Introduction
Cirrhosis is a leading cause of morbidity and mortality and often arises during development of portal hypertension and its related complications. Portal decompression is therefore an important therapeutic objective. Historically, portal decompression was achieved by surgical diversion of blood from the portal vein to the systemic circulation. These major operations were associated with considerable mortality and morbidity, which decreased their utilization. About 25 years ago, a radiologic method for portal decompression, via a transjugular route, was developed to create a track between the intrahepatic portion of the portal vein and the hepatic vein, which was then kept patent using a metal stent. The transjugular intrahepatic portosystemic shunts (TIPS) revived interest in the use of portal decompression for treatment of portal hypertensive complications of cirrhosis. Over the last decade, the metal stents have been coated with materials to prevent occlusion of the lumen by in-growth of tissue from the surrounding liver. We review the clinical utility of TIPS and coated stents for the treatment of portal hypertension.
History of TIPS
Rosch and colleagues first described the creation of a track between the hepatic and portal vein using serial dilators via a cutaneous approach 1. Subsequently, cryoprobes were used to extend the duration of patency of such tracks 2. In 1981, Colapinto and colleagues created the first intrahepatic portosystemic shunt in a patient using balloon angioplasty 3. The ability to keep the track open by placing an expandable metal stent was a major technical breakthrough in the development of TIPS 4. The long-term efficacy of these shunts was however limited by the ingrowth of tissue from the surrounding liver. Due to concerns of stent dysfunction, polytetrafluoroethylene (PTFE) coated stents were introduced 5. The use of PTFE covered stents are currently preferred over bare stents 6.
TIPS Procedure
Optimal outcomes following TIPS depend on a careful selection of the patient, technically proficient placement of the shunt and also the peri-procedural care of the patient. These are briefly reviewed below:
Patient preparation
The initial pre-procedural work up of a patient prior to TIPS placement depends on the clinical circumstances under which the shunt is to be placed. Regardless of whether the shunt is placed electively or emergently, a thorough medical assessment should always be performed to assess procedural risks 7. Specifically, subjects with a prior history of encephalopathy should have the encephalopathy treated and their mental status optimized before elective TIPS placement 8. It is also generally recommended that a large volume paracentesis be performed if tense ascites is present. This allows the liver to drop down and makes it easier for the angiographic catheter to get to the portal vein from the hepatic vein. If there is any suggestion of cardiopulmonary disease, a cardiac assessment is also warranted because of the potential for development of pulmonary edema following TIPS. A Doppler ultrasound exam to document patency of the portal vein should also be performed. The liver function should be assessed prior to the procedure with a bilirubin and INR; subjects with a high bilirubin are at risk of developing progressive liver failure after TIPS and may not be well served by this procedure especially when placed under elective circumstances 7. Finally, antibiotic prophylaxis to cover bacteria resident in skin and in the intestine is often used and is a standard of practice although in a single clinical trial, routine antibiotic prophylaxis was not found to decrease peri-procedural infection 9, 10. There is no role for the routine use of fresh frozen plasma or platelet transfusions in all cases undergoing TIPS.
The TIPS procedure
Using aseptic technique, a cutaneous access to the right jugular vein is obtained. Using this access, a catheter is passed via the right atrium in to the inferior vena cava and then the right hepatic vein 11. The Colapinto needle is extruded and a track created between the hepatic and intrahepatic portion of the portal vein. This is secured by passage of a guidewire in to the portal vein. Balloon angioplasty is performed over the wire to dilate the track and then a stent is positioned across the track. The stent is then deployed and dilated up to achieve a diameter of about 10 mm Hg and to bring the pressure gradient between the portal vein and hepatic vein to less than 12 mm Hg 12.
Post-procedural management
The principal complication in the immediate perioperative period is hemorrhage. This is usually not clinically significant. However, occasionally hemoperitoneum from extrahepatic puncture of the portal vein and a large intrahepatic hematoma can develop. Vital signs are carefully monitored for several hours after the procedure. Shunt patency is assessed by use of Doppler sonography usually in the first four weeks and then at six months to a year; with currently available covered stents, the overall risk of shunt stenosis is approximately 24 % at 2 years 13. The gold standard to evaluate shunt patency is angiography, however, due to cost and invasive nature, it is reserved for patients with stent occlusion on ultrasound or clinical signs of recurrent portal hypertension.
The use of TIPS in specific clinical conditions
The principal physiologic consequence of TIPS is the shunting of blood from the hypertensive portal vein to the hepatic vein thereby decompressing the portal vein. It is therefore used primarily to treat conditions that are directly related to portal hypertension. Variceal hemorrhage and ascites are two major complications of cirrhosis that are due to portal hypertension and TIPS plays a key role in the management of these complications.
Variceal hemorrhage
In-hospital mortality of variceal bleeding has decreased to approximately 15% over the last decade, which is still clinically significant 14. The use of TIPS is likely to have played a key role in this improvement compared to prior literature. However, to use this procedure and obtain optimal outcomes, the role of TIPS must be considered in the context of where in the natural history of variceal hemorrhage it is used.
Primary Prophylaxis
There are no trials that have formally evaluated the value of TIPS for prophylactic portal decompression to keep the HVPG < 10 mm Hg and thus prevent varices and therefore variceal hemorrhage. On the other hand, the side effects of TIPS are well established 15. Therefore, there is no role for TIPS for the prevention of varices or for primary prophylaxis of variceal hemorrhage. It has also been shown that TIPS performed prior to liver transplant or other intraabominal surgery does not reduce operative time or intra-operative blood loss 16. Therefore the use of TIPS for these indications cannot be justified based on the available evidence.
Active hemorrhage
Once bleeding occurs, spontaneous hemostasis occurs in only 50% of subjects compared to over 90% of cases with non-variceal sources of upper gastrointestinal bleeding 17. Those with a HVPG > 20 mm Hg who bleed are more likely to bleed severely and fail initial medical and endoscopic treatment. Patients are often bacteremic at the time of admission with bleeding and the use of prophylactic broad spectrum antibiotics directed towards intestinal flora has been shown to improve survival in the period around active hemorrhage 19. Actively spurting varices, severe anemia and hypotension at the time of admission are also markers identifying subjects less likely to respond to medical therapy. In those where bleeding cannot be quickly controlled, the mortality is high 20. Development of aspiration pneumonia, transfusion of more than 5 units of packed red cells, hypotension and the need for artificial ventilator support are risk factors for mortality 20.
The initial goals of management of active hemorrhage include hemodynamic resuscitation, prevention and treatment of complications and achievement of hemostasis. The principles and practice of hemodynamic resuscitation and management of complications have been reviewed in depth elsewhere and the reader is referred to several excellent reviews for a more detailed discussion of these topics 21. The first-line approach for control of bleeding includes a combination of pharmacologic treatment and endoscopic variceal ligation (EVL) with rubber bands. This combination is superior to the use of pharmacologic or endoscopic treatment alone 21. Endoscopic sclerotherapy may be performed when the bleeding is too severe to allow adequate visualization for band ligation but is rarely needed. Active esophageal variceal hemorrhage can be controlled in about 80% of subjects using such an approach. There are however cases where bleeding cannot be controlled and active bleeding continues or recurs following a brief period of cessation. Over transfusion is a risk factor for such early rebleeding and should be avoided; the target hemoglobin is in the 8–9 gm/dl range in such patients 22.
The failure to control bleeding is usually clinically obvious with large amounts of red blood in the nasogastric tube, hypotension, tachycardia and the need to continually transfuse to maintain the hemoglobin in the target range. This identifies a subset of patients at great risk of dying from their variceal hemorrhage 23. When this occurs in septic subjects who are already hyperbilirubinemic, the mortality is particularly high 24. The development of pneumonia and multiorgan failure are ominous and often pre-terminal events regardless of the outcome of bleeding.
Prior to the availability of TIPS, subjects who failed first-line therapy were managed by surgical approaches to control bleeding. Emergency surgery is a viable option for salvage therapy of variceal hemorrhage especially if the liver function is normal. However, in high risk patients, mortality from emergency portocaval surgery is high at 88% 17. The ability to quickly decompress the portal vein with TIPS without the risks of major abdominal surgery has led to the use of TIPS for such cases. TIPS can be correctly placed and the portal vein decompressed in over 90% of subjects with severe refractory variceal hemorrhage 25. In an early study, subjects deemed to be high risk for surgical portocaval shunting (i.e. those with aspiration pneumonia, severe liver failure (bilirubin >6mg/dl, and prothrombin time >5 seconds than control), tense ascites, severe cardiac, pulmonary or renal disease, or coma were managed by initial control of bleeding with balloon tamponade followed by TIPS within hours 26. The airway was protected by intubation prior to balloon tamponade. Only 2/30 patients rebled due to variceal bleed. The 6 week survival rate was 60%, with aspiration pneumonia as the most common precipitant of multi-organ failure in those patients that died. Analysis of the subgroup of TIPS patients that did not develop aspiration pneumonia, revealed a 6 week survival of 90%. This study demonstrated that patients that have a high-risk of operative mortality can successfully be managed with a TIPS 26. These data have been corroborated by several other studies 27–29.
In a study of subjects with refractory bleeding, the 30 day mortality following TIPS placement was 28% and survival was significantly lower in patients of Child-Pugh Class C, at 48% versus 90% for Childs A or C (P <.001) 30. Subjects who have developed multi-organ failure or have severe hyperbilirubinemia and high model for end-stage liver disease (MELD) scores are likely to die even after successful achievement of hemostasis. It is therefore important to identify when first-line therapy has failed quickly and move to portal decompression with TIPS before complications such as sepsis, acute on chronic liver failure and multi-organ failure set it.
Prevention of rebleeding from varices
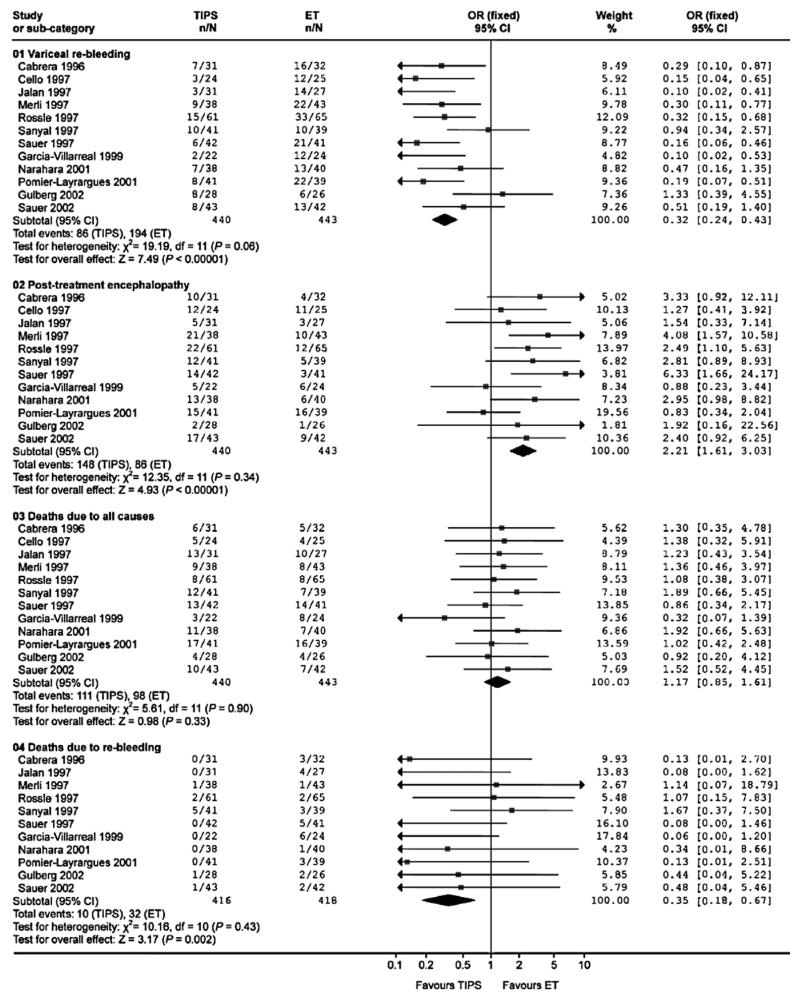
Survivors of an episode of variceal hemorrhage are at high risk of rebleeding if left alone 31. The risk factors for recurrent hemorrhage include increasing age and severity of liver failure 23, 32. The first-line treatment for prevention of rebleeding from esophageal varices is the combined use of EVL and non-selective beta blockers 21. Numerous clinical trials have evaluated the utility of TIPS versus endoscopic therapy for the prevention of recurrent bleeding and are summarize in Table 1. The results of a meta-analysis on TIPS compared to endoscopic therapy for secondary prophylaxis of variceal hemorrhage are displayed in Figure 1 33. In general, most studies used bare stents and the endoscopic therapy used was sclerotherapy in many trials. The routine use of covered stents for TIPS and the use of EVL rather than sclerotherapy should be kept in mind while placing this literature in perspective.
Table 1.
Randomized control trials for the use of TIPS versus endoscopic therapy as secondary prophylaxis of variceal hemorrhage.
| Author, year | Endoscopic | N | Variceal Rebleeding (%) | Hepatic Encephalopathy (%) | Mortality (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TIPS | ET | P value | TIPS | ET | P value | TIPS | ET | P value | |||
| Cabrera, 1996 | ES | 63 | 23 | 52 | <.02 | 33 | 13 | <.05 | 20 | 16 | NS |
| Cello, 1997 | ES | 49 | 13 | 48 | .012 | 58 | 44 | .2 | 42 | 36 | NS |
| Jalan, 1997 | EVL | 58 | 10 | 52 | .0006 | 36 | 33 | NS | 42 | 20 | NS |
| Sanyal, 1997 | ES | 80 | 22 | 21 | NS | 29 | 13 | .001 | 29 | 18 | .03 |
| Sauer, 1997 | ES or EVL | 83 | 23 | 57 | .0001 | 29 | 13 | .041 | 29 | 27 | NS |
| Rossle, 1997 | ES | 46 | 15 | 41 | <.001 | 36 | 18 | .011 | 13 | 12 | NS |
| Merli, 1998 | ES | 81 | 24 | 51 | <.05 | 55 | 26 | .006 | 24 | 19 | .50 |
| Garcia-Villareal, 1999 | ES | 46 | 9 | 50 | .001 | 23 | 25 | NS | 15 | 33 | <.05 |
| Pomier- Layrargues, 2001 | EVL | 80 | 18 | 66 | <.001 | 47 | 44 | NS | 41 | 41 | NS |
| Sauer, 2002 | EVL | 85 | 19 | 30 | NS | 41 | 21 | .041 | 19 | 17 | NS |
| Gulberg, 2002 | EVL | 54 | 33 | 37 | NS | 8 # | 4 # | -- | 20 | 17 | NS |
| Garcia-Pagan, 2010 | EVL | 63 | 3 | 45 | .001 | 25 | 39 | NS | 13 | 38 | <.01 |
ET = Endoscopic therapy, ES = Sclerotherapy, EVL = Endoscopic Variceal Ligation, NS = Not statistically Significant (P value not stated), # at one month
Figure 1.
Forest plot of meta-analysis revealing Odds Ratios for variceal re-bleeding, post-treatment encephalopathy, death due to all causes, and deaths due to re-bleeding comparing TIPS and endoscopic therapy 32.
In general, TIPS is superior to endoscopic treatment for the prevention of rebleeding. This does not however translate in to a survival advantage. TIPS is also associated with an increased risk of hepatic encephalopathy which occurs in about a third of subjects. Bare stents are further limited by the ingrowth of a pseudointima from the surrounding liver and the occlusion of the shunt from hyperplasia of this pseudointima 34, 35. These considerations relegated TIPS to the role of salvage therapy for refractory variceal hemorrhage and especially as a bridge to transplant 26, 28.
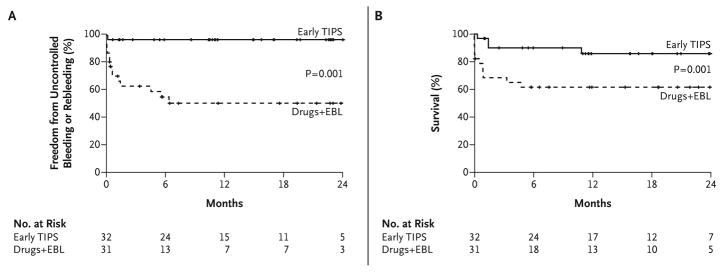
Several recent developments have led to the reassessment of the role of TIPS. First, the problem of shunt stenosis has been largely obviated by the availability of coated stents which are associated with a much improved patency rate (about 76% at 2 years) 36. Also, in a landmark study, Monescillo et al performed hepatic vein catheterization within 12 hours of admission for variceal hemorrhage and randomized subjects with a HVPG > 20 mm Hg to either endoscopic treatment and beta blockers or TIPS 37. Subjects receiving TIPS had significantly less bleeding and improved survival. A recent multi-center study also evaluated an early TIPS approach for long-term control of bleeding 38. This trial also confirmed that TIPS performed within 72 hours of admission confers a survival advantage compared to EVL and pharmacologic treatment with beta blockers and nitrates (in 12/31 subjects). The results of this study are illustrated in Figure 2. While these are very encouraging and lead us to re-evaluate the role of TIPS in the long-term management of variceal bleeding, there are several findings that should give pause. First, the 6 week failure rate with EVL was 35%; this is a high failure rate and more than that expected within this time frame for subjects with a Child Pugh score < 13. The use of nitrates (which has been largely abandoned in routine practice) and its potential role in the failure rate of the EVL plus pharmacologic treatment arm prevents easy generalizability of these data. Finally, this study excluded subjects with a Child Pugh score > 13 and the data are principally applicable for those with lower CPT scores. Despite these caveats, there is now a growing trend towards early assessment of hepatic venous pressure gradients after initial control of bleeding and the use of TIPS within 72 hours especially if the liver function is relatively preserved.
Figure 2.
Panel A: Probability of remaining free from rebleeding or uncontrolled bleeding. Panel B: Survival, according to treatment Group 38.
TIPS has also been compared to surgical shunts to manage rebleeding esophageal varices. A meta-analysis revealed that the 30-day and 1 year survival was equivalent for TIPS and surgical shunts, however at 2 years, surgical shunt patients had greater survival with an Odds Ratio (OR) 2.5 (95% CI 1.2–5.2) 39. Shunt failure was significantly reduced in surgical shunts versus TIPS 39. However, with the advent of covered stents, this is less of an issue.
Recurrence of GI bleeding after TIPS placement
In most instances, TIPS is an effective way to control bleeding varices. There are however occasions when bleeding can recur. When bleeding continues despite adequate portal decompression, one may consider embolization of the left gastric vein 40. On the other hand, if bleeding recurs a few days following TIPS placement, one must consider several possibilities. Immediately after TIPS placement, the stent continues to expand radially and shorten along its long axis. Therefore, if the stent does not protrude in to the portal vein by a few millimeters, the stent can retract in to the parenchymal tract causing it to collapse 34. Another possibility is acute thrombosis of the shunt. This can be evaluated by a variety of imaging modalities. In some cases, recurrent bleeding may represent hemobilia due to a vascular-biliary fistula 15. Finally, we have seen instances where subjects with elevated right heart pressures post-TIPS can reflect these pressures via the open shunt in to the portal system preventing adequate decompression. These possibilities should be considered when clinically significant bleeding recurs after a successful TIPS placement.
Ascites
Another major complication of cirrhosis is ascites. It affects over 60% of subjects with cirrhosis and results from hemodynamic changes triggered by sinusoidal portal hypertension which causes a decrease in effective circulating volume and activates sodium and water retention 41. In the initial stages of ascites, the fluid can be easily managed with sodium restriction and diuretics. However, a proportion of subjects is unresponsive to diuretics or cannot tolerate effective doses of diuretics due to the development of side effects. This is also referred to as refractory ascites 42. Refractory ascites occurs in approximately 10% of patients with cirrhosis 43. Progressive refractoriness to diuretics is a prelude to the onset of renal dysfunction and development of hepatorenal syndrome a dreaded complication of cirrhosis associated with a high mortality. Ascites with a low protein concentration in a subject with advanced liver failure and hyponatremia is at high risk of becoming infected resulting in spontaneous bacterial peritonitis. Spontaneous bacterial peritonitis can drive the progression from medically manageable ascites to refractory ascites and hepatorenal syndrome.
TIPS have been used principally for subjects with refractory ascites. The literature is however confounded by the small sample size in several studies, the failure to use albumin during large volume paracentesis, the widely varying serum albumin levels across trials and the exclusion of subjects with clinically significant hyperbilirubinemia in some but not other trials 44–48. Regardless of these limitations, several facts stand out. TIPS is very effective in causing clinical resolution of ascites over a period of several weeks. This is mainly associated with a natriuresis which is caused by decreased proximal tubular sodium reabsorption as measured by lithium clearance 49. It is also very effective and superior to repeated large volume paracentesis and diuretics for the maintenance of an ascites-free state. It is however noteworthy that most subjects need to continue sodium restriction and the majority of subjects require some diuretic dosing on an ongoing basis to maintain an ascites-free state.
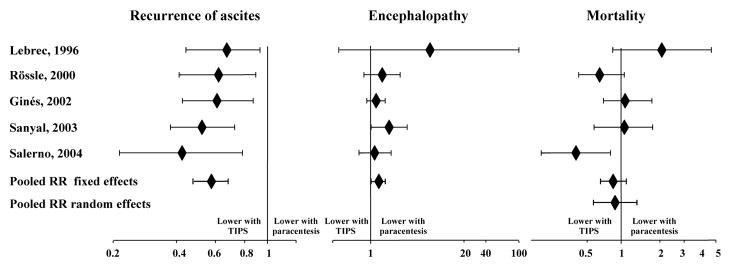
The potential impact of TIPS on survival in subjects with refractory ascites remains controversial. The results of several randomized controlled trials are summarized in Table 2. Three large multicenter trials which were rigorously performed failed to demonstrate a survival advantage 45, 46, 48. However, two studies showed an improvement in survival 44, 47. One of these studies included subjects with more preserved liver function and is not truly comparable to the other studies 44. In the other study, subjects with recurrent ascites despite paracentesis requiring additional taps (recidivant ascites) were included whereas in the earlier trials only subjects with truly refractory ascites were included 47. Based on these data, it is the position of these authors that in subjects with rigorously documented refractory ascites, TIPS can be claimed to improve survival. It is however possible that if TIPS is performed when ascites recurs rapidly but does not fully meet criteria for refractory ascites, it may be beneficial. This possibility is currently under evaluation in a large multi-center clinical trial. Meta-analyses of these trials have been performed and come to divergent conclusions; it is our position that the heterogeneity across studies limits interpretation of these meta-analyses 50–54. The results of one meta-analysis are outlined in Figure 3 50.
Table 2.
Randomized controlled trials to compare TIPS and repeated paracentesis in the management of refractory ascites.
| Author, year | N | Treatment failure | Encephalopathy | Survival – 1 year | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TIPS % | Repeated paracentesis | P value | TIPS % | Repeated paracentesis % | P value | TIPS % | Repeated paracentesis % | P value | ||
| Lebrec, 1996 | 25 | 77 | 92 | -- | 23 | 0 | -- | 29 | 56 | <.05 # |
| Rossle, 2000 | 60 | 16 | 57 | -- | 58 | 48 | NS | 69 | 52 | NS |
| Gines, 2002 | 70 | 49 | 83 | .003 | 77 | 66 | NS * | 41 | 35 | NS |
| Sanyal, 2003 | 109 | 42 | 84 | <.001 | 42 | 23 | NS ** | -- | -- | NS |
| Salerno, 2004 | 66 | 21 | 57 | .012 | 61 | 39 | NS *** | 77 | 52 | .021 |
| Narahara, 2011 | 60 | 13 | 80 | <.001 | 67 | 17 | <.001 | 80 | 49 | <.005 |
severe encephalopathy grade III, IV was significantly higher in TIPS group (P=.03)
higher incidence of moderate to severe HE in TIPS group (P=.058)
number severe HE episodes per patient higher in TIPS: 0.97 versus 0.36 (P=.039)
2-year survival
NS = not statistically significant
Figure 3.
Relative Risks and 95% CI for recurrence of ascites, encephalopathy and mortality comparing TIPS and repeated paracentesis for refractory ascites 50.
Other indications and contraindications for TIPS
TIPS has been used for a number of portal hypertensive complications. There are several anecdotal series of the use of TIPS for the treatment of type 1 hepatorenal syndrome 55, 56. The quality and strength of the evidence from these studies is suspect due to the small numbers of subjects, lack of randomization and retrospective nature of these data. It is also important to remember that the MELD score was originally developed to predict outcomes after TIPS and is driven largely by the serum creatinine 57. At this time, the use of TIPS in such cases should be considered second line and experimental. In most centers, if used, it is used as a last ditch effort when all other means such as vasoconstrictor therapy has failed and liver transplant is not an option. In those who are transplant candidates, renal replacement therapy should be used to bridge them to transplant if they fail vasoconstrictor therapy. The use of TIPS to prevent recurrence of hepatorenal syndrome after an initial response to vasoconstrictors remains experimental.
TIPS has also been used to control recurrent bleeding from portal gastropathy. There are instances where chronic bleeding from portal gastropathy requires repeated transfusions. TIPS have been found in one study to reduce transfusion requirement 58. At this author’s institution, the experience has been mixed with some subjects showing a beneficial response while others have not shown any response. It is also this authors anecdotal experience that subjects with NASH and portal gastropathy are more likely to have troublesome bleeding and that such cases are more likely to have elevated right heart pressures. TIPS has been used for management of gastric varices. One RCT comparing TIPS and cyanoacrylate for secondary prophylaxis demonstrated a significant reduction in rebleeding with TIPS with no difference in survival and one study demonstrated effective use of TIPS as salvage therapy in bleeding gastric varices 59, 60. It was demonstrated that TIPS can effectively stop bleeding from ectopic varices and may be used particularly in cases with stomal variceal hemorrhage in subjects with an enterostomy and portal hypertension 61.
There are several reports of an effective resolution of refractory hepatic hydrothorax following TIPS 62–66. All of the caveats about worsening liver failure should be kept in mind in this population who are quite sick to start with.
TIPS has also been used to effectively manage Budd Chiari syndrome 67–70. In such cases, the hepatic vein cannot be entered and a direct vena cava to portal vein puncture is required. It effectively decompresses the hepatic parenchyma in such cases and can bring liver function back to normal. Budd Chiari syndrome is often associated with a hypercoagulable state and a work up for this is mandatory; TIPS placement is associated with an increased risk of thrombosis in such cases and most centers use anticoagulation routinely for at least six months even in the absence of demonstrable hypercoagulable state. In those with a hypercoagulable state and Budd Chiari syndrome, lifelong anticoagulation is recommended 71. In those with chronic Budd Chiari syndrome, hypertrophy of the caudate lobe can compress the inferior vena cava; in such cases, additional stenting of the vena cava may be required to decompress the intrahepatic vena cava especially if symptomatic.
TIPS has been used in the management of portal vein thrombosis. A recent long-term follow up study on non-tumor related portal vein thrombosis treated with TIPS revealed thrombus resolution in 57% of patients, however many of these patients had non-occlusive thrombus, and TIPS was used as the primary indication in only 4 out of 70 patients72. There are case reports of TIPS placement and direct fibrinolysis of the thrombus followed by systemic anticoagulation 73. These are high risk procedures and associated with considerable morbidity and are not considered routine standard of care procedures. In chronic extrahepatic portal vein obstruction with cavernous transformation, several reports indicate that TIPS can be placed in to one of the collaterals 74, 75. However, whether this translates into clinical benefit remains an open question.
Another potential application of TIPS is the treatment of veno-occlusive disease. There are case reports and small series of subjects who received TIPS for veno-occlusive disease developing after bone marrow transplant 76–78. The use of TIPS for this indication must be individualized given the relative paucity of data for this and the medically complex condition of a subject who has recently received a bone marrow transplant.
Although TIPS has been used in the management of the complications of portal hypertension, care must be taken when selecting appropriate patients to undergo a TIPS. The absolute and relative contraindications to a TIPS are outlined in Table 3 6. The presence of active encephalopathy is a relative contraindication for TIPS when being considered in an elective setting. TIPS should not be considered for the treatment of hepato-pulmonary syndrome or porto-pulmonary syndrome. In the latter condition, the increased venous return to the right heart in the face of a fixed resistance in the pulmonary bed may precipitate severe right heart failure. For similar reasons, severe tricuspid regurgitation and congestive heart failure are contraindications to TIPS, as the TIPS can precipitate pulmonary edema in these conditions 79. Subjects with polycystic liver disease are at high risk of bleeding if these are punctured during a TIPS and the procedure should not be performed in such cases 6. The presence of a hepatocellular cancer especially with portal vein thrombosis is also considered a contraindication unless there is an isolated tumor far away from the track of the shunt. TIPS may be used to treat complications of cirrhosis, however, careful patient selection is important.
Table 3.
Absolute and relative contraindications for TIPS procedure 6
| Absolute Contraindications | Relative Contraindications |
|---|---|
| Congestive Heart Failure | INR > 5 |
| Severe pulmonary hypertension | Platelet < 20000/cm3 |
| Multiple hepatic cysts | Moderate pulmonary hypertension |
| Uncontrolled systemic infection, or sepsis | Portal vein thrombosis |
| Unrelieved biliary obstruction | |
Complications of TIPS
There are three broad categories of complications of TIPS. These include those that are related to the technique, those related to portosystemic shunting and unique complications. These complications are summarized in Table 4.
Table 4.
Complications of TIPS
| Technical Complications | |
|---|---|
| (i) Related to access | Capsule puncture |
| Intraperitoneal bleed | |
| Hepatic infarction | |
| Fistula | |
| Hemobilia | |
|
| |
| (ii) Related to the stent | Thrombosis |
| Occlusion | |
| Stent Migration | |
| Sepsis | |
|
| |
| Related to portosystemic shunting | Hepatic encephalopathy |
| Hemodynamic consequences | |
| Sepsis | |
| Intravascular hemolysis | |
|
| |
| Unique complications | Endotipsitis |
Technical complications are mainly related to the puncture of structures that were not meant to be punctured and those related to malfunction or maldeployment of the stent. Figure 4 illustrates the development of a fistula between the TIPS stent and biliary system 15.
Figure 4.
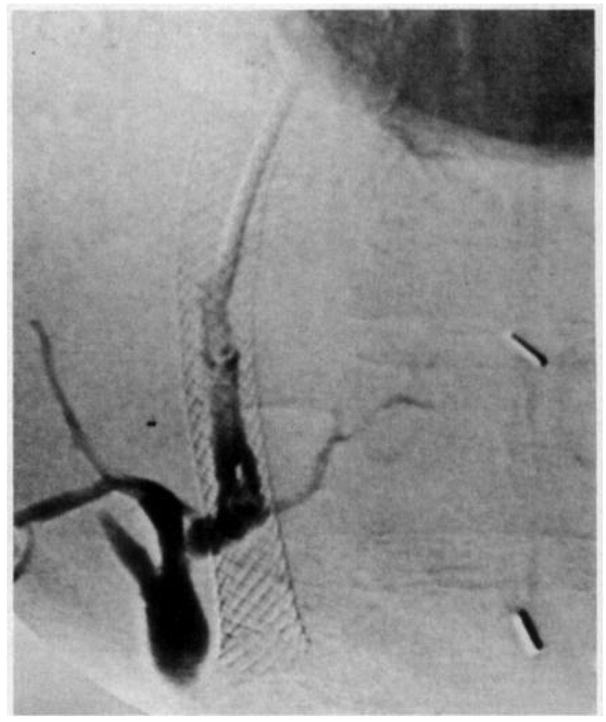
Fistula between stent and biliary system 15.
Hepatic encephalopathy is the most important medical complication after TIPS. It presents typically with acute worsening of mental status within 2–3 weeks of shunt placement 8. Increasing age, liver dysfunction and shunt diameter are key risk factors for the development of encephalopathy after TIPS placement 80, 81. Importantly, studies with bare stents found an increased risk of encephalopathy after TIPS placement for ascites 44, 45, 47, 48. Subsequently, other studies found that the rates of encephalopathy were lower with coated stents compared to bare stents 36, 82. A recent meta-analysis further corroborates this 83. However, there are several issues that limit the robustness of these conclusions: (1) the trials were not designed to test this endpoint, (2) the methodology to evaluate encephalopathy was highly variable across studies and even from one study arm to the other in different studies, and (3) the data did not correct for background encephalopathy and liver function. These data are also somewhat counter-intuitive because one would expect greater shunt patency with coated stents and thus more portosystemic shunting and encephalopathy. These data however raise the intriguing possibility that better portal decompression reduces bowel edema and bacterial translocation thereby reducing the systemic inflammatory state associated with cirrhosis which has been implicated in the genesis of encephalopathy. This remains to be proven.
There are also unique complications of cirrhosis such as TIPS associated hemolysis and vegetative infections associated with TIPS 15, 84, 85. TIPS induced hemolysis is rarely seen with coated stents. Vegetative infections associated with TIPS were also mainly reported with bare stents; these can be treated with prolonged antibiotics. We have in a single case removed the stent 2 months after placement using angiographic methods.
Summary
Since its first clinical application three decades ago, TIPS has become a treatment option for portal decompression. TIPS have been successfully used for secondary prophylaxis of variceal hemorrhage and in salvage therapy for acute variceal hemorrhage. TIPS have also been demonstrated to be superior to repeated large volume paracentesis to manage refractory ascites. However, the use of TIPS comes with the risk of hepatic encephalopathy and no survival benefit for refractory ascites. The survival benefit for TIPS as secondary prophylaxis for variceal hemorrhage is controversial. Future trials should focus on optimizing patient selection to achieve a survival advantage with TIPS.
Acknowledgments
Grant Support: This manuscript was partly supported by a grant from the National Institutes of Health to Dr. Sanyal, (T32DK007150). This work is not being considered for publication elsewhere.
Abbreviations
- TIPS
Transjugular Intrahepatic Portosystemic Shunt
- EVL
Endoscopic Variceal Ligation
Footnotes
Disclosures:
Arun J Sanyal: site investigator for a study of GORE stents
Harjit K Bhogal: screening and consenting for a study of GORE stents
Authors contributions:
Arun J Sanyal: critical revision of the manuscript for important intellectual content
Harjit K Bhogal: drafting of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosch J, Hanafee W, Snow W, et al. Transjugular intrahepatic portacaval shunt. An experimental work. Am J Surg. 1971;121:588–592. doi: 10.1016/0002-9610(71)90147-4. [DOI] [PubMed] [Google Scholar]
- 2.Reich M, Olumide F, Jorgensen E, et al. Experimental cyroprobe production of intrahepatic portocaval shunt. J Surg Res. 1977;23:14–18. doi: 10.1016/0022-4804(77)90183-4. [DOI] [PubMed] [Google Scholar]
- 3.Colapinto RF, Stronell RD, Birch SJ. Creation of an intrahepatic portosystemic shunt with a Gruntzig balloon catheter. Can Med Assoc J. 1982;126:267–268. [PMC free article] [PubMed] [Google Scholar]
- 4.Richter GM, Palmaz JC, Noldge G, et al. The transjugular intrahepatic portosystemic stent-shunt. A new percutaneous method. Radiologe. 1989;29:406–411. [PubMed] [Google Scholar]
- 5.Tripathi D, Redhead D. Transjugular intrahepatic portosystemic stent-shunt: technical factors and new developments. Eur J Gastroenterol Hepatol. 2006;18:1127–1133. doi: 10.1097/01.meg.0000236871.78280.a7. [DOI] [PubMed] [Google Scholar]
- 6.Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 51:306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 7.Ferral H, Patel NH. Selection criteria for patients undergoing transjugular intrahepatic portosystemic shunt procedures: current status. J Vasc Interv Radiol. 2005;16:449–455. doi: 10.1097/01.RVI.0000149508.64029.02. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJFA, Shiffman ML. Portosystemic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt: Results of a Prospective Controlled Study. Hepatology. 1994;20:46–55. doi: 10.1016/0270-9139(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesan AM, Kundu S, Sacks D, et al. Practice Guideline for Adult Antibiotic Prophylaxis during Vascular and Interventional Radiology Procedures. J Vasc Interv Radiol. 2010;21:1611–1630. doi: 10.1016/j.jvir.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Diebert P, Schwarz S, Olschewski M. Risk factors and prevention of early infection after implantation or revision of transjugular intrahepatic portosystemic shunts: results of a randomized study. Dig Dis Sci. 1998;43:1708–1713. doi: 10.1023/a:1018819316633. [DOI] [PubMed] [Google Scholar]
- 11.Conn HO. Portal hypertension, varices, and transjugular intrahepatic portosystemic shunts. Clin Liver Dis. 2000;4:133–150. doi: 10.1016/s1089-3261(05)70100-8. [DOI] [PubMed] [Google Scholar]
- 12.Haskal ZJ, Martin L, Cardella JF, et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2003;14:S265–270. [PubMed] [Google Scholar]
- 13.Bureau CPJ, Layrargues GP, et al. Patency of stents covered with polytetrafluoroehtylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742–747. doi: 10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 14.Carbonell N, Pauwels A, Serfaty L. Improved survival after variceal bleeding in patients with cirrhosis over the past 2 decades. Hepatology. 2004;40:652–9. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 15.Freedman AM, Sanyal AJ, Tisnado J, et al. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13:1185–1210. doi: 10.1148/radiographics.13.6.8290720. [DOI] [PubMed] [Google Scholar]
- 16.Millis JM, Martin P, Gomes S, et al. Transjugular intrahepatic portosystemic shunts: impact on liver transplantation. Liver Transpl Surg. 1995;1:229–233. doi: 10.1002/lt.500010406. [DOI] [PubMed] [Google Scholar]
- 17.Prandi D, Rueff B, Roche-Sicot J, et al. Life-Threatening Hemorrhage of the Digestive Tract in Cirrhotic Patients. Am J Surg. 1976;131:20420–9. doi: 10.1016/0002-9610(76)90098-2. [DOI] [PubMed] [Google Scholar]
- 18.Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626–631. doi: 10.1016/s0016-5085(99)70455-5. [DOI] [PubMed] [Google Scholar]
- 19.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane databse Syst Rev. 2010;8:CD002907. doi: 10.1002/14651858.CD002907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. American Journal of Gastroenterology. 2003;98:653–659. doi: 10.1111/j.1572-0241.2003.07294.x. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. AASLD Practice Guidelines: Prevention and Management of Gastroesophageal Varices and Variceal Hemorrhage in Cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 22.De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800–808. [PubMed] [Google Scholar]
- 24.Vivas S, Rodriguez M, Palacio MA. Presence of bacterial infection in bleeding cirrhotic patients is independently associated with early mortality and failure to control bleeding. Dig Dis Sci. 2001;46:2752–7. doi: 10.1023/a:1012739815892. [DOI] [PubMed] [Google Scholar]
- 25.LaBerge JM, Ring EJ, Gordon RY, et al. Creation of Transjugular Intrahepatic Portosystemic Shunts with the Wallstent Endoprosthesis: Results in 100 Patients. Radiology. 1993;187:413–420. doi: 10.1148/radiology.187.2.8475283. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Freedman AM, Luketic VA, et al. Transjugular intrahepatic portosystemic shunts for patients with active variceal hemorrhage unresponsive to sclerotherapy. Gastroenterology. 1996;111:138–146. doi: 10.1053/gast.1996.v111.pm8698192. [DOI] [PubMed] [Google Scholar]
- 27.Gerbes AL, Gulberg V, Waggershauser T, et al. Transjugular Intrahepatic Portosystemic Shunt (TIPS) for Variceal Bleeding in Portal Hypertension: Comparison of Emergency and Elective Intervention. Dig Dis Sci. 1998;43:2463–2469. doi: 10.1023/a:1026686232756. [DOI] [PubMed] [Google Scholar]
- 28.Chau TN, Patch D, Chan YW, et al. “Salvage” transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998;114:981–987. doi: 10.1016/s0016-5085(98)00640-4. [DOI] [PubMed] [Google Scholar]
- 29.McCormick PA, Dick R, Panagou EB, et al. Emergency transjugular intrahepatic portasystemic stent shunting as salvage treatment for uncontrolled variceal bleeding. Br J Surg. 1994;81:1324–1327. doi: 10.1002/bjs.1800810922. [DOI] [PubMed] [Google Scholar]
- 30.Banares R, Casado M, Rodriquez-Laiz JM, et al. Urgent Transjugular Intrahepatic Portosystemic Shunt for Control of Acute Variceal Bleeding. Am J of Gastroenterol. 1998;93:75–79. doi: 10.1111/j.1572-0241.1998.075_c.x. [DOI] [PubMed] [Google Scholar]
- 31.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 2005;22:332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 32.D’Amico G, De Franchis R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 33.Zheng M, Chen Y, Bai J, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. J Clin Gastroenterol. 2008;42:507–516. doi: 10.1097/MCG.0b013e31815576e6. [DOI] [PubMed] [Google Scholar]
- 34.Sanyal AJ, Freedman AM, Luketic VA, et al. The Natural History of Portal Hypertension After Transjugular Intrahepatic Portosystemic Shunts. Gastroenterology. 1997;112:889–898. doi: 10.1053/gast.1997.v112.pm9041251. [DOI] [PubMed] [Google Scholar]
- 35.Sanyal AJ, Mirshahi F. Endothelial cells lining transjugular intrahepatic portasystemic shunts originate in hepatic sinusoids: implications for pseudointimal hyperplasia. Hepatology. 1999;29:710–718. doi: 10.1002/hep.510290323. [DOI] [PubMed] [Google Scholar]
- 36.Bureau C, Garcia-Pagan JC, Otal P, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469–475. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793–801. doi: 10.1002/hep.20386. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 39.Clark W, Hernandez J, McKeon B, et al. Surgical shunting versus transjugular intrahepatic portasystemic shunting for bleeding varices resulting from portal hypertension and cirrhosis: a meta-analysis. Am Surg. 76:857–864. [PubMed] [Google Scholar]
- 40.Gaba RC, Bui JT, Cotler SJ, et al. Rebleeding rates following TIPS for variceal hemorrhage in the Viatorr era: TIPS alone versus TIPS with variceal embolization. Hepatol Int. 2010;4:749–756. doi: 10.1007/s12072-010-9206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 42.Arroyo V, Gines P, Gerbes AL, et al. Definition and Diagnostic Criteria of Refractory Ascites and Hepatorenal Syndrome in Cirrhosis. Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 43.Planas R, Montoliu S, Balleste B, et al. Natural History of Patients Hospitalized for Management of Cirrhotic Ascites. Clin Gastroenterol Hepatol. 2006;4:1385–1394. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Narahara Y, Kanazawa H, Fukuda T, et al. Transjugular Intrahepatic Portosystemic Shunt Versus Paracentesis plus Albumin in Patients with Refractory Ascites who have good Hepatic and Renal Function: A Prospective Randomized Trial. J Gastroenterol. 2011;46:78–85. doi: 10.1007/s00535-010-0282-9. [DOI] [PubMed] [Google Scholar]
- 45.Sanyal AJ, Genning C, Reddy KR, et al. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124:634–641. doi: 10.1053/gast.2003.50088. [DOI] [PubMed] [Google Scholar]
- 46.Rossle M, Ochs A, Gulberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–7. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 47.Salerno F, Merli M, Riggio O, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629–635. doi: 10.1002/hep.20364. [DOI] [PubMed] [Google Scholar]
- 48.Gines P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–1847. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 49.Wong F, Sniderman K, Liu P, et al. The mechanism of the initial natriuresis after transjugular intrahepatic portosystemic shunt. Gastroenterology. 1997;113:899–907. doi: 10.1053/gast.1997.v112.pm9041252. [DOI] [PubMed] [Google Scholar]
- 50.Albillos A, Banares R, Gonzalez M, et al. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005;43:990–996. doi: 10.1016/j.jhep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 51.D’Amico G, Luca A, Morabito A, et al. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis. Gastroenterology. 2005;129:1282–1293. doi: 10.1053/j.gastro.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Deltenre P, Mathurin P, Dharancy S, et al. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int. 2005;25:349–356. doi: 10.1111/j.1478-3231.2005.01095.x. [DOI] [PubMed] [Google Scholar]
- 53.Saab S, Nieto JM, Ly D, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2004;3:CD004889. doi: 10.1002/14651858.CD004889. [DOI] [PubMed] [Google Scholar]
- 54.Salerno F, Camma C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55–64. doi: 10.1002/hep.20262. [DOI] [PubMed] [Google Scholar]
- 56.Guevara M, Gines P, Bandi JC, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416–422. doi: 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- 57.Angermayr B, Cejna M, Karnel F. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879–885. doi: 10.1136/gut.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamath PS, Lacerda M, Ahlquist DA, et al. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology. 2000;118:905–911. doi: 10.1016/s0016-5085(00)70176-4. [DOI] [PubMed] [Google Scholar]
- 59.Barange K, Peron JM, Imani K, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999;30:1139–1143. doi: 10.1002/hep.510300523. [DOI] [PubMed] [Google Scholar]
- 60.Lo GH, Liang HL, Chen WC, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679–685. doi: 10.1055/s-2007-966591. [DOI] [PubMed] [Google Scholar]
- 61.Kochar N, Tripathi D, McAvoy NC, et al. Bleeding ectopic varcies in cirrhosis: the role of transjugular intrahepatic portosystemic stent shunts. Aliment Pharmacol Ther. 2007;28:294–303. doi: 10.1111/j.1365-2036.2008.03719.x. [DOI] [PubMed] [Google Scholar]
- 62.Gordon FD, Anastopoulos HT, Crenshaw W, et al. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366–1369. doi: 10.1002/hep.510250611. [DOI] [PubMed] [Google Scholar]
- 63.Jeffries MA, Kazanjian S, Wilson M, et al. Transjugular intrahepatic portosystemic shunts and liver transplantation in patients with refractory hepatic hydrothorax. Liver Transpl Surg. 1998;4:416–423. doi: 10.1002/lt.500040506. [DOI] [PubMed] [Google Scholar]
- 64.Siegerstetter V, Deibert P, Ochs A, et al. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long-term results in 40 patients. Eur J Gastroenterol Hepatol. 2001;13:529–534. doi: 10.1097/00042737-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Wilputte JY, Goffette P, Zech F, et al. The outcome after transjugular intrahepatic portosystemic shunt (TIPS) for hepatic hydrothorax is closely related to liver dysfunction: a long-term study in 28 patients. Acta Gastroenterol Belg. 2007;70:6–10. [PubMed] [Google Scholar]
- 66.Dhanasekaran R, West JK, Gonzales PC, et al. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 105:635–641. doi: 10.1038/ajg.2009.634. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Pagan JC, Heydtmann M, Raffa S, et al. TIPS for Budd-Chiari Syndrome: Long-Term Results and Prognostics Factors in 124 Patients. Gastroenterology. 2008;135:808–815. doi: 10.1053/j.gastro.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 68.Rossle M. The Budd-Chairi Syndome: Outcome After Treatment with the Transjugular Intrahepatic Portosystemic Shunt. Surgery. 2003;135:394–403. doi: 10.1016/j.surg.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Mancuso A, Fung K, Mela M, et al. TIPS for acute and chronic Budd-Chiari syndrome: a single-centre experience. J Hepatol. 2003;38:751–754. doi: 10.1016/s0168-8278(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 70.Perello A, Garcia-Pagan JC, Gilabert R, et al. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medical therapy. Hepatology. 2002;35:132–139. doi: 10.1053/jhep.2002.30274. [DOI] [PubMed] [Google Scholar]
- 71.DeLeve LD, Valla D, Garcia-Tsao G. Vascular Disorders of the Liver. Hepatology. 2008;49:1729–1764. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luca A, Miraglia R, Caruso S, et al. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut. 2011;60:846–852. doi: 10.1136/gut.2010.228023. [DOI] [PubMed] [Google Scholar]
- 73.Smalberg JS, Spaander MV, Jie KG, et al. Risks and benefits of transcatheter thrombolytic therapy in patietns with splanchnic venous thrombosis. Thromb Haemost. 2008;100:1084–1088. [PubMed] [Google Scholar]
- 74.Wils A, van der Linden E, van Hoek B, et al. Transjugular intrahepatic portosystemic shunt in patients with chronic portal vein occlusion and cavernous transformation. J Clin Gastroenterol. 2009;43:982–984. doi: 10.1097/MCG.0b013e31819706a4. [DOI] [PubMed] [Google Scholar]
- 75.Senzola M, Tibbals J, Cholangitas E, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without cavernous transformation. Aliment Pharmacol Ther. 2006;23:767–775. doi: 10.1111/j.1365-2036.2006.02820.x. [DOI] [PubMed] [Google Scholar]
- 76.Zenz T, Rossle M, Bertz H. Severe veno-occlusive disease after allogeneic bone marrow or peripheral stem cell transplantation--role of transjugular intrahepatic portosystemic shunt (TIPS) Liver. 2001;21:31–36. doi: 10.1034/j.1600-0676.2001.210105.x. [DOI] [PubMed] [Google Scholar]
- 77.Aoulay D, Castaing D, Lemoine A. Transjugular intrahepatic portosystemic shunt (TIPS) for severe veno-occlusive disease of the liver following bone marrow transplantation. Bone Marrow Transplant. 2000;25:987–992. doi: 10.1038/sj.bmt.1702386. [DOI] [PubMed] [Google Scholar]
- 78.Fried MW, Connaghan DG, Sharma S, et al. Transjugular intrahepatic portosystemic shunt for the management of severe venoocclusive disease following bone marrow transplantation. Hepatology. 1996;24:588–591. doi: 10.1002/hep.510240321. [DOI] [PubMed] [Google Scholar]
- 79.Lotterer E, Wengert A, Fleig WE. Transjugular intrahepatic portosystemic shunt: short-term and long-term effects on hepatic and systemic hemodynamics in patients with cirrhosis. Hepatology. 1999;29:632–639. doi: 10.1002/hep.510290302. [DOI] [PubMed] [Google Scholar]
- 80.Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738–2746. doi: 10.1111/j.1572-0241.2008.02102.x. [DOI] [PubMed] [Google Scholar]
- 81.Rosado B, Kamath PS. Transjugular intrahepatic portosystemic shunts: an update. Liver Transpl. 2003;9:207–217. doi: 10.1053/jlts.2003.50045. [DOI] [PubMed] [Google Scholar]
- 82.Maleux G, Perez-Gutierrez NA, Evrard S, et al. Covered stents are better than uncovered stents for transjugular intrahepatic portosystemic shunts in cirrhotic patients with refractory ascites: a retrospective cohort study. Acta Gastroenterol Belg. 2010;73:336–341. [PubMed] [Google Scholar]
- 83.Yang Z, Han G, Wu Q, et al. Patency and clinical outcomes of transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stents versus bare stents: a meta-analysis. J Gastroenterol Hepatol. 25:1718–1725. doi: 10.1111/j.1440-1746.2010.06400.x. [DOI] [PubMed] [Google Scholar]
- 84.Sanyal AJ, Reddy KR. Vegetative infection of transjugular intrahepatic portosystemic shunts. Gastroenterology. 1998;115:110–115. doi: 10.1016/s0016-5085(98)70371-3. [DOI] [PubMed] [Google Scholar]
- 85.Sanyal AJ, Freedman AM, Purdum PP, et al. The hematologic consequences of transjugular intrahepatic portosystemic shunts. Hepatology. 1996;23:32–39. doi: 10.1002/hep.510230105. [DOI] [PubMed] [Google Scholar]