Abstract
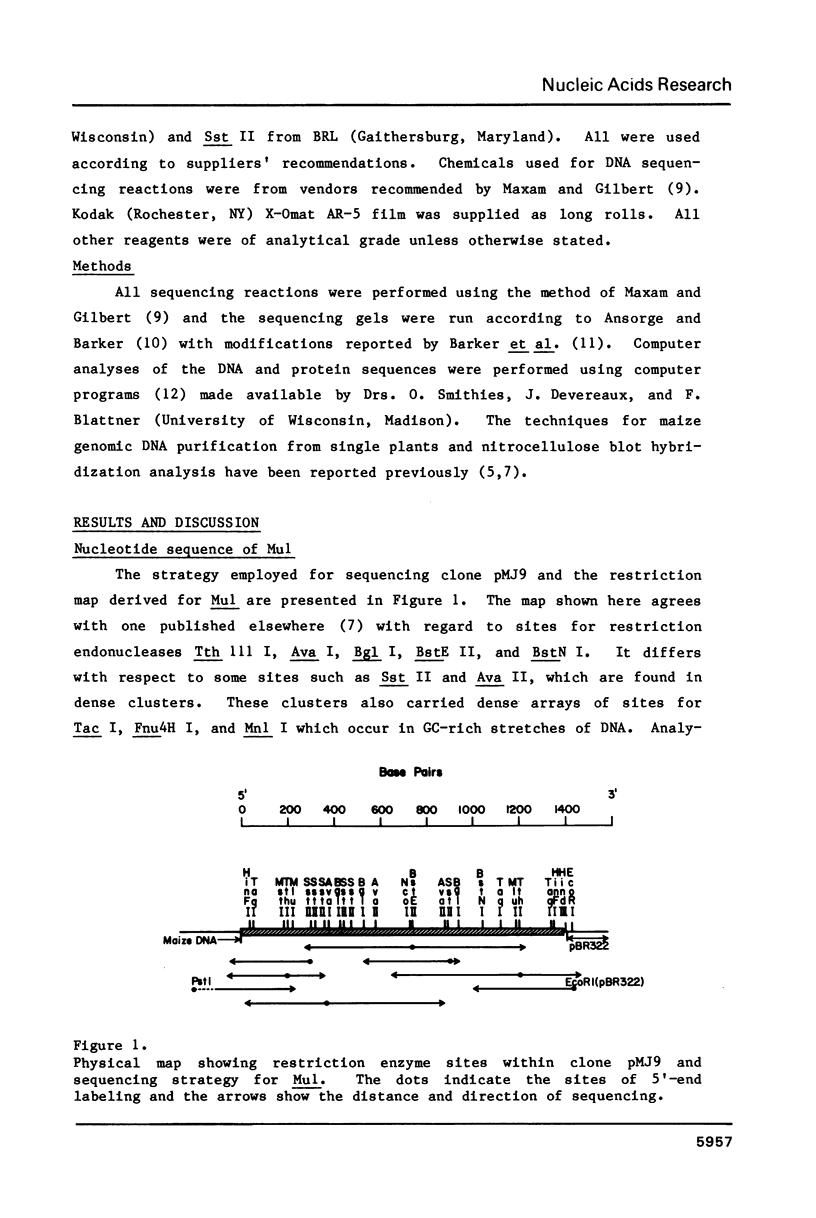
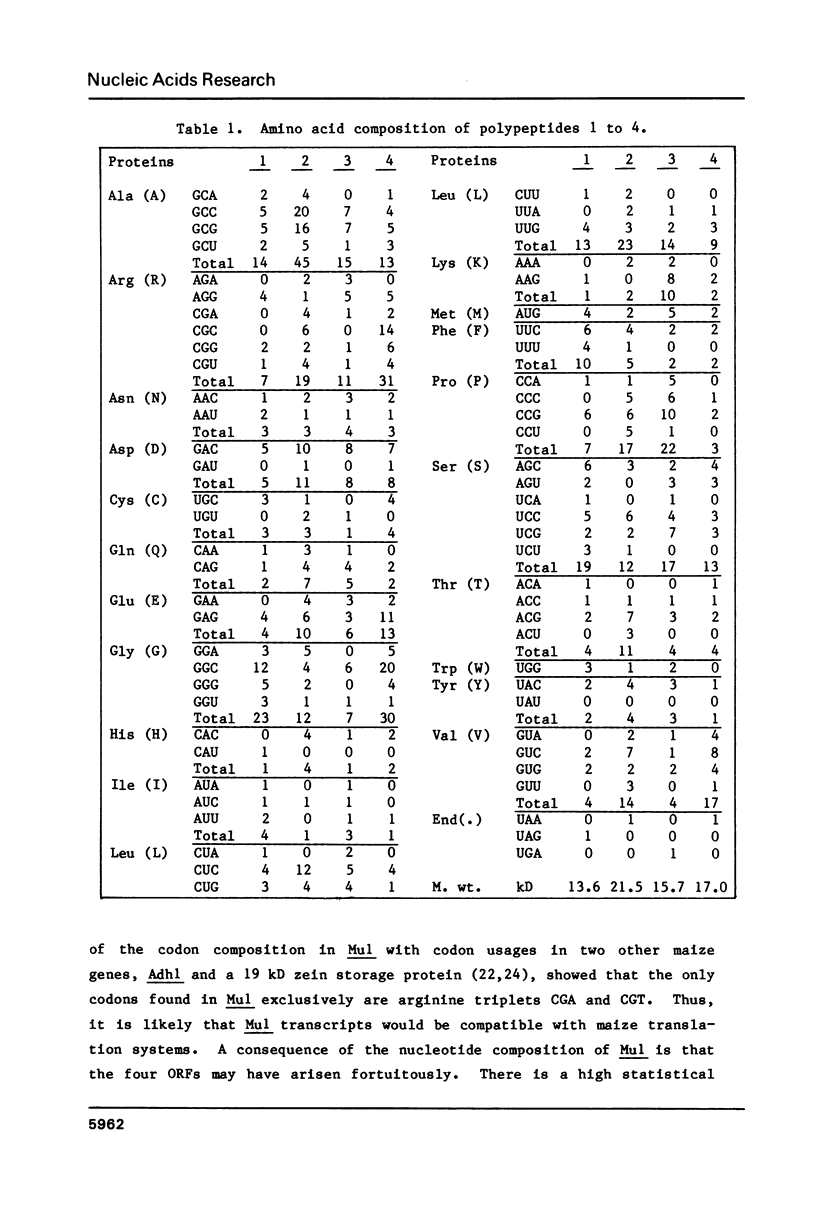
A cloned DNA fragment from the maize allele Adhl-S3034 contains all of Mul, an insertion element involved in Robertson's Mutator activity. The element is 1367 base pairs (bp) long and is flanked by nine bp direct repeats of insertion site DNA. It has inverted terminal repeats of 215 and 213 bp showing 95% homology. Within the element are two direct repeats of 104 bp showing 96% homology. Four open reading frames (ORFs) were found, two in each DNA strand. Mul can be divided into two halves, each containing one terminal inverted repeat, an internal direct repeat, and two overlapping ORFs. The GC content of each half is high (70%), while that of a central 60 base portion of the element is low (26%). The central region contains the only sequence resembling the TAATA Goldberg and Hogness eukaryotic promoter signal. Multiple copies of DNA sequences related to Mul found in Mutator maize plants are generally similar in organization to the cloned element. A larger version containing a discrete 300 to 400 base pair insertion was found in some Mutator lines.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonas U., Sommer H., Saedler H. The 17-kb Tam1 element of Antirrhinum majus induces a 3-bp duplication upon integration into the chalcone synthase gene. EMBO J. 1984 May;3(5):1015–1019. doi: 10.1002/j.1460-2075.1984.tb01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring H. P., Tillmann E., Starlinger P. DNA sequence of the maize transposable element Dissociation. Nature. 1984 Jan 12;307(5947):127–130. doi: 10.1038/307127a0. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Geraghty D., Peifer M. A., Rubenstein I., Messing J. The primary structure of a plant storage protein: zein. Nucleic Acids Res. 1981 Oct 10;9(19):5163–5174. doi: 10.1093/nar/9.19.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cruciform structures in supercoiled DNA. Nature. 1981 Feb 5;289(5797):466–470. doi: 10.1038/289466a0. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Klösgen R. B., Wienand U., Peterson P. A., Saedler H. The Spm (En) transposable element controls the excision of a 2-kb DNA insert at the wx allele of Zea mays. EMBO J. 1984 May;3(5):1021–1028. doi: 10.1002/j.1460-2075.1984.tb01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett M. A., Jones R. S., Potter S. S. Unusual structure of the FB family of transposable elements in Drosophila. Cell. 1981 Jun;24(3):753–763. doi: 10.1016/0092-8674(81)90101-x. [DOI] [PubMed] [Google Scholar]
- Zuker C., Cappello J., Lodish H. F., George P., Chung S. Dictyostelium transposable element DIRS-1 has 350-base-pair inverted terminal repeats that contain a heat shock promoter. Proc Natl Acad Sci U S A. 1984 May;81(9):2660–2664. doi: 10.1073/pnas.81.9.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]




