Abstract
Insulin secretion from pancreatic β cells is stimulated by glucagon-like peptide-1 (GLP-1), a blood glucose-lowering hormone that is released from enteroendocrine L cells of the distal intestine after the ingestion of a meal. GLP-1 mimetics (e.g., Byetta) and GLP-1 analogs (e.g., Victoza) activate the β cell GLP-1 receptor (GLP-1R), and these compounds stimulate insulin secretion while also lowering levels of blood glucose in patients diagnosed with type 2 diabetes mellitus (T2DM). An additional therapeutic option for the treatment of T2DM involves the administration of dipeptidyl peptidase-IV (DPP-IV) inhibitors (e.g., Januvia, Galvus). These compounds slow metabolic degradation of intestinally released GLP-1, thereby raising post-prandial levels of circulating GLP-1 substantially. Investigational compounds that stimulate GLP-1 secretion also exist, and in this regard a noteworthy advance is the demonstration that small molecule GPR119 agonists (e.g., AR231453) stimulate L cell GLP-1 secretion while also directly stimulating β cell insulin release. In this review, we summarize what is currently known concerning the signal transduction properties of the β cell GLP-1R as they relate to insulin secretion. Emphasized are the cyclic AMP, protein kinase A, and Epac2 mediated actions of GLP-1 to regulate ATP-sensitive K+ channels, voltage-dependent K+ channels, TRPM2 cation channels, intracellular Ca2+ release channels, and Ca2+-dependent exocytosis. We also discuss new evidence that provides a conceptual framework with which to understand why GLP-1R agonists are less likely to induce hypoglycemia when they are administered for the treatment of T2DM.
Keywords: GLP-1, glucose, insulin, exocytosis
1. Blood glucose control by incretin hormone GLP-1
Enteroendocrine cells situated in the wall of the intestine act as nutrient sensors, and they release two “incretin hormones” in response to a meal. These hormones are glucagon-like peptide-1 (GLP-1) secreted by L cells, and glucose-dependent insulinotropic peptide (GIP) secreted by K cells. Since the administration of GLP-1 (but not GIP) to patients with type 2 diabetes mellitus (T2DM) results in a reduction of blood glucose concentration, one focus of current drug discovery efforts concerns the identification of small molecule compounds that will replicate the action of GLP-1, and that will reverse the chronic hyperglycemia that is characteristic of T2DM. In this regard, the Class II GTP-binding protein-coupled receptor for glucagon-like peptide-1 (designated as the GLP-1R) is an established target for blood glucose-lowering agents that are insulin secretagogues, and that are now in use for the treatment of T2DM (Ahren, 2009; Lovshin and Drucker, 2009; Nauck, 2011).
The term “incretin effect” refers to the capacity of intestinally released GLP-1 and GIP to exert glucoregulatory effects in which they potentiate the action of orally administered glucose to stimulate pancreatic insulin secretion. These insulin secretagogue actions of GLP-1 and GIP lead to an insulin-dependent reduction of blood glucose levels, and the magnitude of this reduction is greater than what would be measurable if insulin secretion was instead stimulated by intravenous administration of glucose in the absence of intestinally released GLP-1 or GIP (Kieffer and Habener, 1999).
The incretin effect is prominent in healthy individuals, whereas it is reduced in patients with T2DM. However, pharmacological doses of intravenously administered GLP-1 exert an acute stimulatory action to raise levels of plasma insulin, thereby lowering levels of blood glucose in patients with T2DM. Of major clinical significance is the fact that the insulin secretagogue action of GLP-1 at the β cells is glucose-dependent. In fact, GLP-1 is an effective insulin secretagogue only under conditions in which the blood glucose concentration is elevated, as is the case in T2DM. This property of GLP-1 is explained by the fact that under conditions in which the blood glucose concentration is hypoglycemic, GSIS is not initiated, and GLP-1 is therefore incapable of potentiating GSIS. What this means is that the acute insulin secretagogue and blood glucose-lowering actions of GLP-1 are self-terminating as blood glucose levels fall, and for this reason, hypoglycemia is less likely to occur when GLP-1 is administered to patients with T2DM. These properties of GLP-1 contrast with the glucose-independent insulin secretagogue actions of sulfonylureas such as tolbutamide. Sulfonylureas do not exert a self-terminating action to stimulate insulin secretion, and for this reason their use involves a risk for hypoglycemia (Knop et al., 2008).
Studies of mice demonstrate that in addition to its insulin secretagogue action, GLP-1 acts as a β cell growth factor to stimulate insulin gene expression and insulin biosynthesis (Holz and Chepurny, 2003). These studies also demonstrate that GLP-1 stimulates β cell proliferation (mitosis) while slowing β cell death (apoptosis) (Holz and Chepurny, 2005). Although it remains to be demonstrated that such actions of GLP-1 occur in humans, these findings suggest that long-term administration of a GLP-1R agonist might result in a beneficial increase of β cell mass and islet insulin content. The expected outcome would be an increased pancreatic insulin secretory capacity in T2DM patients administered GLP-1R agonists. Such beneficial antidiabetogenic properties are not characteristic of sulfonylureas.
It is also important to recognize that glucoregulation under the control of GLP-1 results not simply from its direct action at pancreatic β cells. Administered GLP-1R analogs act at pancreatic α cells to inhibit glucagon secretion, and this effect is accompanied by a suppression of hepatic glucose production (Hare et al., 2010). Extra-pancreatic actions of GLP-1 lead to a slowing of gastric emptying, a suppression of appetite, and improved cardiovascular performance (Asmar and Holst, 2010). Such actions of GLP-1 are likely to be mediated not only by its Class II GPCR, but also by a non-conventional pathway activated by metabolites of GLP-1 designated as GLP-1(9–36-amide) (Tomas and Habener, 2010) or GLP-1(28–36-amide) (Tomas et al., 2011). Indeed, speculation has centered on whether this as-yet-to-be identified non-conventional pathway allows GLP-1 to exert an “insulin mimetic” action at the liver. It is presently unclear which GLP-1R analogs now in use for the treatment of T2DM have the capacity to exert effects mediated by this non-conventional pathway, and furthermore, it is uncertain whether inhibitors of GLP-1 metabolism exert undesirable side effects as a consequence of their ability to prevent the formation of GLP-1(9–36-amide) and GLP-1(28–36-amide). Therefore, opportunity exists to expand on our present understanding of GLP-1 pharmacology and physiology.
2. GLP-1 based therapies for the treatment of type 2 diabetes
One GLP-1-based strategy for the treatment of T2DM involves the subcutaneous administration of GLP-1R agonists such as Byetta (exenatide; a synthetic form of exendin-4) or Victoza (liraglutide), a modified form of GLP-1. Unlike GLP-1, both Byetta and Victoza are resistant to metabolic degradation catalyzed by dipeptidyl peptidase-IV (DPP-IV), and for this reason these compounds exert prolonged insulin secretagogue actions when they are administered subcutaneously. This is significant because the hydrolytic activity of DPP-IV quickly renders endogenous GLP-1 inactive, thereby making it an unsuitable treatment for T2DM (Holst, 2004; Israili, 2009).
A second GLP-1-based strategy for the treatment of T2DM involves the administration of DPP-IV inhibitors, compounds that have an ability to raise levels of circulating GLP-1, while having no direct stimulatory effect on L-cell GLP-1 secretion. Mechanistically, DPP-IV inhibitors prevent the conversion of GLP-1(7–36-amide) to GLP-1(9–36-amide). Such compounds include Januvia (sitagliptin) and Galvus (vildagliptin), both of which are now in use for the treatment of T2DM. As alluded to above, GLP-1(9–36-amide) may have important actions mediated by a non-conventional pathway, and for this reason it could be that that the actions of GLP-1(9–36-amide) would be absent in T2DM patients administered DPP-IV inhibitors. Despite this uncertainty, DPP-IV inhibitors are an attractive therapeutic option due to the fact that these small molecule compounds can be administered orally (Israili, 2009).
There also appears to be great potential for the development of small molecule compounds that stimulate GLP-1 secretion. In this regard, the best-characterized compounds are designated as GPR119 agonists. GPR119 is a Class I GPCR expressed on L cells, and it mediates stimulatory effects of fatty acid-amides on GLP-1 secretion. Since GPR119 is also expressed on β cells, a GPR119 agonist such as AR231453 exerts multiple effects when it is administered orally in combination with glucose (Chu et al., 2008; Chu et al., 2007). It stimulates L cell GLP-1 secretion, thereby allowing circulating GLP-1 to bind to the β cell GLP-1R and to stimulate insulin secretion. Simultaneously, it directly stimulates insulin secretion by activating GPR119 that is co-expressed with the GLP-1R on β cells. Available evidence indicates that GPR119 agonists exert these secretagogue actions by stimulating cAMP production in the L cells and β cells. The attractiveness of GPR119 agonists is further enhanced by the fact that they are also orally administrable (Jones et al., 2009; Shah and Kowalski, 2010).
3. GLP-1R activation enhances KATP channel closure and depolarizes β cells
Since GLP-1 stimulates cAMP production in pancreatic β cells (Figure 1), and because insulin secretion is stimulated by cAMP-elevating agents such as forskolin, there is good reason to believe that the β cell GLP-1R is coupled to cAMP production and insulin exocytosis. Such effects of GLP-1 are likely to be mediated by protein kinase A (PKA) and the Rap GTPase guanine nucleotide exchange factor known as Epac2 (Figure 1) (Holz, 2004a). In view of the fact that GLP-1 fails to stimulate insulin secretion in the absence of glucose, and since insulin secretion stimulated by glucose does not necessarily require co-administration of GLP-1, it is widely accepted that the physiologically relevant role of GLP-1 is to potentiate GSIS. In this “consensus model” of β cell stimulus-secretion coupling, β cell glucose metabolism is deemed to be the primary stimulus for insulin secretion, whereas cAMP metabolism is viewed as a potentiating factor, one that is capable of interacting with, and possibly modulating, glucose metabolism (Holz, 2004b). Interestingly, GLP-1 is reported to restore the glucose sensitivity of rat β cells that have been rendered metabolically compromised after their exposure to a glucose-free solution (Holz et al., 1993). This induction of “glucose competence” by GLP-1 might be of therapeutic significance since it suggests a capacity of GLP-1 to act through cAMP in order to repair metabolically compromised β cells in the islets of patients with T2DM (Holz and Habener, 1992).
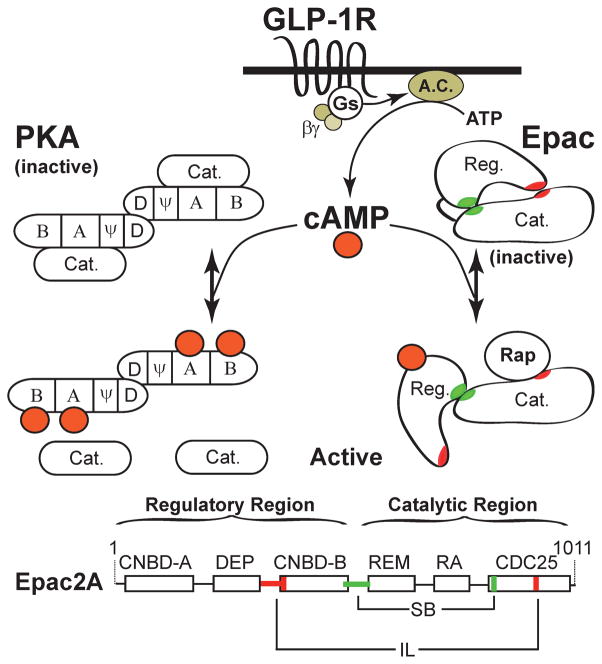
Figure 1. GLP-1 activates PKA and Epac2 in pancreatic β cells.
Binding of GLP-1 to its receptor stimulates the activities of Gs and adenylyl cyclase (A.C.), thereby generating cAMP. In pancreatic β cells the major effectors of cAMP signaling are protein kinase A (PKA) and Epac2, although islets are also known to express low levels of Epac1 (Chepurny et al., 2010; Kelley et al., 2009). The regulatory subunits of PKA contain a dimerization domain (D), a pseudosubstrate domain (ψ), and two cyclic nucleotide binding domains (CNBD, A & B) where cAMP binds and causes the dissociation and activation of the catalytic (Cat.) subunits. In the absence of cAMP, CNBD-A of the regulatory subunit plays an important role in binding the catalytic subunit, whereas CNBD-B reduces the Ka for activation of PKA by cAMP (Sjoberg et al., 2010). The regulatory subunits also bind A-kinase anchoring proteins (AKAPs) that play an important role in the cellular localization of PKA that is under the control of GLP-1 (Lester et al., 1997). The N-terminal of Epac2 contains two CNBDs (A & B) separated by a DEP domain (Dishevelled, Egl-10, Pleckstrin homology domain). This is the regulatory region (Reg.) of Epac2, and it sterically inhibits the C-terminal catalytic region (Cat.). Two interaction zones are formed in the inactive state of Epac2, an ionic latch (IL, red) and a switch board (SB, green). Binding of cAMP to CNBD-B causes a conformational change that allows Rap to bind the CDC25 homology domain that catalyzes GTP/GDP exchange and activation of Rap. The catalytic domain of Epac2 also contains a Ras-exchanger motif (REM) and a Ras-association domain (RA). Figure adapted from (Rehmann et al., 2007). CNBD-A near the N-terminal of Epac2 has low affinity for cAMP and is not important for Epac2 activation, but is instead important for cellular localization of Epac2 (Niimura et al., 2009).
Consistent with the above-summarize concepts, pancreatic insulin secretion is initiated following a simultaneous rise in blood glucose and GLP-1 concentrations. Glucose metabolism in the β cell elevates the cytosolic ATP/ADP concentration ratio and closes ATP-sensitive K+ channels (KATP) resulting in β cell depolarization, Ca2+ influx through voltage-dependent Ca2+ channels (VDCCs), and Ca2+ dependent exocytosis of insulin. This glucose metabolism-dependent closure of β cell KATP channels is potentiated by GLP-1 (Holz et al., 1993), and this modulatory action of GLP-1 appears to involve not only PKA (Light et al., 2002), but also Epac2, a novel cAMP-binding protein that interacts directly with nucleotide binding-fold 1 (NBF-1) of the sulfonylurea receptor 1 (SUR1) subunit of KATP channels (Kang et al., 2006; Kang et al., 2008; Ozaki et al., 2000; Shibasaki et al., 2004b). A role for PKA and Epac2 in the control of KATP channels is indicated because the inhibitory action of GLP-1 at KATP channels is mimicked by the catalytic subunit of PKA (Light et al., 2002), and also by 8-pCPT-2′-O-Me-cAMP, an Epac-selective cAMP analog (ESCA) that does not activate PKA when it is used at appropriately low concentrations (Enserink et al., 2002; Holz et al., 2008a).
In our most recent studies, we find that KATP channel inhibition by ESCAs can best be evaluated using a new and improved cAMP analog (8-pCPT-2′-O-Me-cAMP-AM) that is modified to incorporate an acetoxymethyl ester (AM-ester) moiety, thereby rendering it highly membrane permeable (Chepurny et al., 2009; Vliem et al., 2008). In side-by-side comparisons examining the actions of GLP-1R agonist exendin-4 (Ex-4) and the newly available ESCA-AM, we find that both substances depolarize mouse β cells, as studied in intact islets (Figure 2A–C), or in single β cells (Figure 3A,B). These depolarizing actions of Ex-4 and the ESCA-AM are accompanied by an increase of [Ca2+]i that in intact islets is sometimes accompanied by synchronous oscillations of the membrane potential and [Ca2+]i (Figure 2C). Importantly, all such actions of Ex-4 and the ESCA-AM can be measured under conditions in which islets or β cells are treated with the PKA inhibitor H-89 (Figures 2A–C, 3A,B). Furthermore, such actions of Ex-4 and the ESCA-AM require that islets and β cells be equilibrated in a concentration of glucose that is stimulatory for insulin secretion, as expected if β cell glucose metabolism and Epac2 activation interact synergistically to close KATP channels and to depolarize β cells.
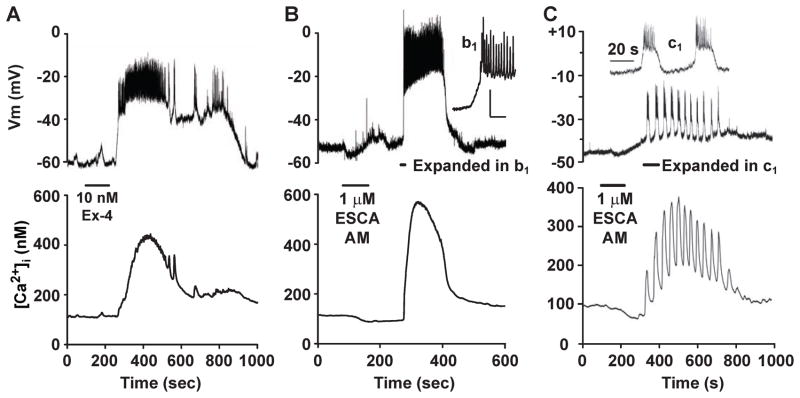
Figure 2. On-islet measurements of membrane potential demonstrate depolarizing actions of GLP-1R agonist Exendin-4 and Epac activator 8-pCPT-2′-O-Me-cAMP-AM.
A. Perforated patch (top trace) measurements of the membrane potential (Vm) were obtained from a cell located on the surface of a mouse islet. Simultaneously, the whole-islet [Ca2+]i was measured using fura 2 (lower trace). A small bolus of test solution containing 10 nM exendin-4 (Ex-4, horizontal bar) was applied directly to the islet, and it stimulated depolarization and an increase of [Ca2+]i. Note that in this experiment, a train of action potentials was superimposed on a plateau depolarization (for methods, see (Holz et al., 1995))
B. Depolarization (top trace) accompanied by an increase of whole-islet [Ca2+]i (lower trace) was also measured when a mouse islet was exposed to 1 μM 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM). The initial burst of action potentials is shown on an expanded time scale in the inset (b1). For A and B, the bath solution contained 7.5 mM glucose and 10 μM H-89, thereby establishing that PKA activity was not required to induce depolarization.
C. Perforated patch measurements of Vm (top trace) and whole-islet [Ca2+]i (lower trace) from a cell located on the surface of a mouse islet. In this cell, application of 1 μM 8-pCPT-2′-O-Me-cAMP-AM (horizontal bar) induced synchronous bursts of action potentials and oscillations of [Ca2+]i. The inset (c1) illustrates the initial bursts of action potentials in response to 8-pCPT-2′-O-Me-cAMP-AM, as displayed on an expanded time scale. For A–C, the measurements of membrane potential and [Ca2+]i were obtained as described previously.
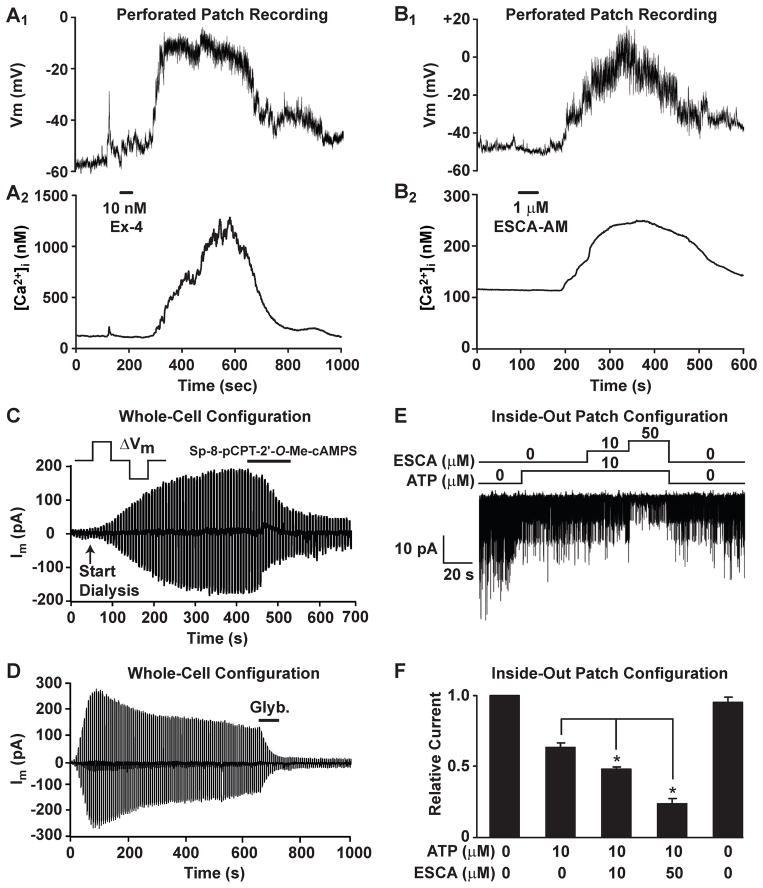
Figure 3. GLP-1R agonist Exendin-4 and Epac activator 8-pCPT-2′-O-Me-cAMP-AM depolarize single mouse β cells by inhibiting KATP channels.
A. Perforated patch recording of the membrane potential from an isolated single β cell equilibrated in buffer containing 7.5 mM glucose and 10 μM H-89 (A1). The [Ca2+]i was simultaneously measured using fura 2 (A2). Application of 10 nM exendin-4 (Ex-4, horizontal bar) induced depolarization and a simultaneous rise of [Ca2+]i.
B. Identical recording conditions as in A except that the β cell was stimulated with 1 μM 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM, horizontal bar). Depolarization accompanied by a rise of [Ca2+]i was observed similar to that elicited by exendin-4.
C,D. The whole-cell KATP current of a mouse β cell was measured under conditions of voltage clamp and dialysis using 0.3 mM ATP (for methods, see (Kang et al., 2006)). This KATP current was inhibited by Sp-8-pCPT-2′-O-Me-cAMPS (100 μM) (C) or glyburide (Glyb., 10 nM) (D) applied extracellularly (horizontal bars). Findings are representative of results obtained using n=6 β cells from two C57BL/6 mice per experimental condition.
E. Single KATP channel activity was measured in excised inside-out patches from mouse β cells (for methods, see (Kang et al., 2008)). Application of ATP (10 μM) to the intracellular surface of the patch inhibited KATP channel activity. Subsequent application of the Epac activator 2′-O-Me-cAMP (ESCA; 10 or 50 μM) further inhibited channel activity.
F. The actions of ATP and 2′-O-Me-cAMP measured in excised patches were quantified as NPo values of KATP channel activity normalized to the KATP channel activity measured at the start of the experiment in the absence of ATP and 2′-O-Me-cAMP (for methods, see (Kang et al., 2008)). Results for F are the means ± s.e.m. using three excised patches from three β cells of three C57BL/6 mice.
To provide additional confirmation that the depolarizing action of the ESCA-AM results from the closure of KATP channels, our studies included measurements of the whole-cell KATP current and single KATP channel activity in mouse β cells since such an analysis has not been reported for mice. We find that the hydrolysis-resistant Epac activator Sp-8-pCPT-2′-O-Me-cAMPS reduces a glyburide-sensitive membrane current that corresponds to the whole-cell KATP current (Figure 3C,D). We also find that the Epac activator 2′-O-Me-cAMP inhibits the activity of single KATP channels, as measured in inside-out excised patches (Figure 3E,F). Such actions of Sp-8-pCPT-2′-O-Me-cAMPS and 2′-O-Me-cAMP are qualitatively identical to what was reported in prior studies examining Epac2 regulated KATP channel activity in human β cells and rat INS-1 cells (Kang et al., 2006; Kang et al., 2008). In these prior studies, Epac2 activation produced a left-shift in the dose-response relationship describing ATP-induced inhibition of KATP channels (Kang et al., 2008). Thus, our findings are consistent with the view that GLP-1 potentiates the inhibitory action of β cell glucose metabolism at KATP channels, thereby explaining why the insulin secretagogue action of GLP-1 is glucose-dependent. Interestingly, Epac2 activation also increases the inhibitory effect of sulfonylureas at KATP channels. This finding may explain the increased incidence of hypoglycemia that is observed in humans when sulfonylureas are administered in combination with GLP-1R agonists (Leech et al., 2010).
To explain prior observations concerning KATP channel regulation, we proposed a model whereby Epac2 activation leads to Rap1 GTPase activation, thereby allowing Rap1 to activate its cognate effector phospholipase C-epsilon (PLCε) (Holz et al., 2006). Since Epac2 is bound to SUR1, this leads to the hydrolysis of phosphatidylinositol 4,5 bisphosphate (PIP2) in the immediate vicinity of KATP channels. By removing the activating effect of PIP2 at KATP channels, the open probability of KATP channels is reduced, thereby leading to an increased apparent affinity of the channels for ATP and sulfonylureas. The net effect is an increased ability of ATP and sulfonylureas to inhibit KATP channels. This model incorporates the concept that SUR1 serves as scaffold at which is located a signaling complex comprised of Epac2, Rap1, and PLCε.
An alternative model, based on studies of vascular smooth muscle, proposes that Epac activation leads to the stimulation of protein phosphatase 2B (PP2B / calcineurin), with consequent dephosphorylation and inhibition of KATP channels (Purves et al., 2009). Such findings are noteworthy since calcineurin inhibitors are used as immunosuppressants after organ transplantation. Since calcineurin inhibitors inhibit insulin secretion and predispose to diabetes (Ozbay et al., 2011), it is possible that their clinical use as immunosuppressants explains the development of post-transplantation diabetes. In this scenario, calcineurin inhibitors antagonize an Epac2-mediated inhibitory effect of GLP-1 at KATP channels, thereby upregulating channel activity so that glucose metabolism is a less effective stimulus for KATP channel closure and insulin secretion.
Since Epac2 binds to SUR1, and since this interaction is disrupted by cAMP (Shibasaki et al., 2004a), it is conceivable that Epac2 exerts a direct effect at KATP channels, and that this effect is independent of Rap1, PLCε, or calcineurin. It is also possible that the interaction of Epac2 with SUR1 enables the formation of a signaling complex that directly controls exocytosis. Such a concept is consistent with the finding that Epac2 binds to Munc13-1 (Kwan et al., 2007), a protein that is important for ATP-dependent “priming” and exocytosis of secretory granules and synaptic vesicles in excitable cells (Sheu et al., 2003). Exocytosis under the control of Munc13-1 is enhanced by the production of endogenous diacylglycerol (DAG) (Sheu et al., 2003). Thus, it is possible that the activation of Epac2 bound to SUR1 results in PLCε activation, with consequent generation of DAG from its precursor PIP2. In this model, PIP2 hydrolysis promotes KATP channel closure and Ca2+ influx, while also providing the DAG that activates Munc13-1. Simultaneously, DAG may activate protein kinase C (PKC), thereby additionally facilitating insulin exocytosis, possibly by increasing the size of a highly Ca2+-sensitive pool of secretory granules (Wan et al., 2004; Yang and Gillis, 2004).
4. GLP-1 inhibits voltage-dependent K+ currents in β cells
Evidence exists that the GLP-1R agonist Ex-4 acts through PKA to inhibit a voltage-dependent K+ current in rat β cells (MacDonald et al., 2002). This effect of Ex-4 is one that should enhance GSIS since the inhibition of voltage-dependent K+ channels (KV) leads to action potential prolongation, thereby allowing increased Ca2+ influx during each action potential. Given that GLP-1 has only a small facilitatory effect on voltage-dependent Ca2+ currents in β cells (Gromada et al., 1998), any action of GLP-1 to inhibit KV channels might be of considerable significance. Interestingly, the PKA-mediated action of Ex-4 to inhibit KV channels in rat β cells is reinforced by a cAMP-independent action of the peptide to signal through the GLP-1R and to activate PI3-kinase (MacDonald et al., 2003). This PI3-kinase-mediated signaling capacity of the GLP-1R is attributed to its ability to promote epidermal growth factor receptor (EGF-R) “transactivation” with consequent activation of β cell growth factor-like signaling pathways (Buteau et al., 2003). Since activation of the EGF-R results in Ras GTPase activation that directly activates PLCε, it could be that a convergent mechanism of GLP-1R signal transduction exists in which PLCε catalyzed hydrolysis of PIP2 underlies inhibitory effects of GLP-1 at KATP and KV channels.
5.1 Ca2+ mobilizing actions of GLP-1 in β cells
GLP-1 not only promotes Ca2+ influx through VDCCs, but it also mobilizes Ca2+ stored in intracellular organelles. Ca2+ released in this manner serves as a direct stimulus for exocytosis in β cells (Dyachok and Gylfe, 2004; Kang and Holz, 2003; Kang et al., 2003). Available evidence indicates that GLP-1 facilitates the opening of intracellular Ca2+ release channels, and that these channels correspond to inositol trisphosphate receptors (IP3R), ryanodine receptors (RYR), and nicotinic acid adenine dinucleotide phosphate receptors (NAADPR). The intracellular sources of Ca2+ include the endoplasmic reticulum (ER), endosomes and lysosomes (“acidic Ca2+ stores”), and possibly the secretory granules. Collectively, these multiple mechanisms of Ca2+ mobilization are considered to be processes of Ca2+-induced Ca2+ release (CICR) in which metabolites such as IP3, cyclic ADP-ribose, or NAADP enhance the ability of cytosolic Ca2+ to activate various Ca2+ release channels located on intracellular organelles (Islam, 2010).
5.2 Evidence that GLP-1 facilitates Ca2+-induced Ca2+ release
GLP-1 facilitates CICR in mouse and human β cells, and also in rat INS-1 cells that secrete insulin (Holz et al., 1999; Kang et al., 2001). This CICR is generated by the opening of intracellular Ca2+ release channels, the various subtypes of which are expressed in a species-specific manner such that the expression of the type-2 isoform of RYR (RYR-2) is higher in human and rat β cells, whereas it is lower in mouse β cells that may instead primarily use the IP3R to mobilize Ca2+ (Beauvois et al., 2004; Dyachok and Gylfe, 2004; Dyachok et al., 2004; Holz et al., 1999). For human and mouse β cells, CICR can be facilitated not only by GLP-1, but also by cAMP analogs that activate either PKA or Epac2 selectively. This finding demonstrates that there is dual PKA and Epac2 regulation of the Ca2+ release process (Kang et al., 2005; Kang et al., 2003). Furthermore, this mechanism of CICR is contingent on equilibration of β cells in stimulatory concentrations of glucose, as expected if intracellular Ca2+ uptake and release are under the control of glucose metabolism. Consistent with this concept, the combined administration of glucose and GLP-1 to β cells induces synchronized oscillations of the cytosolic [Ca2+] and [cAMP], and these oscillations are coupled to exocytosis of insulin (Dyachok et al., 2008; Holz et al., 2008b; Landa et al., 2005).
PKA-dependent facilitation of CICR by GLP-1 is believed to be a consequence of the phosphorylation of intracellular Ca2+ release channels (Dyachok and Gylfe, 2004; Holz et al., 1999). Simultaneously, PKA may synergize with β cell glucose metabolism to promote the uptake of Ca2+ into intracellular organelles, thereby increasing the amount of Ca2+ available for release (Yaekura and Yada, 1998). These PKA mediated actions of GLP-1 are complemented by its ability to activate Epac2 and to stimulate a signal transduction cascade comprised of Rap1, PLCε, PKC, and Ca2+/calmodulin- dependent protein kinase II (CaMK-II) (Dzhura et al., 2010). Activation of this cascade facilitates CICR not only in β cells, but also in cardiomyocytes where CaMK-II ultimately phosphorylates RYR-2 (Oestreich et al., 2009).
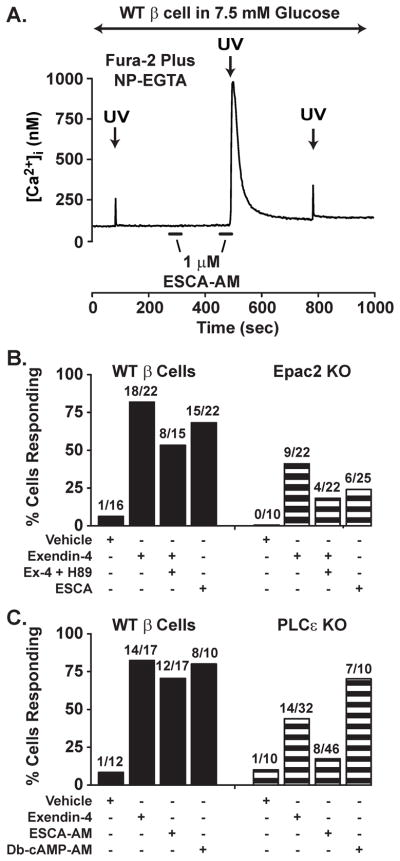
Epac2-dependent mobilization of Ca2+ is most convincingly demonstrated in assays of CICR initiated by the UV flash photolysis-induced uncaging of Ca2+ in β cells loaded with “caged Ca2+” that is bound to NP-EGTA (Figure 4). Thus, when β cells from wild-type (WT) mice are bathed in a solution containing 7.5 mM glucose, flash photolysis releases Ca2+ from NP-EGTA, and this Ca2+ acts as a stimulus for CICR. Under control conditions in which only a small amount of Ca2+ is uncaged, CICR is not initiated, and the small increase of [Ca2+]i that is measured simply reflects the uncaging of Ca2+ (Figure 4A). However, if the experiment is repeated under conditions in which the uncaging of Ca2+ is paired with the application of 8-pCPT-2′-O-Me-cAMP-AM to a β cell, then CICR is generated (Figure 4A). A similar facilitation of CICR is measured in response to Ex-4 or the PKA selective cAMP analog Db-cAMP-AM (Figure 4B,C). By repeating this assay using β cells from Epac2 or PLCε knockout (KO) mice, it is possible to demonstrate that the KO of Epac2 or PLCε nearly abrogates the facilitatory action of 8-pCPT-2′-O-Me-cAMP-AM, while not affecting the action of Db-cAMP-AM (Figure 4B,C). Furthermore, as expected for a GLP-1R agonist that activates both PKA and Epac2, these same two knockouts reduce, but do not abrogate, the facilitatory action of Ex-4 (Figure 4B,C). It may be concluded that in β cells there exists a mechanism of second messenger coincidence detection in which intracellular Ca2+ release channels respond to Ca2+ and cAMP generated as a consequence of GLP-1R stimulation (Kang et al., 2005). Future efforts will be directed at establishing whether these Ca2+ release channels correspond to the IP3R, RYR, or NAADPR.
Figure 4. Ca2+-induced Ca2+ release is facilitated by GLP-1R agonist Exendin-4 in single mouse β cells.
A. A wild-type (WT) mouse β cell was loaded with a fura-2 and NP-EGTA in order to perform the uncaging of Ca2+ while measuring the [Ca2+]i (for methods, see (Kang et al., 2005)). Flash photolysis with brief ultraviolet light (UV, 340 nm) released a small amount of Ca2+ under control conditions in which cells were bathed in 7.5 mM glucose. Note that a 30 s extracellular application of 1 μM 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM, horizontal bar) alone had no effect on [Ca2+]i, whereas application of 8-pCPT-2′-O-Me-cAMP-AM in combination with UV flash photolysis produced a large transient increase of [Ca2+]i due to CICR.
B. A population study of fura-2 and NP-EGTA loaded mouse β cells that were subjected to UV flash photolysis in the presence of exendin-4 (10 nM), exendin-4 plus H-89 (10 μM), or 8-pCPT-2′-O-Me-cAMP-AM (1 μM, ESCA-AM). Note that treatment of wild-type (WT) mouse β cells with either exendin-4 or 8-pCPT-2′-O-Me-cAMP-AM increased the percentage of cells exhibiting CICR. Also note that the action of exendin-4 to facilitate CICR was reduced in β cells of Epac2 knockout (KO) mice, whereas the action of 8-pCPT-2′-O-Me-cAMP-AM was nearly abrogated (for additional information regarding the Epac2 KO mice, see (Dzhura et al., 2010)).
C. A population study as in B except that CICR was evaluated in β cells of WT and PLC-ε KO mice. Note that the action of exendin-4 to facilitate CICR was reduced in the PLC-ε KO β cells, and that the action of 8-pCPT-2′-O-Me-cAMP-AM was nearly abrogated. Also note that the action of a PKA-selective cAMP analog (Db-cAMP-AM, 1 μM) was unaffected by the KO (for additional information regarding the PLC-ε KO mice, see (Dzhura et al., 2010)).
5.3. Non-conventional Ca2+ mobilizing properties of GLP-1 mediated by the IP3R
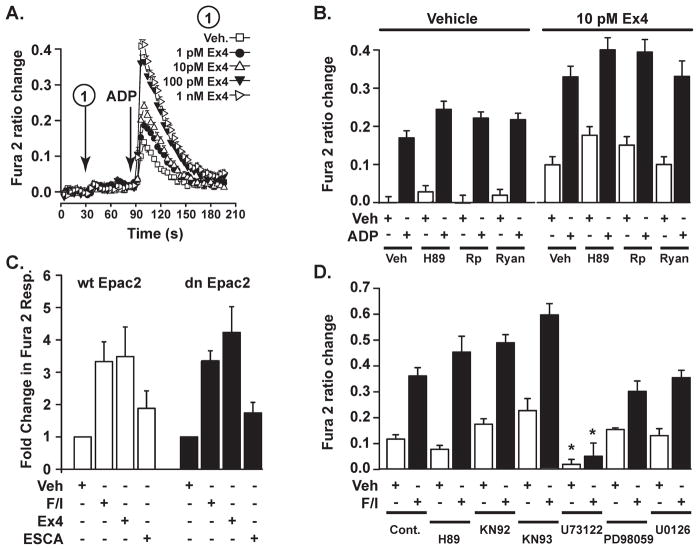
Ca2+ mobilizing properties of GLP-1 can also be studied in cell lines such as HEK293. For our purposes, these cells are engineered to express recombinant human GLP-1 receptors. Although prior studies of HEK-GLP-1R cells provided evidence that GLP-1 facilitates CICR by activating PKA, and by promoting the opening of RYR-2 (Gromada et al. 1995), we used these cells to demonstrate that this is not the only means by which GLP-1 exerts its Ca2+ mobilizing action. Initially, we found that HEK-GLP-1R cells express P2Y purinergic receptors that can be activated by ADP, and that are coupled to IP3R dependent Ca2+ mobilization. Thus, it was possible to analyze the functional interaction of GLP-1R agonists and ADP to stimulate an IP3R dependent increase of [Ca2+]i in these cells. Using this approach, we find that Ex-4 potentiates the action of ADP to mobilize Ca2+ (Figure 5A).
Figure 5. Potentiation of IP3R-mediated Ca2+ mobilization by GLP-1R agonist Exendin-4.
A. Fura-2 determinations of [Ca2+]i were obtained in a 96-well format from monolayers of HEK293 cells that express endogenous P2Y purinergic receptors, and that were engineered to stably express the recombinant human GLP-1R. A 2-step injection protocol was used so that the GLP-1R agonist exendin-4 (Ex-4) could be administered to each well 60 s prior to the administration of P2Y receptor agonist ADP (10 nM). Note that exendin-4 potentiated the action of ADP to mobilize intracellular Ca2+. The vehicle solution (Veh.) was standard extracellular saline (SES) containing 0.1% BSA (for methods, see (Leech et al., 2010)).
B. The action of ADP (10 nM) to mobilize Ca2+ was potentiated by exendin-4 (10 pM), and this action of exendin-4 was not affected by pretreatment of cells with H-89 (5 μM), Rp-8-CPT-cAMPS (Rp, 10 μM), or ryanodine (Ryan., 50 μM). Horizontal lines above the histogram bars indicate the test solutions that were administered during the first injection. The plus or minus symbols under each histogram bar indicate whether a vehicle solution (Veh, 0.1% DMSO) or ADP was administered during the second injection.
C. HEK293 cells were virally transduced with wild-type (wt) or dominant-negative (dn) Epac2 (for details regarding wt and dn Epac2, see (Kang et al., 2001)). A 2-step injection protocol was used so that in the first injection the solution contained either: 1) 0.1% DMS0 vehicle (Veh), or 2) 2 μM forskolin and 50 μM IBMX (F/I), or 3) 10 pM exendin-4 (Ex4), or 4) 3 μM 8-pCPT-2′-O-Me-cAMP-AM (ESCA). 60 s after the first injection, a second injection was performed in which ADP (10 nM) was administered.
D. Potentiation of ADP (10 nM) induced Ca2+ mobilization by forskolin and IBMX (F/I) was unaffected by pretreatment of HEK293 cells with PKA inhibitor H-89 (5 μM), the CaMKII inhibitor KN-93 or its inactive analog KN-92 (1 μM, each), or the MEK inhibitors (PD98059 (25 μM) and U0126 (10 μM). Only the PLC inhibitor U73122 (2 μM) was an effective antagonist in this assay.
Surprisingly, this action of Ex-4 in HEK-GLP-1R cells is not blocked by inhibitors of PKA (H-89, Rp-8-CPT-cAMPS), nor is it antagonized by ryanodine (Figure 5B). Moreover, this action of Ex-4 is mimicked by the cAMP-elevating agents forskolin and IBMX, and these actions are measurable under conditions in which cells are virally transduced with a dominant-negative Epac2 (Figure 5C). Thus, it seems that neither PKA activation nor Epac2 activation can explain how Ex-4 exerts its action in this assay. Such a conclusion is reinforced by the finding that 8-pCPT-2′-O-Me-cAMP-AM has little capacity to potentiate the Ca2+ mobilizing action of ADP (Figure 5C). Since a selective blocker of PLC (U73122) nearly abolishes Ca2+ mobilization in this assay (Figure 5C), it seems clear that cAMP-elevating agents do in fact modulate the activity of the IP3R in this cell type. Remarkably, our findings are nearly identical to what was previously reported in studies of HEK293 cells expressing the parathyroid hormone receptor (PTH-R), a Class II GPCR that is structurally-related to the GLP-1R. In those published studies, it was concluded that cAMP exerts a direct action at the IP3R to facilitate its opening, and that this action of cAMP is independent of both PKA and Epac (Tovey et al., 2010). Taken together, these observations raise the possibility that a non-conventional mechanism of Ca2+ mobilization underlies the ability of GLP-1 to facilitate the release of Ca2+ from IP3R-regulated Ca2+ stores in β cells.
5.4. Potential NAADP receptor-mediated actions of GLP-1
NAADP is a Ca2+-mobilizing metabolite that is reported to bind to and stimulate the opening of “two-pore” Ca2+ channels (TPCs) (Calcraft et al., 2009; Naylor et al., 2009). These TPCs mediate the mobilization of Ca2+ from “acidic Ca2+ stores” (Figure 6A), and this source of Ca2+ may be localized in the lysosomes, endosomes, and possibly secretory granules (Galione et al., 2010). Evidence also exists that the mobilization of Ca2+ from these stores might enhance β cell electrical excitability and insulin secretion due to stimulatory effects of released Ca2+ on plasma membrane cation channels (Arredouani et al., 2010). Thus, it is of interest that GLP-1 is reported to act through PKA and Epac2 to mobilize Ca2+ that is stored within the acidic Ca2+ stores of β cells (Kim et al., 2008).
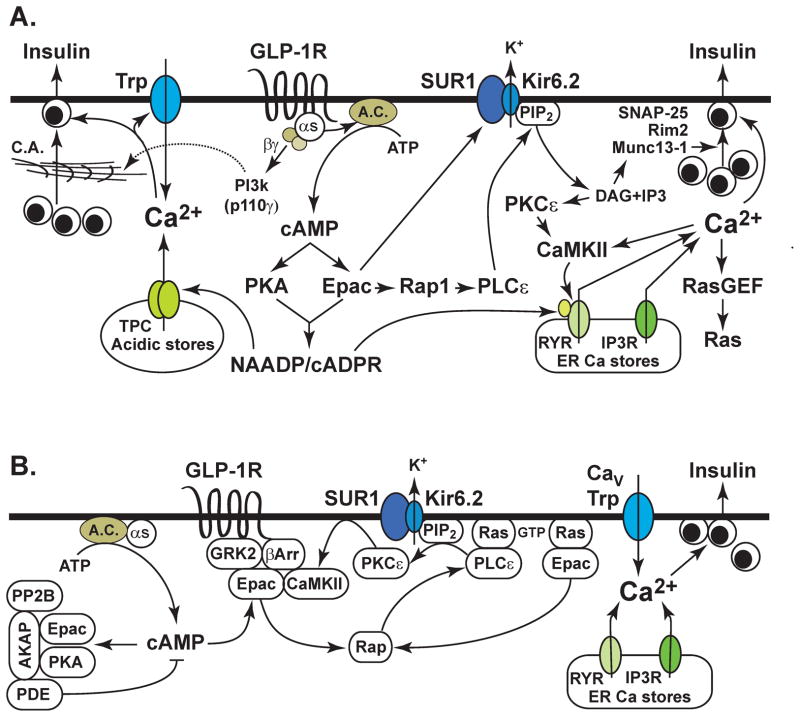
Figure 6. Glucose-stimulated insulin secretion under the control of GLP-1.
A. GLP-1 stimulates cAMP production through its effects mediated by the GLP-1R, Gαs and adenylyl cyclase (A.C.). The βγ subunits of Gs activate PI3-kinase (p110γ) and disrupt cortical actin (CA) in the cytoskeleton in order to promote granule trafficking to the plasma membrane. cAMP activates PKA and Epac2, both of which may play a role in generating NAADP and cADPR. Ca2+ is mobilized from endoplasmic reticulum (ER) Ca2+ stores that contain ryanodine and IP3 receptors (RYR, IP3-R), and also from lysosomal Ca2+ stores that contain two-pore Ca2+ channels (TPC). Mobilized Ca2+ activates Trp channels, and this produces membrane depolarization. Ca2+ also activates CaMKII or RasGEFs that are guanine nucleotide exchange factors that promote Ras activation. Rap1 is activated downstream of Epac2, and it mediates the activation of PLCε that hydrolyzes PIP2 to generate DAG and IP3. KATP chanel activity is regulated by the direct binding of Epac2 to SUR1, and by local effects of PIP2 at the channel. DAG activates PKCε and also Munc13-1 in order to facilitate Ca2+-dependent exocytosis. Interactions between Epac2, SNAP-25, and Rim2 play important roles in cAMP-regulated granule trafficking, docking, and fusion.
B. Compartmentalized cAMP signaling is favored by the assembly of a macromolecular complex comprised of A-kinase anchoring proteins (AKAP) that bind PKA, Epac2, cyclic nucleotide phosphodiesterases (PDE), and protein phosphatase 2B (PP2B). Compartmentalization is also mediated by G protein-coupled receptor kinases (GRK) and β-arrestins that form a complex with Epac2 and CamKII so that these proteins are located close to the GLP-1R. Translocation of Epac2 and PLCε to the plasma membrane is favored by their direct interactions with H-Ras. PKCε also undergoes translocation to the plasma membrane following Epac2 activation. Compartmentalized Ca2+ signaling results from localized Ca2+ release from intracellular Ca2+ stores, and from localized Ca2+ influx that results from the opening of voltage-dependent Ca2+ channels (CaV) and Trp channels.
One way acidic Ca2+ stores are operationally defined is that they are emptied following treatment with bafilomycin. This inhibitor of the vacuolar-type H+-ATPase is responsible for proton transport that acidifies the intra-organellar compartment, thereby enabling sequestration of Ca2+. We have evaluated the potential role of acidic Ca2+ stores in the mobilization of Ca2+ by cAMP, and in this regard we find that treatment of mouse β cells with bafilomycin fails to disrupt the action of 8-pCPT-2′-O-Me-cAMP-AM to facilitate CICR under conditions of NP-EGTA loading and UV flash photolysis (Dzhura et al., 2010). In mark contrast, the facilitatory action of 8-pCPT-2′-O-Me-cAMP-AM is blocked by thapsigargin, an inhibitor of SERCA ATPases that mediate the uptake of Ca2+ into the ER of β cells. Importantly, we also find that thapsigargin disrupts the action of a PKA-selective cAMP analog (6-Bnz-cAMP-AM) to facilitate CICR (Dzhura et al., 2010). Therefore, available evidence indicates that it is the ER from which Ca2+ is released under the experimental conditions used in our assay of CICR. This conclusion is reinforced by our prior finding that in mouse β cells, the ability of forskolin to facilitate CICR is antagonized by heparin, an inhibitor of IP3R dependent ER Ca2+ release (Kang et al., 2005).
6. Trp channels may be under the control of GLP-1
Ca2+-activated non-selective cation (CAN) channels are expressed in β cells but their physiological importance was uncertain since their molecular identities were not previously known. CAN channels were originally described as being activated by high levels of Ca2+, and inhibited by adenine nucleotides (Sturgess et al., 1986). They were later found to be regulated by cyclic nucleotides (Reale et al., 1995). Subsequently, GLP-1 was shown to activate a non-selective cation current in β cells (Holz et al., 1995). This current appears to be identical to the current activated by maitotoxin (Leech and Habener, 1998), and it is generated by the opening of CAN channels that have a single channel conductance of 25 – 30 pS (Leech and Habener, 1998). The properties of these CAN channels resemble those of TRPM4 channels, a class of CAN channels that play a role in glucose-stimulated and arginine-vasopressin-regulated insulin secretion (Cheng et al., 2007; Marigo et al., 2009). In addition to TRPM4, the closely related CAN channel TRPM5 also plays a role in glucose-stimulated insulin secretion (Brixel et al., 2010; Colsoul et al., 2010). Thus, an attractive hypothesis it is that TRPM4 and/or TRPM5 might be under the control of GLP-1, and that activation of these channels would lead to membrane depolarization, Na+ and Ca2+ influx, and increased β cell excitability.
TRPM2 is also a non-selective cation channel, and it is activated by heat and ADP-ribose in β cells. Its activation may explain some stimulatory effects of glucose and GLP-1 on insulin secretion. In this regard, cyclic-ADP-ribose may mediate these effects (Lange et al., 2009; Togashi et al., 2006; Uchida et al., 2011). Evidence also exists that TRPM2 participates in cell death initiated by reactive oxygen species-induced release of Ca2+ from lysosomes (Lange et al., 2009). Finally, it should be noted that TRPV1 and TRPV2 are expressed in β cells, and that these capsaicin-activatable channels seem to play some role in the dual control of insulin secretion and β cell growth (Akiba et al., 2004). Thus, it will be particularly interesting to assess whether any of these Trp channel variants are under the control of GLP-1.
7. GLP-1 exerts a direct stimulatory effect on Ca2+-dependent exocytosis
GLP-1 also exerts a direct action at “late steps” of β cell stimulus-secretion coupling in order to facilitate Ca2+-dependent exocytosis of insulin. This capacity is understandable because PKA activity increase the sizes of a highly Ca2+ sensitive pool (HCSP) of secretory granules in β cells (Wan et al., 2004; Yang and Gillis, 2004). Conceivably, this action of PKA may allow CICR to efficiently trigger insulin exocytosis (Kang and Holz, 2003). Since PKC activity also increases the size of the HCSP (Wan et al., 2004; Yang and Gillis, 2004), and since GLP-1 acts through PLC to activate PKC (Dzhura et al., 2010; Suzuki et al., 2006), it seems likely that the dual regulation of the HCSP by PKA and PKC underlies some stimulatory effects of GLP-1 on insulin secretion. Given that the HCSP is not prepositioned adjacent to VDCCs at the plasma membrane, this pool of secretory granules may undergo exocytosis in response to Ca2+ released from intracellular organelles.
Evidence also exists that GLP-1 acts through PKA to recruit secretory granules from a reserve pool into a readily releasable pool (RRP) that is docked and primed in close proximity to VDCCs (Renstrom et al., 1997). These secretory granules undergo exocytosis in response to high concentrations (10–20 μM) of Ca2+ that form in microdomains at the inner mouths of VDDCs (Barg et al., 2002; Bokvist et al., 1995). Thus, in the presence of GLP-1, and under conditions in which PKA is activated, Ca2+ influx through VDCCs initiates exocytosis of the RRP. Simultaneously, exocytosis of the HCSP may be initiated by CICR that is itself triggered by Ca2+ influx. This concept is consistent with the observation that CICR generates a global increase of cytosolic [Ca2+] that is comparatively small in magnitude (0.5–1.0 μM), but that may be capable of releasing the HCSP. One interesting prediction of this model is that in the absence of GLP-1, exocytosis stimulated by glucose metabolism will release a small number of secretory granules located within the RRP without stimulating exocytosis of the HCSP. However, in the presence of GLP-1, both the RRP and the HCSP undergo exocytosis, thereby establishing that exocytosis initiated by CICR constitutes an amplification mechanism that enables GLP-1 to potentiate GSIS (Kang and Holz, 2003).
A “post-priming” step in insulin exocytosis is also under the control of PKA, and it may mediate the action of GLP-1 to potentiate GSIS (Takahashi et al., 1999). This finding is understandable if in β cells there exists PKA-mediated phosphorylation of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, and their protein-interacting partners. Such proteins may include Snapin, a PKA substrate that interacts with the SNARE protein SNAP-25, and that regulates a direct association of the Ca2+ sensor synaptotagmin with the core SNARE complex (Chheda et al., 2001; Ilardi et al., 1999). More speculative is the potential role of PKA substrate Rim2. It is a SNAP-25 and Mun13-1 interacting protein that acts at an earlier step in exocytosis, one that supports the ATP-dependent priming of secretory granules, a step that renders the granules release competent (Kwan et al., 2007).
There is also substantial evidence that Epac2 participates in the regulation of exocytosis by GLP-1. For example, Epac2 interacts with Piccolo (a Ca2+-sensor), and Rim2 (a Rab3 effector), both of which play a role in exocytosis (Shibasaki et al., 2004a). Moreover, the knockdown of Epac2 expression is reported to diminish GLP-1-stimulated insulin secretion by ca. 50% (Kashima et al., 2001). In these assays, the action of GLP-1 mediated by Epac2 is also demonstrated to be mediated by Rim2 (Kashima et al., 2001), and the importance of Rim2 to insulin exocytosis is now quite clear (Kwan et al., 2007; Yasuda et al., 2010). Interestingly, the SNARE protein SNAP-25 also binds Epac2, and the formation of an Epac2, SNAP-25, and Rim2 complex may mediate additional stimulatory actions of GLP-1 on insulin exocytosis (Vikman et al., 2009). Finally, it is important to note that Epac2 activation promotes secretory granule acidification, a step necessary for granule priming and release competence (Eliasson et al., 2003). Since this action of Epac2 is absent in SUR1 knockout mice, it appears that cAMP-dependent granule acidification is contingent on the interaction of Epac2 with SUR1. This might explain why the insulin secretagogue action of GLP-1 is reduced in SUR1 knockout mice (Nakazaki et al., 2002; Shiota et al., 2002).
Additional insights concerning the stimulatory action of GLP-1 on insulin secretion have been obtained in studies examining interactions of Ca2+ channel subunits with SNARE-associated proteins. Both Piccolo and Rim2 are reported to bind to the α1.2 subunit of L-type Ca2+ channels in β cells (Shibasaki et al., 2004a), and this interaction may anchor L-type Ca2+ channels in lipid rafts to form a signaling complex important for glucose and GLP-1 regulated insulin secretion (Jacobo et al., 2009b). The intracellular II-III loop of CaV1.2 immunoprecipitates with Rim2 (Jacobo et al., 2009b), consistent with previous data showing that Rim2 specifically binds CaV1.2 through its C2 domain (Shibasaki et al., 2004a). These findings correlate with the fact that that the knockdown of CaV1.2 expression in INS-1 cells disrupts GSIS (Nitert et al., 2008).
Studies performed with transfected INS-1 cells expressing dyhydropyridine-insensitive Ca2+ channel mutants seem to indicate that GSIS under the control of GLP-1 also requires the expression of CaV1.3 (Liu et al., 2003). This approach has revealed that influx of Ca2+ through CaV1.3 stimulates exocytosis that can be potentiated by 8-pCPT-2′-O-Me-cAMP, and that this effect of the Epac activator requires permissive PKA activity since it is blocked by H-89 (Jacobo et al., 2009a; Liu et al., 2003). Similarly, the insulin secretagogue action of 8-pCPT-2′-O-Me-cAMP-AM in human islets is blocked by Rp-8-CPT-cAMPS, an antagonist of PKA activation (Chepurny et al., 2010). Importantly, the PKA dependent insulin secretagogue action of 8-pCPT-2′-O-Me-cAMP-AM is not explained by any unexpected action of this cAMP analog to activate PKA. This fact was demonstrated in assays of β cells expressing AKAR3, a biosensor that reports PKA activation (Chepurny et al., 2010). What remains unclear is the nature of the permissive PKA activity that supports insulin secretion. Available evidence suggest that permissive PKA activity might be the consequence of β cell glucose metabolism (Hatakeyama et al., 2006). Such findings lend credibility to the idea that PKA plays a critical role in support of insulin exocytosis that is under the dual control of glucose metabolism and GLP-1.
8. Current controversies regarding Epac2 and PKA
Despite direct evidence that GSIS can be potentiated by 8-pCPT-2′-O-Me-cAMP-AM (Chepurny et al., 2010), there exists controversy concerning the exact role Epac2 plays in the control of exocytosis. An important role for Epac2 is indicated since β cells of Epac2 KO mice exhibit a secretory defect in which the action of cAMP to potentiate first phase GSIS is reduced (Shibasaki et al., 2007). What is surprising is that two-photon extracellular polar-tracer (TEP) imaging studies of β cells do not substantiate a role for Epac2 in the control of insulin secretion (Hatakeyama et al., 2007). However, it is important to emphasize that these TEP studies were performed under conditions in which exocytosis was stimulated by the uncaging of Ca2+. Exocytosis studied in this manner is not representative of GSIS due to the fact that this approach bypasses early steps of stimulus-secretion coupling that involve KATP channel closure. Since first phase GSIS is primarily a KATP channel dependent mechanism of exocytosis (Henquin, 2000), and since the KO of Epac2 disrupts first phase GSIS, it could be that under true physiological conditions, the primary role of Epac2 is to mediate cAMP-dependent closure of KATP channels, thereby depolarizing β cells. This concept is consistent with the therapeutically important action of GLP-1 to restore the missing first phase component of GSIS in patients with T2DM. In this regard, it is noteworthy that the action of GLP-1 to potentiate GSIS is strongly attenuated in mice in which there is a knockout of the KATP channel SUR1 or Kir6.2 subunits (Miki et al., 2005; Nakazaki et al., 2002; Shiota et al., 2002). These findings are interpretable if an Epac2-mediated action of GLP-1 to close KATP channels plays an important role in conferring stimulatory effects of GLP-1 on insulin secretion. This concept is further supported by studies of Kcnj11Y12STOP mice in which the insulin secretagogue action of GLP-1 is reduced (Hugill et al., 2010). In these mutant mice, the gene coding for Kir6.2 (Kcnj) contains an inactivating tyrosine to stop codon point mutation (Y12STOP) so that KATP channel activity is undetectable in islet β cells. Thus, in β cells with no active KATP channels, the insulin secretagogue action of GLP-1 is nearly absent, thereby establishing KATP channels to be critically important intermediaries linking GLP-1R activation to islet insulin secretion (Hugill et al., 2010).
9. Summary
The remarkable effectiveness of incretin-based therapies for the treatment of T2DM has spurred continued efforts to pinpoint exactly how GLP-1 exerts its stimulatory effects at the β cells. In this regard, ongoing efforts are primarily devoted to understanding the differential, yet complementary roles, PKA and Epac2 play in the potentiation of GSIS. An outgrowth of recent studies is that it is increasingly appreciated that GLP-1 may act through PKA and Epac2 to modulate the ADP and ATP sensitivities of K+ channels (Light et al. 2002; Kang et al. 2008). This action of GLP-1 may allow β cell glucose metabolism to more efficiently close these channels, thereby stimulating insulin secretion. To what extent GLP-1 has any capacity to enhance oxidative glucose metabolism in the β cell is a topic of considerable interest. However, discrepant findings concerning this possibility remain to be resolved (Peyot et al., 2009; Tsuboi et al., 2003). In conclusion, the biochemical and molecular basis for beneficial insulin secretagogue and blood glucose-lowering actions of incretin mimetics, GLP-1R analogs, and DPP-IV inhibitors remains a topic of considerable interest to the diabetes research community.
Acknowledgments
G.G.H. acknowledges the support of the NIH (R01-DK045817; R01-DK069575). C.A.L. acknowledges the support of the American Diabetes Association (Basic Science Research Grant Award).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B. Clinical results of treating type 2 diabetic patients with sitagliptin, vildagliptin or saxagliptin--diabetes control and potential adverse events. Best Pract Res Clin Endocrinol Metab. 2009;23:487–98. doi: 10.1016/j.beem.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321:219–25. doi: 10.1016/j.bbrc.2004.06.149. [DOI] [PubMed] [Google Scholar]
- Arredouani A, Evans AM, Ma J, Parrington J, Zhu MX, Galione A. An emerging role for NAADP-mediated Ca2+ signaling in the pancreatic beta-cell. Islets. 2010;2:323–330. doi: 10.4161/isl.2.5.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmar M, Holst JJ. Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: new advances. Curr Opin Endocrinol Diabetes Obes. 2010;17:57–62. doi: 10.1097/MED.0b013e3283339051. [DOI] [PubMed] [Google Scholar]
- Barg S, Eliasson L, Renstrom E, Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 2002;51(Suppl 1):S74–82. doi: 10.2337/diabetes.51.2007.s74. [DOI] [PubMed] [Google Scholar]
- Beauvois MC, Arredouani A, Jonas JC, Rolland JF, Schuit F, Henquin JC, Gilon P. Atypical Ca2+-induced Ca2+ release from a sarco-endoplasmic reticulum Ca2+-ATPase 3-dependent Ca2+ pool in mouse pancreatic beta-cells. J Physiol. 2004;559:141–56. doi: 10.1113/jphysiol.2004.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokvist K, Eliasson L, Ammala C, Renstrom E, Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO J. 1995;14:50–7. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D. TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch. 2010;460:69–76. doi: 10.1007/s00424-010-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–32. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium. 2007;41:51–61. doi: 10.1016/j.ceca.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG, Holz GG. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–33. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepurny OG, Leech CA, Kelley GG, Dzhura I, Dzhura E, Li X, Rindler MJ, Schwede F, Genieser HG, Holz GG. Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2′-O-Me-cAMP-AM. J Biol Chem. 2009;284:10728–36. doi: 10.1074/jbc.M900166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda MG, Ashery U, Thakur P, Rettig J, Sheng ZH. Phosphorylation of Snapin by PKA modulates its interaction with the SNARE complex. Nat Cell Biol. 2001;3:331–8. doi: 10.1038/35070000. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, Jones RM, Behan DP, Leonard J. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149:2038–47. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–9. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci U S A. 2010;107:5208–13. doi: 10.1073/pnas.0913107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachok O, Gylfe E. Ca2+-induced Ca2+ release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic beta-cells. J Biol Chem. 2004;279:45455–61. doi: 10.1074/jbc.M407673200. [DOI] [PubMed] [Google Scholar]
- Dyachok O, Idevall-Hagren O, Sagetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjarvi G, Gylfe E, Tengholm A. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008;8:26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Dyachok O, Tufveson G, Gylfe E. Ca2+-induced Ca2+ release by activation of inositol 1,4,5-trisphosphate receptors in primary pancreatic beta-cells. Cell Calcium. 2004;36:1–9. doi: 10.1016/j.ceca.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Chepurny OG, Kelley GG, Leech CA, Roe MW, Dzhura E, Afshari P, Malik S, Rindler MJ, Xu X, Lu Y, Smrcka AV, Holz GG. Epac2-dependent mobilization of intracellular Ca2+ by GLP-1 receptor agonist Exendin-4 is disrupted in beta cells of PLC-{epsilon} knockout mice. J Physiol. 2010 doi: 10.1113/jphysiol.2010.198424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic β-cells. J Gen Physiol. 2003;121:181–97. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–6. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Galione A, Morgan AJ, Arredouani A, Davis LC, Rietdorf K, Ruas M, Parrington J. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem Soc Trans. 2010;38:1424–31. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide 1 (7–36) amide stimulates exocytosis in human pancreatic β-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998;47:57–65. doi: 10.2337/diab.47.1.57. [DOI] [PubMed] [Google Scholar]
- Hare KJ, Vilsboll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59:1765–70. doi: 10.2337/db09-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol. 2006;570:271–82. doi: 10.1113/jphysiol.2005.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse β-cells. J Physiol. 2007;582:1087–98. doi: 10.1113/jphysiol.2007.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–60. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- Holst JJ. Treatment of type 2 diabetes mellitus with agonists of the GLP-1 receptor or DPP-IV inhibitors. Expert Opin Emerg Drugs. 2004;9:155–66. doi: 10.1517/eoed.9.1.155.32952. [DOI] [PubMed] [Google Scholar]
- Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta cell. Diabetes. 2004a;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic beta-cells. Horm Metab Res. 2004b;36:787–94. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10:2471–83. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG. Diabetes outfoxed by GLP-1? Sci STKE. 2005;2005:pe2. doi: 10.1126/stke.2682005pe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal. 2008a;20:10–20. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: pancreatic beta cells and the glucose competence concept. Trends Biochem Sci. 1992;17:388–93. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Heart E, Leech CA. Synchronizing Ca2+ and cAMP oscillations in pancreatic beta-cells: a role for glucose metabolism and GLP-1 receptors? Focus on “regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic beta-cell: a computational approach”. Am J Physiol Cell Physiol. 2008b;294:C4–6. doi: 10.1152/ajpcell.00522.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kuhtreiber WM, Habener JF. Pancreatic beta cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37) Nature. 1993;361:362–5. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Habener JF. Activation of a cAMP-regulated Ca2+-signaling pathway in pancreatic beta cells by the insulinotropic hormone glucagon-like peptide-1. J Biol Chem. 1995;270:17749–57. [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7–37) J Biol Chem. 1999;274:14147–56. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugill A, Shimomura K, Ashcroft FM, Cox RD. A mutation in KCNJ11 causing human hyperinsulinism (Y12X) results in a glucose-intolerant phenotype in the mouse. Diabetologia. 2010;53:2352–6. doi: 10.1007/s00125-010-1866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilardi JM, Mochida S, Sheng ZH. Snapin: a SNARE-associated protein implicated in synaptic transmission. Nat Neurosci. 1999;2:119–24. doi: 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- Islam MS. Calcium signaling in the islets. Adv Exp Med Biol. 2010;654:235–59. doi: 10.1007/978-90-481-3271-3_11. [DOI] [PubMed] [Google Scholar]
- Israili ZH. Advances in the Treatment of Type 2 Diabetes Mellitus. Am J Ther. 2009 doi: 10.1097/MJT.0b013e3181afbf51. [DOI] [PubMed] [Google Scholar]
- Jacobo SM, Guerra ML, Hockerman GH. Cav1.2 and Cav1.3 are differentially coupled to glucagon-like peptide-1 potentiation of glucose-stimulated insulin secretion in the pancreatic beta-cell line INS-1. J Pharmacol Exp Ther. 2009a;331:724–32. doi: 10.1124/jpet.109.158519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo SM, Guerra ML, Jarrard RE, Przybyla JA, Liu G, Watts VJ, Hockerman GH. The intracellular II-III loops of Cav1.2 and Cav1.3 uncouple L-type voltage-gated Ca2+ channels from glucagon-like peptide-1 potentiation of insulin secretion in INS-1 cells via displacement from lipid rafts. J Pharmacol Exp Ther. 2009b;330:283–93. doi: 10.1124/jpet.109.150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Leonard JN, Buzard DJ, Lehmann J. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin Ther Pat. 2009;19:1339–59. doi: 10.1517/13543770903153878. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β cells. J Physiol. 2001;536:375–85. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J Physiol. 2006;573:595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β cells. J Physiol. 2005;566:173–88. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Holz GG. Amplification of exocytosis by Ca2+-induced Ca2+ release in INS-1 pancreatic beta cells. J Physiol. 2003;546:175–89. doi: 10.1113/jphysiol.2002.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta cells. J Biol Chem. 2003;278:8279–85. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor Epac as a determinant of K-ATP channel ATP sensitivity in human pancreatic beta cells and rat INS-1 cells. J Physiol. 2008;586:1307–19. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII--Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–53. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- Kelley GG, Chepurny OG, Leech CA, Roe MW, Li X, Dzhura I, Dzhura E, Afshari P, Holz GG. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM. Islets. 2009;1:1–6. doi: 10.4161/isl.1.3.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, Kim UH. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes. 2008;57:868–78. doi: 10.2337/db07-0443. [DOI] [PubMed] [Google Scholar]
- Knop FK, Holst JJ, Vilsboll T. Replacing SUs with incretin-based therapies for type 2 diabetes mellitus: challenges and feasibility. IDrugs. 2008;11:497–501. [PubMed] [Google Scholar]
- Kwan EP, Xie L, Sheu L, Ohtsuka T, Gaisano HY. Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes. 2007;56:2579–88. doi: 10.2337/db06-1207. [DOI] [PubMed] [Google Scholar]
- Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta cell line. J Biol Chem. 2005;280:31294–302. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech CA, Dzhura I, Chepurny OG, Schwede F, Genieser HG, Holz GG. Facilitation of β cell KATP channel sulfonylurea sensitivity by a cAMP analog selective for the cAMP-regulated guanine nucleotide exchange factor Epac. Islets. 2010;2:72–81. doi: 10.4161/isl.2.2.10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech CA, Habener JF. A role for Ca2+-sensitive nonselective cation channels in regulating the membrane potential of pancreatic beta cells. Diabetes. 1998;47:1066–73. doi: 10.2337/diabetes.47.7.1066. [DOI] [PubMed] [Google Scholar]
- Lester LB, Langeberg LK, Scott JD. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc Natl Acad Sci U S A. 1997;94:14942–7. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol. 2002;16:2135–44. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- Liu G, Dilmac N, Hilliard N, Hockerman GH. Ca v 1.3 is preferentially coupled to glucose-stimulated insulin secretion in the pancreatic beta-cell line INS-1. J Pharmacol Exp Ther. 2003;305:271–8. doi: 10.1124/jpet.102.046334. [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–9. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Salapatek AM, Wheeler MB. Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K+ currents in beta-cells: a possible glucose-dependent insulinotropic mechanism. Diabetes. 2002;51(Suppl 3):S443–7. doi: 10.2337/diabetes.51.2007.s443. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Wang X, Xia F, El-kholy W, Targonsky ED, Tsushima RG, Wheeler MB. Antagonism of rat beta-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem. 2003;278:52446–53. doi: 10.1074/jbc.M307612200. [DOI] [PubMed] [Google Scholar]
- Marigo V, Courville K, Hsu WH, Feng JM, Cheng H. TRPM4 impacts on Ca2+ signals during agonist-induced insulin secretion in pancreatic beta-cells. Mol Cell Endocrinol. 2009;299:194–203. doi: 10.1016/j.mce.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Miki T, Minami K, Shinozaki H, Matsumura K, Saraya A, Ikeda H, Yamada Y, Holst JJ, Seino S. Distinct effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 on insulin secretion and gut motility. Diabetes. 2005;54:1056–63. doi: 10.2337/diabetes.54.4.1056. [DOI] [PubMed] [Google Scholar]
- Nakazaki M, Crane A, Hu M, Seghers V, Ullrich S, Aguilar-Bryan L, Bryan J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes. 2002;51:3440–9. doi: 10.2337/diabetes.51.12.3440. [DOI] [PubMed] [Google Scholar]
- Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med. 2011;124:S3–18. doi: 10.1016/j.amjmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–6. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T, Seino S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol. 2009;219:652–8. doi: 10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- Nitert MD, Nagorny CL, Wendt A, Eliasson L, Mulder H. CaV1.2 rather than CaV1.3 is coupled to glucose-stimulated insulin secretion in INS-1 832/13 cells. J Mol Endocrinol. 2008;41:1–11. doi: 10.1677/JME-07-0133. [DOI] [PubMed] [Google Scholar]
- Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–22. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–11. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Ozbay L, Smidt K, Mortensen D, Carstens J, Jorgensen K, Rungby J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br J Pharmacol. 2011;162:136–146. doi: 10.1111/j.1476-5381.2010.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyot ML, Gray JP, Lamontagne J, Smith PJ, Holz GG, Madiraju SR, Prentki M, Heart E. Glucagon-like peptide-1 induced signaling and insulin secretion do not drive fuel and energy metabolism in primary rodent pancreatic beta-cells. PLoS One. 2009;4:e6221. doi: 10.1371/journal.pone.0006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves GI, Kamishima T, Davies LM, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) mediates cAMP-dependent but protein kinase A-insensitive modulation of vascular ATP-sensitive potassium channels. J Physiol. 2009;587:3639–50. doi: 10.1113/jphysiol.2009.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale V, Hales CN, Ashford ML. Regulation of calcium-activated nonselective cation channel activity by cyclic nucleotides in the rat insulinoma cell line, CRI-G1. J Membr Biol. 1995;145:267–78. doi: 10.1007/BF00232718. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Wittinghofer A, Bos JL. Capturing cyclic nucleotides in action: snapshots from crystallographic studies. Nat Rev Mol Cell Biol. 2007;8:63–73. doi: 10.1038/nrm2082. [DOI] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and - independent stimulation of exocytosis by cAMP in mouse pancreatic β cells. J Physiol. 1997;502 (Pt 1):105–18. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah U, Kowalski TJ. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders. Vitam Horm. 2010;84:415–48. doi: 10.1016/B978-0-12-381517-0.00016-3. [DOI] [PubMed] [Google Scholar]
- Sheu L, Pasyk EA, Ji J, Huang X, Gao X, Varoqueaux F, Brose N, Gaisano HY. Regulation of insulin exocytosis by Munc13-1. J Biol Chem. 2003;278:27556–63. doi: 10.1074/jbc.M303203200. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004a;279:7956–61. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004b;53(Suppl 3):S59–62. doi: 10.2337/diabetes.53.suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A. 2007;104:19333–8. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277:37176–83. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- Sjoberg TJ, Kornev AP, Taylor SS. Dissecting the cAMP-inducible allosteric switch in protein kinase A RIalpha. Protein Sci. 2010;19:1213–21. doi: 10.1002/pro.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess NC, Hales CN, Ashford ML. Inhibition of a calcium-activated, non-selective cation channel, in a rat insulinoma cell line, by adenine derivatives. FEBS Lett. 1986;208:397–400. doi: 10.1016/0014-5793(86)81056-0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Zhang H, Saito N, Kojima I, Urano T, Mogami H. Glucagon-like peptide 1 activates protein kinase C through Ca2+-dependent activation of phospholipase C in insulin-secreting cells. J Biol Chem. 2006;281:28499–507. doi: 10.1074/jbc.M604291200. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kadowaki T, Yazaki Y, Ellis-Davies GC, Miyashita Y, Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic β cells. Proc Natl Acad Sci U S A. 1999;96:760–5. doi: 10.1073/pnas.96.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–15. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab. 2010;21:59–67. doi: 10.1016/j.tem.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Stanojevic V, Habener JF. GLP-1-Derived Nonapeptide GLP-1(28–36)amide Targets To Mitochondria and Suppresses Glucose Production and Oxidative Stress in Isolated Mouse Hepatocytes. Regul Pept. 2011 doi: 10.1016/j.regpep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Tovey SC, Dedos SG, Rahman T, Taylor EJ, Pantazaka E, Taylor CW. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J Biol Chem. 2010;285:12979–89. doi: 10.1074/jbc.M109.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 β cells. Biochem J. 2003;369:287–99. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, Yada T, Minokoshi Y, Tominaga M. Lack of TRPM2 Impaired Insulin Secretion and Glucose Metabolisms in Mice. Diabetes. 2011;60:119–26. doi: 10.2337/db10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, Eliasson L. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2009;297:E452–61. doi: 10.1152/ajpendo.90585.2008. [DOI] [PubMed] [Google Scholar]
- Vliem MJ, Ponsioen B, Schwede F, Pannekoek WJ, Riedl J, Kooistra MR, Jalink K, Genieser HG, Bos JL, Rehmann H. 8-pCPT-2′-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. Chembiochem. 2008;9:2052–4. doi: 10.1002/cbic.200800216. [DOI] [PubMed] [Google Scholar]
- Wan QF, Dong Y, Yang H, Lou X, Ding J, Xu T. Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+ J Gen Physiol. 2004;124:653–62. doi: 10.1085/jgp.200409082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaekura K, Yada T. [Ca2+]i-reducing action of cAMP in rat pancreatic beta-cells: involvement of thapsigargin-sensitive stores. Am J Physiol. 1998;274:C513–21. doi: 10.1152/ajpcell.1998.274.2.C513. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gillis KD. A highly Ca2+-sensitive pool of granules is regulated by glucose and protein kinases in insulin-secreting INS-1 cells. J Gen Physiol. 2004;124:641–51. doi: 10.1085/jgp.200409081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Shibasaki T, Minami K, Takahashi H, Mizoguchi A, Uriu Y, Numata T, Mori Y, Miyazaki J, Miki T, Seino S. Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab. 2010;12:117–29. doi: 10.1016/j.cmet.2010.05.017. [DOI] [PubMed] [Google Scholar]