Abstract
Multiple System Atrophy (MSA) is a neurodegenerative disease presenting with motor abnormalities including akinesia, rigidity and postural instability. Whilst improved diagnostic criteria have aided accurate diagnosis of MSA, understanding of neuropathological aspects underlying MSA was bolstered by the identification of alpha-synuclein (α-syn) as the primary constituent of the abnormal protein aggregations observed in the brains of MSA patients. The generation of transgenic animal models of MSA coupled with an increasing understanding of the biochemical structure and function of α-syn has highlighted a number of key pathological pathways thought to underlie the neurodegeneration observed in MSA. This review will summarize key findings in the field, discuss current areas of debate and describe current experimental approaches towards disease-modifying therapies.
INTRODUCTION
A common feature among a number of neurological disorders is the abnormal aggregation of a natively soluble protein as observed with amyloid beta in Alzheimer’s disease1 and huntingtin protein in Huntington’s Disease2. Similarly, a particular group of neurodegenerative disorders is characterized by the abnormal accumulation of α-synuclein (α-syn); termed the ‘α-synucleinopathies’, this group includes Parkinson’s Disease (PD), Dementia with Lewy Bodies (DLB) and Multiple System Atrophy (MSA)3. While PD and DLB have received much scientific attention in recent years, research into MSA has continued in the relative background. A stark indication of the relative levels of research being conducted into these different disorders can be seen in a simple PubMed search, which recently returned close to 60,000 results when searching for “Parkinson’s Disease” but a mere 3,765 results for “Multiple System Atrophy”. However, despite this apparent underrepresentation of MSA, a considerable amount of progress has been made in this field, largely due to the cross-disease application of the understanding of key neurodegenerative pathways, and in particular, the pathological role of α-syn.
This review will focus on MSA and describe recent efforts to improve the clinical diagnosis of the disorder, as well as discussing the impact that transgenic animal models have had on unraveling key pathological mechanisms underlying the disease. Finally, we will discuss how a greater understanding of these mechanisms is advancing the development of disease-modifying therapeutic interventions.
The clinico-pathological characteristics of MSA
The term MSA was coined in 1969 to encompass three previously distinct neurodegenerative disorders, striatonigral degeneration, olivopontocerebellar ataxia, and “Shy-Drager syndrome”4. MSA is a progressive neurodegenerative disorder with an estimated annual world-wide incidence of about 0·6/100 000, rising to 3/100 000 in the 50 years+ population5–7. The mean age of disease onset is around 60 years and the mean survival ranges from 7–9 years following the appearance of clinical symptoms8. Since its initial classification as a single, albeit pleomorphic, disease entity a great deal of research has gone into understanding the clinical characteristics of MSA.
MSA is characterized clinically by symptoms that can be subdivided into pyramidal, extrapyramidal, cerebellar and autonomic categories. Extrapyramidal motor abnormalities such as bradykinesia, rigidity and postural instability, are classed as either Parkinsonian-type (MSA-P) or cerebellar (MSA-C) and reflect damage to the basal ganglia (striato-nigral degeneration) or cerebellum (olivo-pontocerebellar atrophy), respectively6, 9–13. Additionally, MSA patients develop behavioral alterations such as depression and executive dysfunction that suggest frontal lobe impairment14–18. Recent epidemiological studies in North America6 and Japan19 have suggested an ethnic variation with regards to the incidence rates of MSA-P or MSA-C. The North American Study reported 60% of their patients as having MSA-P and 13% exhibiting MSA-C6. In contrast, the Japanese study reported a much higher percentage of patients (83.8%) exhibiting MSA-C features with only 16.2% of patients being categorized as MSA-P19. The underlying cause of this variability remains undetermined but may involve genetic or environmental factors, or a combination thereof. Autonomic dysfunction, most commonly urogenital, gastrointestinal and cardiovascular dysfunction in the form of orthostatic hypotension, eventually develops in both MSA-P and MSA-C patients20.
Current therapeutic interventions for MSA are aimed at the treatment of symptoms such as hypotension, erectile dysfunction and gastrointestinal dysfunction, rather than at the underlying pathology itself 21–25. Many MSA-P patients present to the clinic with symptoms resembling PD including postural or resting tremor. Such patients are often prescribed Levodopa to which they are largely irresponsive, although it should be noted that some reports have suggested a minimal, if short-lived, beneficial effect of Levodopa administration in some MSA patients20, 26–28.
Given the wide range of clinical features associated with MSA, a great deal of effort has been focused on the development of reliable clinical diagnostic criteria for the disease. The Consensus Criteria currently employed to aid in the diagnosis of MSA was first proposed in 199810 and allowed for the diagnosis of definite, probable, and possible MSA based on a combination of neuropathology and the presentation of autonomic and cerebellar dysfunction and parkinsonian features. The MSA Consensus Criteria also included a list of exclusion criteria, including family history of a similar disorder. The presence of criteria listed in the Diagnostic and Statistical Manual of Mental Disorders for dementia or hallucinations unrelated to medication also ruled out a diagnosis of MSA10. These MSA Consensus Criteria were revised and updated in 2008 to include the use of neuroimaging criteria to aid in the diagnosis of possible MSA23. In addition to criteria used to diagnose MSA, the development of the unified MSA rating scale (UMSARS) in 200429 allowed clinicians to monitor and evaluate disease progression in patients.
Whilst possible and probable MSA can be diagnosed on the basis of clinical presentations, the diagnosis of definite MSA requires neuropathological confirmation. MSA is characterized by widespread neuronal loss in the basal ganglia, cerebellum, pons, inferior olivary nuclei, and spinal cord, accompanied by gliosis. However, the cardinal neuropathological hallmark of MSA is the presence of argyrophilic filamentous glial cytoplasmic inclusions (GCIs)9, 30, 31. These GCI are predominantly found in oligodendrocytes, the myelin producing cells of the central nervous system (CNS), however, inclusions have also been reported in oligodendroglial nuclei and neuronal cytoplasm (NCI) and nuclei (NNI) and in neurites32–36. In 1998 multiple groups reported that α-syn, a predominantly neuronal presynaptic protein37–39, was the major component of GCIs40–43. In addition to α-syn, GCIs have been reported to be immunoreactive for tau, tubulin, ubiquitin, αB-crystallin, cyclin-dependent kinase 5, transferin, Leu-7 and microtubule-associated protein 530, 33, 35, 44. In addition to the CNS, α-syn immunoreactive inclusions have also been reported in autonomic neurons20.
The identification of α-syn as a key component of the inclusions found in MSA linked MSA to PD and DLB, other disorders characterized by the pathological aggregation of α-syn 45, 46. In PD and DLB, α-syn forms neuronal inclusions called Lewy Bodies in contrast to the oligodendroglial inclusions found in MSA47. Interestingly, α-syn load in MSA is much higher than that found in PD and DLB48. Moreover, MSA has higher levels of soluble α-syn48, in contrast to PD and DLB, which are characterized by higher levels of insoluble α-syn49. The reasons for and implications of this differential α-syn expression pattern remain undefined. In addition to the α-synucleinopathies, α-syn is also found as the non-amyloid component (NAC) in the amyloid plaques that form the classical hallmarks of Alzheimer’s disease (AD)50, 51.
Animal models of MSA
In recent years, a number of neurotoxic and transgenic (tg) animal models have been developed to better understand the mechanisms underlying the oligodendrocytic accumulation of α-syn in MSA (Table 1). Although the precise origin of α-syn in the oligodendrocytes remains unknown, the presence of α-syn in these cells is a key pathological hallmark of this disease and therefore many groups have developed transgenic animal models of MSA that express human α-syn (hα-syn) under the regulatory control of oligodendroglial-specific promoters52–54. These models display accumulation of α-syn in oligodendrocytes with concomitant demyelination and degeneration, especially in the substantia nigra and spinal cord52, 54. Transgenic mice that express hα-syn under the regulatory control of the proteolipid protein (PLP) promoter52 (PLP-α-syn tg mice) have also been reported to exhibit neurodegeneration and GCI-like inclusions in brain regions associated with autonomic failure including the intermediolateral columns, nucleus ambiguus and Onuf’s nucleus, suggesting that this model may replicate both motor and non-motor aspects of MSA55. Given the fact that patients with MSA also develop behavioral alterations that suggest frontal lobe involvement20, including attention deficits 56, 57, the effects of hα-syn accumulation in neurodegeneration in cortical brain regions also deserves consideration. In this context, it is interesting that by 6 months of age transgenic mice that express hα-syn under the regulatory control of the myelin basic protein (MBP)53 (MBP1hα-syn tg mice) develop abundant hα-syn immunoreactive inclusions in oligodendrocytes in the neocortex in addition to the basal ganglia, cerebellum and brainstem with concomitant myelin and neuronal damage53. These mice also display motor deficits, including ataxia and impaired performance on the rotarod and pole tests, in comparison to age-matched non-transgenic control mice. Such findings support a more general role of α-syn accumulation in the pathogenesis of MSA53. Among the neurotoxic models, challenge with the mitochondrial toxin 3-nitroproprionic acid (3NP) has been shown to enhance the motor deficits and neurodegeneration in transgenic mice models of MSA over and above those observed in saline-treated transgenic mice58, 59.
Table 1.
Summary of selected transgenic mouse models of MSA
| PHENOTYPE | ||||
|---|---|---|---|---|
| TRANSGENE | PROMOTER | NEUROPATHOLOGY | BEHAVIOR | REFS |
| Human α-syn | PLP | Dopaminergic cell loss in substantia nigra, loss of neurons in spinal cord and axonal degeneration. Delayed loss of cholinergic neurons and neurons in areas associated with autonomic failure. GCI-like inclusion comprised of insoluble α-syn, hyperphosphorylated at S129 |
Shorted stride length. The administration of 3NP in these mice results in progressive motor disability as assessed by the Bordeaux motor behavior scale, impaired rearing, hindlimb strength and pole test performance. | 52,55,58,105 |
| Human α-syn | 2',3'-cyclic nucleotide 3' phosphodiesterase (CNP) | Neuronal loss in hippocampus and cortex, demyelination and axonal degeneration. GCI-like inclusions of insoluble α-syn in addition to accumulation of α-syn in the neuropil |
Impaired motor performance, as assessed in the rotarod and hanging wire tests | 54 |
| Human α-syn | MBP | Loss of dopaminergic fibers in the basal ganglia, astrogliosis, loss of dendritic density, demyelination and mitochondrial alterations. Progressive accumulation of α-syn and S129 phosphorylated α-syn immunoreactive inclusions in oligodendrocytes along axonal tracts in brainstem, basal ganglia, cerebellum, corpus callosum and neocortex. The administration of 3NP in these mice enhances the oxidative modifications of α-syn |
Impaired motor behavior as assessed by pole test and rotarod. Impaired olfaction. | 53, 59, 84 |
In addition to unraveling the mechanisms underlying oligodendrocytic α-syn aggregation and neurodegeneration in MSA and investigating the interactions between environmental toxins and α-syn, animal models with face and construct validity for MSA provide a means to develop and test experimental therapeutic interventions for MSA. The rapid progression of the disease, coupled with the fact that all the MSA treatments currently available are aimed solely at controlling the symptoms for MSA, means there is great need for the identification and development of disease-modifying therapies.
Insights into the key mechanisms underlying neurodegeneration from transgenic models of MSA
A number of recent studies have identified pathways such as mitochondrial dysfunction/oxidative stress and abnormal posttranslational modifications of α-syn (including phosphorylation, ubiquitination and carboxyl-terminal cleavage60–63), as being involved in the aggregation and toxicity of α-syn. However, the mechanisms underlying how α-syn, a predominantly neuronal protein, reaches the oligodendrocytes in MSA and leads to cellular dysfunction and neurodegeneration remain unclear. Much of the research on α-syn has been conducted in animal models of PD, however some key findings in MSA tg models have progressed the understanding of the role of α-syn in this disorder. These findings and experimental disease-modifying interventions for MSA are discussed below.
The origin of oligodendrocytic α-syn
The presence of oligodendrocytic α-syn protein in MSA has drawn a lot of attention, especially in light of a number of studies that have reported no alterations in mRNA levels between the brains of control and MSA patients 64–66, suggesting that the protein may have an ectopic origin. A possible explanation for this aberrant oligodendroglial expression of α-syn protein in MSA comes from recent studies with tissue grafted into human PD patients which have highlighted the possibility that α-syn may be able travel from one cell to another67–69. Neuronal and glial cells have been reported to endocytose α-syn from the surrounding media and transmit it to neighboring neurons, glial cells or neuronal precursor cells forming Lewy-like inclusions70. α-syn has also been reported to be transmitted from affected neurons to engrafted neuronal precursor cells in a tg mouse model of PD-like pathology71. A recent study reported that α-syn is able to propagate from host cells in the striatum of α-syn over-expressing mice to donor embryonic mesencephalic neurons from wild-type mice72. Furthermore, it was demonstrated that the propagated α-syn was able to seed further α-syn aggregation in a cell culture system72.
Collectively these findings highlight the possibility of the cell-to-cell transmission of α-syn and suggest a possible mechanism by which α-syn may travel from neurons to the oligodendrocytes in the MSA brain. However, it should be noted that the exact mechanisms underlying this transfer and the relative contributions of soluble and oligomeric species of α-syn in this propagation require further elucidation69.
Factors effecting oligodendrocytic α-syn aggregation
Whatever its origin, α-syn is present in the oligodendrocytes of MSA patients and transgenic models of MSA. Typically, the α-syn present in oligodendrocytes has undergone posttranslational modifications, including oxidative modifications and phosphorylation, and is often found in inclusions comprised of insoluble α-syn9, 34, 35, 43, 48, 52–54, 61, 73–75. A number of mechanisms have been proposed to exacerbate the propensity of α-syn to aggregate. One such mechanism involves oxidative stress and mitochondrial dysfunction - a key contributor to aberrant levels of oxidative stress76.
Oxidative stress is associated with an increased production of reactive oxygen species (ROS), such as oxygen (O2)-derived free radicals, the hydroxyl radical (•OH) and non-radical derivatives of O2 such as hydrogen peroxide (H2O2), or a significant decrease in the activity of antioxidant defenses. Increased production or inadequate clearance of ROS can result in high levels of ROS, which can then go on to damage many components of the cell including proteins, lipids, and DNA. Oxidative stress has been linked to many neurodegenerative diseases, including AD and PD76. Epidemiological studies have suggested that increased environmental exposure to pesticides may be linked to the occurrence of sporadic PD77 and it is thought that this exposure may increase levels of oxidative stress and play a role in the toxic conversion of α-syn in such sporadic cases of PD. Many PD-linked mutations are thought to effect mitochondrial function78–80. Pesticide exposure has also been linked to an increased incidence of MSA81. Moreover, a recent Japanese study reported genetic associations between MSA and candidate genes involved in oxidative stress [ie. CCAAT/enhancer-binding protein-beta, sequestosome 1 (SQSTM1), cysteinyl-tRNA synthetase, solute carrier family 1A4 (SLC1A4) and eukaryotic translation initiation factor 4E-binding protein 1 (EIF4EBP1)], suggesting a possible genetic basis for the association of oxidative stress with the pathogenesis of MSA82.
In addition to the human epidemiological data, a number of studies using tg models of MSA have highlighted the role of mitochondrial dysfunction and oxidative stress in MSA. The mitochondrial toxin 3NP is known to enhance the motor deficits and neurodegeneration in tg mice models of MSA58, 59. Administration of 3NP to the PLP-α-syn tg mice exacerbated motor deficits in these mice and was associated with MSA-like striatonigral neurodegeneration and olivopontocerebellar atrophy63. Dopaminergic cell loss was also exacerbated upon 3NP exposure58. 3NP administration also exaggerated neurological deficits in the MBP1hα-syn tg mice, resulting in widespread neuronal degeneration and behavioral impairment. Administration of 3NP in these mice also altered levels of nitrated and oxidized α-syn, while not affecting global levels of total α-syn59. The toxic effects of 3NP were shown to be dependent on the presence of α-syn since α-syn knockout (KO) mice, although susceptible to 3NP-induced oxidative stress, displayed reduced neuronal loss and dendritic pathology in comparion to 3NP-treated MBP1hα-syn tg mice83. Furthermore, the α-syn KO mice were resistant to 3NP-induced motor deficits and displayed attenuated loss of tyrosine hydroxylase and dopamine transporter striatal immunoreactivity83. This suggests that deficits in MSA are not solely due to general oxidative protein modifications, but in addition, may be related to specific α-syn modifications84.
A large number of studies aimed at ameliorating α-syn toxicity have focused on modulating levels of oxidative stress in α-synucleinopathies85–87. A number of anti-oxidant compounds have been identified and shown to reduce α-syn oxidation88, resulting in reduced α-syn aggregation and an amelioration of neuropathological deficits in tg mice over-expressing α-syn. Recent findings have demonstrated that administration of rifampicin, an antibiotic commonly prescribed for the treatment of tuberculosis and leprosy, results in a reduction of monomeric and oligomeric α-syn in the MBP1hα-syn tg mouse model, and a reduction in phosphorylated (S129) and nitrosylated α-syn upon rifampicin treatment89. This reduction in α-syn aggregation was accompanied by reduced neurodegeneration. On the basis of its anti-aggregenic properties, rifampicin may have therapeutic potential for MSA89.
Collectively these studies demonstrate a key role for oxidative stress in MSA pathology and underscore the therapeutic potential of anti-oxidant compounds for this disorder. However oxidative stress is unlikely to be the only mechanism responsible for α-syn aggregation in MSA. This is highlighted by a recent study that described a role for p25α [also called tubulin polymerization promoting protein (TPPP)], an oligodendroglial-specific protein involved in myelination90, in MSA. Under physiological conditions, p25α resides in the myelin sheath, however in patients with MSA it relocalizes from the myelin sheath to the oligodendroglial cytoplasm where it is found in the GCI, associated with insoluble aggregates of α-syn90, 91. Furthermore, p25α has been reported to stimulate α-syn aggregation in an oligodendroglial cell culture model92; this aggregation was accompanied by caspase 3 activation and apoptotic cell death. Inhibition of sirtuin 2 (SIRT2), a tubulin deacetylase, partially rescued cell death in this model92.
It is likely that multiple mechanisms interact to result in the abnormal accumulation of α-syn in MSA subjects. Continued work with transgenic models of MSA, human samples, and lessons from other α-synucleinopathies, will no doubt shed new light on these mechanisms and increase the likelihood of developing disease-modifying interventions for MSA.
Effects of oligodendroglial α-syn accumulation - neurotrophic factor disturbance
A key question in MSA research has been how accumulation of α-syn in predominantly oligodendrocytes can lead to death of another cell type, namely neurons. Oligodendrocytes have many roles in the support of neuronal function, the most notable of these being myelination. Oligodendrocytes have also been reported to express neurotrophic factors [including glial-derived neurotrophic factor (GDNF)93, 94, brain-derived neurotrophic factor (BDNF)95, and insulin-like growth factor 1 (IGF-1)96] that are involved in the maintenance and survival of neuronal populations. One possible explaination for how oligodendroglial accumulation of α-syn may result in neuronal death is that altered communication between oligodendrocytes and neurons, perhaps by a perturbation of this neurotrophic support, may contribute to neurodegeneration84. In support of this notion, a recent study has demonstrated that postmortem tissue from MSA subjects displayed decreased levels of GDNF in the white matter of the frontal cortex as compared to controls, and to a lesser degree in the cerebellum compared to normal controls84. The MBP1h-αsyn tg mice have also been shown to display a specific decrease in GDNF protein expression in total brain lysates84. Furthermore, intracerebroventricular infusion of GDNF improved behavioral deficits and ameliorated neurodegenerative pathology in these mice compared to vehicle-treated mice84. Collectively, these results suggest that α-syn expression in oligodendrocytes may impact the trophic support provided by oligodendrocytes for neurons, thereby perhaps contributing to neurodegeneration.
GDNF-based therapeutic approaches have received a lot of attention in relation to PD. However, early clinical trials of GDNF infusion resulted in multiple side-effects, including nausea, weight loss, and psychotic manifestations97 and the results from these trials remain inconclusive98. Many of the problems with the early trials have been linked to the long-term administration and delivery of GDNF. However a recent study using a gene therapy approach for the delivery of GDNF in monkeys has shown promising results 99. In this study, GDNF was packaged into a the adeno-associated virus (AAV) and injected into the putamen of monkeys treated with MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a toxin used to induce parkinsonian-like symptoms. This delivery method allowed a more precise delivery of GDNF and resulted in symptomatic improvements in the treated animals over sham-operated controls 99. A clinical trial using AAV-GDNF in PD patients is being planned; meanwhile AVV-Neurturin (a member of the GDNF family) is currently in clinical trials for PD (NCT00985517; www.clinicaltrials.gov).
An alternative to the continued administration of GDNF through the use of infusion or the grafting of GDNF-secreting cells is the use of a compound that can be delivered orally, and which will enhance GDNF levels in a manner in which the exact dose and treatment duration can be tailored. A potential candidate for this type of therapy is rasagiline, a novel selective irreversible monoamine oxidase-B (MAO-B) inhibitor. Rasagiline has ben shown to be effective in animal models of PD100, 101 and is currently showing promise in clinical trials of PD102, 103. Recent reports have suggested that rasagiline may work by inducing expression of neurotrophic factors such as GDNF and BDNF104. Rasagiline treatment has been shown to ameliorate motor deficits in PLP-α-syn transgenic mice that had been given 3NP to mimic full-blown MSA-like neurodegeneration105. Immunohistochemical analysis of these animals showed significant reductions in neuronal loss in the striatum, substantia nigra pars compacta, cerebellar cortex, pontine nuclei and inferior olives105. Taken together, these results indicate that rasagiline may enhance neuroprotection in a transgenic model of MSA by the induction of neurotrophic support. Thus, it may be a promising disease-modifying candidate for MSA patients.
Current Clinical Trials for MSA
Multiple clinical trials for MSA are planned or currently underway (Table 2). These trials are predominantly focused on the investigation of symptom-alleviating therapies, however some are aimed at targeting the underlying neurodegenerative dysfunction. Based on their efficacy in animal models of MSA, rasagiline84, 104, 105 and rifampicin89, 106 may represent two such potentially disease-modifying therapies. Another clinical trial currently underway involves the use of autologous mesenchymal stem cells (NCT00911365). This trial follows-on from promising preclinical studies, which demonstrated that grafts of human mesenchymal stem cells were able to increase neuronal survival in the substantia nigra and striatum of a double-toxin-induced (MPTP and 3NP) model of MSA-P107. The reduced neurodegeneration in these mice was accompanied by an improvement in motor behavior107.
Table 2.
Summary of selected MSA clinical trials
| INDICATION | DRUG | MODE OF ACTION | CLINICAL TRIAL ID1 |
|
|---|---|---|---|---|
|
SYMPTOM MANAGEMENT |
Mood | Fluoxetine | Selective serotonin reuptake inhibitor | NCT01146548 |
| Lithium | Mood-stabilizer drug | NCT00997672 | ||
| Autonomic Failure-Hypotention | Droxidopa | Orally active synthetic precursor of norepinephrine |
NCT01370512 NCT00004478 NCT00547911 |
|
| Pyridostigmine | Parasympathomimetic and reversible cholinesterase inhibitor | NCT01370512 | ||
| Autonomic Failure-Supine hypertension | Nebivolol | Beta1-receptor blocker | NCT01044693 | |
|
POTENTIAL DISEASE MODIFICATION |
Modification of α-syn aggregation | Rifampicin | Antibiotic drug used to treat tuberculosis | NCT01287221 |
| Rasagiline | Irreversible MAO-B inhibitor | NCT00977665 | ||
| OTHER | Autologous Mesenchymal Stem Cells | - - | Neuroprotective action and possible replacement of lost/damaged cells | NCT00911365 |
Source: www.clinicaltrials.gov
Transplantation of embryonic striatal allografts into a double-lesion rat model of MSA-P has also been reported to improve dopaminergic responsiveness and restore responsiveness to Levadopa108. The outcome of MSA graft studies will be interesting in light of the emerging hypothesis concerning the cell-to-cell transfer of α-syn and recent findings that demonstrate that oligodendrocytes expressing host-specific α-syn are able to migrate into graft tissue in 3NP-treated PLP-αsyn tg mice grafted with embryonic striatal allografts109. It is possible that the grafts in MSA patients may be faced with potential host-donor transmission problems, however it is important to point out that although host-to-graft α-syn transmission has been observed in PD patients who received transplants of embryonic mesencephalic neurons, it did not appear to occur till more than a decade after transplantation 67, 110, 111. Furthermore, although the grafted cells displayed α-syn pathology, they did not appear to be functionally impaired and therefore it may be possible for PD graft recipients to experience long-term symptomatic relief 67, 110–112; a similar situation may well be the case for MSA graft recipients.
As more is learnt about mechanisms underlying the accumulation and abnormal aggregation of α-syn it is envisioned that an increasing number of disease-modifying therapies will make their way from the lab to the clinical setting in the coming years. Many of the current experimental therapies aimed at α-synucleinopathies are aimed at reducing α-syn aggregation or increasing its clearance from cells and have been primarily tested in models of PD - it will be interesting to see if, and how, these translate to a clinical setting and whether or not they will be applicable to MSA.
Past successes and future directions
The discovery of α-syn as the main component of GCIs was a major milestone in research into the underlying neuropathological mechanisms of MSA. Other important milestones have been the development and characterization of valid animal models of the disease and the use of these models to elucidate and understand the mechanisms underlying pathogenesis in MSA. The continued use of these animal models for the pre-clinical testing of potentially therapeutically relevant compounds has also shown great promise 89, 100, 103, 105. Despite the intensified focus of many researchers into MSA, a number of fundamental questions remain regarding both the biochemical nature of the disease and its clinical treatment (Box 1).
BOX 1 – OUTSTANDING QUESTIONS.
-
What is the origin of the oligodendrocytic α-syn in MSA patients?
Given that α-syn has been reported as being a presynaptic neuronal protein, how does it end up in the oligodendrocytes in the MSA brain? Determination of the origin of α-syn will not only aid in understanding key disease-related mechanisms but may also impact the development of therapeutic interventions.
-
How does the accumulation of α-syn in oligodendrocytes lead to neuronal cell death?
Despite the predominantly oligodendrocytic accumulation of α-syn in MSA there is widespread neurodegeneration across the basal ganglia, cerebellum, pons, inferior olivary nuclei and spinal cord. It is possible that α-syn may disrupt the support and maintenance of the neurons usually provided by the oligodendrocytes - this is consistent with studies showing demylination31 and altered neurotrophic factor levels84.
-
What are the mechanisms underlying the aggregation of α-syn in MSA? Are they the same as in PD?
It is possible that conditions in MSA may favor the oligodendrocytic accumulation and/or aggregation of α-syn. Interesting, a recent study has shown that CSF from MSA patients promotes α-syn fibril formation in vitro to a greater extent than CSF from patients with PD or DLB114, suggesting that the CNS environment may be particularly adapted to α-syn fibrillation in MSA.
-
What are the toxic species of α-syn in MSA? Are they the same as in PD? Does the α-syn in MSA differ biochemically from that found in PD?
A recent study reported increased levels of membrane-associated, detergent-soluble α-syn in post-mortem samples from MSA subjects that were over and above those observed in PD or progressive supranuclear palsy (PSP), whereas PD and PSP brains exhibited different patterns of α-syn accumulation48. Such findings highlight a potential disease-specific accumulation pattern of particular α-syn species and suggest that there may be differences in the aggregenic mechanisms involved in each disorder. This would be an important area for further investigation as it would increase our understanding of the potentially disease-specific biochemical aspects of α-syn but may also allow the development of disease-specific aimed at particular toxic species of α-syn.
-
Will drugs currently being developed for PD have any effect on MSA?
Since many key pathological mechanisms are common between PD, MSA and other neurodegenerative disorders, it may be possible to develop drugs that target specific pathways (eg. the proteasome system, mitochondrial function) and that may have broad-spectrum application. However, further investigation is necessary to determine the relative roles of each of these pathways/mechanisms in the different neurodegenerative disorders.
-
Will it be possible to identify biomarkers that will allow the early and accurate identification of MSA and differentiate it from other disorders with a similar clinical appearance?
Emerging research has begun to identify CSF biomarkers that may be specific for particular α-synucleinopathies115. Coupled with imaging techniques, such as the magnetic resonance imaging (MRI) identification of the ‘hot-cross bun’ sign, characteristic of MSA117, it may be possible to differentiate MSA from other disorders.
A central concept in future research into MSA should be clarification of the precise origin of α-syn in the oligodendrocytes of MSA patients. Currently, a number of possible explanations exist for the presence of oligodendrocytic α-syn, including its pathological disease-related up-regulation in oligodendrocytes, or its translocation from neurons to oligodendrocytes, with each explanation hinting at different underlying mechanisms. A clearer understanding of the origin of oligodendrocytic α-syn will not only inform researchers as to the physiological and pathophysiological properties of α-syn but will also open up new therapeutic avenues. For example, if oligodendrocytic α-syn is a result of pathological up-regulation it may be therapeutically susceptible to oligodendrocyte-specific compounds that interfere with its transcription/translocation. However, given that a number of studies have reported no difference in oligodendrocytic α-syn mRNA levels between control and MSA human brains 64–66, it is becoming increasingly apparent that the α-syn protein observed in MSA may originate in another cell-type. If oligodendrocytic α-syn is indeed a result of cell-to-cell transfer of α-syn it implies that α-syn, a normally cytoplasmic protein, traverses into the extracellular space. This proposed extracellular location of α-syn is consistent with a number of studies 68–72 and suggests that it may be liable to immunotherapy approaches, consistent with recent findings in a transgenic model of DLB113. Other key questions regarding α-syn such as its toxic species, biochemical properties, aggregenic mechanisms, and whether these differ in MSA from other α-synucleinopathies, also remain topics of continued research48, 114. Concomitant with a greater appreciation of the pathological role of α-syn will be an increased understanding and emphasis on the development of disease-modifying therapies rather than the symptomatic control that is the mainstay current of MSA therapeutic regimens.
Given the rapid progression of MSA, early and accurate diagnosis is imperative to enable timely initiation of therapeutic interventions. The identification of disease-specific biomarkers for MSA (or for other disorders that can then be used as exclusion criteria for MSA) is receiving considerable attention of late, and a recent study has identified cerebrospinal fluid (CSF) biomarkers that may be used to distinguish MSA from PD 115. Coupled with an increased knowledge of the neuropathology underlying clinical variability among synucleinopathies 74, the identification of disease-specific biomarkers will assist in tailoring therapeutic interventions to particular disorders within this disease family.
Finally, despite the seemingly daunting tasks ahead, the outlook remains promising, as MSA will continue to prosper not only from the work of dedicated MSA researchers but also from studies examining α-syn in relation to PD and DLB, as well as from research into other disorders characterized by abnormal protein accumulation, which may shed light on common protein-misfolding pathways. The more that is learnt about each particular neurodegenerative disorder the more we discover that certain mechanisms re-appear across the neurodegenerative disease spectrum. An increasing appreciation of this phenomenon will only aid future research into disease-modifying therapies, not only for MSA but also for all neurodegenerative disorders characterized by abnormal protein accumulation.
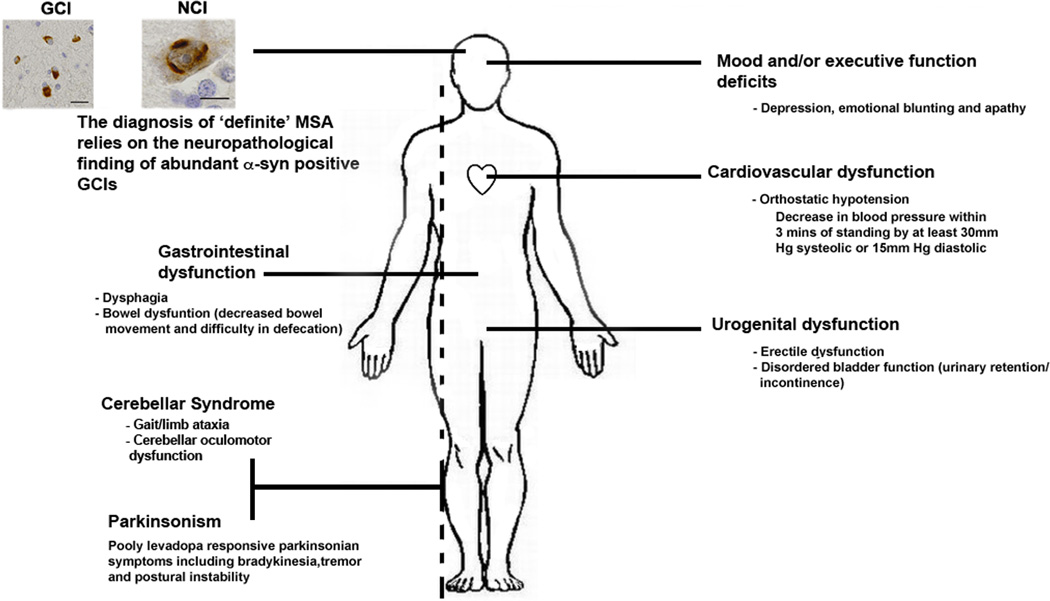
Figure 1. Clinical and Neuropathological Characteristics of MSA.
A number of clinical features are assessed before making a diagnosis of probable or possible MSA, including mood/cognitive alternations, ataxia/tremor, cardiovascular dysfunction and/or urogential dysfunction. However, a definitive diagnosis of MSA relies upon the neuropathological finding of α-syn positive inclusions in oligodendroglial cells, termed glial cytoplasmic inclusion (GCI). The presence of α-syn positive neuronal inclusions, termed neuronal cytoplasmic inclusions (NCI), has also been reported in the brains of MSA patients. Images of GCI and NCI reproduced, with permission, from 116.GCIs are visible as pale eosinophilic inclusions by hematoxylin and eosin staining in oligodendrocytes of post-mortem cortical tissue. Scale bars = 10µM.
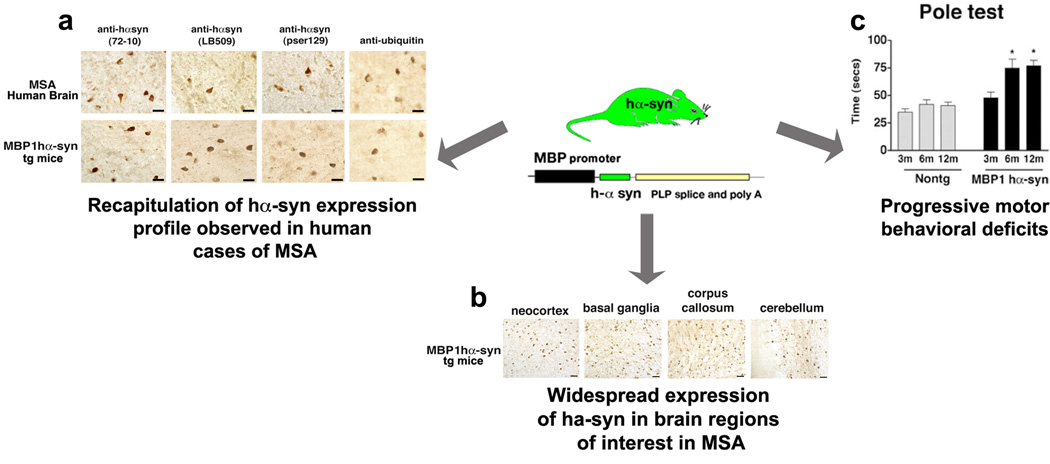
Figure 2. Typical features of a transgenic mouse model of MSA.
The central image is a schematic representation of a transgene in a typical transgenic (tg) model of MSA. In this particular model, human α-syn (hα-syn) is being driven by the oligodendrocyte-specific promoter MBP (myelin basic protein). (a) Comparison of the glial cell inclusions observed in post-mortem brain samples from MSA subjects and MBP hα-syn tg mice. Images are from the white matter tracts in the basal ganglia of a human case with typical MSA and MBP hα-syn tg mice (4 months of age). GCIs in the MSA case and the MBP hα-syn tg animal were positively immunostained with a polyclonal antibody against hα-syn (72–10), a monoclonal antibody against hα-syn (LB509), a monoclonal antibody against phospho-serine129 hα-syn (pser129) and an antibody against ubiquitin. Scale bar = 20µm. (b) Immunoreactivity of hα-syn in various brain regions in MBP hα-syn tg mice (4-months -of age), demonstrating widespread expression of hα-syn across these brain regions. Scale bars = 10µm. (c) Motor assessment in the pole test demonstrated only mild deficits in the MBP hα-syn tg mice at 3 months of age compared to controls (Nontg); however, at 6 months of age, these deficits were accentuated and remained similar at 12 months of age. Error bars are mean±SEM. *Significant difference compared with controls (p<0.05).
Panels a–c adapted, with permission, from 53
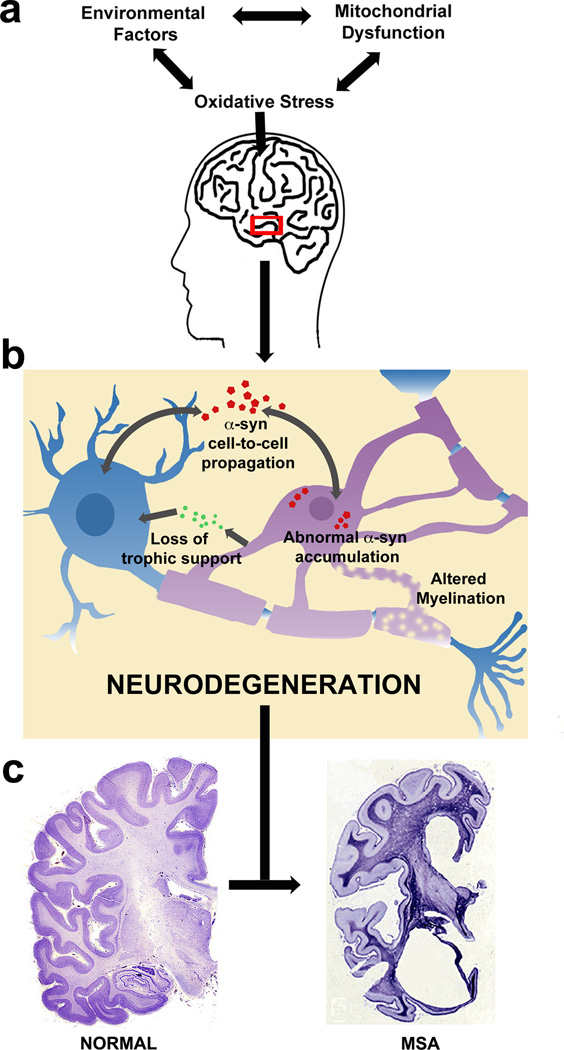
Figure 3. Key mechanisms underlying neurodegeneration in MSA.
(a) The precise mechanisms underlying neurodegeneration in MSA remain to be resolved, however, emerging results from studies on MSA and other α-synucleinopathies have highlighted a number of key pathways, including oxidative stress, perhaps as a result of mitochondrial dysfunction, environmental exposure to toxins, or a combination of both. (b) The pathological accumulation of oligodendroglial α-syn, possibly a result of cell-to-cell transfer of the protein, may potentiate neurodegeneration by a lack of trophic support for the neuron or by altered myelination. (c) The eventual macroscopic effect of these mechanisms is widespread atrophy in MSA brains. Image of the control brain is Nissel stained and adapted with permission from http://www.brains.rad.msu.edu, (supported by the US National Science Foundation). The image of the MSA brain is stained with Holzer stain, and reproduced, with permission from 116.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Selkoe D. Amyloid β-protein deposition as a seminal pathogenic event in AD: an hypothesis. Neurobiol.Aging. 1990;11:299. [Google Scholar]
- 2.Vonsattel JP, et al. Huntington's disease - neuropathology. Handb Clin Neurol. 2011;100:83–100. doi: 10.1016/B978-0-444-52014-2.00004-5. [DOI] [PubMed] [Google Scholar]
- 3.Kahle PJ. alpha-Synucleinopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:87–95. doi: 10.1007/s00401-007-0302-x. [DOI] [PubMed] [Google Scholar]
- 4.Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1969;32:28–34. doi: 10.1136/jnnp.32.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geser F, et al. The European Multiple System Atrophy-Study Group (EMSA-SG) J Neural Transm. 2005;112:1677–1686. doi: 10.1007/s00702-005-0328-y. [DOI] [PubMed] [Google Scholar]
- 6.Gilman S, et al. The North American Multiple System Atrophy Study Group. J Neural Transm. 2005;112:1687–1694. doi: 10.1007/s00702-005-0381-6. [DOI] [PubMed] [Google Scholar]
- 7.Stefanova N, et al. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A, et al. Survival in multiple system atrophy. Mov Disord. 2008;23:294–296. doi: 10.1002/mds.21839. [DOI] [PubMed] [Google Scholar]
- 9.Burn DJ, Jaros E. Multiple system atrophy: cellular and molecular pathology. Mol Pathol. 2001;54:419–426. [PMC free article] [PubMed] [Google Scholar]
- 10.Gilman S, et al. Consensus statement on the diagnosis of multiple system atrophy. American Autonomic Society and American Academy of Neurology. Clin Auton Res. 1998;8:359–362. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- 11.Kosaka K. Lewy bodies in cerebral cortex. Report of three cases. Acta Neuropathol.(Berl) 1978;42:127–134. doi: 10.1007/BF00690978. [DOI] [PubMed] [Google Scholar]
- 12.Kosaka K. Diffuse Lewy body disease in Japan. J.Neurol. 1990;237:197–204. doi: 10.1007/BF00314594. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka K, et al. Diffuse type of Lewy body disease. Progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree - A new disease? Clin.Neuropathol. 1984;3:183–192. [PubMed] [Google Scholar]
- 14.Schrag A, et al. Measuring health-related quality of life in MSA: the MSA-QoL. Mov Disord. 2007;22:2332–2338. doi: 10.1002/mds.21649. [DOI] [PubMed] [Google Scholar]
- 15.Schrag A, et al. A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Mov Disord. 2010;25:1077–1081. doi: 10.1002/mds.22794. [DOI] [PubMed] [Google Scholar]
- 16.Dujardin K, et al. Executive function differences in multiple system atrophy and Parkinson's disease. Parkinsonism Relat Disord. 2003;9:205–211. doi: 10.1016/s1353-8020(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 17.Fetoni V, et al. Affective symptoms in multiple system atrophy and Parkinson's disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry. 1999;66:541–544. doi: 10.1136/jnnp.66.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benrud-Larson LM, et al. Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord. 2005;20:951–957. doi: 10.1002/mds.20450. [DOI] [PubMed] [Google Scholar]
- 19.Yabe I, et al. MSA-C is the predominant clinical phenotype of MSA in Japan: analysis of 142 patients with probable MSA. J Neurol Sci. 2006;249:115–121. doi: 10.1016/j.jns.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer RF. Multiple system atrophy. Handb Clin Neurol. 2007;84:305–326. doi: 10.1016/S0072-9752(07)84046-2. [DOI] [PubMed] [Google Scholar]
- 21.Mathias CJ. Multiple system atrophy and autonomic failure. J Neural Transm Suppl. 2006:343–347. doi: 10.1007/978-3-211-45295-0_52. [DOI] [PubMed] [Google Scholar]
- 22.Kollensperger M, et al. Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord. 2010;25:2604–2612. doi: 10.1002/mds.23192. [DOI] [PubMed] [Google Scholar]
- 23.Gilman S, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flabeau O, et al. Multiple system atrophy: current and future approaches to management. Ther Adv Neurol Disord. 2010;3:249–263. doi: 10.1177/1756285610375328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7:451–458. doi: 10.1016/S1474-4422(08)70088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parati EA, et al. Response to L-DOPA in multiple system atrophy. Clin Neuropharmacol. 1993;16:139–144. doi: 10.1097/00002826-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Boesch SM, et al. Dystonia in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;72:300–303. doi: 10.1136/jnnp.72.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christine CW, Aminoff MJ. Clinical differentiation of parkinsonian syndromes: prognostic and therapeutic relevance. Am J Med. 2004;117:412–419. doi: 10.1016/j.amjmed.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Wenning GK, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004;19:1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 30.Papp MI, et al. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 31.Jellinger KA, Lantos PL. Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol. 2010;119:657–667. doi: 10.1007/s00401-010-0672-3. [DOI] [PubMed] [Google Scholar]
- 32.Kato S, Nakamura H. Cytoplasmic argyrophilic inclusions in neurons of pontine nuclei in patients with olivopontocerebellar atrophy: immunohistochemical and ultrastructural studies. Acta Neuropathol. 1990;79:584–594. doi: 10.1007/BF00294235. [DOI] [PubMed] [Google Scholar]
- 33.Kato S, et al. Argyrophilic ubiquitinated cytoplasmic inclusions of Leu-7-positive glial cells in olivopontocerebellar atrophy (multiple system atrophy) Acta Neuropathol. 1991;82:488–493. doi: 10.1007/BF00293383. [DOI] [PubMed] [Google Scholar]
- 34.Murayama S, et al. Immunocytochemical and ultrastructural studies of neuronal and oligodendroglial cytoplasmic inclusions in multiple system atrophy. 2. Oligodendroglial cytoplasmic inclusions. Acta Neuropathol. 1992;84:32–38. doi: 10.1007/BF00427212. [DOI] [PubMed] [Google Scholar]
- 35.Arima K, et al. Immunocytochemical and ultrastructural studies of neuronal and oligodendroglial cytoplasmic inclusions in multiple system atrophy. 1. Neuronal cytoplasmic inclusions. Acta Neuropathol. 1992;83:453–460. doi: 10.1007/BF00310020. [DOI] [PubMed] [Google Scholar]
- 36.Papp MI, Lantos PL. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J Neurol Sci. 1992;107:172–182. doi: 10.1016/0022-510x(92)90286-t. [DOI] [PubMed] [Google Scholar]
- 37.Iwai A, et al. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 38.Maroteaux L, et al. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinreb PH, et al. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 40.Wakabayashi K, et al. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 41.Spillantini MG, et al. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 42.Arima K, et al. NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol. 1998;96:439–444. doi: 10.1007/s004010050917. [DOI] [PubMed] [Google Scholar]
- 43.Tu PH, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 44.Nakazato Y, et al. Oligodendroglial microtubular tangles in olivopontocerebellar atrophy. J Neuropathol Exp Neurol. 1990;49:521–530. doi: 10.1097/00005072-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Goedert M. Parkinson's disease and other alpha-synucleinopathies. Clin Chem Lab Med. 2001;39:308–312. doi: 10.1515/CCLM.2001.047. [DOI] [PubMed] [Google Scholar]
- 46.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 47.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 48.Tong J, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–188. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- 49.Klucken J, et al. Clinical and biochemical correlates of insoluble alpha-synuclein in dementia with Lewy bodies. Acta Neuropathol. 2006;111:101–108. doi: 10.1007/s00401-005-0027-7. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto M, Masliah E. Alpha-synuclein in Lewy body disease and Alzheimer's disease. Brain Pathol. 1999;9:707–720. doi: 10.1111/j.1750-3639.1999.tb00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masliah E, et al. Altered presynaptic protein NACP is associated with plaque formation and neurodegeneration in Alzheimer's disease. Am J Pathol. 1996;148:201–210. [PMC free article] [PubMed] [Google Scholar]
- 52.Kahle PJ, et al. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shults CW, et al. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yazawa I, et al. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 55.Stemberger S, et al. Targeted overexpression of human alpha-synuclein in oligodendroglia induces lesions linked to MSA-like progressive autonomic failure. Exp Neurol. 2010;224:459–464. doi: 10.1016/j.expneurol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meco G, et al. Attentional functions in multiple system atrophy and Parkinson's disease. J Neurol Neurosurg Psychiatry. 1996;60:393–398. doi: 10.1136/jnnp.60.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbins TW, et al. Cognitive performance in multiple system atrophy. Brain. 1992;115(Pt 1):271–291. doi: 10.1093/brain/115.1.271. [DOI] [PubMed] [Google Scholar]
- 58.Stefanova N, et al. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol. 2005;166:869–876. doi: 10.1016/s0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ubhi K, et al. Mitochondrial inhibitor 3-nitroproprionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res. 2009;87:2728–2739. doi: 10.1002/jnr.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dufty BM, et al. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–1738. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beyer K. Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers. Acta Neuropathol. 2006;112:237–251. doi: 10.1007/s00401-006-0104-6. [DOI] [PubMed] [Google Scholar]
- 62.Gorbatyuk OS, et al. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oueslati A, et al. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: implications for Parkinson's disease pathogenesis and therapies. Prog Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 64.Jin H, et al. Analyses of copy number and mRNA expression level of the alpha-synuclein gene in multiple system atrophy. J Med Dent Sci. 2008;55:145–153. [PubMed] [Google Scholar]
- 65.Miller DW, et al. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- 66.Ozawa T, et al. Analysis of the expression level of alpha-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol. 2001;102:188–190. doi: 10.1007/s004010100367. [DOI] [PubMed] [Google Scholar]
- 67.Kordower JH, et al. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 68.Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 69.Lee SJ, et al. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol. 2010;6:702–706. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee HJ, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansen C, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beyer K, Ariza A. Protein aggregation mechanisms in synucleinopathies: commonalities and differences. J Neuropathol Exp Neurol. 2007;66:965–974. doi: 10.1097/nen.0b013e3181587d64. [DOI] [PubMed] [Google Scholar]
- 74.Halliday GM, et al. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- 75.Ischiropoulos H. Oxidative modifications of alpha-synuclein. Ann N Y Acad Sci. 2003;991:93–100. doi: 10.1111/j.1749-6632.2003.tb07466.x. [DOI] [PubMed] [Google Scholar]
- 76.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 77.Franco R, et al. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson's disease. Chem Biol Interact. 2010;188:289–300. doi: 10.1016/j.cbi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie W, et al. New insights into the role of mitochondrial dysfunction and protein aggregation in Parkinson's disease. Biochim Biophys Acta. 2011;1802:935–941. doi: 10.1016/j.bbadis.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 79.McCoy MK, Cookson MR. DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy. 2011;7 doi: 10.4161/auto.7.5.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akundi RS, et al. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS One. 2011;6:e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanna PA, et al. Multiple system atrophy: the putative causative role of environmental toxins. Arch Neurol. 1999;56:90–94. doi: 10.1001/archneur.56.1.90. [DOI] [PubMed] [Google Scholar]
- 82.Soma H, et al. Associations between multiple system atrophy and polymorphisms of SLC1A4, SQSTM1, and EIF4EBP1 genes. Mov Disord. 2008;23:1161–1167. doi: 10.1002/mds.22046. [DOI] [PubMed] [Google Scholar]
- 83.Ubhi K, et al. Alpha-synuclein deficient mice are resistant to toxin-induced multiple system atrophy. Neuroreport. 2010;21:457–462. doi: 10.1097/WNR.0b013e328338ba6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ubhi K, et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30:6236–6246. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norris EH, et al. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- 87.Norris EH, et al. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- 88.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J Neurochem. 2006;97:105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 89.Ubhi K, et al. Rifampicin reduces alpha-synuclein in a transgenic mouse model of multiple system atrophy. Neuroreport. 2008;19:1271–1276. doi: 10.1097/WNR.0b013e32830b3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song YJ, et al. p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol. 2007;171:1291–1303. doi: 10.2353/ajpath.2007.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lindersson E, et al. p25alpha Stimulates alpha-synuclein aggregation and is co-localized with aggregated alpha-synuclein in alpha-synucleinopathies. J Biol Chem. 2005;280:5703–5715. doi: 10.1074/jbc.M410409200. [DOI] [PubMed] [Google Scholar]
- 92.Hasegawa T, et al. Role of TPPP/p25 on alpha-synuclein-mediated oligodendroglial degeneration and the protective effect of SIRT2 inhibition in a cellular model of multiple system atrophy. Neurochem Int. 2010;57:857–866. doi: 10.1016/j.neuint.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci Res. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 94.Wilkins A, et al. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai X, et al. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilkins A, et al. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia. 2001;36:48–57. doi: 10.1002/glia.1094. [DOI] [PubMed] [Google Scholar]
- 97.Nutt JG, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 98.Hong M, et al. GDNF therapy for Parkinson's disease. Expert Rev Neurother. 2008;8:1125–1139. doi: 10.1586/14737175.8.7.1125. [DOI] [PubMed] [Google Scholar]
- 99.Kells AP, et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J Neurosci. 2010;30:9567–9577. doi: 10.1523/JNEUROSCI.0942-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blandini F, et al. Neuroprotective effect of rasagiline in a rodent model of Parkinson's disease. Exp Neurol. 2004;187:455–459. doi: 10.1016/j.expneurol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 101.Zhu W, et al. Comparison of neuroprotective and neurorestorative capabilities of rasagiline and selegiline against lactacystin-induced nigrostriatal dopaminergic degeneration. J Neurochem. 2008;105:1970–1978. doi: 10.1111/j.1471-4159.2008.05330.x. [DOI] [PubMed] [Google Scholar]
- 102.Olanow CW, et al. A randomized, double-blind, placebo-controlled, delayed start study to assess rasagiline as a disease modifying therapy in Parkinson's disease (the ADAGIO study): rationale, design, and baseline characteristics. Mov Disord. 2008;23:2194–2201. doi: 10.1002/mds.22218. [DOI] [PubMed] [Google Scholar]
- 103.Sampaio C, Ferreira JJ. Parkinson disease: ADAGIO trial hints that rasagiline slows disease progression. Nat Rev Neurol. 2010;6:126–128. doi: 10.1038/nrneurol.2010.2. [DOI] [PubMed] [Google Scholar]
- 104.Weinreb O, et al. Induction of neurotrophic factors GDNF and BDNF associated with the mechanism of neurorescue action of rasagiline and ladostigil: new insights and implications for therapy. Ann N Y Acad Sci. 2007;1122:155–168. doi: 10.1196/annals.1403.011. [DOI] [PubMed] [Google Scholar]
- 105.Stefanova N, et al. Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol. 2008;210:421–427. doi: 10.1016/j.expneurol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 106.Li J, et al. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11:1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 107.Park HJ, et al. Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy-parkinsonism. Cell Transplant. 2010 doi: 10.3727/096368910X540630. [DOI] [PubMed] [Google Scholar]
- 108.Kollensperger M, et al. Striatal transplantation in a rodent model of multiple system atrophy: effects on L-Dopa response. J Neurosci Res. 2009;87:1679–1685. doi: 10.1002/jnr.21972. [DOI] [PubMed] [Google Scholar]
- 109.Stefanova N, et al. Striatal transplantation for multiple system atrophy--are grafts affected by alpha-synucleinopathy? Exp Neurol. 2009;219:368–371. doi: 10.1016/j.expneurol.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 110.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 111.Mendez I, et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brundin P, et al. Research in motion: the enigma of Parkinson's disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 113.Masliah E, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hirohata M, et al. Cerebrospinal fluid from patients with multiple system atrophy promotes in vitro alpha-synuclein fibril formation. Neurosci Lett. 2011;491:48–52. doi: 10.1016/j.neulet.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Shi M, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 117.Kollensperger M, Wenning GK. Assessing disease progression with MRI in atypical parkinsonian disorders. Mov Disord. 2009;24 Suppl 2:S699–S702. doi: 10.1002/mds.22582. [DOI] [PubMed] [Google Scholar]