Abstract
Articular cartilage cannot repair itself in response to degradation from injury or osteoarthritis. As such, there is a substantial clinical need for replacements of damaged cartilage. Tissue engineering aims to fulfill this need by developing replacement tissues in vitro. A major goal of cartilage tissue engineering is to produce tissues with robust biochemical and biomechanical properties. One technique that has been proposed to improve these properties in engineered tissue is the use of non-enzymatic glycation to induce collagen crosslinking, an attractive solution that may avoid the risks of cytotoxicity posed by conventional crosslinking agents such as glutaraldehyde. The objectives of this study were 1) to determine whether continuous application of ribose would enhance biochemical and biomechanical properties of self-assembled articular cartilage constructs, and 2) to identify an optimal time window for continuous ribose treatment. Self-assembled constructs were grown for 4 weeks using a previously established method and were subjected to continuous 7-day treatment with 30 mM ribose during culture weeks 1, 2, 3, or 4, or for the entire 4-week culture. Control constructs were grown in parallel, and all groups were evaluated for gross morphology, histology, cellularity, collagen and sulfated glycosaminoglycan (GAG) content, and compressive and tensile mechanical properties. Compared to control constructs, it was found that treatment with ribose during week 2 and for the entire duration of culture resulted in significant 62% and 40% increases in compressive stiffness, respectively; significant 66% and 44% increases in tensile stiffness; and significant 50% and 126% increases in tensile strength. Similar statistically significant trends were observed for collagen and GAG. In contrast, constructs treated with ribose during week 1 had poorer biochemical and biomechanical properties, although they were significantly larger and more cellular than all other groups. We conclude that non-enzymatic glycation with ribose is an effective method for improving tissue engineered cartilage and that specific temporal intervention windows exist to achieve optimal functional properties.
Keywords: Articular cartilage, tissue engineering, biomechanics, crosslinking, extracellular matrix
1. Introduction
Articular cartilage covers the surfaces of diarthrodial joints and serves to reduce friction and distribute loads during joint motion. Structurally, articular cartilage is an avascular, hypocellular tissue with an abundant extracellular matrix (ECM) rich in collagen type II and glycosaminoglycans (GAGs), which give rise to the tissue's tensile and compressive properties, respectively. Due to its avascularity and hypocellularity, cartilage lacks an intrinsic capacity to repair itself after painful destruction brought on by trauma or osteoarthritis [1]. Thus, there is a substantial clinical need for suitable replacements of damaged cartilage. The field of tissue engineering aims to fulfill this need by developing biologic replacements in vitro for eventual in vivo implantation. A fundamental objective of cartilage tissue engineering is to produce tissues with robust biochemical and biomechanical properties [2].
To address this objective, our laboratory has developed a self-assembly process for engineering cartilage constructs [3]. The self-assembly process involves seeding chondrocytes at a high density into pre-formed, non-adherent, cylindrical wells. Cells coalesce into disc-shaped constructs and, over time, undergo biochemical and biophysical changes that approximate in vivo cartilage development [4]. Unlike traditional tissue engineering strategies, the self-assembly process does not employ a biomaterial scaffold, thereby circumventing the typical challenges associated with scaffold use, such as toxicity, biodegradability, and inhibited cellular signaling [3]. An important advantage of the self-assembly process is that, since it is a strictly cell-mediated phenomenon, it can serve as a model system for examining the direct effects of biochemical [5–7] and biophysical [8–10] stimuli on cell physiology and ECM development. Although progress has been made in identifying beneficial stimuli for self-assembly, the functional properties of self-assembled cartilage constructs still fall short of native tissue values. Therefore, it is imperative that additional treatment modalities be evaluated.
One technique that has been proposed to improve engineered tissue is the use of non-enzymatic glycation to induce collagen crosslinking [11–14]. Non-enzymatic glycation involves three steps [15]. First, the aldehyde group of a reducing sugar, such as ribose, reacts with the nucleophilic ε-amino residue of lysine in the collagen polypeptide to form an unstable, reversible Schiff base. One advantage of using ribose is that formation of the Schiff base occurs more rapidly with ribose compared to other sugars (e.g., 17× faster than glucose), largely due to its preferentially open-chain configuration [15]. In the second step of non-enzymatic glycation, the Schiff base undergoes Amadori rearrangement, producing a stable, less reversible ketone. Finally, over time, the Amadori products are degraded to form a variety of advanced glycation end-products (AGEs) [16], which accumulate in the ECM. Traditionally, the accumulation of AGEs has been understood to be an unwanted manifestation of aging and diabetes [15,17,18]. However, researchers have begun to recognize the potential benefits of non-enzymatic glycation as a tool to improve engineered tissue properties, especially without the risk of cytotoxicity posed by conventional crosslinking methods like glutaraldehyde fixation [11–14,19,20].
In a recent experiment from our laboratory, self-assembled cartilage constructs were treated for 3.5 h with one of four exogenous crosslinking agents on the final day of construct culture (t=28 days) [11]. A head-to-head comparison of treatments revealed that ribose produced the greatest improvements in tensile stiffness and strength, beating glutaraldehyde, genipin, and methylglyoxal. Encouraged by these results and other studies in the literature, we decided to examine the effects of continuous ribose treatment on self-assembled cartilage constructs at different time windows throughout 4 weeks of culture.
The objectives of this study were 1) to determine whether continuous application of ribose would enhance the biochemical and biomechanical properties of self-assembled articular cartilage constructs, and 2) to identify an optimal time window for continuous ribose treatment. Constructs were self-assembled from bovine chondrocytes and subjected to continuous 7-day treatment with ribose during culture weeks 1, 2, 3, or 4, or for the entire 4-week duration of culture. Control constructs were grown in parallel. It was hypothesized that 1) ribose would improve construct biochemical and biomechanical properties, 2) an optimal treatment time window exists for which constructs undergo greatest improvement, and 3) ribose treatment for the entire duration of culture would produce the greatest effect on constructs. Assessments included gross morphology, histology, quantitative biochemistry, and biomechanical testing.
2. Materials and Methods
2.1. Media
This study employed two medium formulations: “control medium” and “ribose medium.” Control medium is a chondrogenic medium described extensively by our group [5,6,8]. Ribose medium is control medium plus 30 mM D-ribose (Sigma). This concentration of ribose was selected from literature that demonstrated beneficial effects of 30 mM ribose on specimen mechanical properties, with no effects on cell viability [11,12]. Our chosen concentration of 30 mM ribose is far below the 250 mM ribose shown to be tolerated well by chondrocytes in vitro [14].
2.2. Chondrocyte isolation
Cartilage harvested from the distal femur of 1-week-old male calves (Research 87) was digested in 0.2% collagenase type II (Worthington) for 24 h [7]. Each leg came from a different animal, and cells from 8 legs were pooled. Cells were counted and then frozen at −80°C in DMEM containing 20% fetal bovine serum and 10% dimethyl sulfoxide.
2.3. Preparation of agarose wells for construct self-assembly
Cylindrical, non-adherent wells were prepared using a technique adapted from previous work [3,4]. A stainless steel mold consisting of 5 mm diameter cylindrical prongs was placed into molten 2% agarose in a 48-well plate. The agarose solidified at room temperature, and the mold was removed. Two changes of control medium were used to saturate the agarose before cell seeding.
2.4. Self-assembly and culture of cartilage constructs
Cells were thawed and counted within 5 days of freezing. Viability was >90%. To create each construct, 5.5 million cells in 100 μL control medium were seeded into each cylindrical agarose well, followed by addition of 400 μL control medium after 4 h. Cells coalesced into free-floating disc-shaped constructs; t=1 day was defined as 24 h after seeding. Constructs were cultured in the agarose wells until t=10 days, at which point they were unconfined and transferred to 48-well plates unrestricted by circumferential confinement. Constructs received 500 μL medium change every 24 h and remained in culture until t=28 days. All culture was at 37°C and 10% CO2.
This study examined six groups: Control, Week 1, Week 2, Week 3, Week 4, and All Weeks. Controls received control medium during t=1–28 days. Ribose-treated constructs received ribose medium during t=1–7 days (Week 1), t=8–14 days (Week 2), t=15–21 days (Week 3), t=22–28 days (Week 4), or t=1–28 days (All Weeks). Ribose-treated constructs received control medium when not undergoing ribose treatment.
2.5. Gross morphology and histology
At t=28 days, constructs were removed from culture. Photographs were taken, and diameters were measured from photographs using ImageJ software. Wet weights (WW) were recorded, and constructs were portioned for analysis. A 3 mm diameter punch was taken from the construct's center for indentation testing. The remaining outer ring was split into portions for histology, quantitative biochemistry, and tensile testing. For histology, constructs were cryoembedded and sectioned at 14 μm. Samples were fixed in 10% formalin and stained with Safranin-O/fast green (GAG) and picrosirius red (collagen).
2.6. Quantitative biochemistry
Biochemistry samples were weighed, frozen, and lyophilized. Dry weights (DW) were measured, after which samples were digested with 125 μg/mL papain (Sigma) for 18 h at 65°C. Total DNA was assessed with a PicoGreen Assay (Invitrogen), and cell number was estimated assuming 7.7 pg DNA per cell. Sulfated GAG was quantified using the Blyscan Glycosaminoglycan Assay (Biocolor). Following hydrolysis with 4 N sodium hydroxide for 20 min at 110°C, total collagen was quantified with a chloramine-T hydroxyproline assay [21]. Sircol collagen standard (Biocolor) was used such that the standard curve reflected collagen amount, eliminating the need to convert hydroxyproline to collagen. Total collagen and sulfated GAG were normalized to WW and DW for making comparisons.
2.7. Creep indentation testing
A creep indentation apparatus was used to determine compressive behavior of each construct [22]. Each sample was affixed to a stainless steel surface and equilibrated for 20 min in PBS. A 0.7 g (0.007 N) mass was applied with a 0.8 mm diameter flat, porous indenter tip, and specimens crept until equilibrium. Specimen thickness was measured from photographs. Aggregate modulus, a measure of compressive stiffness, was calculated using a semi-analytical, semi-numeric, linear biphasic model [22].
2.8. Tensile testing
Tensile specimens were cut into dog-bone shapes with 1-mm gauge length. Specimen thickness and width were measured from photographs using ImageJ software. Specimens were then affixed with glue to paper tabs outside the gauge length, and these tabs were gripped during testing. A uniaxial materials testing system (Instron Model 5565) was employed to determine tensile properties. Tensile tests were performed until failure at a strain rate of 1% of the gauge length per second. Stress-strain curves were calculated by normalizing force-displacement to specimen dimensions. Young's modulus, a measure of tensile stiffness, was determined by least squares fitting of the linear region of the stress-strain curve. The ultimate tensile strength (UTS) was determined as the maximum stress reached during a test.
2.9. Statistical analysis
All quantitative assessments were made using n=5–9. Numerical data are represented as means ± standard deviations. To compare among treatment groups, single-factor ANOVA was employed, with Fisher LSD post hoc testing when warranted. Significance was defined as p<0.05.
3. Results
3.1. Gross appearance and histology
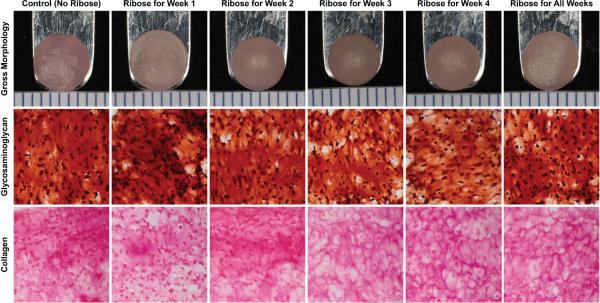
At t=28 days, constructs from every group had similar flat surfaces with no surface abnormalities (Figure 1). Diameter and WW are provided in Table 1. Week 1 constructs had significantly greater diameter (1.06× control) and WW (1.34× control) compared to all other groups. On histology, all constructs stained positively for collagen and GAG (Figure 1).
Figure 1.
Gross morphology and histology of self-assembled constructs at 4 weeks. From left to right: representative images of constructs from Control, Week 1, Week 2, Week 3, Week 4, and All Weeks ribose treatment groups. Constructs had a similar flat circular appearance with no surface abnormalities (top row). All constructs stained positively for GAG (middle row) and collagen (bottom row). Color images available in online version of this article.
Table 1.
Diameter, wet weight, cell number, collagen/dry weight, and GAG/dry weight of self-assembled constructs. Ribose treatment during Week 1 had the largest diameter (6% > control), wet weight (34% > control), and cell number (63% > control), with no other significant differences among the other groups. Week 2 and All Weeks had the highest collagen/DW (50% and 53% > control, respectively), and All Weeks had the highest GAG/DW (23% > control). Data are presented as means ± standard deviations. Asterisks and lowercase letters denote significant differences within a column; groups not connected by the same letter are significantly different (p<0.05).
| Dia. (mm) | WW (mg) | Cells (×106) | Collagen/DW (mg/mg) | GAG/DW (mg/mg) | |
|---|---|---|---|---|---|
| Control | 5.92 ± 0.22 | 22.0 ± 1.3 | 5.39 ± 1.20 | 0.34 ± 0.08 b | 0.30 ± 0.03 b |
| Week 1 | 6.28 ± 0.25 * | 29.4 ± 1.8 * | 8.78 ± 2.51 * | 0.25 ± 0.06 c | 0.24 ± 0.02 c |
| Week 2 | 5.78 ± 0.10 | 20.4 ± 1.3 | 4.89 ± 0.68 | 0.51 ± 0.08 a | 0.33 ± 0.05 ab |
| Week 3 | 5.80 ± 0.17 | 20.6 ± 1.7 | 4.68 ± 1.66 | 0.44 ± 0.11 ab | 0.32 ± 0.04 ab |
| Week 4 | 5.93 ± 0.13 | 21.9 ± 1.5 | 5.39 ± 1.00 | 0.31 ± 0.07 c | 0.29 ± 0.04 b |
| All Weeks | 5.92 ± 0.23 | 20.5 ± 0.2 | 6.01 ± 1.33 | 0.52 ± 0.07 a | 0.37 ± 0.06 a |
3.2. Quantitative biochemistry
Cell number is shown in Table 1. Week 1 had a significantly higher cell number (1.63× control), with no other differences between groups.
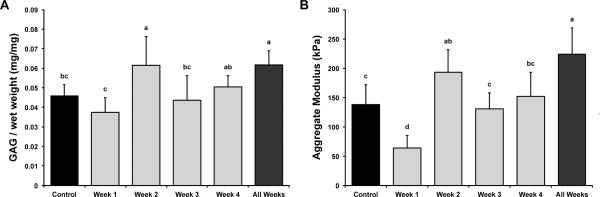
GAG/WW is shown in Figure 2A. GAG/WW values for control, Week 1, Week 2, Week 3, Week 4, and All Weeks were 4.6±0.6%, 3.8±0.7%, 6.2±1.5%, 4.4±1.3%, 5.0±0.6%, and 6.2±0.7%, respectively. Week 2 and All Weeks had the greatest GAG/WW (1.34× and 1.35× control, respectively). Week 1 had the least GAG/WW (0.82× control). GAG per dry weight (DW), provided in Table 1, showed a similar trend to that observed for GAG/WW.
Figure 2.
Glycosaminoglycan content and compressive stiffness of self-assembled constructs. (A) GAG/WW for all groups. Ribose treatment during Week 2 and All Weeks had the greatest GAG/WW (34% and 35% > control, respectively). Week 1 had the least GAG/WW (18% < control). (B) Compressive stiffness for all groups. The highest aggregate moduli were found in All Weeks (62% > control), followed by Week 2 (40% > control). Week 1 had the lowest aggregate moduli (54% < control). As expected, trends for aggregate moduli followed GAG/WW trends. Data are presented as means ± standard deviations. Groups not connected by the same letter are significantly different (p<0.05).
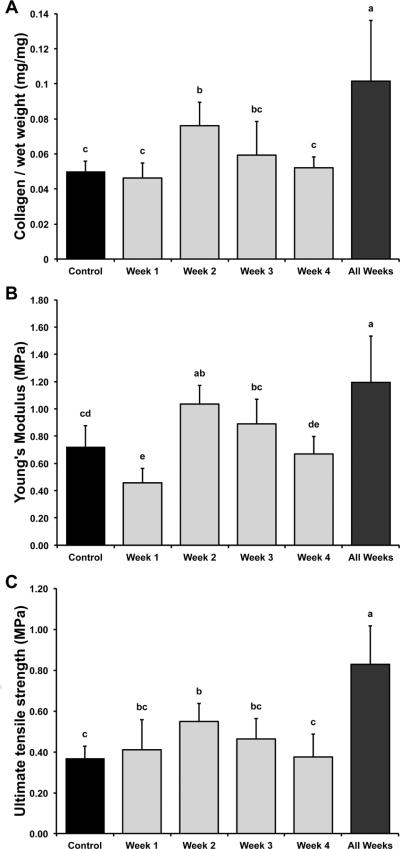
Collagen/WW is shown in Figure 3A. Collagen/WW values for control, Week 1, Week 2, Week 3, Week 4, and All Weeks were 5.0±0.6%, 4.6±0.9%, 7.6±1.3%, 5.9±1.9%, 5.2±0.6%, and 10.1±3.4%, respectively. All Weeks had the highest collagen/WW (2.04× control), followed by Week 2 (1.53× control). Week 1, Week 3, and Week 4 were no different from control. Similar trends were observed for collagen/DW (Table 1), though Week 2 and All Weeks did not differ from each other in collagen/DW.
Figure 3.
Collagen content, tensile stiffness, and tensile strength of self-assembled constructs. (A) Collagen/WW for all groups. Ribose treatment during All Weeks had the highest collagen/WW (104% > control), followed by Week 2 (53% > control). (B) Tensile stiffness for all groups. The highest Young's moduli were found in All Weeks (66% > control), followed by Week 2 (44% > control). Week 1 had the lowest Young's moduli (36% < control). (C) Tensile strength for all groups. All Weeks had the highest UTS (126% > control), followed by Week 2 (50% > control). UTS trends appeared to reflect collagen/WW trends. Data are presented as means ± standard deviations. Groups not connected by the same letter are significantly different (p<0.05).
3.3. Biomechanics
Compressive stiffness, represented by aggregate modulus, is shown in Figure 2B. Aggregate moduli for control, Week 1, Week 2, Week 3, Week 4, and All Weeks were 138±34, 64±22, 193±38, 131±27, 152±41, and 224±45 kPa, respectively. Trends for aggregate moduli followed those of GAG/WW. The highest aggregate moduli were found in All Weeks (1.62× control), followed by Week 2 (1.40× control). Week 1 had the lowest aggregate moduli (0.46× control).
Tensile stiffness, represented by Young's modulus, and UTS are shown in Figure 3. Young's moduli for control, Week 1, Week 2, Week 3, Week 4, and All Weeks were 717±160, 457±106, 1034±141, 888±181, 669±130, and 1192±340 kPa, respectively. All Weeks had the highest Young's moduli (1.66× control), followed by Week 2 (1.44× control). Week 1 had the lowest Young's moduli (0.64× control). UTS values for control, Week 1, Week 2, Week 3, Week 4, and All Weeks were 366±61, 411±147, 551±147, 465±101, 376±110, and 830±188 kPa, respectively. Trends for UTS appeared to reflect trends in collagen/WW. All Weeks had the highest UTS (2.26× control), followed by Week 2 (1.50× control).
4. Discussion
This study examined the effects of continuous ribose treatment over various time windows during self-assembly of articular cartilage constructs. Experimental results supported the hypotheses motivating the study: 1) treatment of self-assembled constructs with ribose produced significant increases in biochemical and biomechanical properties; 2) week 2 was identified as the optimal treatment time window to produce the greatest improvements in constructs; and 3) continuous ribose treatment for the entire duration of culture had the greatest effect on construct properties, notably producing a 62% increase in compressive stiffness, a 66% increase in tensile stiffness, and a 126% increase in tensile strength compared to control. To the best of our knowledge, this is the first study not only to systematically compare ribose treatment over various time windows during in vitro tissue development, but also to examine the direct effects of ribose treatment on both cells and their surrounding ECM during tissue engineering. This study demonstrates the effectiveness of ribose as an agent to improve tissue engineered materials.
It was found that the optimal time window for ribose treatment is during week 2 (t=8–14 days). Compared to controls, week 2 constructs exhibited significant improvements in GAG/WW (34% increase), collagen/WW (53% increase), compressive stiffness (40% increase), tensile stiffness (44% increase), and tensile strength (50% increase). To understand why intervening during week 2 can lead to such dramatic improvements in construct properties, it is important to consider the developmental milestones of self-assembling constructs. A previous study from our laboratory characterized matrix development during self-assembly [4]. A principal finding was that collagen production reaches a plateau between days 10–14 of culture, after which GAG production predominates. It is thought that rapid production of GAG with no new collagen contributes to pre-stress within the fledgling ECM, thereby compromising the engineered tissue's tensile properties [6,23]. Altering this imbalance between GAG and collagen has been shown to improve the tensile properties of self-assembled constructs [6]. During week 2, before collagen production halts and GAG production ramps up, the developing ECM may be more susceptible to interventions like ribose that either reinforce existing matrix or induce new matrix biosynthesis. The beneficial effects of week 2 ribose treatment are corroborated by previous work showing that other stimuli also produce their maximal effects when applied to constructs during week 2 [5,7,8].
One interesting finding is that the results for collagen content reflected trends seen for tensile strength but do not track as closely with tensile stiffness. It is understood that the tensile properties of cartilage are conferred by the tissue's collagen network. Collagen networks have complex structure-function relationships governed by peptide abundance, fibril organization, and crosslink presence [24]. A possible explanation for the fidelity between tensile strength and collagen content is that collagen abundance may preferentially determine the tissue's failure point (and thus strength), whereas individual crosslinks within the network may determine the fiber bundle response to strain (and thus stiffness). Future studies on native cartilage should be undertaken to tease out structure-function relationships between collagen abundance, crosslinks, tensile stiffness, and tensile strength.
Increased GAG and compressive stiffness in ribose-treated constructs may be explained by the effects of crosslinking, as well. Glycation-mediated crosslinking may be trapping GAGs within the crosslinked network, thereby preventing GAG loss during culture. Higher compressive stiffness may be explained by tighter packing of GAG within the crosslinked network. One way to test this hypothesis in the future may be to examine the ratio of GAG to pentosidine, a molecule derived from ribose that is responsible for crosslinks between lysine and arginine residues in collagen [24]. By studying correlations between GAG and pentosidine, inferences can be made about the effect of collagen crosslinking on GAG retention or loss.
Although the guiding principle underlying this study is that ribose induces crosslinking of the ECM, the observed changes in biochemistry and biomechanics may be further explained by cellular metabolism or osmotic stress. Ribose is known to affect cellular metabolism [15] and therefore may influence chondrocyte biosynthesis during self-assembly. Additionally, ribose supplementation increases medium osmolarity. Cells undergo shrinkage in a hyperosmotic environment; such cellular strain is thought to alter chromatin condensation and nucleocytoplasmic transport [25]. The downstream effect of these nuclear changes may be increased GAG or collagen. To test this concept in the future, one could examine the use of a non-reducing sugar such as sucrose to modulate osmolarity while preventing glycation.
Of note is that constructs treated with ribose during week 1 exhibited decreases in biochemical and biomechanical properties but increases in size and cell number compared to all other groups. During week 1, in a process resembling chondrogenesis in vivo [26], our laboratory has shown that the nascent construct's efforts are focused primarily on cell condensation through N-cadherin upregulation rather than on ECM synthesis [4]. As such, there is very little ECM available as a substrate for glycation. Thus, the effect of early ribose treatment may be metabolic or osmotic, rather than crosslink forming. Metabolically, ribose may have shunted cellular biosynthesis towards proliferation [15], which may explain the 63% increase in cell number after week 1 treatment. Thus it is not surprising that, given the greater cell number, week 1 treated constructs are larger. Despite the greater cell number and size, these constructs were unable to keep up with ECM synthesis observed in other groups. Similar results were obtained in a previous study that investigated initial cell seeding density in self-assembly and revealed an upper limit for effective tissue engineering [27].
Constructs treated with ribose throughout the entire 4 weeks exhibited the greatest improvements. It is possible that the beneficial effects seen with week 2 treatment were able to mitigate the negative effects seen with week 1 treatment. Most importantly, however, the success of these constructs demonstrates that treatment with 30 mM ribose can be used safely in tissue engineering strategies with no risk of in vitro cytotoxicity. Future work is warranted to assess biocompatibility in vivo, since previous work has suggested that AGEs may mark ECM proteins for targeted proteolysis [15].
This work provides evidence that continuous treatment with ribose can significantly enhance the biochemical and biomechanical properties of self-assembled cartilage constructs. We have identified an optimal time window for ribose application. Additionally, we provide evidence that 30 mM ribose can be used safely in vitro without risk of cell death or other deleterious effects. Finally, a major innovation of this study is that it evaluated ribose application during self-assembly, a purely cell-mediated phenomenon, from which direct effects on both cells and ECM were ascertained for the first time.
-
>
Cartilage constructs were engineered using self-assembly over 4 weeks.
-
>
Ribose treatment was evaluated directly on cells and developing ECM.
-
>
Ribose enhanced mechanical properties of engineered tissue.
-
>
Effects were time dependent.
-
>
Ribose did not exhibit detrimental effects when used over 4 weeks.
Acknowledgements
We acknowledge funding from NIH NIAMS R01AR053286 and NIH NIGMS T32GM007730. We thank Jerry Hu for his assistance with cell culture during this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Buckwalter JA. Articular cartilage: injuries and potential for healing. The Journal of orthopaedic and sports physical therapy. 1998;28:192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- [2].Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- [3].Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- [4].Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis and rheumatism. 2010;62:1097–1107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Natoli R, Revell CM, Athanasiou K. Chondroitinase ABC Treatment Results in Increased Tensile Properties of Self-Assembled Tissue Engineered Articular Cartilage. Tissue Eng Part A. 2009;15:3119–3128. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114–123. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue engineering. 2009;Part A 15:1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26:238–246. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Elder BD, Mohan A, Athanasiou KA. Beneficial effects of exogenous crosslinking agents on self-assembled tissue engineered cartilage construct biomechanical properties. J Mech Med Biol. 2011;11:433–443. doi: 10.1142/S0219519410003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Girton TS, Oegema TR, Tranquillo RT. Exploiting glycation to stiffen and strengthen tissue equivalents for tissue engineering. J Biomed Mater Res. 1999;46:87–92. doi: 10.1002/(sici)1097-4636(199907)46:1<87::aid-jbm10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- [13].Lima EG, Tan AR, Tai T, Marra KG, DeFail A, Ateshian GA, Hung CT. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res. 2009;Part A 91:692–700. doi: 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roy R, Boskey AL, Bonassar LJ. Non-enzymatic glycation of chondrocyte-seeded collagen gels for cartilage tissue engineering. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26:1434–1439. doi: 10.1002/jor.20662. [DOI] [PubMed] [Google Scholar]
- [15].Brownlee M. Nonenzymatic glycosylation of macromolecules. Prospects of pharmacologic modulation. Diabetes. 1992;41(Suppl 2):57–60. doi: 10.2337/diab.41.2.s57. [DOI] [PubMed] [Google Scholar]
- [16].Nagai R, Ikeda K, Kawasaki Y, Sano H, Yoshida M, Araki T, Ueda S, Horiuchi S. Conversion of Amadori product of Maillard reaction to Nepsilon-(carboxymethyl)lysine in alkaline condition. FEBS letters. 1998;425:355–360. doi: 10.1016/s0014-5793(98)00263-4. [DOI] [PubMed] [Google Scholar]
- [17].King GL, Brownlee M. The cellular and molecular mechanisms of diabetic complications. Endocrinology and metabolism clinics of North America. 1996;25:255–270. doi: 10.1016/s0889-8529(05)70324-8. [DOI] [PubMed] [Google Scholar]
- [18].Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Howard EW, Benton R, Ahern-Moore J, Tomasek JJ. Cellular contraction of collagen lattices is inhibited by nonenzymatic glycation. Experimental cell research. 1996;228:132–137. doi: 10.1006/excr.1996.0308. [DOI] [PubMed] [Google Scholar]
- [20].Roy R, Boskey A, Bonassar LJ. Processing of type I collagen gels using nonenzymatic glycation. Journal of biomedical materials research. 2010;Part A 93:843–851. doi: 10.1002/jbm.a.32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Archives of biochemistry and biophysics. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- [22].Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop Relat Res. 1995;316:254–266. [PubMed] [Google Scholar]
- [23].Schwartz MH, Leo PH, Lewis JL. A microstructural model for the elastic response of articular cartilage. J Biomech. 1994;27:865–873. doi: 10.1016/0021-9290(94)90259-3. [DOI] [PubMed] [Google Scholar]
- [24].Responte DJ, Natoli RM, Athanasiou KA. Collagens of articular cartilage: structure, function, and importance in tissue engineering. Critical reviews in biomedical engineering. 2007;35:363–411. doi: 10.1615/critrevbiomedeng.v35.i5.20. [DOI] [PubMed] [Google Scholar]
- [25].Finan JD, Leddy HA, Guilak F. Osmotic stress alters chromatin condensation and nucleocytoplasmic transport. Biochemical and biophysical research communications. 2011;408:230–235. doi: 10.1016/j.bbrc.2011.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. The International journal of developmental biology. 1995;39:881–893. [PubMed] [Google Scholar]
- [27].Revell CM, Reynolds CE, Athanasiou KA. Effects of Initial Cell Seeding in Self Assembly of Articular Cartilage. Ann Biomed Eng. 2008;36:1441–1448. doi: 10.1007/s10439-008-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]