Abstract
This study was undertaken to determine the role of adenosine signalling in the development of age-related hearing loss (ARHL). We and others have shown previously that adenosine signalling via A1 receptors is involved in cochlear protection from noise-induced cochlear injury. Here we demonstrate that enhanced adenosine signalling in the cochlea provides partial protection from ARHL in C57BL/6J mice. We targeted adenosine kinase (ADK), the key enzyme in adenosine metabolism, using a treatment regime with the selective ADK inhibitor ABT-702 (1.5 mg/kg intraperitoneally twice a week) commencing at the age of three months or six months. This treatment, intended to increase free adenosine levels in the cochlea, was maintained until the age of nine months and hearing thresholds were evaluated monthly using auditory brainstem responses (ABR). At nine months, when C57BL/6J mice normally exhibit significant ARHL, both groups treated with ABT-702 showed lower ABR threshold shifts at 10 and 16 kHz compared to control animals receiving the vehicle solution. The better thresholds of the ABT-702-treated mice at these frequencies were supported by increased survival of hair cells in the apical region of the cochlea. This study provides the first evidence that ARHL can be mitigated by enhancing adenosine signalling in the cochlea.
Keywords: Presbyacusis, Adenosine, Adenosine kinase inhibitor, Hearing loss, Cochlear protection, Purinergic signalling
1. Introduction
Age-related hearing loss (ARHL), or presbyacusis, is the most common sensory deficit (Morton, 1991). It is characterised by a decline in hearing sensitivity and speech discrimination, delayed central processing of acoustic information, and impaired localisation of sound sources (Gates and Mills, 2005). Various cochlear pathologies related to ARHL have been observed, including loss of sensory hair cells and spiral ganglion neurones, and degeneration of secretory and supporting tissues (stria vascularis and spiral ligament) that are responsible for maintenance of electrochemical homeostasis of cochlear fluids (Schucknecht and Gacek, 1993). Mechanical changes to the basilar membrane have also been proposed to explain the deterioration in the audiogram (Schucknecht and Gacek, 1993). Secondary changes in the central auditory pathways also contribute to the hearing deficits (Syka, 2002).
Multiple mechanisms have been proposed for age-related cochlear degeneration, and it appears that both genetic and environmental factors play a role (Van Eyken et al., 2007). A dominant theory is that accumulated oxidative stress causes cochlear damage (Seidman et al., 1999; Staecker et al., 2001; Jiang et al., 2007), possibly as a consequence of impaired blood flow (Dai et al., 2004) or environmental factors such as excessive noise (Ohlemiller et al., 2000a). This results in membrane and mitochondrial DNA damage in cochlear tissues leading to cell death and hearing loss (Fischel-Ghodsian et al., 1997; Ueda et al., 1998; Seidman et al., 1999; Fischel-Ghodsian, 2003; Pickles, 2004; Yin et al., 2007; Niu et al., 2007).
Much of our understanding of the mechanisms of ARHL comes from animal models (Zheng et al., 1999; Ohlemiller, 2006; Bielefeld et al., 2008). The variance seen in susceptibility to hearing loss both within and between genetic models is similar to that seen in susceptibility to both noise- and age-related hearing loss in humans, which may include individual differences in resistance to environmental stress (Duvdevany and Furst, 2007). Inbred mice often show early onset ARHL (Zheng et al., 1999), and the C57BL/6J mouse is the most established model of accelerated ARHL exhibiting behavioural and functional changes similar to those in the aging human ear (Prosen et al., 2003; Francis et al., 2003). Akin to humans, the hearing loss progresses from the high to low frequencies and the damage comprises loss of sensory cells and neurons starting in the base and progressing to the apex of the cochlea (Spongr et al., 1997; Bartolome et al., 2002). Other studies have shown that cochlear pathology also includes degeneration of the stria vascularis and fibrocytes of the spiral ligament (Ichimiya et al., 2000; Hequembourg and Liberman, 2001), reinforcing the utility of the C57BL/6J mouse as a model of mixed presbyacusis in humans (Ohlemiller, 2006). The C57BL/6J mouse is also more susceptible to the damaging effects of noise exposure, ototoxic drugs and hypoxia (Ohlemiller et al., 2000a; Ohlemiller, 2006), suggesting diminished protective or reparative processes compared to mouse strains that do not show early onset ARHL.
The ARHL locus (ahl) that contributes to the hearing loss in the C57BL/6J mouse has been mapped to chromosome 10 (Erway et al., 1993; Johnson et al., 1997). It has been shown that strains susceptible to early onset ARHL carry a specific mutation in the cadherin 23 gene (Cdh23753A), which encodes a component of the hair cell stereocilia tip-link associated with the mechanical-to-electrical transduction channels (Noben-Trauth et al., 2003). There is also evidence that the ahl locus on chromosome 10 is not the only region involved in the development of hearing loss in inbred mice (Johnson and Zheng, 2002; Keithley et al., 2004; Mashimo et al., 2006; Zheng et al., 2009). Ahl3 on chromosome 17, for example, contributes to susceptibility of C57BL/6J mice to age- and noise-induced hearing loss (Morita et al., 2007). In keeping with the mitochondrial theory of aging (Loeb et al., 2005), it was proposed that the ahl locus at mouse chromosome 10 mediates a decrease in protective anti-oxidant enzymes and consequently increased impact of oxidative stress on tissues (Staecker et al., 2001). This notion has been confirmed in a recent study (Someya et al., 2009) demonstrating that Bak-mediated mitochondrial apoptosis in response to oxidative stress is a key mechanism of ARHL in C57BL/6J mice. The Cdh23753A allele thus affects the age of onset of ARHL, but the basic mechanisms of cochlear aging such as oxidative imbalance appear to be similar in early and late onset ARHL mouse strains (Someya et al., 2009).
As the prevalence of hearing impairment increases with an aging population (Gates and Mills, 2005), there is a demand for novel treatment strategies that would target the principal mechanisms of ARHL and reduce the impairment. We and others have shown that the adenosine signalling system in the cochlea has an important role in its protection from oxidative stress (for review, see Vlajkovic et al., 2009). For example, the administration of A1 adenosine receptor agonists onto the round window membrane (a membrane separating the middle ear from the perilymph of the cochlea) can prevent cochlear injury from noise (Hu et al., 1997; Hight et al., 2003) or partially reverse hearing loss after noise exposure (Wong et al., 2010; Vlajkovic et al., 2010a). In addition, selective A1 adenosine receptor agonists can reduce cisplatin-induced auditory threshold shifts (Whithworth et al., 2004), most likely by promoting the antioxidant defense system (Ford et al., 1997).
Adenosine signalling is known to decline in the aging brain (Cunha, 2005), and a similar process has been postulated to occur in the aging cochlea (Vlajkovic et al., 2009). Given the evidence of an otoprotective effect of adenosine described above, restoring adenosine signalling may protect the cochlea from age-related degeneration. Adenosine kinase (ADK) is the primary route for adenosine metabolism and the principal negative regulator of intracellular and extracellular adenosine concentrations in the brain (Boison, 2006) and the cochlea (Vlajkovic et al., 2010b). We have previously demonstrated that physiological reduction of ADK expression is associated with an increase in endogenous adenosine in the brain (Pignataro et al., 2008); conversely, experimental overexpression of ADK in the brain is associated with a reduced concentration of adenosine (Fedele et al., 2005). Subsequently, we demonstrated that ADK-expression levels are key determinants for adenosine-based neuroprotection in the brain (Li et al., 2008; Pignataro et al., 2007; Theofilas et al., 2011). Drawing on this background, it is reasonable to speculate that activity of ADK and the resultant enhancement of endogenous adenosine levels in the cochlea has potential to ameliorate ARHL. In this study, we measured auditory thresholds and hair cell loss in C57BL/6J mice in the period spanning 3–9 months of age (by which point this strain of mice develops significant ARHL) to investigate the otoprotective potential of the selective ADK inhibitor ABT-702. This study provides the first evidence that a manipulation of the adenosine signalling system in the cochlea can delay the onset of ARHL.
2. Materials and methods
2.1. Animals
Male C57BL/6J inbred mice were used in this study. The mice were housed under standard conditions at the animal unit at the University of Auckland for the duration of the study (up to 6 months). All experimental procedures described in this study were approved by the University of Auckland Animal Ethics Committee.
2.2. Adenosine kinase immunohistochemistry
Adenosine kinase (ADK) immunostaining in cochlear tissues of 3-month-old C57BL/6 mice was visualised by laser scanning confocal microscopy. Mice were euthanized with sodium pentobarbital (100 mg/kg i.p.) and perfused transcardially with 4% paraformaldehyde (PFA) in a 0.1 M phosphate buffer. Mouse cochleae were fixed overnight in 4% PFA, and then decalcified in 5% EDTA solution for 7 days. After overnight cryoprotection in 30% sucrose, they were rinsed in 0.1 M phosphate-buffer (PB, pH 7.4), snap-frozen in isopentane at −80°C and cryosectioned at 30 μm. The sections were placed in 24-well plates (Nalge Nunc Int., Rochester, NY, USA) in sterile 0.1 M PBS. The tissues were permeabilised with 1% Triton X-100 for 1 hr, and non-specific binding sites blocked with 5% bovine serum albumin (BSA) and 5% normal goat serum (Vector Laboratories, Burlingame, CA, USA). Primary ADK antibody used in this study (a polyclonal rabbit antibody raised against recombinant mouse ADK) was previously characterised in tissues of AdK+/+ and AdK−/− mice (Gouder et al., 2004) and in the rat cochlea (Vlajkovic et al., 2010b). The antibody was diluted (1:500) and applied overnight at 4°C. In control experiments, the primary antibody was omitted. The sections were then incubated with the secondary antibody (Alexa 488 goat anti-rabbit IgG, dilution 1:400; Molecular Probes, Eugene, OR, USA) for 2 hr at room temperature. The sections were rinsed several times in PBS, mounted in Citifluor (Citifluor Ltd, London, UK) and screened for ADK labelling using a high-resolution laser scanning confocal microscopy (Olympus FluoView FV1000, Olympus Corporation, Tokyo, Japan) with 488 nm excitation from an argon ion laser. Image acquisition was controlled by FluoView software (FV10-ASW version 1.5, Olympus) with a bandpass emission of 520 nm. A series of 6–10 optical sections were collected for each specimen, and image analysis was performed on an optical section from the centre of the stack. Five cochleae obtained from different animals were analysed and the representative images are shown.
2.3. Treatment with ABT-702
A selective ADK inhibitor 4-Amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido[2,3-d]pyrimidine (ABT-702) was obtained from Sigma-Aldrich. ABT-702 (5 mg) was dissolved in 0.25 mL of DMSO (20 mg/mL) and then in 9.75 mL of distilled (MilliQ) water to prepare a 0.5 mg/mL stock solution. The solutions were aliquoted and stored at −20°C for later use. When required, the aliquots were warmed in a 37°C water bath for 30 minutes before intraperitoneal administration. An equivalent volume of vehicle solution was administered to the control animals. C57BL/6J mice used in this study were assigned to four groups shown in Table 1. All animals were euthanised at 9 months, and their cochleae harvested for hair cell counting.
Table 1.
Experimental groups
| Group | Age at start | Treatment | Regime | Tissue taken |
|---|---|---|---|---|
| 1 | 3 months | ABT-702 (1.5 mg/kg) | Twice a week, 6 months | - |
| 2 | 3 months | Vehicle | Twice a week, 6 months | - |
| 3 | 6 months | ABT-702 (1.5 mg/kg) | Twice a week, 3 months | ✓ |
| 4 | 6 months | Vehicle | Twice a week, 3 months | ✓ |
n = 8 animals per group
2.4. Auditory Brainstem Responses (ABR)
Auditory thresholds in all mice were evaluated monthly using auditory brainstem responses (ABR). ABR represents the activity of the auditory nerve and the central auditory pathways (brainstem/mid-brain regions) in response to transient sounds (acoustic clicks or tone pips). All ABR measurements were performed in a sound attenuating chamber (Shelburg Acoustics, Sydney, Australia). Mice were anaesthetised with a mixture of ketamine (75 mg/kg) and xylazine (10 mg/kg) intraperitoneally, and then placed onto a heating pad, to maintain body temperature at 37°C. ABRs were obtained by placing fine platinum electrodes subdermally at the mastoid region of the ear of interest (active electrode), scalp vertex (reference) and mastoid region of the opposite ear (ground electrode). The acoustic stimuli for ABR were produced and the responses recorded using a Tucker-Davis Technologies auditory physiology System II workstation (Alachua, FL). Auditory clicks (10 μsec square wave, alternating polarity) or tone pips (5 ms duration, 1.5 ms rise and fall times, 4 – 28 kHz) were presented. The output of this closed field acoustic stimulus system was calibrated regularly using a sound level meter (Bruel and Kjaer model 2235, Naerum, Denmark) and a 1/8th inch microphone (Bruel and Kjaer model 4138). The output of the sound level meter was passed to a spectrum analyser (Stanford Instruments model SR760) to record the frequency response and amplitude of the stimuli and to an oscilloscope or TDT input to capture the stimulus waveform. The Beyer headphone has a flat frequency response to approximately 20 kHz.
The threshold of the ABR complex (waves I – V) was determined by progressively attenuating the sound intensity in 5 dB steps from 90 dB SPL until the wave I–V complex of the averaged ABR waveforms was no longer distinguishable from noise floor in recorded traces. Wave I is the most robust wave of the mouse ABR and is considered to be generated by auditory nerve activity (Zheng et al., 1999). The ABR threshold was defined as the lowest intensity (to the nearest 5 dB) at which a response could be visually detected above the noise floor (approximately 0.5 μV). Repeat waveforms were analysed at each frequency to determine the consistency of the responses and to identify the recurring peaks. Waveforms were saved and analysed off-line to determine the amplitude of wave 1 (voltage measured from baseline to peak).
2.5. Hair cell counts
After the last ABR measurement, 9-month-old C57BL/6J mice were euthanised with an overdose of anaesthetic (pentobarbitone, 100 mg/kg i.p.) and cochleae removed for histology. After overnight fixation in 4% paraformaldehyde (PFA), the cochleae were decalcified for 7 days in 5% EDTA, decapsulated and the organ of Corti removed. The organ of Corti was separated into two pieces representing the apical and basal turns, and the tissues permeabilised with 1% Triton-X 100 for 1 hour. Alexa Fluor 488 phalloidin (Invitrogen) dissolved in 0.1 M phosphate-buffered saline (PBS, pH 7.4) was used to stain F-actin in the hair cells and their stereocilia to aid quantitative analysis of the hair cell population. Tissues were incubated in 1:400 phalloidin in 0.1 M PBS for 1 hour, washed with 0.1 M PBS several times and mounted onto glass slides using Citifluor. The slides were visualised using a Zeiss epifluorescence microscope equipped with an Axiocam camera and controlled by Axiovision v3.1 software. Images were taken from the entire length of the cochlea, and the number of missing inner and outer hair cells was counted for the apical and basal turns respectively, and presented as a percentage of total number of hair cells for each turn. Hair cell counting was performed for groups 3 and 4 only (Table 1).
2.6. Statistical Analysis
ABR thresholds and amplitude input-output functions were analysed using a one-way ANOVA followed by Tukey’s multiple comparison test. Hair cell loss was analysed using a Student’s unpaired t-test assuming unequal variances; data met the normal distribution criterion. The α level was set at P = 0.05.
Results
3.1. ADK distribution in the adult mouse cochlea
There was extensive distribution of ADK immunofluorescence in the adult C57BL/6J mouse cochlea, which was reminiscent of the nuclear/cytoplasmic ADK immunolabelling seen in the adult rat cochlea (Vlajkovic et al., 2010b). ADK immunofluorescence was observed in the spiral ganglion neurones and satellite cells, fibrocytes and interdental cells in the spiral limbus, epithelial cells of the auditory sensory organ (Deiters’ cells, inner and outer sulcus cells), fibrocytes in the spiral ligament (mostly Type 3 fibrocytes bordering the otic capsule) and marginal cells of the stria vascularis (Fig. 1). No qualitative differences were noted in the ADK expression along the cochlea, hence the mid-cochlear turn was taken as representative of the entire length of the cochlea. Immunolabelling was absent when the primary antibody was omitted (Fig. 1 inset).
Fig. 1.
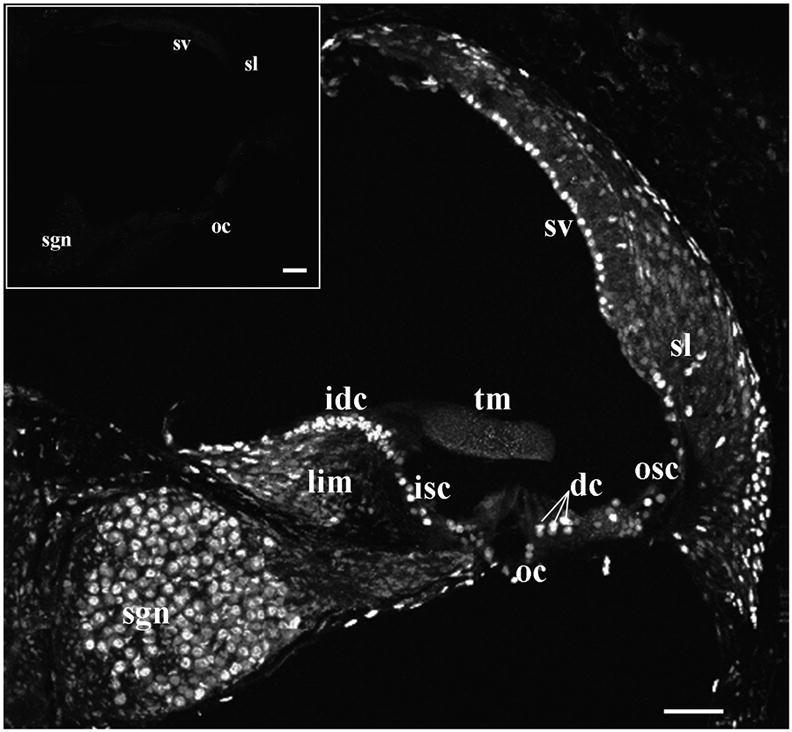
Adenosine kinase distribution in the adult C57BL/6J mouse cochlea (confocal immunofluorescence). ADK immunolabelling is present in the spiral ligament (sl), stria vascularis (sv), outer sulcus cells (osc), supporting Deiters’ cells (dc) in the organ of Corti (oc), inner sulcus cells (isc), interdental cells (idc) of the spiral limbus (lim) and spiral ganglion neurones (sgn), but absent from the tectorial membrane (tm). No immunofluorescence was observed in the absence of the primary antibody (inset). The figure is representative of five individual images from the middle turn of the cochlea. Scale bars: 50 μm.
3.2. Inhibition of adenosine kinase mitigates age-related hearing loss in C57BL/6J mice
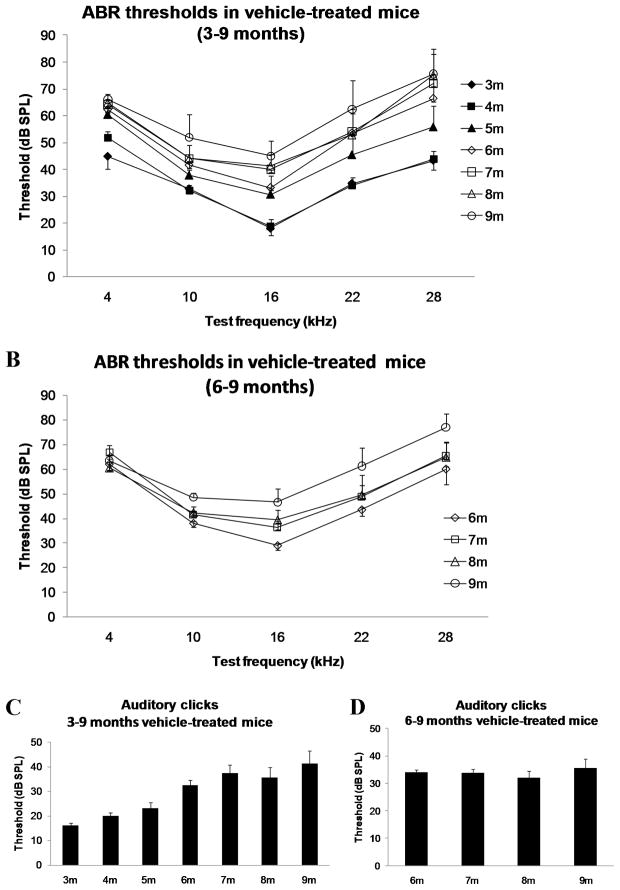
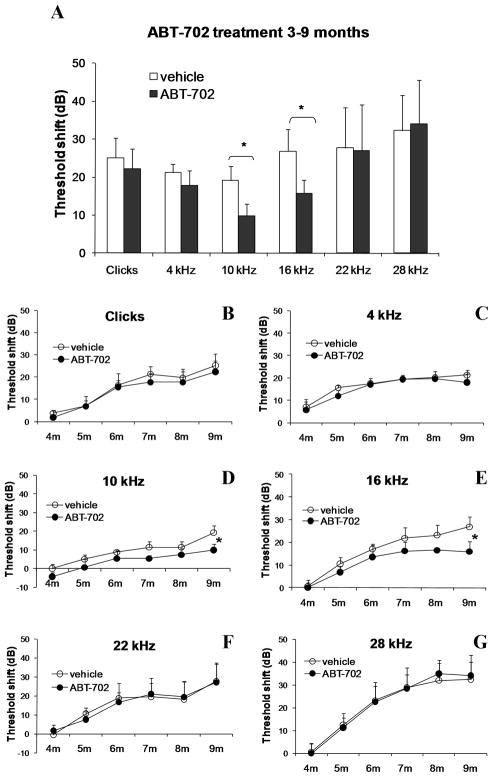
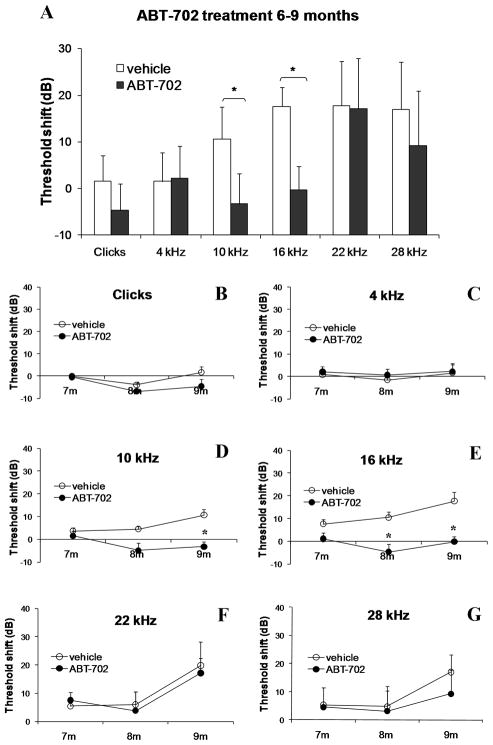
Extensive distribution of ADK in cochlear tissues of adult C57BL/6J mice supports the potential role of ADK as a principal regulator of adenosine signalling in the cochlea. Therefore, we investigated the effect of blocking ADK activity with a selective ADK inhibitor, ABT-702, on the development of ARHL. This experimental treatment was designed to boost adenosine levels in cochlear fluids by reducing ADK activity and thereby decreasing the uptake of adenosine from the extracellular space. The treatment of C57BL/6J mice with ABT-702 (1.5 mg/kg i.p.) commenced at the age of 3 months or 6 months and continued twice a week for a period of 6 months (Group 1, Table 1) or 3 months (Group 3, Table 1) respectively. Auditory thresholds were evaluated using auditory brainstem responses (ABR) once a month. Figure 2 establishes the model of ARHL by showing the increase in ABR thresholds with age at test frequencies ranging from 4–28 kHz (Figs. 2A,B) and auditory clicks broadly representative of the more sensitive region of hearing (Figs. 2C,D). At the age of 9 months, both early and late treatments with ABT-702 significantly (p < 0.05) reduced ABR threshold shifts at frequencies of 10 and 16 kHz (region of most sensitive hearing) compared to control animals receiving the vehicle solution (Figs. 3A and 4A). The development of hearing loss over time in treated and control animals is further demonstrated in Figs. 3B–G and 4B–G. Animals treated with ABT-702 from 3 to 9 months of age show delayed progression of hearing loss at 10 and 16 kHz (Figs. 3D,E), however ABR threshold shifts at other frequencies were not significantly affected (Figs. 3B,C,F,G). A selective improvement of ABR thresholds was also demonstrated in animals treated with ABT-702 from 6 to 9 months of age (Fig. 4). ABR thresholds for auditory clicks and at 4 kHz remained stable between 6 and 9 months both in control and ABT-702-treated animals (Fig. 4B,C). At higher frequencies, ABR thresholds were elevated in control animals (Figs. 4D–G). Treatment with ABT-702 reduced threshold shifts at 10 and 16 kHz (Figs. 4D,E), but did not prevent hearing loss at 22 and 28 kHz (Figs. 4F,G). Whilst ABR thresholds are a sensitive measure of hair cell damage, they are less sensitive to the amount of primary neuronal degeneration (Kujawa and Liberman, 2009). Suprathreshold measures, such as wave 1 amplitude, may be a more sensitive index of the severity of neuronal injury (Kujawa and Liberman, 2009). Thus to evaluate the effect of ABT-702 treatment on survival of spiral ganglion neurones in the mid-cochlear region, we have measured the amplitudes of the ABR wave 1 responses at 10 and 16 kHz, the two frequencies substantially affected by ABT-702 treatment. ABR wave 1 amplitudes are presented in Fig. 5 for early (3–9 months) and late (6–9 months) treatments. Baseline wave 1 amplitudes at 10 and 16 kHz measured before the start of ABT-702 or vehicle treatment were similar between the groups at 3 months (Figs. 5A,C) and at 6 months of age (Figs. 5E,G). At 9 months, suprathreshold amplitudes of ABR wave 1 significantly (p < 0.05) increased in ABT-702-treated mice at 10 and 16 kHz (Figs. 5B,D,F,H), lending further support to the otoprotective role of ABT-702 extending to both hair cells and their primary afferent innervation provided by the spiral ganglion neurones.
Fig. 2.
Age-related changes of ABR thresholds in control vehicle-treated C57BL/6J mice. (A) For tone pips at 3–9 months of age; (B) for tone pips at 6–9 months; (C) for auditory clicks at 3–9 months; (D) for auditory clicks at 6–9 months. Data are expressed as means ± SEM (n = 8).
Fig. 3.
ABR threshold shifts in C57BL/6J mice treated with a selective ADK inhibitor ABT-702 or control vehicle solution for 6 months (early treatment). (A) ABR threshold shifts for auditory clicks and tone pips (4–28 kHz) were calculated as the difference between ABR thresholds at 9 months and baseline ABR thresholds at 3 months. (B–G) ABR threshold shifts at monthly intervals (4–9 months) relative to baseline ABR thresholds measured at 3 months. Data are expressed as means ± SEM (n = 8). *P = 0.045 (10 kHz); *P = 0.034 (16 kHz).
Fig. 4.
ABR threshold shifts in C57BL/6J mice treated with a selective ADK inhibitor ABT-702 or control vehicle solution for 3 months (late treatment). (A) ABR threshold shifts for auditory clicks and tone pips (4–28 kHz) were calculated as the difference between ABR thresholds at 9 months and baseline ABR thresholds at 6 months. (B–G) ABR threshold shifts at 7, 8 and 9 months relative to baseline ABR thresholds measured at 6 months. Data are expressed as means ± SEM (n = 8). *P = 0.034 (10 kHz); *P = 0.003 (16 kHz).
Fig. 5.
The input-output functions of ABR responses (wave 1 amplitudes at 10 and 16 kHz) in ABT-702-treated and control vehicle-treated C57BL/6J mice (early and late treatments). Only those data where a repeatable wave 1 could be measured above the noise floor were plotted. Baseline wave 1 amplitudes measured before the start of ABT-702 or vehicle treatment were similar between the groups at 3 months (A,C) and at 6 months of age (E,G). At 9 months, suprathreshold amplitudes of ABR wave 1 increased in ABT-702-treated mice at 10 kHz (B,F) and 16 kHz (D,H). Data are expressed as means ± SD (n = 8); *P < 0.05.
3.3. ABT-702 reduces hair cell loss in the aging C57BL/6J cochlea
The hair cells were counted in the cochlea of 9-month-old C57BL/6J mice (Groups 3 and 4; Table 1) and the hair cell loss was compared between ABT-702- and vehicle-treated animals. The loss of hair cells was accentuated in the basal turn of the cochlea corresponding to frequencies >20 kHz, however the treatment with ABT-702 did not improve hair cell survival in that region (Fig. 6). Increased survival of hair cells was, however, observed in the apical turn of the cochlea (Fig. 6) corresponding to the frequencies below 20 kHz (Viberg and Canlon, 2004). The number of missing hair cells in the apical cochlear turn was reduced by 50% in animals treated with ABT-702 compared to control vehicle-treated animals (4% vs 8%; p<0.05).
Fig. 6.
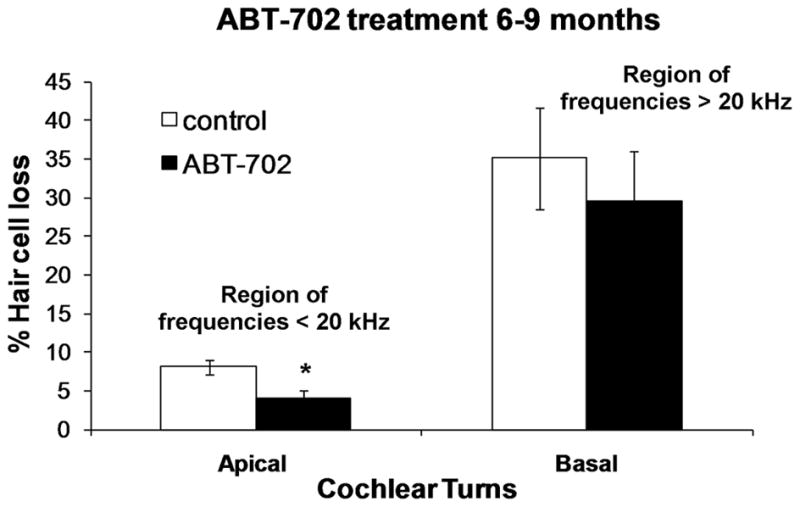
The percentage hair cell loss in cochlea of C57BL/6J mice treated with ABT-702 or control vehicle solution between 6 and 9 months of age (late treatment). Data are presented as means ± SEM. (n = 8). *P = 0.01, unpaired t-test. Scale bars: 50 μm.
3. Discussion
This study demonstrates that treatment with a selective adenosine kinase inhibitor ABT-702, thereby enhancing extracellular adenosine, can provide partial protection from age-related hearing loss in C57BL/6J mice. Chronic treatment with ABT-702, commencing at the age of 3 months or 6 months, improved auditory thresholds, suprathreshold responses and hair cell survival in aging (9-month-old) mice. This implies that ABT-702 enhances the survival of both sensory hair cells and spiral ganglion neurones in the aging mouse cochlea. The treatment appears to be more effective in the apical, lower frequency range, compared with the more basal high frequencies. Given there was no difference in the distribution of ADK along the cochlea, this difference in response may indicate differential injury processes along the cochlea, or perhaps differences in the distribution of adenosine receptors or ABT-702. Oxidative stress may be a prevalent mechanism causing the age-related degeneration in the low-mid frequency region. However, structural abnormalities of hair cell tip-links, which facilitate the opening of hair cell mechanical transduction channels, could be the principal mechanism leading to hair cell degeneration in the high-frequency region, which would render this region less susceptible to manipulation of adenosine receptor signalling. Potential base-to-apex gradients in the distribution of adenosine receptors may also result in differential sensitivity to ADK inhibition in the high- and low-frequency regions of the cochlea. It should also be noted that with systemic drug administration, differential drug access to the cochlea and the distribution within the cochlea can be expected (Sha et al., 2001).
Age-related hearing loss (ARHL) is the most common sensory deficit in senescence, and yet the prevention of ARHL is a relatively novel research field (Bielefeld et al., 2010). To date, several strategies have been proposed to mitigate ARHL: increasing the anti-oxidant defence system (Finkel and Holbrook, 2000), caloric restriction (Sweet et al., 1988; Seidman, 2000; Someya et al., 2010), induction of heat shock proteins (Mikuriya et al., 2008) and electrical stimulation to restore the endocochlear potential (Schmiedt, 1993). The cochlear anti-oxidant system can be strengthened endogenously in the enhanced acoustic environment (Tanaka et al., 2009) or exogenously using a variety of anti-oxidant treatments such as vitamin C and E (Seidman et al., 2000) and lecithin (Seidman et al., 2002). In addition, animals with genetic deletion of anti-oxidant enzymes such as superoxide dismutase 1 and 2 (SOD1, SOD2) or glutathione peroxidase show accelerated ARHL (McFadden et al., 1999; Ohlemiler et al., 2000b; Keithley et al., 2005). Caloric restriction (CR) delays the onset of many age-related diseases in animal models, and it was postulated that the delayed ARHL could be a consequence of the prolonged lifespan (Bielefeld et al., 2010). It is more likely, however, that CR suppresses apoptotic cell death in the aging mammalian cochlea by down-regulating stress-related intracellular signalling pathways and up-regulating molecular pathways essential for mitochondrial function and DNA repair (Someya et al., 2007).
Based on the demonstrated tight inverse correlation of ADK expression levels and the ambient tone of adenosine (Li et al., 2008; Pignataro et al., 2007, 2008; Theofilas et al., 2011) we postulate that increased endogenous adenosine levels in cochlear fluids resulting from ADK inhibition leads to the better preservation of the sensory cells and delayed onset of hearing loss in the mid-frequency region. It is not clear from this study whether treatment with ABT-702 has any off-target effects, and how these effects could contribute to the improvement of hearing thresholds observed in this study.
As for the role of adenosine kinase in tissue protection, previous studies have demonstrated that ADK may have a pivotal role in the brain response to injury (Boison, 2006, 2008). Transient down-regulation of ADK after acute brain injury protects the brain from further seizures and cell death, whilst up-regulation of ADK is associated with epileptogenesis and neuronal cell loss (Li et al., 2008). Regulation of adenosine levels by ADK may also have a key role in brain protection from ischemic injury (Pignataro et al., 2008). Pharmacological inhibition of ADK is effective therapeutically with an improved side-effect profile compared with A1 receptor agonists (Kowaluk and Jarvis, 2000; Jarvis et al., 2002; Gouder et al., 2004). ABT-702 is a new generation ADK inhibitor (McGaraughty et al., 2005) lacking the off-target effects of classical ADK inhibitors such as 5′-amino-5′-deoxyadenosine or 5′-deoxy-5-iodotubercidin. ABT-702 was shown to readily cross the blood brain barrier and brain levels of ABT-702 are approximately one third of plasma levels (Suzuki et al., 2001). This orally active drug has an exceptional potency in thermal hyperalgesia (Jarvis et al, 2000; Lee et al., 2001) and neuropathic pain (Suzuki et al., 2001). In our study, no toxic effects or overt behavioural changes were associated with chronic administration of ABT-702 over the period of 3–6 months. Body weight of mice increased evenly in ABT-702-treated and control groups during the measurement period.
Adenosine receptors activate a number of signalling pathways involved in tissue survival including several mitogen-activated protein kinases (Fredholm et al., 2001). The common feature of all adenosine receptors is the positive coupling to ERK1/2, whilst A2B and A3 receptors can also activate the stress-activated protein kinases JNK and p38 (Fredholm et al., 2001; Hammarberg et al., 2004). ERK1/2 are prosurvival signals (Datta et al., 1999), whilst JNK is a proapoptotic signal (Lin and Dibling, 2002). A2A receptors are coupled with the activation of adenylyl cylase and generation of cAMP in a canonical way (Fredholm et al., 2001). Stimulation of the A2A receptors leads to activation of the cAMP-dependent protein kinase A (PKA) which in turn may phosphorylate a range of different proteins and transcription factors such as the cAMP response element binding protein (CREB) (Lynge et al., 2003). Phosphorylated CREB in turn activates transcription of a number of prosurvival genes (Wilson et al., 1996; Riccio et al., 1999). In addition to the activation of anti-apoptotic signalling pathways, released adenosine may increase cochlear blood flow and oxygen supply via A2A receptors, and enhance anti-oxidant defences via A1 receptors (Vlajkovic et al., 2009). This implies that the balanced activation of A1 and A2A receptors resulting from pharmacological inhibition of ADK is likely required for the survival and proper functioning of critical tissues in the aging cochlea.
In summary, ADK inhibition shows potential as an experimental strategy for therapeutic management of ARHL. Increasing endogenous adenosine levels in the aging cochlea potentially has multiple benefits which minimise the risk of hearing loss in senescence.
Research highlights.
Age-related hearing loss (ARHL) is the most common sensory deficit
We investigated the role of adenosine signalling in the development of ARHL in mice
Adenosine kinase (ADK) is the primary route for adenosine metabolism in the cochlea
ADK inhibition provides partial protection of C57BL/6J mice from ARHL
ARHL can be mitigated by enhancing adenosine signalling in the cochlea
Acknowledgments
This study was supported by the NZ Lottery Grants Board, Royal National Institute for Deaf People (RNID, UK), Deafness Research Foundation (NZ), Lodge Discovery 501 (NZ) and grant NS061844 from the National Institutes of Health (NIH, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartolomé MV, del CE, López LM, Carricondo F, Poch-Broto J, Gil-Loyzaga P. Effects of aging on C57BL/6J mice: an electrophysiological and morphological study. Adv Oto-Rhino-Laryngol. 2002;59:106–111. doi: 10.1159/000059247. [DOI] [PubMed] [Google Scholar]
- Bielefeld EC, Coling D, Chen GD, Li M, Tanaka C, Hu BH, Henderson D. Age-related hearing loss in the Fischer 344/NHsd rat substrain. Hear Res. 2008;241(1–2):26–33. doi: 10.1016/j.heares.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld EC, Tanaka C, Chen GD, Henderson D. Age-related hearing loss: Is it a preventable condition? Hear Res. 2010;264(1–2):98–107. doi: 10.1016/j.heares.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27(12):652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8(1):2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A(2A) receptor blockade. Purinergic Signal. 2005;1(2):111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, Yang W, Jiang S, Gu R, Yuan H, Han D, Guo W, Cao J. Correlation of cochlear blood supply with mitochondrial DNA common deletion in presbyacusis. Acta Oto-Laryngol. 2004;124:130–136. doi: 10.1080/00016480410016586. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Duvdevany A, Furst M. The effect of longitudinal noise exposure on behavioural audiograms and transient-evoked otoacoustic emissions. Int J Audiol. 2007;46:119–127. doi: 10.1080/14992020600937402. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65(1–2):125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N. Mitochondrial deafness. Ear Hear. 2003;24:303–313. doi: 10.1097/01.AUD.0000079802.82344.B5. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N, Bykhovskaya Y, Taylor K, Kahen T, Cantor R, Ehrenman K, Smith R, Keithley E. Temporal bone analysis of patients with presbyacusis reveals high frequency of mitochondrial mutations. Hear Res. 1997;110:147–154. doi: 10.1016/s0378-5955(97)00077-4. [DOI] [PubMed] [Google Scholar]
- Ford MS, Nie Z, Whitworth C, Rybak LP, Ramkumar V. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear Res. 1997;111(1–2):143–152. doi: 10.1016/s0378-5955(97)00103-2. [DOI] [PubMed] [Google Scholar]
- Francis HW, Ryugo DK, Gorelikow MJ, Prosen CA, May BJ. The functional age of hearing loss in a mouse model of presbyacusis. II. Neuroanatomical correlates. Hear Res. 2003;183(1–2):29–36. doi: 10.1016/s0378-5955(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbyacusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gouder N, Scheurer L, Fritschy JM, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24(3):692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg C, Fredholm BB, Schulte G. Adenosine A3 receptor-mediated regulation of p38 and extracellular-regulated kinase ERK1/2 via phosphatidylinositol-3′-kinase. Biochem Pharmacol. 2004;67(1):129–134. doi: 10.1016/j.bcp.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6J mice. J Assoc Res Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hight NG, Sandra LM, Henderson D, Robert F, Burkard F, Nicotera T. Noise- induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21–32. doi: 10.1016/s0378-5955(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Hu BH, Zheng XY, McFadden SL, Kopke RD, Henderson D. R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res. 1997;113:198–206. doi: 10.1016/s0378-5955(97)00143-3. [DOI] [PubMed] [Google Scholar]
- Ichimiya I, Suzuki M, Mogi G. Age-related changes in the murine cochlear lateral wall. Hear Res. 2000;139(1–2):116–122. doi: 10.1016/s0378-5955(99)00170-7. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Yu H, Kohlhaas K, Alexander K, Lee CH, Jiang M, Bhagwat SS, Williams M, Kowaluk EA. ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther. 2000;295(3):1156–1164. [PubMed] [Google Scholar]
- Jarvis MF, Mikusa J, Chu KL, Wismer CT, Honore P, Kowaluk EA, McGaraughty S. Comparison of the ability of adenosine kinase inhibitors and adenosine receptor agonists to attenuate thermal hyperalgesia and reduce motor performance in rats. Pharmacol Biochem Behav. 2002;73(3):573–581. doi: 10.1016/s0091-3057(02)00840-7. [DOI] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28(10):1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY. Ahl2, a second locus affecting age-related hearing loss in mice. Genomics. 2002;80(5):461–464. [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114(1–2):83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hear Res. 2004;188(1–2):21–28. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005;209(1–2):76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaluk EA, Jarvis MF. Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs. 2000;9(3):551–564. doi: 10.1517/13543784.9.3.551. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Jiang M, Cowart M, Gfesser G, Perner R, Kim KH, Gu YG, Williams M, Jarvis MF, Kowaluk EA, Stewart AO, Bhagwat SS. Discovery of 4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido[2,3-d]pyrimidine, an orally active, non-nucleoside adenosine kinase inhibitor. J Med Chem. 2001;44(13):2133–2138. doi: 10.1021/jm000314x. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1(2):112–116. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Loeb LA, Wallace DC, Martin GM. The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci U S A. 2005;102(52):18769–18770. doi: 10.1073/pnas.0509776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynge J, Schulte G, Nordsborg N, Fredholm BB, Hellsten Y. Adenosine A2B receptors modulate cAMP levels and induce CREB but not ERK1/2 and p38 phosphorylation in rat skeletal muscle cells. Biochem Biophys Res Commun. 2003;307(1):180–187. doi: 10.1016/s0006-291x(03)01125-2. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Erven AE, Spiden SL, Guénet JL, Steel KP. Two quantitative trait loci affecting progressive hearing loss in 101/H mice. Mamm Genome. 2006;17(8):841–850. doi: 10.1007/s00335-004-2438-5. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20(1):1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Cowart M, Jarvis MF, Berman RF. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem. 2005;5(1):43–58. doi: 10.2174/1568026053386845. [DOI] [PubMed] [Google Scholar]
- Mikuriya T, Sugahara K, Sugimoto K, Fujimoto M, Takemoto T, Hashimoto M, Hirose Y, Shimogori H, Hayashida N, Inouye S, Nakai A, Yamashita H. Attenuation of progressive hearing loss in a model of age-related hearing loss by a heat shock protein inducer, geranylgeranylacetone. Brain Res. 2008;1212:9–17. doi: 10.1016/j.brainres.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Morita Y, Hirokawa S, Kikkawa Y, Nomura T, Yonekawa H, Shiroishi T, Takahashi S, Kominami R. Fine mapping of Ahl3 affecting both age-related and noise-induced hearing loss. Biochem Biophys Res Commun. 2007;355(1):117–121. doi: 10.1016/j.bbrc.2007.01.115. [DOI] [PubMed] [Google Scholar]
- Morton NE. Genetic epidemiology of hearing loss. Ann NY Acad Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Niu X, Trifunovic A, Larsson NG, Canlon B. Somatic mtDNA mutations cause progressive hearing loss in the mouse. Exp Cell Res. 2007;313(18):3924–3934. doi: 10.1016/j.yexcr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35(1):21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise- related hearing loss. Brain Res. 2006;1091(1):89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Heidbreder AF. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: a dose-response approach in CBA/J, C57BL, and BALB inbred strains. Hear Res. 2000a;149(1–2):239–247. doi: 10.1016/s0378-5955(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000b;1(3):243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO. Mutation in mitochondrial DNA as a cause of presbyacusis. Audiol NeuroOtol. 2004;9:23–33. doi: 10.1159/000074184. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2008;28(1):17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Dore DJ, May BJ. The functional age of hearing loss in a mouse model of presbyacusis. I. Behavioral assessments. Hear Res. 2003;183(1–2):44–56. doi: 10.1016/s0378-5955(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286(5448):2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Cochlear potentials in quiet-aged gerbils: does the aging cochlea need a jump start? In: Verrillo R, editor. Sensory Research: Multimodal Perspective. Erlbaum; Hillsdale, NJ: 1993. pp. 91–104. [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbyacusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Seidman MD. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope. 2000;110(5 Pt 1):727–738. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Bai U, Khan MJ, Quirk WS. Mitochondrial DNA deletions associated with aging and presbyacusis. Archiv Otolaryngol Head Neck Surg. 1999;123:1039–1045. doi: 10.1001/archotol.1997.01900100009001. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Bai U, Shirwany N, Quirk WS. Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol. 2000;21(2):161–167. doi: 10.1016/s0196-0709(00)80003-4. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Tang WX, Quirk WS. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg. 2002;127(3):138–144. doi: 10.1067/mhn.2002.127627. [DOI] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155(1–2):1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging. 2007;28(10):1613–1622. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci USA. 2009;106(46):19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Tanokura M, Weindruch R, Prolla TA, Yamasoba T. Effects of caloric restriction on age-related hearing loss in rodents and rhesus monkeys. Curr Aging Sci. 2010;3(1):20–25. doi: 10.2174/1874609811003010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA/J and C57BL/6J mice throughout their life spans. J Acoustic Soc Am. 1997;101(6):3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Staecker H, Zheng QY, Van De Water TR. Oxidative stress in aging in the C57B16/J mouse cochlea. Acta Otolaryngol. 2001;121(6):666–672. doi: 10.1080/00016480152583593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Stanfa LC, Kowaluk EA, Williams M, Jarvis MF, Dickenson AH. The effect of ABT-702, a novel adenosine kinase inhibitor, on the responses of spinal neurones following carrageenan inflammation and peripheral nerve injury. Br J Pharmacol. 2001;132(7):1615–1623. doi: 10.1038/sj.bjp.0703972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RJ, Price JM, Henry KR. Dietary restriction and presbyacusis: periods of restriction and auditory threshold losses in the CBA/J mouse. Audiol. 1988;27(6):305–312. doi: 10.3109/00206098809081601. [DOI] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82(3):601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Bielefeld EC, Chen GD, Li M, Henderson D. Ameliorative effects of an augmented acoustic environment on age-related hearing loss in middle-aged Fischer 344/NHsd rats. Laryngoscope. 2009;119(7):1374–1379. doi: 10.1002/lary.20244. [DOI] [PubMed] [Google Scholar]
- Theofilas P, Brar S, Stewart KA, Shen HY, Sandau US, Poulsen DJ, Boison D. Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia. 2011;52(3):589–601. doi: 10.1111/j.1528-1167.2010.02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Oshima T, Ikeda K, Abe K, Aoki M, Takasaka T. Mitochondrial DNA deletion is a predisposing cause for sensorineural hearing loss. Laryngoscope. 1998;108:580–584. doi: 10.1097/00005537-199804000-00022. [DOI] [PubMed] [Google Scholar]
- Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol. 2007;12(6):345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- Viberg A, Canlon B. The guide to plotting a cochleogram. Hear Res. 2004;197(1–2):1–10. doi: 10.1016/j.heares.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Housley GD, Thorne PR. Adenosine and the auditory system. Curr Neuropharmacol. 2009;7:246–256. doi: 10.2174/157015909789152155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajkovic SM, Lee KH, Wong A, Guo CX, Gupta R, Housley GD, Thorne PR. Adenosine amine congener mitigates noise-induced cochlear injury. Purinergic Signaling. 2010a;6(2):273–281. doi: 10.1007/s11302-010-9188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajkovic SM, Guo CX, Dharmawardana N, Wong ACY, Boison D, Housley GD, Thorne PR. The role of adenosine kinase in cochlear development and response to noise. J Neurosci Res. 2010b;88:2598–2609. doi: 10.1002/jnr.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth CA, Ramkumar V, Jones B, Tsukasaki N, Rybak LP. Protection against cisplatin ototoxicity by adenosine agonists. Biochem Pharmacol. 2004;67:1801–1807. doi: 10.1016/j.bcp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol. 1996;16(10):5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Guo CX, Gupta R, Housley GD, Thorne PR, Vlajkovic SM. A1 adenosine receptor agonists attenuate noise-induced hearing loss. Hear Res. 2010;260(1–2):81–88. doi: 10.1016/j.heares.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Yin S, Yu Z, Sockalingam R, Bance M, Sun G, Wang J. The role of mitochondrial DNA large deletion for the development of presbyacusis in Fischer 344 rats. Neurobiol Dis. 2007;27(3):370–377. doi: 10.1016/j.nbd.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Ding D, Yu H, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol Aging. 2009;30(10):1693–1705. doi: 10.1016/j.neurobiolaging.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]