needle) for the different study protocols (forearm injection, dorsum injection). The vertical lines extend from the upper and lower boundary of the box to the maximum and minimum data points. The hollow square inside the box represents the mean pain score for each treatment procedure. The horizontal line inside each box represents the median for the corresponding treatment procedure. Microneedle injection was significantly less painful than hypodermic needle injection for both the forearm and dorsum of the hand procedures. * p<0.05
Depth of numbness
To test the depth of anesthesia, a 26-gauge, 9.5 mm-long hypodermic needle was inserted into the forearm skin at the site of lidocaine injection until subjects indicated they felt a sensation (Figure 2e). There were no significant differences in the depth of anesthesia for the two injection methods across all time points (repeated measures two-way ANOVA: p > 0.05).
Efficacy of injection method based on venous cannulation
To further test the efficacy of microneedle-based lidocaine administration for use in clinical applications, an IV was inserted into a vein on the dorsum of the hand immediately following lidocaine injection near the vein (Table 1). Microneedles were as effective as hypodermic-needles in administering rapid dermal anesthesia for venous cannulation, as there was no significant difference between the IV insertion pain scores for the two treatment methods (Student's t-test: p=0.130). Notably, 82% of the subjects considered the IV insertion post-microneedle treatment to be painless and 91% considered cannulation following hypodermic-needle treatment to be painless.
Onset of anesthesia
As seen from Figure 2, the area and depth of numbness were the same at t = 0 min for both the microneedle and hypodermic-needle injections. Further, there were no significant differences in the IV catheter placement pain scores for the two treatment methods immediately after lidocaine injection.
Preference of treatment method
In an attempt to determine whether microneedles would be clinically accepted by patients, at the end of each protocol (forearm and dorsum of the hand), subjects were asked which treatment method they would prefer (first versus second procedure) to receive in a clinical setting. For the forearm injection protocol, 78% of subjects reported they would prefer microneedles. For the dorsum protocol, treatment preference was assessed for the overall procedure (i.e. lidocaine injection and IV catheter insertion) with 82% of subjects reporting they would prefer microneedle injection. Thus, even though the subjects were blinded to the treatments, a large majority of subjects preferred microneedle-based lidocaine injection over hypodermic-needles. Preference rates could be still higher during non-blinded procedures in which the fear associated with seeing needles is reduced through the use of microneedles.
Local skin reaction and adverse events
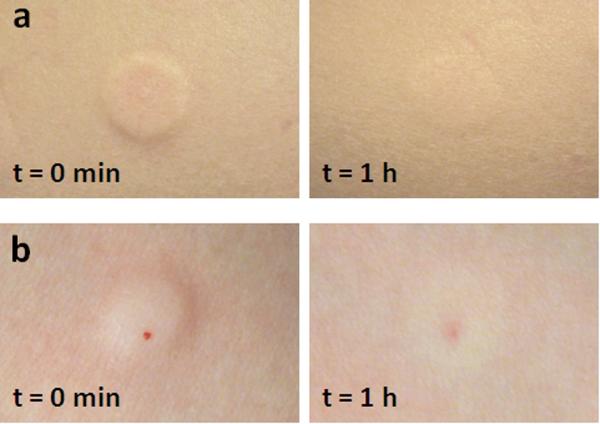
To study the effect of microneedles on skin irritation, the treatment sites were visually examined for local skin reactions. Immediately after injection, a distinct raised skin wheal confirming intradermal delivery was observed at the treatment site (Figure 5). In subjects with light skin, very mild erythema and slight skin blanching were observed. Both the skin wheal and erythema were either reduced or gone within 1 h of treatment. Within 24 h, subjects self-reported that there were no signs of erythema or edema.
Figure 5.
Representative images of the forearm injection sites immediately after lidocaine administration and 1 h after injection. a) Microneedle: a distinct symmetric skin wheal and slight erythema are seen immediately after injection. There is no evidence of blood. One hour post injection, there is a faint wheal still present. b) Hypodermic needle: a distinct raised skin wheal with slight blanching of the skin and a drop of blood at the needle insertion point is seen immediately after injection.
Corresponding treatment with hypodermic needles resulted in a similar raised skin wheal which was either reduced or had disappeared within 1 h. In contrast, hypodermic-needle insertions led to the presence of a drop of blood at the injection site immediately after treatment which was never seen after microneedle injections. Punctate redness at the hypodermic-needle insertion point also often appeared 1 h after treatment. Subjects reported no wheal after 24 h, although in some cases, subjects still observed punctate redness at the hypodermic-needle injection site. There were no adverse events observed or reported during or after the study. Overall, skin responses to microneedle and hypodermic needle injection were similar, although hypodermic needle caused minor bleeding and appeared to cause more lasting local trauma to the skin.
Discussion
This study supports the hypothesis that minimally invasive microneedles cause less pain during injection of lidocaine, but induce local anesthesia in human subjects with the same rapid onset and efficacy as intradermal injections using hypodermic needles. Microneedles were less painful than hypodermic needles for both the forearm and dorsum protocols as indicated by the injection VAS pain scores. Previous studies have shown that the minimum difference in VAS scores for results to be considered clinically significant is between 9 and 13 mm (14,15). Therefore, our results with differences of 16.4 mm and 17.2 mm between the average pain scores for the two treatment methods for the forearm and hand-dorsum procedures were also significant from a clinical standpoint.
We hypothesize that the reduced pain associated with the microneedle is due to its small size and shallow insertion depth into the skin. However, another factor could be the slower injection rate when using microneedles. While 100–200 μl was injected using the hypodermic needle in 3–5 s, injection of the same volumes using a microneedle required 20–40 s at the flow rate of 300 μl/min used in this study. We believe, however, that the slower infusion rate was only a secondary factor, because separate studies injecting insulin (6) and sterile saline (data not shown) showed that increasing flow rate approximately 3-fold to 1 ml/min did not significantly affect pain scores.
Both microneedle and hypodermic needle-based lidocaine injections induced anesthesia immediately after injection, indicating rapid onset time as seen from the comparable area and depth of numbness at t=0 and from the comparable VAS scores for IV catheter placement. The results of this study also indicated no significant differences in the depth of anesthesia induced by the two devices. This depth of anesthesia is almost two times greater than that induced by EMLA (5 mm) (16) and similar to depths induced by iontophoresis, which are 6–10 mm (17). Thus, by inserting a microneedle 500 μm deep into skin, we were able to generate numbness to a depth almost 20 times the needle length.
An advantage of hypodermic needle injection is that it is fast, requiring just 3–5 s to inject 100–200 μl. Microneedle injection required 20–40 s to inject 100–200 μl in this study, which used a flow rate of 300 μl/min. The flow rate could be increase, for example to 1 ml/min as we did in our previous study of insulin delivery (6), and thereby reduce injection time to 6–12 s. Thus, microneedle injection times are not much longer than hypodermic needle injections, which is important to acceptance in busy clinical environments. In contrast, non-invasive lidocaine delivery methods, such as iontophoresis, ultrasound, and jet injectors, have application and onset times varying between 2–15 min, while topical creams and gels have onset times that can exceed 1 h (3).
With respect to cost, microneedles can be manufactured at very low cost expected to be just a few cents each in mass production (18). Therefore, microneedles are likely to be cost competitive with other disposable/consumable systems such hypodermic needles (which also cost a few cents) and topical anesthetics, and significantly cheaper than more complex technologies like iontophoresis, ultrasound and jet injection systems, which can cost hundreds to thousands of dollars (19). Thus, microneedles appear to be a cost-effective solution for reducing time to anesthesia onset in a minimally invasive and significantly less painful manner.
The potential medical significance of this work is that microneedles can provide a less painful yet efficacious alternative to current local anesthesia administration systems. The ability of microneedles to induce significantly less painful and rapid anesthesia prior to intravenous cannulation as shown in this study can reduce pain associated with venipuncture and IV cannulation. This impact can be more significant amongst children who consider these two procedures to be the most common sources of pain in the hospital and the second most common cause of worst pain during hospitalization (second only to the patient's underlying disease) (20,21). Further, the small size of microneedles makes the needle visually less apparent and may well be a suitable alternative to hypodermic needles for patients with needle phobia. Moreover, commercially manufactured microneedles would require less user training than hypodermic needles as their short depth will ensure reliable intradermal needle insertion as opposed to the Mantoux technique which requires training to achieve reliable intradermal needle placement (22). Additionally, the rapid anesthesia onset makes microneedles attractive for use in busy clinical settings.
A limitation of this study is that the microneedles and microneedle-insertion device used in this pilot study were lab prototypes that are still under development. Glass microneedles were used in this study, because they were easy to fabricate. The experimental nature of the prototype device also led to leakage of lidocaine during some injections resulting in incomplete injections. To address these limitations, we have recently fabricated metal microneedles out of medical-grade stainless steel mounted on a conventional luer lock using methods suitable for mass production, and demonstrated reliable intradermal injection using these needles (data not shown).
Conclusions
This study demonstrates for the first time that minimally invasive microneedles cause less pain during injection while resulting in local anesthesia in human subjects with the same speed of onset and efficacy as intradermal lidocaine injection using hypodermic needles. Consequently, we believe that microneedles can provide an enabling technology for administering local anesthesia that is acceptable to patients, in terms of pain and efficacy, and acceptable to physicians in terms of speed, simplicity, and cost.
Figure 3.
Map of the area of numbness from the injection site (0,0) extending along the four axes (T = top, B = bottom, L = left, R = right) for a) t = 0 min, b) t = 7.5 min, and c) t = 15 min after lidocaine injection. The axes are in millimeters. The distances from the injection site to the point of first sensation felt upon pin-prick testing were measured as radii along each axis for each time point. d) Total area of numbness for the three measurement periods. The radii for each treatment method at each time point were averaged to produce an average radius for each period. The average radius was used to calculate the average numb area using the equation for a circle (πr2, where r is the average radius). e) Depth of numbness for the three measurement periods ■ = microneedle; □ = hypodermic needle.
Figure 4.
Box plot representation of the visual analog pain scores associated with IV placement following lidocaine injection on the dorsum of the hand. There was no significant difference between the pain scores for the microneedle and hypodermic needle, indicating the anesthetic effect was similar for both injection procedures.
Acknowledgements
This project was supported in part by the National Institutes of Health grant R01 EB000260 to MRP. Experiments were carried in the Department of Anesthesiology at Emory University. Analyses were carried out at the Institute for Bioengineering and Bioscience and the Center for Drug Design, Development and Delivery at The Georgia Institute for Technology. We thank Donna Bondy for administrative support.
Funding: Supported in part by the National Institutes of Health grant number R01EB006369
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
MRP serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This potential conflict of interest has been disclosed and is being managed by the Georgia Institute of Technology and Emory University.
References
- 1.Houck CS, Sethna NF. Transdermal analgesia with local anesthetics in children: review, update and future directions. Expert Rev Neurother. 2005;5:625–34. doi: 10.1586/14737175.5.5.625. [DOI] [PubMed] [Google Scholar]
- 2.Holmes HS. Choosing a local anesthetic. Dermatol Clin. 1994;12:817–23. [PubMed] [Google Scholar]
- 3.Zempsky WT. Pharmacologic approaches for reducing venous access pain in children. Pediatrics. 2008;122:S140–S53. doi: 10.1542/peds.2008-1055g. [DOI] [PubMed] [Google Scholar]
- 4.Prausnitz MR, Langer R. Trandermal drug delivery. Nat Biotech. 2008;26:1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivamani RK, Stoeber B, Wu GC, Zhai H, Liepmann D, Maibach H. Clinical microneedle injection of methyl nicotinate: stratum corneum penetration. Skin Res Technol. 2005;11:152–6. doi: 10.1111/j.1600-0846.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 6.Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in type 1 diabetic subjects using hollow microneedles. Diabetes Technol Ther. 2009;11:329–37. doi: 10.1089/dia.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, Weber F, Saville M. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 8.Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: A randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, Morrissey A, Birchall JC. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 10.Gill HS, Denson DD, Prausnitz MR. Effect of microneedle design on pain in human subjects. Clin J Pain. 2008;24:585–94. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92:502–4. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 12.Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–42. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–7. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 14.Todd KH, Funk KG, Funk JP. R. B. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–9. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 15.Kelly A-M. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998;5:1086–90. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 16.Bjerring P, Arendt-Nielsen L. Depth and duration of skin analgesia to needle insertion after topical application of EMLA cream. Br J Anaesth. 1990;64:173–7. doi: 10.1093/bja/64.2.173. [DOI] [PubMed] [Google Scholar]
- 17.Zempsky WT. Iontophoresis for local anesthesia. In: Harahap M, Abadir AR, editors. Anesthesia and Analgesia in Dermatologic Surgery. Informa; New York: 2008. pp. 163–70. [Google Scholar]
- 18.Prausnitz MR, Gill HS, Park JH. Microneedles for drug delivery. In: Rathbone MJ, Hadgraft J, Roberts MS, Lane ME, editors. Modified Release Drug Delivery. 2nd edition Informa Press; New York: 2008. pp. 295–309. [Google Scholar]
- 19.Young KD. What's new in topical anesthesia. Clin Ped Emerg Med. 2007;8:232–9. [Google Scholar]
- 20.Cummings EA, Reid GJ, Finley GA, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain. 1996;68:25–31. doi: 10.1016/S0304-3959(96)03163-6. [DOI] [PubMed] [Google Scholar]
- 21.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- 22.Mikszta J, Laurent PE. Cutaneous delivery of prophylactic and therapeutic vaccines: historical perspective and future outlook. Expert Rev Vaccines. 2008;7:1329–39. doi: 10.1586/14760584.7.9.1329. [DOI] [PubMed] [Google Scholar]