Abstract
Purpose
We evaluated the effect of local recurrence (LR) and regional recurrence (RR) on distant metastasis and survival in patients treated with breast conservation therapy (BCT).
Methods
We analyzed 907 patients who were treated for invasive breast cancer between 1993 and 2006. With 53 months of follow-up, 28 patients (3.1%) developed LR in the breast and 12 patients (1.3%) developed RR before distant metastasis. LR and RR were separated into four patterns to determine the prognostic relevance of recurrence site and time to recurrence: LR within 3 years (early LR), LR after 3 years (late LR), RR within 3 years (early RR), and RR after 3 years (late RR).
Results
Early LR (hazard ratio [HR], 4.76; p=0.003) and early RR (HR, 18.16; p<0.001) were independent predictors of distant metastasis. In terms of overall survival, early LR (HR, 5.24; p=0.002), and early RR (HR, 18.80; p<0.001) were significantly related with poor survival. Patients with late LR/RR had a similar favorable prognosis compared with patients who never experienced LR/RR.
Conclusion
The result suggests that time to LR/RR following BCT is a significant predictor developing a distant metastasis and surviving.
Keywords: Breast conservation therapy, Distant metastasis, Local recurrence, Regional recurrence, Survival
INTRODUCTION
A substantial increase in local recurrence (LR) of the treated breast has become a significant issue with the increased use of breast conservation therapy (BCT). The incidence of LR in the remnant breast varies between 2-10% after 5 years [1,2], and LR continues to occur at about 1% per year following BCT [3]. Isolated regional lymph node recurrence (RR) is an uncommon pattern of recurrence, and occurs in only 1-3% of patients with early stage breast carcinoma after a mastectomy or BCT [1,4,5]. Because of its rarity, relatively few data are available regarding the effect of LR and RR on the development of distant metastasis and survival in patients treated with BCT.
Studies on the prognostic effect of time on LR and RR after BCT have reported conflicting results. While some studies have reported that prognosis of patients with late recurrence of more than 2-3 years is significantly better than that of patients with early recurrence within 2-3 years [6-8], another study found no significant effect of the interval from BCT to regional nodal recurrence on prognosis [9].
We hypothesized that the recurrence site (LR vs. RR) and time to recurrence (<3 vs. >3 years) might have prognostic relevance. In this study, the occurrence of LR and RR and time to LR and RR were considered together, and we tested for their ability to predict subsequent development of distant metastasis and survival.
METHODS
Study population
Between January 1993 and December 2006, 1,103 consecutive patients with breast cancer were treated with BCT at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. We retrospectively reviewed the data. Among the patients, we excluded 82 (7.4%) with ductal carcinoma in situ, one (0.1%) with a T4 lesion, four (0.4%) with stage IV disease at diagnosis, two (0.2%) with LR/RR diagnosed less than 3 months after initial surgery, and 32 (2.9%) with non-epithelial originating tumors (phyllodes, sarcoma, or lymphoma). Thirty-nine (3.5%) patients received neoadjuvant chemotherapy did not include to exclude the confounding effect of neoadjuvant chemotherapy. We also excluded 33 patients (3.0%) who did not receive radiation therapy, and three patients (0.2%) who did not receive appropriate axillary dissection. Finally, 907 patients comprised the study population.
BCT consisted of a wide resection of the primary tumor with an attempted margin of at least 1 cm and axillary staging followed by definitive breast radiation with or without systemic therapy. Microscopic margins were outperformed as routine clinical practice, and the primary site was re-excised for patients with microscopically involved margins. Sentinel lymph node biopsy (SLNB) using radioisotope was started in early 2000 at our medical center, so SLNB was performed in 279 patients (30.7%). Among them, we did not perform an axillary dissection in 209 patients with a negative sentinel lymph node, and they were considered N0.
Patients were treated with radiotherapy using tangential fields directed at the whole breast after conservative surgery. The whole breast dose was 50.4 Gy (range, 45-55.8 Gy) with 1.8-2 Gy per fraction. A tumor bed boost was performed with a 9-12 MeV electron beam. The median boost dose was 10 Gy (range, 10-20 Gy). In cases with a close or positive resection margin, the total radiation dose including the boost dose was increased to 65 Gy. As a rule, the irradiated volume involved the breast alone in patients with less than three positive lymph nodes. Cases with extracapsular lymph node extension or more than four positive lymph nodes were irradiated in the axilla or subclavicular fossa. The internal mammary lymph node was not routinely irradiated if there was no evidence of tumor involvements by radiological tests.
Adjuvant chemotherapy was indicated for patients who were node positive and had high-risk node negative disease. Between 1993 and 2004, patients with node negative disease received six cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), whereas those with node positive disease received six cycles of 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC). From 2005 until the end of the study, four cycles of doxorubicin plus cyclophosphamide (AC) were administered to those with node positive disease and four cycles of doxorubicin plus cyclophosphamide followed by four cycles of taxane (AC→T) were administered to those with node positive disease. Among 798 patients with positive estrogen (ER) or progesterone receptors (PR), only 539 patients (63.0%) received adjuvant endocrine therapy, because premenopausal women were excluded from endocrine therapy in the early period of this study.
Tested variables
We retrospectively reviewed the patients' clinicopathological data including age, tumor stage, lymph node status, histological type, histological grade, ER/PR status, adjuvant systemic therapy, and follow-up data. LR was defined as histological evidence of a new tumor occurring in the treated breast or overlying skin more than 3 months after the completion of definitive surgery. The definition of RR was any biopsy-proven carcinoma found in the axilla or supraclavicular lymph nodes. Patient diagnosed with both LR and RR simultaneously were defined as RR. To evaluate the prognostic effect of LR and RR and time to recurrence, LR and RR were separated into four patterns: LR within 3 years (early LR), LR after 3 years (late LR), RR within 3 years (early RR), and RR after 3 years (late RR). Clinicopathological variables and patterns of LR and RR were tested for their ability to predict distant relapse free survival (DRFS) and overall survival (OS). All data were extracted from the Severance Hospital breast cancer registry which is a prospectively maintained database and includes clinical information, pathological information, treatment modalities, and outcome details.
Statistics
The primary end points of this analysis were DRFS and OS. Events determining DRFS were distant recurrence and death before recurrence. OS was death from any cause. Survival time was defined as the length of time from the date of surgery to the date of an event or the date last known to be alive. Survival rates were calculated by the Kaplan-Meier method, and the log-rank test was used to compare the groups. Multivariate analyses were performed using Cox proportional hazard regression model to determine whether LR and RR patterns were independent predictors of DRFS and OS. All reported p-values were two-sided, and a p-value <0.05 was considered significant.
RESULTS
Patient characteristics
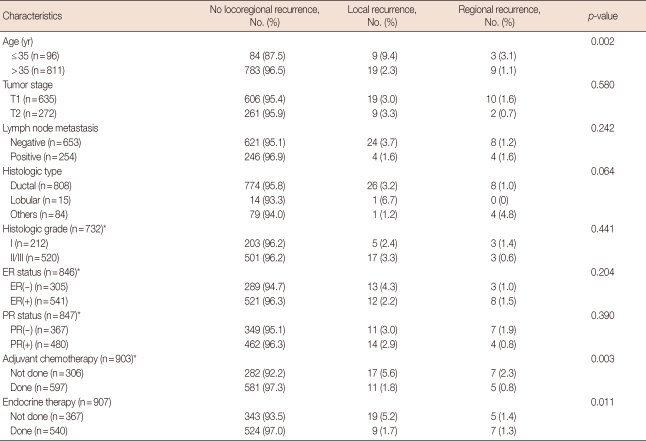
The clinicopathological characteristics of the study population and LR and RR patterns are summarized in Table 1. At the time of initial diagnosis, 96 patients (10.6%) were under the age of 35, and 272 patients (30.0%) had T2 lesions. At the time of initial surgery, 254 patients (28.0%) had a positive axillary lymph node. Almost 90% of patients had invasive ductal carcinoma (808 of 907, 89.9%). Information on histological grade, ER and PR status, and adjuvant chemotherapy was available in 732, 846, 847, and 903 patients, respectively. Approximately two-thirds of patients received adjuvant systemic chemotherapy and endocrine therapy. No significant differences were observed in the clinicopathological characteristics between LR and RR.
Table 1.
Clinicopathologic, treatment characteristics, and patterns of local/regional recurrence
ER=estrogen receptor; PR=progesterone receptor.
*Histologic grade, ER and PR status, and adjuvant chemotherapy did not know whole data. The numbers in parentheses are data that can be known as the chart review.
Twenty-eight patients (3.1%) developed LR and 12 patients (1.3%) developed RR as the first event, with a median follow-up time of 53 months (range, 4-179 months). None of the patients showed histological or radiological signs of distant metastasis at the time of LR and RR diagnosis (Table 1). The distribution of early LR, late LR, early RR, and late RR was 12 (1.3%), 16 (1.8%), 5 (0.6%), and 7 (0.8%), respectively. Recurrent sites were an ipsilateral breast (n=28, 70.0%), axillary lymph node (n=2, 5.0%), supraclavicular lymph node (n=9, 22.5%), and internal mammary lymph node (n=1, 2.5%).
Among 653 patients with axillary node-negative disease, 209 patients (32.0%) performed only SLNB and the rest 444 patients (68.0%) underwent axillary node dissection. Early LR, late LR, early RR, and late RR occurred in 1, 1, 0, and 1 patient among those who performed only SLNB, respectively, and 9, 13, 2, and 4 patients among those who underwent ALND, respectively. Among patients with node-negative disease, SLNB alone did not lead to inferior locoregional control than ALND.
At the time of the LR and RR diagnosis, 30 patients (75%) underwent surgical treatment including simple mastectomy with or without axillary dissection, or axillary dissection alone. Twelve patients (30%) were treated with additional radiotherapy to regional lymph node. Chemotherapy was administered to 22 patients (55%), and 16 patients (40%) received endocrine therapy. Two patients (5%) received herceptin.
Effect of LR and RR on DRFS and OS
Of the 40 patients who underwent LR and RR, 13 (32.5%) experienced subsequent distant recurrence. The distribution of early LR, late LR, early RR, and late RR was four (5.0%), three (3.8%), four (5.0%), and two (2.5%). The distance metastatic sites were bone (n=5), lung (n=7), liver (n=4), central nervous system (n=4), and other visceral organ (n=3). Eleven patients (27.5%) died after detection of LR and RR. Of 867 patients who did not undergo LR and RR, 67 (7.7%) experienced distance recurrence. Distance metastasis sites were bone (n=38), lung (n=37), liver (n=18), central nervous system (n=7), and other visceral organ (n=7). Fifty-seven patients (6.7%) died during the following-up period. The following factors were analyzed for their ability to predict DRFS and OS: age, T stage, lymph node status, histological type, histological grade, ER/PR status, adjuvant chemotherapy, endocrine therapy, and LR and RR patterns.
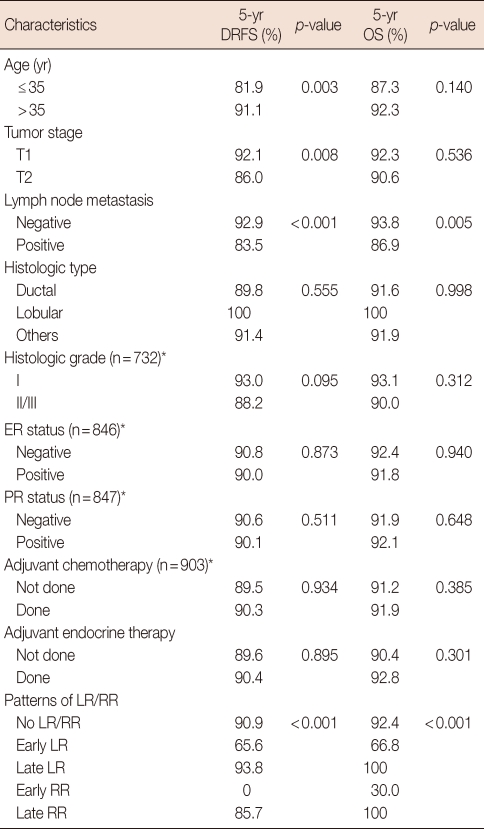
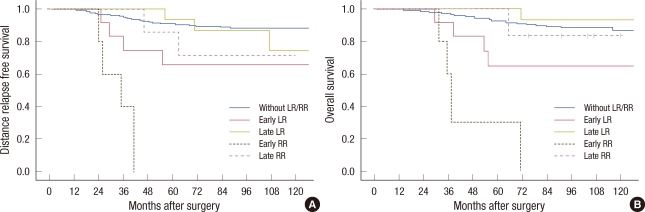
In a univariate analysis, younger age (≤35 years) at diagnosis (p=0.003), larger tumor size of more than 2 cm (p=0.008), positive lymph node (p<0.001), and early LR and early RR development (p<0.001) had a significant effect on DRFS. Positive lymph node status (p=0.005) and early LR and early RR development (p<0.001) had a significant effect on poor survival (Table 2, Figure 1). The 5-year DRFS for patients presenting with early LR and early RR was 65.6% and 0%, respectively. The 5-year OS of early LR and early RR was 66.8% and 30.0%, respectively. The 5-year DRFS and OS of late LR and late RR were similar compared with patients who never experienced LR and RR (Table 2).
Table 2.
Univariate analysis for predicting 5 year distant relapse free survival and overall survival
DRFS=distant relapse free survival; OS=overall survival; ER=estrogen receptor; PR=progesterone receptor; Early LR=local recurrence within 3 years; Late LR=local regional after 3 years; Early RR=regional recurrence within 3 years; Late RR=regional recurrence after 3 years.
*Histologic grade, ER and PR status, and adjuvant chemotherapy did not know whole data. The numbers in parentheses are data that can be known as the chart review.
Figure 1.
Kaplan-Meier 5-year distant relapse free survival (A) and overall survival (B) estimate according to the patterns of local recurrence and regional recurrence. Early local recurrence (LR), local recurrence within 3 years; Late LR, local regional after 3 years; Early regional recurrence (RR), regional recurrence within 3 years; Late RR, regional recurrence after 3 years.
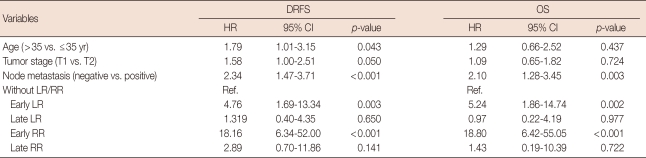
In multivariate analysis, younger age (≤35 years) (hazard ratio [HR], 1.79; 95% confidence interval [CI], 1.01-3.15; p=0.043), positive lymph node (HR, 2.34; 95% CI, 1.47-3.71; p<0.001), early LR (HR, 4.76; 95% CI, 1.69-13.34; p=0.003), and early RR (HR, 18.16; 95% CI, 6.34-52.00; p<0.001) were significantly related with lower DRFS, whereas tumor size (HR, 1.58; 95% CI, 1.00-2.51; p=0.05) had marginal significance. In terms of OS, a positive lymph node (HR, 2.10; 95% CI, 1.28-3.45; p=0.003), early LR (HR, 5.24; 95% CI, 1.86-14.74; p=0.002), and early RR (HR, 18.80; 95% CI, 6.24-55.05; p<0.001) were significantly related to lower OS. No apparent difference in DRFS and OS was found between patients who experienced late LR and RR and those who did not (Table 3).
Table 3.
Hazard ratios and 95% confidence intervals by multivariate Cox model
DRFS=distance relapse free survival; OS=overall survival; HR=hazard ratio; CI=confidence interval; LR=local recurrence; RR=regional recurrence; Early LR=local recurrence within 3 years; Late LR=local regional after 3 years; Early RR=regional recurrence within 3 years; Late RR=regional recurrence after 3 years; Ref.=reference.
DISCUSSION
The magnitude of problem of LR is substantial as many women choose BCT for initial management. RR is a relatively uncommon event, and few data are available regarding the association among RR, DRFS, and OS. However, as the number of long term survivors has increased, analysis of treatment outcomes has revealed that the LR and RR problem has a potential impact on DRFS and OS [10,11]. Evaluation of the prognostic effect of LR and RR on the development of DR and survival following BCT may help to determine the treatment modality.
Several studies have focused on LR and RR as the first event after BCT and reported that LR and RR are significant predictors of subsequent distant metastases and survival [4,12-16]. Additionally, it seems that the prognosis after RR is much worse than that after LR. According to an analysis of five trials conducted by the National Surgical Adjuvant Breast and Bowel Project, the risks of distant disease and death were greater after RR than LR in each trial [17]. However, whether time to recurrence is a marker for distant metastasis remains controversial. Several studies have identified the time to LR and RR from initial surgery as an additional important prognostic factor for DRFS and OS [5,12-14,17-20], whereas another study reported that the time to regional nodal recurrence did not have a significant effect on prognosis [9].
We separated LR and RR into four patterns-(early LR, late LR, early RR, and late RR) to evaluate the prognostic relevance of site of recurrence (LR vs. RR) and time to LR and RR (early vs. late) after BCT. The data presented here confirm that patients who experience early LR and early RR within the first few years following their original diagnosis have a poor prognosis. LR and RR within 3 years was a strong predictor of developing distant recurrence and death. Early RR showed the worst prognostic patterns and the outcomes in these patients at 5 years showed a DRFS of 0% and an OS of 30%, followed by an early LR. Although a trend was seen for higher rates of distant metastases in patients with late RR, it was not statistically significant (HR, 2.89; 95% CI, 0.70-11.86; p=0.141). These findings suggest that time to LR and RR is a more important prognostic factor for distant metastases and survival than site of recurrence. We assumed that more rapidly recurring tumors have higher biological aggressiveness, and that patients who sustain early LR and early RR tend to display worse clinical behavior and a relatively unfavorable prognosis.
Interestingly, the prognosis of patients who experienced late LR was not that different from that of patients who never experienced recurrence. A possible explanation for this finding is that a significant portion of patients who experience late LR following BCT develop new primary tumors as opposed to true LR [21]. True recurrence and a new primary tumor may have a different natural history, different biological behavior and a different prognosis. Theoretically, true recurrent tumors maybe more radio-resistant and more drug-insensitive than a new primary tumor [17,21].
Prevention and prediction of LR and RR at the time of initial diagnosis of primary tumor might be an important issue if LR and RR, as the first event after BCT, influences DRFS and OS. Patients at high risk for LR and RR can benefit from initial aggressive surgery [22], but, no robust marker predicts the risk of locoregional failure, which would be helpful when selecting an ideal initial therapy [23]. A major effort is underway to predict LR and RR using molecular markers in genome-wide association studies.
In the absence of data from prospective randomized trials, we suggest that the decision should be tailored to the risk of the individual patient based on the knowledge of a demonstrated benefit of adjuvant systemic therapy in patients with primary cancer. Our data suggests that patients with early LR and early RR need to be considered as a clinically distinct group compared with those who develop late LR and late RR. The high incidence of distant metastasis and death in patients who experienced early LR and early RR justifies considering more aggressive systemic therapy at the time of the LR and RR diagnoses. Age, tumor size, and axillary lymph node status were also significantly related to distant recurrence and all should be considered for further adjuvant therapy.
We noted that patients who experienced late RR had a greater than 2.89 fold relative risk of developing distant metastasis without statistical significance. Because the limited power of this study with a small number of patients was a possible cause for the failure to demonstrate a difference, our data could not provide definitive answers regarding the role of systemic treatment for patients with late RR. It seems reasonable to select systemic treatment strategies on an individual basis for patients who experience late LR and late RR.
We included patients who had received appropriate radiation, and standard axillary surgery and excluded patients who were administered inappropriate locoregional treatment. However, similar to other studies, an important limitation of this retrospective study was the small number of patients, as is apparent from the broadness of the confidence intervals. Interpretation of the results is also hampered by the heterogeneity of the study population because of the long study duration and different adjuvant systemic therapies used after initial surgery. Additionally, treatment strategies at the time of LR and RR were highly individualized given the lack of prospective data to make decisions regarding patients with LR and RR.
Early LR and early RR within 3 years following BCT was a strong independent predictor of DRFS and OS, whereas survival of patients with late LR and late RR was not significantly different from those who never experienced LR and RR. Other prognostic factors (age, tumor size, positive axillary lymph node) were also important predictors of DRFS and OS. Aggressive systemic treatment should be considered for patients who experience early LR and early RR, whereas systemic treatment for patients with late LR and late RR could be determined on an individual basis. A multi-institutional study is necessary to establish a standard treatment protocol for patients who suffer from LR and RR following BCT. It may be possible in the future to tailor initial surgery for a primary tumor using biological marker that estimates the risk of local and regional failure.
Footnotes
This work was supported by the Brain Korea 21 Project for Medical Science, Yonsei University, and in part by a grant-in-aid from Novartis Korea Co., Astra Zeneca Korea Co., Dong-A Pharmaceutical Co., and Sanofi-Aventis Pharmaceutical Co.
All authors declare no conflicts of interest.
References
- 1.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Arriagada R, Lê MG, Guinebretière JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol. 2003;14:1617–1622. doi: 10.1093/annonc/mdg452. [DOI] [PubMed] [Google Scholar]
- 4.Harris EE, Hwang WT, Seyednejad F, Solin LJ. Prognosis after regional lymph node recurrence in patients with stage I-II breast carcinoma treated with breast conservation therapy. Cancer. 2003;98:2144–2151. doi: 10.1002/cncr.11767. [DOI] [PubMed] [Google Scholar]
- 5.Moran MS, Haffty BG. Local-regional breast cancer recurrence: prognostic groups based on patterns of failure. Breast J. 2002;8:81–87. doi: 10.1046/j.1524-4741.2002.08202.x. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson I, Liljegren G, Arnesson LG, Emdin SO, Palm-Sjövall M, Fornander T, et al. Local recurrence in the breast after conservative surgery--a study of prognosis and prognostic factors in 391 women. Eur J Cancer. 2002;38:1860–1870. doi: 10.1016/s0959-8049(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 7.Fourquet A, Campana F, Zafrani B, Mosseri V, Vielh P, Durand JC, et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys. 1989;17:719–725. doi: 10.1016/0360-3016(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 8.Elkhuizen PH, Hermans J, Leer JW, van dE Vijver MJ. Isolated late local recurrences with high mitotic count and early local recurrences following breast-conserving therapy are associated with increased risk on distant metastasis. Int J Radiat Oncol Biol Phys. 2001;50:387–396. doi: 10.1016/s0360-3016(01)01469-9. [DOI] [PubMed] [Google Scholar]
- 9.Lukens JN, Vapiwala N, Hwang WT, Solin LJ. Regional nodal recurrence after breast conservation treatment with radiotherapy for women with early-stage breast carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1475–1481. doi: 10.1016/j.ijrobp.2008.06.1955. [DOI] [PubMed] [Google Scholar]
- 10.Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. N Engl J Med. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 11.Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher B, Anderson S, Fisher ER, Redmond C, Wickerham DL, Wolmark N, et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet. 1991;338:327–331. doi: 10.1016/0140-6736(91)90475-5. [DOI] [PubMed] [Google Scholar]
- 13.Doyle T, Schultz DJ, Peters C, Harris E, Solin LJ. Long-term results of local recurrence after breast conservation treatment for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:74–80. doi: 10.1016/s0360-3016(01)01625-x. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro Grillo I, Jorge M, Marques Vidal P, Ortiz M, Ravasco P. The effect of locoregional recurrence on survival and distant metastasis after conservative treatment for invasive breast carcinoma. Clin Oncol (R Coll Radiol) 2005;17:111–117. doi: 10.1016/j.clon.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Cowen D, Jacquemier J, Houvenaeghel G, Viens P, Puig B, Bardou VJ, et al. Local and distant recurrence after conservative management of "very low-risk" breast cancer are dependent events: a 10-year follow-up. Int J Radiat Oncol Biol Phys. 1998;41:801–807. doi: 10.1016/s0360-3016(98)00144-8. [DOI] [PubMed] [Google Scholar]
- 16.Voogd AC, van Tienhoven G, Peterse HL, Crommelin MA, Rutgers EJ, van de Velde CJ, et al. Local recurrence after breast conservation therapy for early stage breast carcinoma: detection, treatment, and outcome in 266 patients. Dutch Study Group on Local Recurrence after Breast Conservation (BORST) Cancer. 1999;85:437–446. doi: 10.1002/(sici)1097-0142(19990115)85:2<437::aid-cncr23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE, Jr, Jeong JH, Tan-Chiu E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 18.Haffty BG, Reiss M, Beinfield M, Fischer D, Ward B, McKhann C. Ipsilateral breast tumor recurrence as a predictor of distant disease: implications for systemic therapy at the time of local relapse. J Clin Oncol. 1996;14:52–57. doi: 10.1200/JCO.1996.14.1.52. [DOI] [PubMed] [Google Scholar]
- 19.Whelan T, Clark R, Roberts R, Levine M, Foster G. Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: results from a randomized trial. Investigators of the Ontario Clinical Oncology Group. Int J Radiat Oncol Biol Phys. 1994;30:11–16. doi: 10.1016/0360-3016(94)90513-4. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz JM, Amalric R, Brandone H, Ayme Y, Jacquemier J, Pietra JC, et al. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer. 1989;63:1912–1917. doi: 10.1002/1097-0142(19890515)63:10<1912::aid-cncr2820631007>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Smith TE, Lee D, Turner BC, Carter D, Haffty BG. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–1289. doi: 10.1016/s0360-3016(00)01378-x. [DOI] [PubMed] [Google Scholar]
- 22.Fatouros M, Baltoyiannis G, Roukos DH. The predominant role of surgery in the prevention and new trends in the surgical treatment of women with BRCA1/2 mutations. Ann Surg Oncol. 2008;15:21–33. doi: 10.1245/s10434-007-9612-4. [DOI] [PubMed] [Google Scholar]
- 23.Roukos DH. Genetics and genome-wide association studies: surgery-guided algorithm and promise for future breast cancer personalized surgery. Expert Rev Mol Diagn. 2008;8:587–597. doi: 10.1586/14737159.8.5.587. [DOI] [PubMed] [Google Scholar]