Abstract
Background
Asthma and related respiratory tract allergic diseases are among the most common chronic diseases of adults and children. Despite their importance, disease course cannot be predicted and treatment remains non-specific and potentially hazardous, with no means for cure. Improved clinical management of asthma will require an improved understanding of the fundamental factors that initiate allergic inflammation, especially T helper type 2 (TH2) cell induction.
Scope of Review
In this review, we explore the Proteinase Hypothesis of allergic airway disease, considering specifically how organismal proteinases contribute to the expression of allergic disease and potentially important proteinase signaling pathways.
Major Conclusions
Proteinases from diverse sources (bacteria, fungi, plants) may cause occupational asthma by acting as immune adjuvant factors that specifically elicit TH2 cell-dependent allergic inflammation. However, more conventional allergic airway diseases (asthma, allergic sinusitis) are more likely to arise from contained fungal or viral infections of the airway in which proteinases are produced and serve as major virulence factors. Proteinases may elicit allergic disease by disrupting numerous cellular proteins, potentially including Toll like receptor (TLR) 4, but critical proteinase-activated signaling pathways remain largely unknown.
General Significance
Clarification of how proteinases cause allergic disease, specifically confirming an infectious basis for airway proteinase exposure, will likely radically advance how asthma and related respiratory tract disorders are diagnosed and treated.
Asthma: a disease potentially driven by exogenous proteinases
Asthma afflicts roughly 300 million adults and children world-wide, making it one of the most common causes of human suffering[1, 2]. In the United States, nearly 23 million people currently suffer from asthma, conferring an annual economic disease burden of approximately $30 billion[3–5]. As with all diseases, genetic susceptibility is a critically important factor driving the expression of asthma. However, genetic changes in large populations are far too slow to explain the large increase in asthma incidence observed over the last several decades. Rather, such relatively sudden increases in disease frequency indicate more important changes occurring within the environment.
The pathophysiology of asthma is best viewed as a combination of two fundamental elements, a physiological aspect in which airway obstruction increases in response to a wide variety of provocative stimuli, and an allergic immunological component in which increased eosinophilia, immunoglobulin E (IgE) production, and T helper cell type 2 (TH2) activity are observed. A critical advance in asthma research was the demonstration through experimental systems is that rather than distinct elements of asthma pathophysiology, allergic inflammation and airway obstruction were intimately linked[6]. Additional observations from mice confirmed that TH2 cytokines such as interleukins (IL) -4, IL-5, and IL-13 mediate both inflammation and airway obstruction in the setting of allergen challenge[7–10]. Since these seminal observations, efforts have increasingly focused on determining the environmental causes of asthma, i.e., those elements that when (presumably) inhaled elicit TH2 cell responses and allergic lung disease.
Asthma has long been known to be associated with atopy, the predilection to making antigen-specific antibodies (IgE and IgG) against environmental allergens that can mediate hypersensitivity reactions, especially immediate skin reactions[11, 12]. The Hygiene Hypothesis posits that TH2 responses, atopy and allergic inflammation arise in individuals who have been relatively under-exposed to infectious organisms that typically elicit Th1-predominant immune responses[13, 14]. Putatively, humans are naturally born with a predilection to making TH2 responses and atopy, but this tendency is re-directed to producing Th1 responses after sufficient encounters with viruses, bacteria and other microbes that either suppress TH2 responses or favor the emergence of T regulatory cells with the potential to suppress allergic inflammation[13, 15]. Despite its popularity, a detailed review of the relevant literature failed to find support for the Hygiene Hypothesis[16], suggesting that asthma pathophysiology remains both more complex and obscure than previously thought.
We explore here an alternative concept for the development of allergic disease that is based on exposure to proteinases. Intrinsic to the “Proteinase Hypothesis” is the concept that immunological responses are triggered by exposure to specific danger signals, or adjuvant moieties, possessing two fundamental properties. The first is an ability to induce effector immunity, i.e., active inflammation, and second is an additional capacity to determine the direction or bias of the immune response, generally TH1-, TH17- or TH2-predominant. Much is currently known about TH1- and TH17-inducing adjuvants, which consist largely of pathogen associated molecular patterns (PAMPs) that trigger TH1 and TH17 immune responses by signaling through Toll like receptors (TLRs) and related signaling pathways[17]. In this review, we will consider an entirely distinct type of TH2-inducing adjuvant factor based on proteinase activity and how developing this concept has the potential to revolutionize the clinical management of diverse allergic airway diseases.
Proteinases are linked to human asthma
Abundant circumstantial evidence links proteinases to human asthma. A direct relation between proteinase inhalation and human allergic lung disease has been shown through occupational exposure to microbial and plant proteinases used in industrial settings. Workers handling or directly exposed to proteinases such as Bacillus subtilis-derived proteinases (subtilisin) that are added to detergents[18, 19], pepsin[20], bromelain[21], and papain[22] were more likely to develop asthma than unexposed workers. Furthermore, asthmatic subjects frequently exhibit atopy, i.e., specific IgE and IgG antibodies, to antigens that contain proteinase activity. For example, several of the most common human allergens, including Der p 1, the major dust mite allergen, and Fel d 1, the major allergen of the domestic cat, are proteinases (Table 1; http://fermi.utmb.edu/cgi-bin/SDAP/sdap_07?dB_Type=0&Code=10). The sheer abundance of proteinase in virtually all organisms suggests that finding active proteinases in the environment is expected and not necessarily relevant to expression of allergic disease. However, allergens tend to derive from micron-sized sources that contain concentrated proteinase activity and which are readily dispersed through aerosols. Foremost among these unique sources with relevance to asthma are pollens and fungi[23, 24]. Upon contact with the female reproductive structures of the flower, pollens must release potent proteinases that are required to breach proteinaceous protective layers to reach the egg for fertilization. Fungi must secrete active proteinases in order to obtain necessary amino acids from the environment. Thus, evidence exists to support proteinases as potent factors driving allergic disease, but a true causal relationship can only be established through controlled experimentation.
Table 1.
Exemplary proteinases linked to human and experimental asthma and the major organisms producing them.
| Proteinase | Source | Link to asthma | Mechanism of action |
|---|---|---|---|
| Subtilisin | Bacillus spp. | Induces human asthma | Unknown |
| Der p 1 | Dermatophagoides pteronyssinus (common dust mite) | Most common human allergen; induces experimental asthma | Cleave of CD23[53], CD25[52]; alters IL-4/IFN cytokine balance[54]; induction of allergic cytokine secretion[51, 78] |
| Fel d 1 | Felis domesticus (domestic cat) | Common allergen | Unknown |
| Bromelain | Ananas comosus (pineapple) | Induces human asthma | Unknown |
| Papain | Carica papaya (papaya fruit) | Induces human and experimental asthma | Promotes basophil and airway epithelial activation; production of TSLP[28, 45, 60] |
| Aspergillo pepsin I | Aspergillus spp. | Induces experimental asthma | Activation of dendritic cells [46]; induction of IL-25, TSLP, and allergy-related chemokines[37, 42, 43] |
| Proteinase 2A | rhinoviruses | Induces experimental asthma | Unknown |
Proteinases induce allergic lung disease in experimental mice by bypassing lung-intrinsic tolerogenic mechanisms
The most commonly used model of asthma involves intraperitoneal or cutaneous injections of chicken egg ovalbumin (OVA) prepared with an aluminum salt-based vehicle to induce sensitization and ovalbumin-specific T and B cell responses[25]. Subsequent inhalation of ovalbumin will then result in robust lung TH2 responses, allergic inflammation and airway obstruction resembling asthma. This two-stage protocol is necessary because ovalbumin given strictly through the airway induces only antigen tolerance, i.e., T regulatory cell (Treg) responses that suppress antigen-specific IgE and T cell responses and lung inflammation[26]. Because allergic airway inflammation presumably arises through direct inhalation of antigens without peripheral sensitization, other models are needed to understand how more relevant allergens elicit inflammation.
In contrast to ovalbumin, our laboratory established that diverse proteinases derived from ragweed pollen and multiple fungi were potent inducers of lung TH2 responses and asthma-like allergic lung disease through direct inhalation, i.e., prior intraperitoneal immunization was not required for proteinases to induce allergic disease[27]. Similarly, inhalation of the papaya fruit proteinase papain is sufficient to elicit allergic lung disease (Table 1; [28]). Most recently, we have shown that viral proteinases, especially those derived from human rhinovirus (HRV), are potent inducers of lung TH2 responses and allergic lung disease (Table 1; [29]). Regarding structure, binding site specificity, proteinase class (cysteine, serine, metallo, etc.) and sources, the described allergenic proteinases all differ greatly, with no consistent features other than proteinase activity that suggest a specific link to allergic disease (Table 1; http://fermi.utmb.edu/cgi-bin/SDAP/sdap_07?dB_Type=0&Code=10). Similarly, allergens lack structural motifs that uniquely induce TH2 and IgE responses[30]. These observations suggest therefore that proteinases are allergenic not due to their structural features, but rather due to their proteinase activity. Indeed, proteinase activity has been shown experimentally to be required for allergic responses due to proteinases[27]. Proteinases should therefore be viewed as immune adjuvant factors or danger signals that both activate the immune system and determine its bias, i.e., predominant TH2 character. Allergens, in contrast, have none of these properties, but rather serve predominantly to elicit T and B cell memory allergic responses. Despite such insights, the precise molecular mechanisms by which allergenic proteinases elicit allergic disease are complex and poorly understood as discussed below. However, one essential feature of proteinase action is to bypass the normal tolerogenic immune processes mediated by Tregs that are triggered by non-proteinase allergens such as ovalbumin[27].
Environmental proteinases associating with human allergic disease are largely fungal in origin
Abundant experimental evidence thus establishes proteinases as potent allergenic adjuvants in rodents, but far less data exist to link such proteinases to typical human allergic lung diseases outside of the occupational setting. To gain further insight into the causes of perennial childhood asthma, we determined if active proteinases exist in house dust of pediatric subjects with asthma living in Houston. A critical aspect of these studies was to discover proteinases that remained active as we have previously shown that enzymatic activity is critical for allergenicity[27]. Proteinases linked to allergic disease such as Der p 1 are already known to be widespread in house dust, but no studies have determined if Der p 1 exits in native or denatured forms in situ. Using a combination of total proteinase activity assays and zymography, we determined that numerous active proteinases exist in typical household dust samples, but the majority of proteinase activity appeared to reside in an ~85 kD multimer of aspergillopepsin I, the major secreted proteinase of many members of the Aspergillus genus. Of particular interest was whether proteinase activity occurring at ~23 kD, corresponding to Der p 1 and other mite-derived proteinases, would be found. However, no such proteinase activity was observed in this molecular size range in over 40 dust samples tested, although Der p 1 was readily detected in many of these samples by immunoassay. We further were unable to reactivate the apparently denatured Der p 1 by treatment under reducing conditions. These findings confirmed that fungi, particularly from the Aspergillus genus, comprised the predominant source of active household proteinases[31].
The unexpected discovery of the dominance of fungi as sources of active proteinases in domestic human environments raised interesting questions regarding the mechanism by which proteinases might induce allergic disease. Studies from mice and analysis of human occupational proteinase exposures clearly indicate that direct inhalation of proteinase is sufficient to cause disease under some conditions. However, active proteinases exist in household dust only in trace quantities, probably far less than what is required to elicit allergic disease through inhalation[31]. Thus, rather than inhalation of proteinase alone, a plausible alternative possibility is that fungal spores are inhaled and proteinase is secreted directly in the airway following germination of the fungi, i.e., proteinase is produced in situ during contained airway fungal infection. In support of this, we have demonstrated that fungal isolates (e.g., A. niger) responsible for house dust proteinases are highly infectious for the normal mouse airway and produce allergic lung disease that strongly resembles human allergic asthma[31]. We further demonstrated that production of proteinases was required for A. niger to induce allergic lung disease in the setting of contained, i.e., non-invasive, airway infection[31].
Additional evidence documents the ability of A. niger and related fungi to produce allergic airway disease involving both the upper and lower human airways. Chronic upper respiratory tract allergic disease often manifests as allergic fungal rhinosinusitis (AFRS), a fungal infectious disease of the sinuses in which one or more filamentous fungi can be detected from the sinus secretions of affected individuals[32]. AFRS is often accompanied by difficulty breathing involving the upper and lower airways (i.e., asthma in the latter instance), fungus-specific IgE, and peripheral blood eosinophilia. Moreover, peripheral blood T cells from AFRS patients strongly react to fungal antigens added in vitro by producing TH2 cell cytokines[33]. A fungal infectious etiology for human allergic airway disease is further supported by allergic bronchopulmonary aspergillosis, a lung disease in which Aspergillus species can be found actively infecting the human lower respiratory tract and causing a disease syndrome that strongly overlaps with asthma[34]. The link between contained fungal infection and asthma is further supported by observations in two separate randomized clinical trials involving antifungal antibiotics that demonstrated improved quality of life in fungal sensitive asthmatics[35, 36]. Together, these observations suggest a general mechanism by which a subset of allergic asthmatics develops disease through the TH2 adjuvant effect of secreted fungal proteinases during contained infection of either the upper or lower airway.
Potential mechanisms of proteinase adjuvant effect
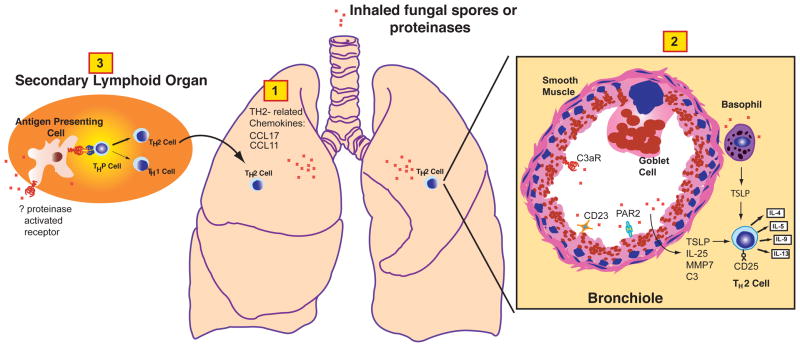
A fascinating challenge in immunology is to determine the molecular mechanisms by which proteinases induce allergic inflammation. Analysis of microbial- and plant-derived allergenic proteinases has revealed a complex, airway epithelial-centered mechanism in which multiple epithelial cytokines including thymic stromal lymphopoietin (TSLP) and IL-25 are induced and lead to robust TH2 cell commitment and allergic responses[28, 37–42] (Figure 1). Moreover, fungal proteinases induce expression of airway matrix metalloproteinase (MMP) 7, which is required for activation of IL-25, as well as multiple airway chemokines such as CCL17 and CCL11 that are important for recruitment of TH2 cells and other allergic effector cells to the airway[43, 44]. The basophil has been proposed as a key cellular target of airway proteinases for production of TSLP and antigen presentation in the airway[28, 45], but other studies suggest that airway epithelial cells may also be relevant cellular proteinase targets[37]. Dendritic cells are also directly activated by proteinases to induce TH2 responses, although the molecular targets of this response are unknown [46](Figure 1).
Figure 1. Mechanisms of allergenic proteinase-dependent induction of allergic airway disease.
Inhaled proteinases or proteinase sources (e.g., fungal spores) initiate a series of molecular events in discrete lung compartments and involving distinct cell types that induce predominant airway TH2 responses that coordinate both the allergic inflammation and physiological changes that typify allergic respiratory tract disease. Initial innate immune responses induced by proteinases include induction of airway chemokines that favor recruitment of allergic effector cells including TH2 cells (1). Likely airway cellular targets of proteinases include basophils, airway epithelial cells and possibly airway smooth muscle cells (2). Activation of these cells by cleavage of cell surface receptors such as PAR2 and CD23 potentially leads to activation of these cells to produce pro-allergic cytokines such as TSLP and IL-25, the latter of which is activated by MMP7, an endogenous proteinase also induced by allergenic proteinases. Allergenic proteinases also likely act on soluble substrates such as complement, especially C3 to generate C3a, the ligand for the C3aR. CD25 is another immune receptor present on T cells that can be cleaved by proteinases, potentially to favor TH2 cytokine secretion. Finally, proteinases act directly on antigen presenting cells such as dendritic cells through an unknown mechanism in secondary lymphoid organs such as lymph nodes to promote their maturation in a manner that favors TH2 cell differentiation from naïve precursor (THP) T cells (3).
Equally important are the earliest airway events activated by allergenic proteinases that lead to downstream allergic immune activation, but these are less well understood. A long-standing concept is that allergens enhance permeabilization of the respiratory epithelium through their proteinase activity to permit the efficient detection of inhaled antigens that would otherwise fail to be perceived[47]. In support of this, proteolytic disruption of airway epithelial tight junctions has been demonstrated experimentally with Der p 1[48], but this effect is likely to be seen with virtually any inhaled proteinase. A major argument against disruption of tight junctions as being required for antigen recognition is that ovalbumin administered to the respiratory tract in the absence of proteinases can nonetheless be readily detected immunologically, leading to induction of ovalbumin-specific Tregs[26, 49] and antigen-specific IgE tolerance[50]. Thus, antigen recognition through the respiratory tract does not require airway epithelial disruption by proteinases; rather, the importance of proteinases lies in their ability to redirect tolerogenic immunity as discussed above to TH2-driven allergic inflammation through their proteinase activity[27].
Another important concept of proteinase-induced allergic disease is that proteinases disrupt normal airway immune homeostasis by cleaving essential immune effector molecules, including CD25 and CD23, that lead to a pro-allergic immune environment, including altered expression of TH1 and TH2 cytokines[51–54] (Figure 1). Proteolytic activation of proteinase activated receptor 2 (PAR-2) has also been proposed as an initiating factor in allergic disease. PAR2 is activated by tryptase and other chymase-like proteinases found in the gut and airway[55]. Analysis of PAR2 in non-proteinase-dependent allergic lung disease models has suggested both pro- and anti-inflammatory roles for this receptor in allergic inflammation[55–60], suggesting a complex role for this receptor in allergic disease. Allergenic proteinases also activate soluble macromolecules such as complement protein 3 (C3), specifically leading to generation of the C3a anaphylatoxin. Binding of C3a to its endogenous receptor, C3aR is required for proteinase-dependent allergic lung disease and robust TH2 responses [61, 62].
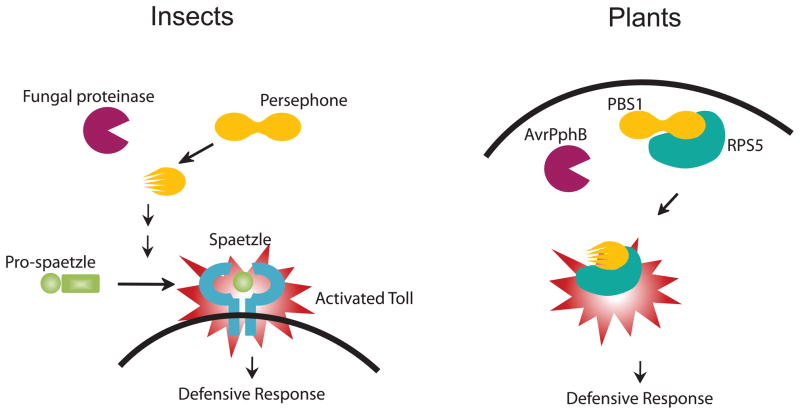
A major hindrance to determining the immune mechanisms of exogenous proteinases is the marked complexity of the mammalian immune system. A potentially promising means of reconciling this complexity is to determine in greater detail the earliest immune events triggered by allergenic proteinases in model organisms with far simpler immune systems. Recognition of fungal invasion in Drosophila melanogaster, the common fruit fly, is accomplished by molecular pattern recognition and proteolytic activity detection, both of which converge to activate the singularly important immune sensor of infection, Drosophila Toll (dToll)[63]. Whereas most mammalian dToll homologues recognize diverse pathogen-associated molecular patterns (PAMPs) directly, dToll is activated indirectly by the cleaved fragments derived from an endogenous circulating ligand, spätzle (Figure 2) [63]. Gram-negative binding protein 3 (GNBP3) is a pattern recognition receptor found in insect hemolymph (the equivalent of blood) that recognizes β-(1, 3)-glucans, which are present in the chitinous fungal cell wall[64, 65]. Infection studies have demonstrated that GNBP3 is required for activation of Toll in response to the yeast Candida albicans[64]. However, GNBP3 is dispensable for responses to filamentous, entomopathogenic (pathogenic for insects) fungi, which secrete proteinases during invasion. Detection of entomopathogenic fungal infection is instead dependent on a serine proteinase present in hemolymph, Persephone (Psh)[63]. After cleavage and activation by exogenous fungal protease PR1A, Psh triggers an endogenous proteolytic cascade that ultimately leads to activation of spätzle and the Toll pathway[64].
Figure 2. Proteinase-activated host defense reactions of insects and plants.
Invasion of insects such as Drosophila melanogaster by fungal hyphae initiates a proteinase-activated enzymatic cascade involving the endogenous proteinase Persephone that terminates in the cleavage of Pro-spaetzle to yield spaetzle, the final common ligand for Toll. Activation of Toll induces a broadly effective anti-microbial defensive response against fungi, bacteria and other organisms. Similarly, bacterial proteinases such as AvrPphB can activate plant proteins such as PBS1 and RPS5 to induce a defense response against bacterial invasion.
Moreover, GNBP3, but not Psh, is required to detect killed fungal spores that lack proteinase activity, suggesting that Psh senses only exogenous proteinase activity in hemolymph. Persephone has also been shown to be activated by bacterial-derived proteinases and is required for the tracheal melanization cascade[66], an essential component of the insect immune response. Intriguingly, the paradigm of proteinase-based activation of immunity is not confined to animals. In the plant Arabidopsis, cleavage by cysteine proteinase AvrPphB from Pseudomonas syringae, a bacterial plant pathogen, activates PBS1, a protein kinase, leading to activation of membrane protein RPS5 and a downstream defensive response[67, 68]. Thus, foreign proteolytic activity qualifies as a “danger signal” indicative of early fungal infection in diverse species (Figure 2).
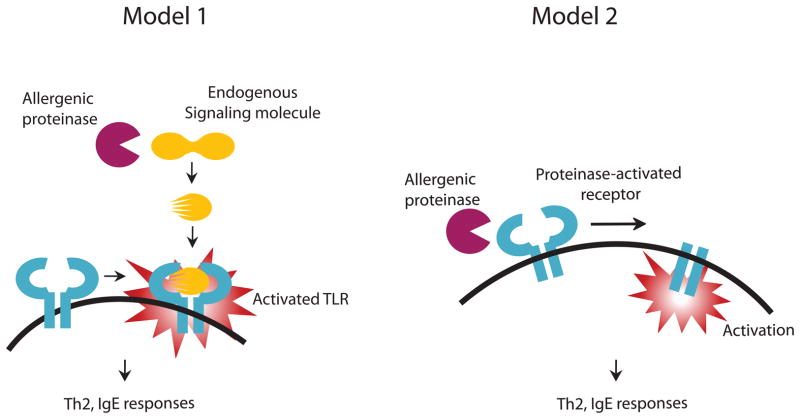
Insects and plants do not possess adaptive immune cells and are therefore incapable of mounting TH2 or allergic immune responses. Only in mammals are proteinase activated TH2 immune responses found that are protective against contained fungal infection in a manner analogous to the innate immune responses of insects and plants. It is possible, therefore, that the central role played by dToll in defense against fungi might extend to mammals. Indeed, recent studies suggest that TLR4 signaling is required for induction of asthma-like disease in mice, although it is not clear if proteinase activity was involved[69, 70]. On the other hand, TLR4 and its principal PAMP ligand endotoxin have also been shown to inhibit allergic lung disease and promote Th1 responses[71–73]. Clearly, much further work is required, but early studies suggest the intriguing possibility that TLR4 and perhaps other TLRs [74] may through a variety of possible proteinase-related mechanisms mediate TH2 responses (Figure 3).
Figure 3. Possible mechanisms by which allergenic proteinases may induce allergic responses through Toll like receptors.
Similar to insect host defense reactions involving Toll, allergenic proteinases may induce enzymatic cascades leading to a common cleavage product that is capable of binding to one or more Toll-like Receptors (TLRs) and initiating essential allergic immune responses such as TH2 differentiation and IgE secretion (Model 1). Alternatively, allergenic proteinases may cleave and directly activate distinct immune receptors such as CD23, CD25, and PAR2 to induce allergic responses (Model 2).
Conclusions and future directions
The Proteinase Hypothesis of allergic disease is attractive because it is now broadly supported by extensive human and experimental data. Moreover, highly allergenic fungal proteinases have been shown to be widely prevalent in human households. The fungi responsible for most household proteinase activity are further able to infect the mouse airway and produce asthma-like disease through production of proteinase directly in the airway. These findings together point to a contained fungal infectious etiology for asthma and related respiratory syndromes, a concept that is further supported by the known link between contained fungal infection of the respiratory tract and allergic diseases such as allergic fungal rhinosinusitis (AFRS)[32, 33] and allergic bronchopulmonary aspergillosis (ABPA)[75]. Respiratory tract viral infections, especially due to human rhinovirus, are also linked to exacerbations of asthma [76], providing a clinically important correlation with the observation that HRV proteinases are potent allergenic adjuvant factors in mice [29].
In addition to the remaining challenge of determining in molecular detail how allergenic proteinases trigger allergic responses, it is now time to begin converting these largely experimental observations into genuine benefit for those suffering from allergic diseases of all kinds. Perhaps foremost of these tasks is determining the true extent to which proteinases contribute to diverse allergic processes and how, i.e., potentially through infection with organisms such as fungi or respiratory viruses. The role of organismal proteinases must further be reconciled with that of emerging allergic disease adjuvant factors such as chitin[77].
Clarification of these issues will prove crucial to improving current medical practice for allergic disease. Current pharmacological therapy for asthma is entirely non-specific, providing mechanical bronchodilation and immunosuppression that, though effective in a majority of patients, is directed at no specific etiologic agent and is potentially hazardous. There is further no means of prognosing asthma and current therapy offers no prospect for cure. These serious limitations of asthma clinical practice would largely evaporate for many patients if, for example, a fungal infectious etiology could be shown. Novel therapeutic approaches suggested by analysis of the mechanisms of proteinase-dependent allergic inflammation are summarized in Table 2. Preliminary studies have already demonstrated that antifungal antibiotics are salutary in fungal-sensitized asthmatics[35, 36]. Thus, clarification of the clinical issues framing the Proteinase Hypothesis is essential to spurring much needed improvements in the care for those suffering from asthma and related afflictions.
Table 2.
Potential therapies for proteinase-dependent allergic disease.
| Potential Approach | Mechanism of action |
|---|---|
| Serine proteinase inhibitors (Serpins) | Inhibit activity of inhaled or in situ-generated fungal proteinases |
| Neutralization of proteinase-activated receptors (TLR, PAR2, CD23, C3aR), soluble substrates (C3) or proteinase-induced allergy-promoting factors (CCL17, CCL11, TSLP, IL-25) | Disrupts key signaling pathways potentially underlying allergic inflammation. |
| Anti-fungal antibiotics (e.g., itraconazole) | Resolves the airway infection that may serve as a source of in situ proteinase that promotes disease. |
Research Highlights.
Proteinases are now recognized as important allergic disease virulence factors.
Fungi and viruses are potentially important sources of allergenic proteinases.
Proteinase-activated signaling mechanisms are poorly understood.
Management of allergic disease will improve with clarification of proteinase biology.
Acknowledgments
Supported by NIH grants HL75243, AI057696 (to D.B.C.), and AI070973 (F.K., D.B.C., G.L.D., and S.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant EN, Wagner R, Weiss KB. Observations on emerging patterns of asthma in our society. J Allergy Clin Immunol. 1999;104:S1–9. doi: 10.1016/s0091-6749(99)70268-x. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. World Health Organization; 2007. [Google Scholar]
- 3.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma-United States, 1980–1999. MMWR. 2002;51:1–13. [PubMed] [Google Scholar]
- 4.Beasley R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol. 2002;109:S482–S489. doi: 10.1067/mai.2002.122716. [DOI] [PubMed] [Google Scholar]
- 5.Kamble S, Bharmal M. Incremental direct expenditure of treating asthma in the United States. J Asthma. 2009;46:73–80. doi: 10.1080/02770900802503107. [DOI] [PubMed] [Google Scholar]
- 6.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 8.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 10.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 12.Peat JK, Salome CM, Sedgwick CS, Kerrebijn J, Woolcock AJ. A prospective study of bronchial hyperresponsiveness and respiratory symptoms in a population of Australian schoolchildren. Clin Exp Allergy. 1989;19:299–306. doi: 10.1111/j.1365-2222.1989.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 13.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 14.Weiss ST. Eat dirt--the hygiene hypothesis and allergic diseases. N Engl J Med. 2002;347:930–931. doi: 10.1056/NEJMe020092. [DOI] [PubMed] [Google Scholar]
- 15.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey CD, Celedon JC. The hygiene hypothesis and asthma. Curr Opin Pulm Med. 2005;11:14–20. doi: 10.1097/01.mcp.0000145791.13714.ae. [DOI] [PubMed] [Google Scholar]
- 17.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 18.Pepys J. Immunological and clinical findings in workers and consumers exposed to enzymes of Bacillus subtilis. Proc Royal Soc Med. 1973;66:930–932. [PMC free article] [PubMed] [Google Scholar]
- 19.Pepys J, Longbottom JL, Hargreave FE, Faux J. Allergic reactions of the lungs to enzymes of Bacillus subtilis. Lancet. 1969;1:1181–1184. doi: 10.1016/s0140-6736(69)92166-7. [DOI] [PubMed] [Google Scholar]
- 20.Cartier A, Malo JL, Pineau L, Dolovich J. Occupational asthma due to pepsin. J Allergy Clin Immunol. 1984;73:574–577. doi: 10.1016/0091-6749(84)90513-x. [DOI] [PubMed] [Google Scholar]
- 21.Gailhofer G, Wilders-Truschnig M, Smolle J, Ludvan M. Asthma caused by bromelain: an occupational allergy. Clin Allergy. 1988;18:445–450. doi: 10.1111/j.1365-2222.1988.tb02894.x. [DOI] [PubMed] [Google Scholar]
- 22.Novey HS, Keenan WJ, Fairshter RD, Wells ID, Wilson AF, Culver BD. Pulmonary disease in workers exposed to papain: clinico-physiological and immunological studies. Clin Allergy. 1980;10:721–731. doi: 10.1111/j.1365-2222.1980.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC PulmMed. 2005;5:4–14. doi: 10.1186/1471-2466-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007;120:610–617. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR, Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997;155:661–669. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]
- 26.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–2600. [PubMed] [Google Scholar]
- 27.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 28.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, Lee SH, Porter P, Xu C, Ohno A, Atmar RL, Greenberg SB, Bandi V, Gern J, Amineva S, Aminev A, Skern T, Smithwick P, Perusich S, Barrow N, Roberts L, Corry DB, Kheradmand F. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2010;125:1369–1378. doi: 10.1016/j.jaci.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–238. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 31.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, Vaidya S, Sur S, Ongeri V, Yang T, Delclos GL, Abramson S, Kheradmand F, Corry DB. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luong A, Marple BF. Allergic fungal rhinosinusitis. Curr Allergy Asthma Rep. 2004;4:465–470. doi: 10.1007/s11882-004-0013-5. [DOI] [PubMed] [Google Scholar]
- 33.Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009;23:281–287. doi: 10.2500/ajra.2009.23.3311. [DOI] [PubMed] [Google Scholar]
- 34.Denning DW, O'Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 35.Ward GW, Jr, Woodfolk JA, Hayden ML, Jackson S, Platts-Mills TA. Treatment of late-onset asthma with fluconazole. J Allergy Clin Immunol. 1999;104:541–546. doi: 10.1016/s0091-6749(99)70321-0. [DOI] [PubMed] [Google Scholar]
- 36.Denning DW, O'Driscoll BR, Powell G, Chew F, Atherton GT, Vyas A, Miles J, Morris J, Niven RM. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 37.Angkasekwinai P, Park H, Wang Y-H, Wang Y-H, Chang SH, Corry DB, Liu Y-J, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu Y-J. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y-H, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu Y-J. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YH, Ito T, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Yu HS, Angkasekwinai P, Chang SH, Chung Y, Dong C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J Korean Med Sci. 2010;25:829–834. doi: 10.3346/jkms.2010.25.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, Amar S, Kheradmand F, Corry DB. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song L-z, Redding D, Singh B, Sur S, Woodruff P, Dong C, Corry DB, Kheradmand F. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamhamedi-Cherradi S-E, Martin RE, Ito T, Kheradmand F, Corry DB, Liu Y-J, Moyle M. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180:6000–6009. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohashi Y, Motojima S, Fukuda T, Makino S. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am Rev Respir Dis. 1992;145:1469–1476. doi: 10.1164/ajrccm/145.6.1469. [DOI] [PubMed] [Google Scholar]
- 48.Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsitoura DC, Blumenthal RL, Berry G, Dekruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–246. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 50.Seymour BW, Gershwin LJ, Coffman RL. Aerosol-induced immunoglobulin (Ig)-E unresponsiveness to ovalbumin does not require CD8+ or T cell receptor (TCR)-gamma/delta+ T cells or interferon (IFN)-gamma in a murine model of allergen sensitization. J Exp Med. 1998;187:721–731. doi: 10.1084/jem.187.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghaemmaghami AM, Robins A, Gough L, Sewell HF, Shakib F. Human T cell subset commitment determined by the intrinsic property of antigen: the proteolytic activity of the major mite allergen Der p 1 conditions T cells to produce more IL-4 and less IFN-gamma. Eur J Immunol. 2001;31:1211–1216. doi: 10.1002/1521-4141(200104)31:4<1211::aid-immu1211>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 52.Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med. 1998;187:271–275. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–316. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 54.Comoy EE, Pestel J, Duez C, Stewart GA, Vendeville C, Fournier C, Finkelman F, Capron A, Thyphronitis G. The house dust mite allergen, Dermatophagoides pteronyssinus, promotes type 2 responses by modulating the balance between IL-4 and IFN-gamma. J Immunol. 1998;160:2456–2462. [PubMed] [Google Scholar]
- 55.Schechter NM, Brass LF, Lavker RM, Jensen PJ. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–373. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 56.Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 57.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 58.Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg M, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–630. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 59.D'Agostino B, Roviezzo F, De Palma R, Terracciano S, De Nardo M, Gallelli L, Abbate GF, D'Aiuto E, Russo M, Cirino G, Rossi F. Activation of protease-activated receptor-2 reduces airways inflammation in experimental allergic asthma. Clin Exp Allergy. 2007;37:1436–1443. doi: 10.1111/j.1365-2222.2007.02793.x. [DOI] [PubMed] [Google Scholar]
- 60.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 62.Drouin SM, Corry DB, Kildsgaard J, Wetsel RA. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2001;167:4141–4145. doi: 10.4049/jimmunol.167.8.4141. [DOI] [PubMed] [Google Scholar]
- 63.Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart J-M. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- 64.Gottar M, Gobert V, Matskevich AA, Reichhart J-M, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishima Y, Quintin J, Aimanianda V, Kellenberger C, Coste F, Clavaud C, Hetru C, Hoffmann JA, Latge JP, Ferrandon D, Roussel A. The N-terminal domain of Drosophila Gram-negative binding protein 3 (GNBP3) defines a novel family of fungal pattern recognition receptors. J Biol Chem. 2009;284:28687–28697. doi: 10.1074/jbc.M109.034587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El Chamy L, Leclerc V, Caldelari I, Reichhart J-M. Sensing of 'danger signals' and pathogen-associated molecular patterns defines binary signaling pathways 'upstream' of Toll. Nat Immunol. 2008;9:1165–1170. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ade J, DeYoung BJ, Golstein C, Innes RW. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc Natl Acad Sci USA. 2007;104:2531–2536. doi: 10.1073/pnas.0608779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, Mengiste T, Zhang Y, Zhou JM. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuipers H, Hijdra D, De Vries VC, Hammad H, Prins JB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Lipopolysaccharide-induced suppression of airway Th2 responses does not require IL-12 production by dendritic cells. J Immunol. 2003;171:3645–3654. doi: 10.4049/jimmunol.171.7.3645. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003;278:42361–42368. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- 73.Bortolatto J, Borducchi E, Rodriguez D, Keller AC, Faquim-Mauro E, Bortoluci KR, Mucida D, Gomes E, Christ A, Schnyder-Candrian S, Schnyder B, Ryffel B, Russo M. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clin Exp Allergy. 2008;38:1668–1679. doi: 10.1111/j.1365-2222.2008.03036.x. [DOI] [PubMed] [Google Scholar]
- 74.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 75.Zhaoming W, Lockey RF. A review of allergic bronchopulmonary aspergillosis. J Investig Allergol Clin Immunol. 1996;6:144–151. [PubMed] [Google Scholar]
- 76.Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB. Respiratory tract viral infections in inner-city asthmatic adults. Arch Int Med. 1998;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 77.Reese TA, Liang H-E, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161:3645–3651. [PubMed] [Google Scholar]