Abstract
Purpose
Lymphovascular invasion (LVI) is an important prognostic factor in patients with lymph node-negative patients with invasive breast cancer. However, the prognostic value of LVI it is unclear and controversial about its prognostic value in patients with lymph node-positive breast cancer patients. So, we report the an analysis of the prognostic significance of LVI in a large cohort study of patients with lymph node-positive patients with invasive breast cancer.
Methods
We retrospectively reviewed 967 patients with invasive breast cancer that had undergone surgical treatment at our hospital, from January 2004 to December 2007. Among these thempatients, 349 patients with lymph node-positive breast cancer patients are were included in this study. We evaluated clinical and pathological data in these patients, we compared with 5-year overall survival and disease-free survival between an LVI-present group and an LVI-absent group.
Results
The median follow-up was 48 months (range, 12-78 months), and the mean age of the patients was 48 years (range, 23-78 years). LVI was present in 192 patients (55%) of with tumors and was associated with age ≤40 years (p=0.009), high histologichistological grade (p=0.007), estrogen receptor status (p=0.001), tumor size ≥2 cm (p<0.001), and number of involved lymph nodes (p<0.001), but not with progesterone receptor status, HER2 status, p53 status, or tumor multiplicity. LVI was a significant independent prognostic factor for disease-free survival (p<0.001) and overall survival (p=0.006). By multivariate analysis revealed that LVI (p=0.003), number of involved lymph nodes (≥4; p=0.005), and high histological grade (II and III; p=0.02) was were an independent significant predictors of disease-free survival and overall survival in the whole group of patients.
Conclusion
In this case, we demonstrated that LVI is a significant predictor of poor prognosis in patients with lymph node-positive patients with primary invasive breast cancer, LVI is a significant predictive predictor value of poor prognosis. So, LVI should be considered in the therapeutic strategy as a decision making tool in the adjuvant chemotherapy setting.
Keywords: Breast neoplasms, Lymph node metastasis, Lymphovascular invasion, Prognostic factor
INTRODUCTION
One of the most important issues for treating patients with early stage breast cancer is determining those who could benefit from adjuvant chemotherapy and which chemotherapy regimen would provide the best results. Prognostic factors can be used to predict the natural history of breast cancer, so they are included in the decision to apply adjuvant systemic chemotherapy for patients with breast cancer. Currently, the presence of axillary lymph node involvement, tumor size, nuclear grade, hormone receptor status, and patient age are well-known prognostic factors for patients with operable invasive breast cancer [1]. These prognostic factors are widely used to determine whether to apply adjuvant chemotherapy in patients with breast cancer. Among the prognostic factors, axillary lymph node involvement is the most significant [1,2]. Because the presence of lymph node involvement shows that the cancer has already developed the ability for distant metastasis [2-4]. Thus, the presence of axillary lymph node involvement predicts the choice of adjuvant chemotherapy and radiation therapy after surgery for primary breast cancer [5].
Lymphovascular invasion (LVI) is defined as tumor emboli present within a definite endothelial-lined space in the breast surrounding invasive carcinoma [6,7]. The existence of LVI may help identify who is at increased risk for axillary lymph node and distant metastasis [2,3] Although LVI has been accepted as an important prognostic factor in patients with lymph node-negative invasive breast cancer [8], LVI is controversial regarding prognostic value in patients with lymph node-positive breast cancer [3,9-11]. The aim of this study was to analyze the prognostic significance of LVI in a large cohort study of patients with lymph node-positive invasive breast cancer.
METHODS
Patients and methods
From January 2004 to December 2007, we retrospectively reviewed 989 patients with invasive breast cancer that had undergone surgical treatment at the Department of Surgery, Chonnam National University Hospital. In total, 596 lymph node-negative and 371 lymph-node positive patients were enrolled. Among the 371 lymph-node positive patients, 22 (six patients with a previous or a concurrent contralateral breast cancer, two males with breast cancer, and 14 patients who underwent neoadjuvant chemotherapy) were excluded. Thus, 349 patients with lymph node-positive breast cancer were included. Among the 349 patients, 11 were treated with sentinel lymph node biopsy only because the frozen biopsy was negative, whereas permanent sections revealed positive findings.
We evaluated the 349 patients with lymph-node positive invasive breast cancer during a mean follow-up of 48 months (range, 12-78 months). These patients had been surgically treated with either a modified radical mastectomy or wide local tumor resection, with sentinel lymph node biopsy or axillary lymph node dissection, followed by postoperative radiation therapy. The need for adjuvant systemic therapy (chemotherapy and hormonal therapy) was determined according to axillary lymph node status, hormone receptor status, and menopausal status. All patients visited the hospital every 6 months for 5 years, then once per year.
Pathologic examination
We analyzed the patient's clinicopathological data including age, tumor size, hormone receptor status, axillary lymph node status, nuclear grade, and presence of LVI. Diameter of the tumor was measured on pathological specimens that were hematoxylin and eosin (H&E) stained. Nuclear grade was identified by a modified Scarff-Bloom-Richardson grading system. Lymph nodes were stained with H&E and examined for tumor cell metastasis. Hormone receptor (estrogen [ER] and progesterone [PR] receptors) and HER2 status was determined by immunohistochemical (IHC) analysis using a tissue microarray [13]. The immunohistochemical analyses used an ER antibody (1D5; DAKO, Carpinteria, USA), a PR antibody (PgR636; DAKO), and a HER2 antibody (4B5; DAKO). Hormone receptors were considered positive if expression was ≥10%. The HER2 expression results by IHC analysis were scored as negative, 1+, 2+, or 3+, according to the manufacturer's recommendations. Patients with a score of 3+ were considered HER2 positive. HER2/chromosome 17 fluorescence in situ hybridization was performed on patients in which the IHC analysis score was 2+.
LVI was assessed on H&E-stained slides, as defined by Rosen and Oberman [6]. LVI was defined as carcinoma cells present within a definite endothelial-lined space, at a distance from the tumors, in the breast surrounding the invasive carcinoma. [6-8] That is, LVI must be assessed outside the boundaries of the main breast tumor (at least more than one high power microscopic field away) to avoid misinterpretation [9]. When interpretation results were vague, the immunostaining markers D2-40 and CD34 were used to confirm that LVI by H&E staining was truly present.
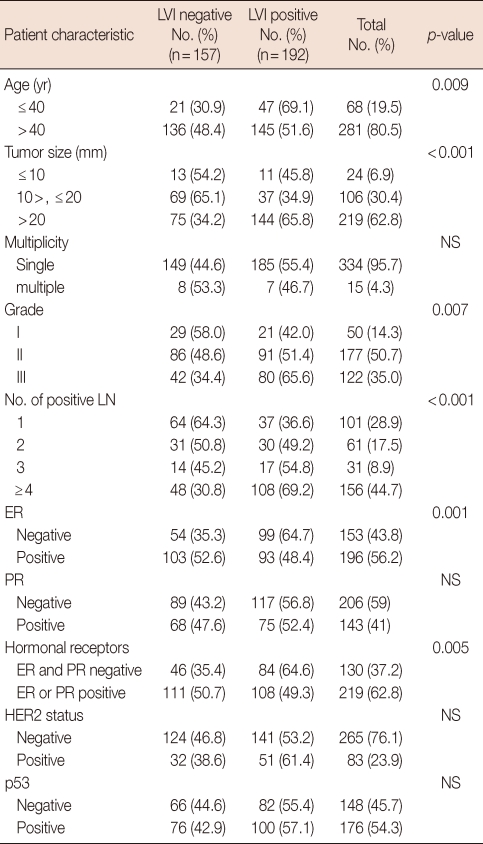
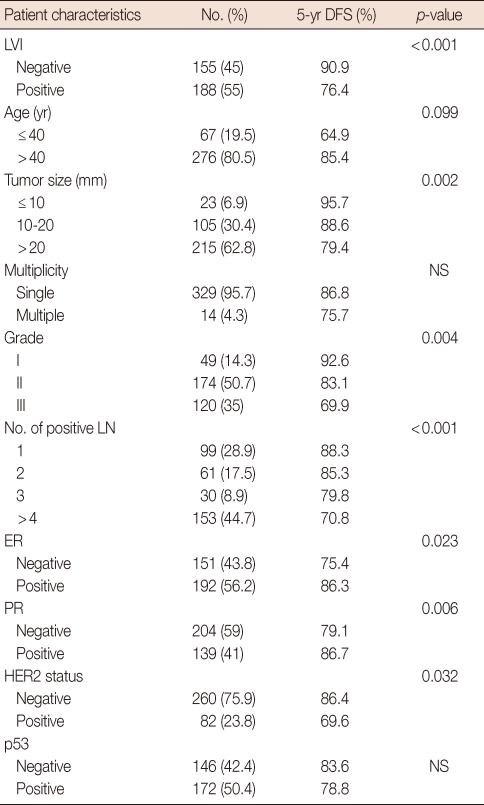
The clinicopathological data and systemic or locoregional treatment of the 349 patients are summarized in Tables 1 and 2. Clinicopathological data were analyzed according to the presence of LVI to establish the relationship between LVI and other factors.
Table 1.
Clinicopathological data of patients
LVI=lymphovascular invasion; NS=not significant; LN=lymph node; ER=estrogen receptor; PR=progesterone receptor.
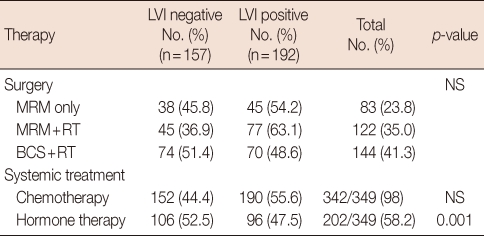
Table 2.
Locoregional and systemic adjuvant therapy
LVI=lymphovascular invasion; MRM=modified radical mastectomy; RT=radiotherapy; BCS=breast conserving surgery; NS=not significant.
Statistical analysis
Correlations between LVI and patient clinicopathological data were analyzed with the chi-square test. Overall survival (OS), disease-free survival (DFS), and metastasis-free survival (MFS) rates were evaluated using the Kaplan-Meier method from the date of surgery to death, recurrence, or distant metastasis. Univariate analyses were performed with the log-rank test. Multivariate analyses were performed using the Cox regression model. A p-value<0.05 was considered significant.
RESULTS
The mean age of the patients at the time of surgery was 48 years (range, 23-78 years), and the median follow-up period was 48 months (range, 12-78 months). The number of distant metastases with or without locoregional recurrence was 61 (17.5%). The number of locoregional recurrences or distant metastases was 72 (20.9%). Thirty-six of 343 (10.3%) patients died because of breast cancer at the time of the study. Five-year OS, MFS, and DFS were 93%, 89.1%, and 87%, respectively.
LVI was present in 192 of 349 (55%) patients and was significantly associated with patient age ≤40 years (p=0.009), high histological grade (grades II and III, p=0.007), tumor diameter >20 mm (p=0.001), number of involved lymph nodes ≥4 (p=0.001), and negative ER (p=0.001) tumors. But it was not related to tumor multiplicity, PR status, HER2 receptor status, or p53 status (Table 1). Patients with negative LVI tumors were more frequently treated with adjuvant endocrine therapy (52.5% vs. 47.5, p=0.001). Surgical treatment and adjuvant chemotherapy were not significantly associated with the presence of LVI (Table 2).
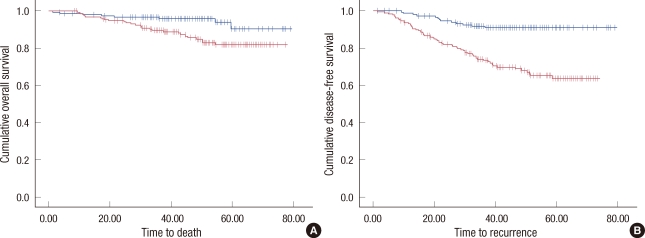
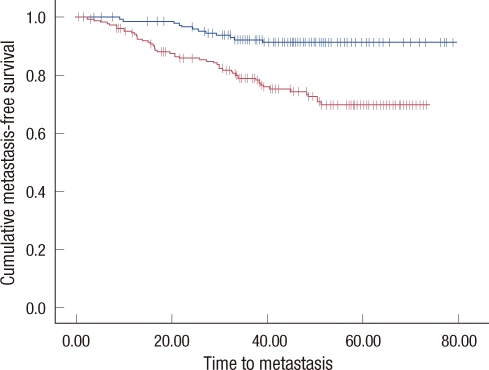
In a univariate survival analysis, both 5-year OS and 5 year DFS were significantly different in patients with or without LVI: 88.8% vs. 94.1% (p=0.007) for OS (Figure 1A) and 76.4% vs. 90.9% (p<0.001) for DFS (Figure 1B). Furthermore, 5-year MFS was shorter in patients with LVI: 80.1% vs. 91.5% than those without LVI (p<0.001) (Figure 2).
Figure 1.
Overall survival and disease-free survival of whole patients. (A) Overall survival curves are shown according to presence or absence of lymphovascular invasion (LVI). (B) Disease-free survival curves according to presence or absence of LVI are shown.
Figure 2.
Metastasis-free survival of whole patients. Metastasis-free survival curves are shown according to presence or absence of lymphovascular invasion (LVI).
In the univariate analysis, presence of LVI, tumor size (>20 mm), high histological grade (grades II and III), number of involved lymph nodes (≥4), hormone receptor status (negative ER and PR), and HER2 receptor status (positive) were associated with poor DFS. But age, tumor multiplicity, and p53 status did not influence DFS (Table 3).
Table 3.
Univariate analysis of disease-free survival and clinicopathological data
DFS=disease-free survival; LVI=lymphovascular invasion; NS=not significant; LN=lymph node; ER=estrogen receptor; PR=progesterone receptor.
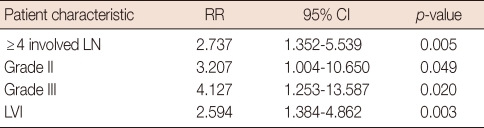
LVI was an independent prognostic factor for DFS in all patients (relative risk [RR], 2.59; 95% confidence interval [CI], 1.35-5.53; p=0.005). Furthermore, histological grade II (RR, 3.20; 95% CI, 1.00-10.65; p=0.049), histological grade III (RR, 4.12; 95% CI, 1.25-13.57; p=0.02), and a high number of involved lymph nodes (≥4; RR, 2.73; 95% CI, 1.35-5.53; p=0.003) were independent predictors in all patients. However, age, tumor size, hormone receptor status, and HER2 status were not significant independent factors (Table 4).
Table 4.
Disease-free survival: multivariate analysis in the whole group of patients
RR=relative risk; CI=confidence interval; LN=lymph node; LVI=lymphovascular invasion.
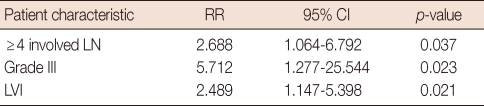
In the hormone receptor-positive group (n=218), positive LVI (p=0.021), number of involved lymph nodes (p=0.037) and histological grade III (p=0.023) were also independent factors for a poor prognosis (Table 5).
Table 5.
Disease-free survival: multivariate analysis in the hormone receptor-positive group of patients
RR=relative risk; CI=confidence interval; LN=lymph node; LVI=lymphovascular invasion.
DISCUSSION
Previous well-designed studies have analyzed the prognostic factors in patients with invasive breast cancer. Axillary lymph node involvement, younger age, high histological grade, large tumor size, and negative hormone receptor status were significantly associated with a poor prognosis (DFS and OS) [1]. Previous studies have also shown that LVI is an independent poor prognostic factor in patients with invasive breast cancer [2,3,14]. Nevertheless, although LVI is an important prognostic factor in patients with lymph node-negative invasive breast cancer, its prognostic value in patients with lymph node-positive breast cancer is unclear and controversial [3,9-11]. So, we report the analysis of the prognostic significance of LVI in a large cohort study of lymph node-positive patients with invasive breast cancer.
In our retrospective study, LVI was observed in 55% (n=192) of all patients (n=349), which was similar to other series of lymph node-positive cancer. LVI added prognostic information for patients with lymph node-positive breast cancer with a RR of 2.59 and a p-value of 0.003. These results contrast with those reported by Colleoni et al. [10] This discrepancy may be explained by the short-term follow-up period and analysis limitations in patients with 1 to 3 metastatic lymph nodes in their study.
In addition to LVI, our study demonstrated that high histological grade, number of lymph nodes involved, and both factors constituting the Nottingham Prognostic Index were other significant independent clinicopathological factors. Because LVI appears to be independent from these pathological factors, our results propose that LVI should be considered in the prognostic index for patients with lymph node-positive breast cancer as well as in the group of patients with lymph node-negative breast cancer [15]. However, tumor size and patient age were not significant independent prognostic factors in our study. This result may be explained by the shorter follow-up period compared to that in previous studies.
We also assessed whether LVI had an effect on the hormone receptor-positive subgroup of patients as an independent prognostic factor. This analysis was restricted to the large subgroup of 218 patients who were hormone receptor-positive. In this subgroup, with a RR of 2.48 and a p-value of 0.021, LVI was an significant prognostic factor in patients who were hormone receptor-positive. These results suggest that LVI and other pathological factors may be useful to determine the need for adjuvant treatment in some patients for whom adjuvant chemotherapy could be dangerous despite axillary lymph node involvement. Eventually this may be applicable to the elderly or fragile patients (patients with severe underlying disease) with other treatment options such as endocrine and trastuzumab therapy.
Our analysis had three limitations. First, our analysis had a short follow-up period, and it was retrospective in nature. Second, not all patients with HER2-positive breast cancer were treated with a trastuzumab-based regimen. Third, LVI was assessed by H&E staining, and the selective endothelial cell markers such as D2-40, CD34 were not used in the routine pathological evaluation, but use of such markers could potentially have improved the accuracy of detecting LVI [16].
In conclusion, this study emphasized the role of pathologic-almetastasis analysis in the prognostic evaluation of breast cancer, particularly those factors that cannot be subjected to molecular analyses, such as size, lymph node status, histological grade, and LVI. These pathological factors are useful to evaluate prognostic and predictive factors for more effective and convenient clinical decision-making tools during adjuvant treatment.
The presence of LVI was an independent significant prognostic factor in patients with lymph node-positive breast cancer as well as patients with lymph node-negative breast cancer. The existence of LVI alone cannot be used to decide to omit adjuvant chemotherapy in all patients with lymph-node positive breast cancer but, it may be considered in the adjuvant treatment decision in a specific subgroup of patients for whom chemotherapy is contraindicated. We suggest that patients with LVI-positive breast cancer require a shorter follow up and additional management.
Footnotes
All authors declare no conflicts of interest.
References
- 1.Clark GM. Prognostic and predictive factors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 489–514. [Google Scholar]
- 2.Woo CS, Silberman H, Nakamura SK, Ye W, Sposto R, Colburn W, et al. Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am J Surg. 2002;184:337–340. doi: 10.1016/s0002-9610(02)00950-9. [DOI] [PubMed] [Google Scholar]
- 3.Davis BW, Gelber R, Goldhirsch A, Hartmann WH, Hollaway L, Russell I, et al. Prognostic significance of peritumoral vessel invasion in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Hum Pathol. 1985;16:1212–1218. doi: 10.1016/s0046-8177(85)80033-2. [DOI] [PubMed] [Google Scholar]
- 4.Page DL, Anderson TJ, Connelly JL, Schnitt SF. Miscellaneous features of carcinoma. In: Page DL, Anderson TJ, editors. Diagnostic Histopathology of the Breast. Edinburgh: Churchill Livingstone; 1987. pp. 283–284. [Google Scholar]
- 5.Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Rosai J, Sobin LH, editors. Tumors of the Mammary Gland. Washington, DC: Armed Forces Institute of Pathology; 1993. [Google Scholar]
- 7.de Mascarel I, MacGrogan G, Debled M, Sierankowski G, Brouste V, Mathoulin-Pthoulin- S, et al. D2-40 in breast cancer: should we detect more vascular emboli? Mod Pathol. 2009;22:216–222. doi: 10.1038/modpathol.2008.151. [DOI] [PubMed] [Google Scholar]
- 8.de Mascarel I, Bonichon F, Durand M, Mauriac L, MacGrogan G, Soubeyran I, et al. Obvious peritumoral emboli: an elusive prognostic factor reappraised. Multivariate analysis of 1320 node-negative breast cancers. Eur J Cancer. 1998;34:58–65. doi: 10.1016/s0959-8049(97)00344-4. [DOI] [PubMed] [Google Scholar]
- 9.Yildirim E, Berberoglu U. Lymph node ratio is more valuable than level III involvement for prediction of outcome in node-positive breast carcinoma patients. World J Surg. 2007;31:276–289. doi: 10.1007/s00268-006-0487-5. [DOI] [PubMed] [Google Scholar]
- 10.Colleoni M, Rotmensz N, Maisonneuve P, Sonzogni A, Pruneri G, Casadio C, et al. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann Oncol. 2007;18:1632–1640. doi: 10.1093/annonc/mdm268. [DOI] [PubMed] [Google Scholar]
- 11.MacGrogan G, Desrousseaux M, de Mascarel I. Prognostic value of Mib1 in a tissue microarray of 855 invasive breast carcinomas; 5th European Breast Cancer Conference; 2006. p. Abstract #261. [Google Scholar]
- 12.Truong PT, Berthelet E, Lee J, Kader HA, Olivotto IA. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer. 2005;103:2006–2014. doi: 10.1002/cncr.20969. [DOI] [PubMed] [Google Scholar]
- 13.Rosen PP. Tumor emboli in intramammary lymphatics in breast carcinoma: pathologic criteria for diagnosis and clinical significance. Pathol Annu. 1983;18 Pt 2:215–232. [PubMed] [Google Scholar]
- 14.McCready DR, Chapman JA, Hanna WM, Kahn HJ, Murray D, Fish EB, et al. Factors affecting distant disease-free survival for primary invasive breast cancer: use of a log-normal survival model. Ann Surg Oncol. 2000;7:416–426. doi: 10.1007/s10434-000-0416-z. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31:1825–1833. doi: 10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Goodison S, Li J, Liu L, Farmerie W. Improved breast cancer prognosis through the combination of clinical and genetic markers. Bioinformatics. 2007;23:30–37. doi: 10.1093/bioinformatics/btl543. [DOI] [PMC free article] [PubMed] [Google Scholar]