Abstract
Purpose
This study evaluated the prognostic impact of the lymph node ratio (LNR; i.e., the ratio of positive to dissected lymph nodes) on recurrence and survival in breast cancer patients with positive axillary lymph nodes (LNs).
Methods
The study cohort was comprised of 330 breast cancer patients with positive axillary nodes who received postoperative radiotherapy between 1987 and 2004. Ten-year Kaplan-Meier locoregional failure, distant metastasis, disease-free survival (DFS) and disease-specific survival (DSS) rates were compared using Kaplan-Meier curves. The prognostic significance of the LNR was evaluated by multivariate analysis.
Results
Median follow-up was 7.5 years. By minimum p-value approach, 0.25 and 0.55 were the cutoff values of LNR at which most significant difference in DFS and DSS was observed. The DFS and DSS rates correlated significantly with tumor size, pN classification, LNR, histologic grade, lymphovascular invasion, the status of estrogen receptor and progesterone receptor. The LNR based classification yielded a statistically larger separation of the DFS curves than pN classification. In multivariate analysis, histologic grade and pN classification were significant prognostic factors for DFS and DSS. However, when the LNR was included as a covariate in the model, the LNR was highly significant (p<0.0001), and pN classification was not statistically significant (p>0.05).
Conclusion
The LNR predicts recurrence and survival more accurately than pN classification in our study. The pN classification and LNR should be considered together in risk estimates for axillary LNs positive breast cancer patients.
Keywords: Breast neoplasms, Lymph nodes, Prognosis
INTRODUCTION
The involvement of axillary lymph nodes (LNs) is the most important prognostic factor in operable primary breast cancer and is strongly associated with both disease-free and overall survival [1,2]. After curative breast surgery, the involvement of axillary nodes is examined to determine the use of adjuvant systemic therapy, which is strongly indicated in patients with axillary LN metastases. The absolute number of nodes involved is also considered when deciding on the use of radiotherapy according to current guidelines based on the tumor-node-metastasis system; for example, if a patient has a pT2N1M0 cancer, radiation therapy is frequently omitted after modified radical mastectomy (MRM), even in node positive breast cancer patients. The field of radiation therapy is also influenced by the number of involved nodes; where four or more axillary LNs are involved, the radiation field is extended to include the supraclavicular area. Hence, radiation oncologists have severe doubts as to whether the absolute number of positive nodes is a suitable criterion for assessing the axillary nodal status to guide therapeutic choices and predict the prognosis of breast cancer patients.
Lymph node status is assessed by axillary lymph node dissection (ALND) and, often in daily practice, the extent of axillary dissection varies according to the surgeon. There is also heterogeneity in node examination. The Comprehensive Cancer Center North-Netherlands (CCCN) reviewed 4,806 axillary dissections. The number of reported positive nodes varied significantly between pathology laboratories. Generally, a more extensive surgical axillary dissection or histopathologic examination of the specimen resulted in a higher number of positive nodes [3].
Several authors have noted this confusion and have suggested the use of a proportion or percentage of involved nodes [4]. A growing number of studies have found that a ratio-based classification of node involvement is a superior prognostic factor than the absolute numbers of involved nodes in breast cancer [4-11]. To evaluate this issue, we examined the impact of the number of positive nodes, the number of dissected nodes and the proportion of involved nodes among all dissected nodes, i.e., the lymph node ratio (LNR), on recurrence and survival in breast cancer after other known prognostic factors had been taken into account.
METHODS
Patient population and treatment methods
The patients in this study were identified from a database of patients who received postoperative radiotherapy in Yeouido St. Mary's Hospital between 1987 and 2004. Eligible patients were those with primary breast cancer with positive axillary LNs after adequate ALND. Three hundred thirty females were enrolled in this study. None of the patients had evidence of distant metastases at the time of diagnosis, and all underwent breast conserving surgery or MRM including at least level l-ll ALND. The adjuvant treatment was a combination of chemotherapy, hormonal therapy and radiotherapy. The adjuvant treatment was done after operation and none of patients received neoadjuvant chemotherapy. The external beam radiation therapy was done in all patients using photon or electron beams. The ipsilateral breast or chestwall was irradiated. When 4 or more positive axillary nodes were presented, supraclavicular area was also treated. The internal mammary area was treated when preoperative radiological imaging defined the involvement of internal mammary LN. The Institutional Review Board of Yeouido St. Mary's Hospital approved this retrospective study (The number of approval: SC10RESI0019).
Prognostic variables
In each patient, the following data were available from the medical records: age, menopausal status, type of operation, chemotherapeutic agents, hormonal and radiotherapy and pathology reports on the surgical specimen. The tumor factors analyzed were the histologic type, histologic grade, tumor size, the status of margin, lymphovascular invasion, the status of estrogen receptor (ER), progesterone receptor (PR) and c-erbB2 receptor. The LN factors analyzed were the number of positive axillary nodes, the number of dissected nodes and the LNR. The pathologic stage was classified according the 6th edition of the American Joint Committee on Cancer (AJCC) staging manual [12].
The treatment outcomes were also evaluated. Local recurrence means recurrence in the ipsilateral breast or chest wall. Regional recurrence means recurrence in ipsilateral axillary, supraclavicular, infraclavicular or internal mammary nodes. The survival end event was defined as death from breast cancer.
Statistical analyses
Univariate analyses of survival were performed by the method of Kaplan and Meier. The curves for locoregional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS) and disease-specific survival (DSS) were plotted. The significance of outcome differences was compared by the log-rank test.
After ascertaining that the LNR was significantly associated with DFS and DSS, various LNR cutoffs were evaluated, ranging from 0.05 to 0.95 at intervals of 0.05, by the minimum p-value approach. We selected cutoff points at which the most significant difference in DFS and DSS was observed. The number of dissected nodes was also evaluated in the same way to find the most significant cutoff value that correlated with recurrence and survival.
Multivariate analysis was performed using Cox proportional hazard modeling with or without LNR as a covariate. A p-value of less than 0.05 was considered significant. Analyses were performed with SAS software version 9.1 (SAS Institute Inc., Cary, USA).
RESULTS
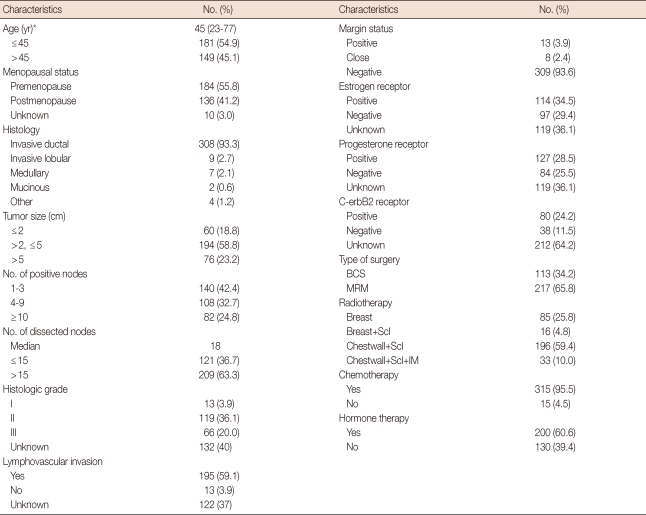
The median follow up was 7.5 years (range, 0.3-21.8 years). Table 1 summarizes the data on tumor and treatment characteristics of this study. The median age was 45 years (range, 23-77 years). The median number of nodes removed was 18 (range, 7-61 years). Most patients (95.5%) had at least 10 axillary LNs removed. The median number of involved nodes was 4 (range, 1-43). The median LNR was 0.28 (range, 0.03-1). Among 330 patients, adjuvant systemic therapy was completed in 328 patients (chemotherapy alone, 38.8%; hormone therapy alone, 3.9%; both chemotherapy and hormone therapy, 56.7%). The current treatment guideline consists of chemotherapy +/- trastuzumab +/- hormone therapy depending on hormoneand c-erbB2 receptor status. However, the status of c-erbB2 receptor was not routinely examined before 2000 in our institution and trastuzumab could not be considered for adjuvant therapy in this study period. All patients completed the course of radiotherapy. The median dose of radiation therapy was 50.4 Gy (range, 50.4-64.8 Gy).
Table 1.
Clinical and pathologic features of the patients
BCS=breast conserving surgery; MRM=modified radical mastectomy; Scl=supraclavicular area; IM=internal mammary.
*Median (range).
Recurrence and survival outcomes
Overall, 28 patients (8.5%) experienced local recurrence, and 8 patients (2.4%) experienced regional recurrence. The combined LRR rate was 10.9% (n=36 patients). DM occurred in 133 patients (40.3%). The corresponding 10-year Kaplan-Meier estimates (±standard error) were 89.2±1.7% for LRRFS and 62.3±2.7% for DMFS. The 10-year Kaplan-Meier DFS and DSS were 60.0±2.7% and 55.5±3.4%, respectively.
Cutoff values for LNR and dissected nodes
By the minimum p-value approach, 0.25 and 0.55 were selected as the most significant LNR levels correlating with DFS and DSS (p<0.0001). The patients were classified into three groups: patients with LNR≤0.25, LNR 0.26-0.55, and LNR>0.55, which represented 48.2%, 24.8%, and 27.0% of the study cohort, respectively. With respect to the numbers of dissected nodes, we could not find any value that correlated significantly with DFS and DSS in this study.
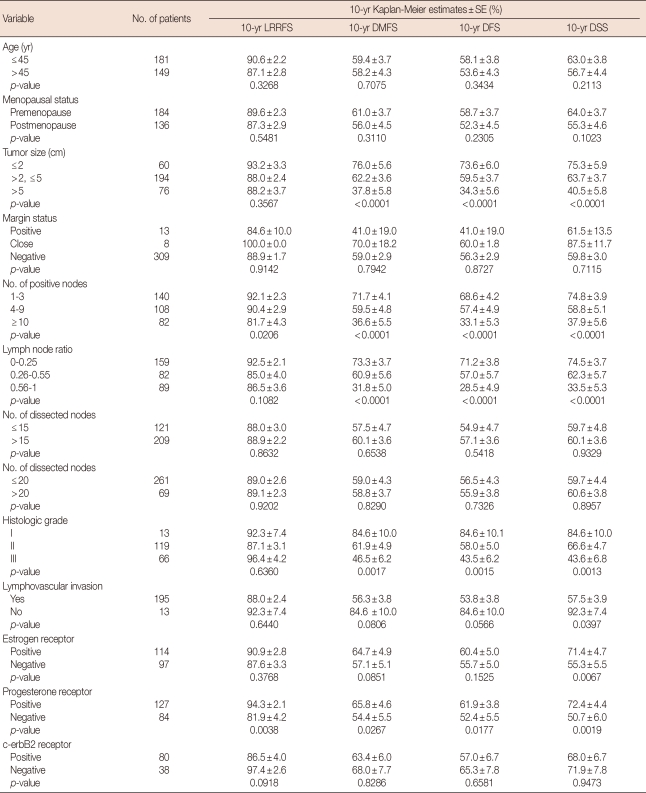
Univariate analysis
Table 2 presents the comparisons of 10-year Kaplan-Meier LRRFS, DMFS, DFS, and DSS stratified by various prognostic factors. The LNR based classification was tested to predict the treatment outcome and survival. The LRRFS decreased with higher numbers of positive nodes and PR-negative status. The status of margin was not correlated with the LRRFS. The DMFS decreased with larger tumor size, higher number of positive nodes, higher LNR, higher histologic grade and PR-negative status. Larger tumor size, higher number of positive nodes, higher LNR, higher histologic grade, presence of lymphovascular invasion and PR-negative status were associated with decreased DFS and DSS. ER-negative status was correlated with decreased DSS but not with DFS.
Table 2.
Ten-year Kaplan-Meier locoregional recurrence-free survival, distant metastasis-free survival, disease-free survival, disease-specific survival according to the prognostic factors
SE=standard error; LRRFS=locoregional recurrence-free survival; DMFS=distant metastasis-free survival; DFS=disease-free survival; DSS=disease-specific survival.
Analysis of survival outcome by pN classification vs. LNR
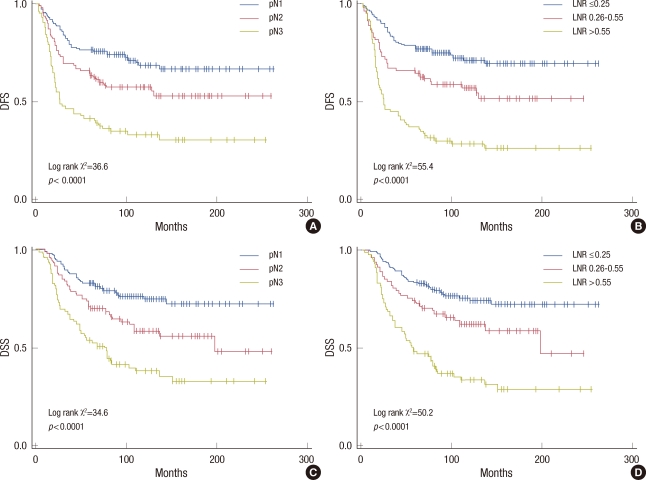
The univariate Kaplan-Meier DFS estimates were compared between the groups defined by pN staging (Figure 1A) or by LNR (Figure 1B). The groups categorized by LNR yielded a significantly larger separation of the DFS curves compared with pN staging. The log-rank χ2 associated with the LNR (p<0.0001, log-rank χ2=55.4) was larger than that of pN (p<0.0001, log-rank χ2=36.6), indicating a higher significance. Ten-year DFS was 71.2±3.8% among patients with LNR ≤0.25, compared with 57.0±5.7% and 28.5±4.9% for those with LNRs of 0.26-0.55 and >0.55, respectively (p<0.0001). The DSS estimates were also compared between the groups stratified by pN staging (Figure 1C) or by LNR (Figure 1D). The log-rank χ2 associated with the LNR (p<0.0001, log-rank χ2=50.2) was larger than that of pN (p<0.0001, log-rank χ2=34.6) and 10-year DSS was 74.5±3.7% among patients with LNR ≤0.25, compared with 62.3±5.7% and 33.5±5.3% for those with LNRs of 0.26-0.55 and >0.55, respectively (p<0.0001).
Figure 1.
Disease-free survival (DFS) and disease-specific survival (DSS) of all patients stratified by pN stage and lymph node ratio (LNR), respectively. The LNR based classification (B, D) yields a statistically larger separation of the curves (larger χ2) compared to absolute number of the nodes (A, C).
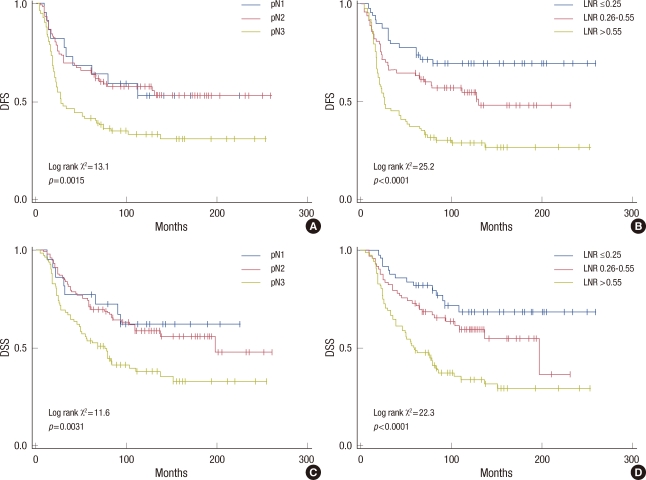
In addition, for stage III patients, the LNR based classification can distinguish subgroups more accurately than pN stage for DFS and DSS (Figure 2). The statistical power of the LNR based classification (p<0.0001, log-rank χ2=25.2) for DFS was larger than pN staging classification (p=0.0015, log-rank χ2=13.1). The pN classification graphically showed a poorer prognostic separation. The pN1 and pN2 survival curves are close to one another (Figure 2A), but the ratio-based curves are distinct from the early course of follow-up and remain separated, even at follow-up exceeding 20 years (Figure 2B). For DSS, similar findings were observed that the LNR based classification (p<0.0001, log-rank χ2=22.3) showed better stratification than the pN classification (p=0.0031, log-rank χ2=11.6).
Figure 2.
Disease-free survival (DFS) and disease-specific survival (DSS) of stage lll patients stratified by pN stage and lymph node ratio (LNR) based classification, respectively. The LNR based classification (B, D) can distinguish subgroups more clearly than pN stage (A, C).
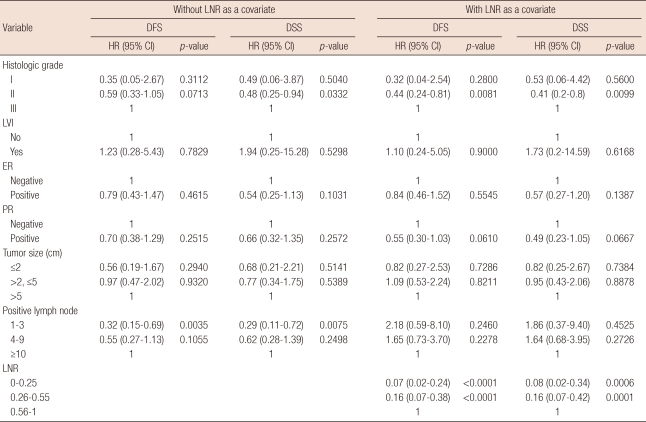
Multivariate analysis
Cox regression analysis was performed to evaluate whether the LNR was associated with DFS and DSS. The results of multivariate analyses are presented in Table 3. Histologic grade and the number of positive nodes were significant prognostic factors when the LNR was not included in the analysis. However, when the LNR was included in the model as a covariate, the LNR was highly significant (p<0.0001) and the number of positive nodes lost its significance (p>0.05). This means that, in this analysis, the LNR is a more significant prognostic factor than the absolute number of nodes.
Table 3.
Multivariate analysis of disease-free survival and disease-specific survival
LNR=lymph node ratio; DFS=disease-free survival; DSS=disease-specific survival; HR=hazard ratio; CI=confidence interval; LVI=lymphovascular invasion; ER=estrogen receptor; PR=progesterone receptor.
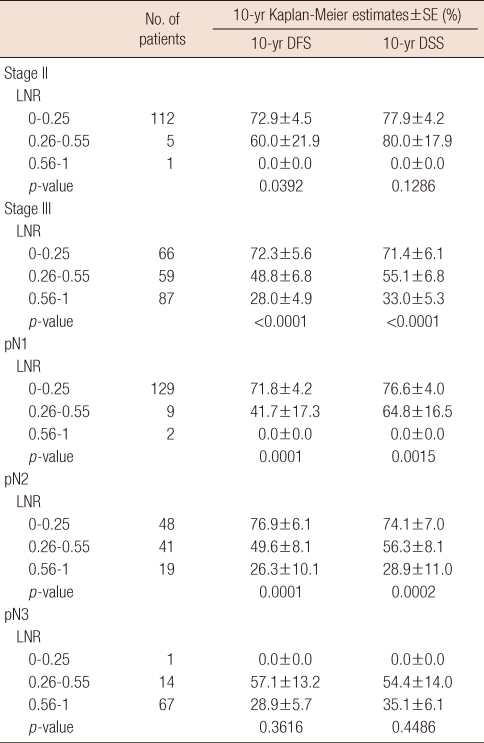
Survival analysis by LNR in each stage
To determine whether the LNR had a prognostic significance on survival in each stage, we performed the survival analysis based on the LNR in patients with stage II and stage III, respectively. The LNR had a prognostic impact on DFS in both patients with stage II (p=0.0392) and stage III (p<0.0001). For the patients with stage III, the DSS was also significantly correlated with the LNR (p<0.0001).
The survival outcome was analyzed by the LNR in each pN stage. The LNR had a prognostic significance on the DFS and DSS in both patients with pN1 and pN2. However, for the patients with pN3, the LNR was not associated with the survival. It might be attributed to that the group with pN3 and the LNR ≤0.25 consisted of only 1 patient. The results of above analyses were presented in Table 4.
Table 4.
Disease-free survival and disease-specific survival rate by lymph node ratio in each stage
SE=standard error; DFS=disease-free survival; DSS=disease-specific survival; LNR=lymph node ratio.
DISCUSSION
The lymphatic drainage of the breast moves primarily superiorly and laterally toward the axillary nodes. Lymphatic metastases to the axilla occur in an orderly fashion, with level I preceding level II, followed by level III. It is relatively uncommon to see a patient with level III node involvement in the absence of node involvement at lower levels [13]. Therefore, ALND is extended to level III nodes only if gross disease is apparent in the level I or II nodes.
To accurately stage the axilla, it is commonly recommended that ALND should clear the level I and II nodes with at least 10 nodes sampled. However, the recommendation to examine at least 10 nodes suffices only to determine nodal positivity or negativity. A mathematical model based on 1,446 complete axillary dissections has suggested that a minimum of 10 nodes needs to be removed to have a 93% predictive value that the remaining modes are clear [14].
There is a tendency for a higher number of dissected nodes to be associated with an increased chance of finding tumor positive nodes [3,7,8]. Kuru and Bozgul [15] reported that level IIII ALND to remove more than 20 nodes is needed for assessing the axillary nodal status by removal of more positive nodes. However, extensive ALND may result in pain, numbness, impairment of the range of motion of the shoulder or arm edema, all of which severely impair the patients' quality of life [16]. Thus, the extent of ALND has been more limited recently.
This limited extent of axillary dissection combined with radiation therapy was compared with the traditional full ALND. In the United Kingdom, axillary node sampling (ANS), which is widespread in clinical practice, needs at least four nodes removed from the axillary tail and the lower axillary fat to obtain 95% accuracy in staging the axilla. In the Edinburgh randomized trial, ANS showed a comparable axillary recurrence and overall survival with ALND up to level III. For node positive patients, the ANS group received postoperative radiotherapy. There was no significant difference in axillary recurrence between ALND and ANS followed by radiotherapy. This suggested that the involved axilla could be effectively treated by radiotherapy. Morbidity associated with full ALND was decreased in the ANS group. Modern simulation techniques and shielding of the shoulder joint and capsule are likely to reduce shoulder morbidity after radiotherapy to the axilla [17-19].
Since its recent introduction by Krag et al. [20], the more targeted procedure of sentinel lymph node biopsy (SLNB) has become a safe and acceptable technique in patients with T1-2 clinically node negative breast cancer. If a positive sentinel node (SN) is found, it is recommended to continue with ALND. Even in locally advanced breast cancer, the feasibility and accuracy of SLNB has been investigated after neoadjuvant chemotherapy.
Because the absolute number of dissected nodes is decreasing because of the trend to minimize the extent of ALND, physicians could be concerned about underestimating the real number of involved nodes. Thus, an appropriate method to assess the nodal status is needed to allow the choice of the correct adjuvant therapy and predict the treatment outcome.
From the Surveillance, Epidemiology, and End Results (SEER) population data, the importance of LNR has been shown for many cancer sites including the esophagus [21], colon [22], and corpus uteri [23]. In breast cancer there is growing evidence establishing the prognostic value of the LNR. Woodward et al. [5] conducted a systematic review of 24 reports published between 1994 and 2005 that implicate a prognostic role for the LNR, using both prospective and retrospectively collected data sets. The LNR was confirmed to be superior to the number of involved nodes as a prognostic indicator.
However, there is no clear consensus about the cutoff points that are required for a staging classification. The cutoff points to classify patients into two groups are 0.2 [7,10] or 0.25 [8], which were most significant for recurrence or overall survival. Several authors divided patients into three groups using cutoffs such as 0.33/0.67 [11], 0.1/0.5 [4] or 0.25/0.5 [9]. The process for deciding such cutoff points was not described in their articles. Vinh-Hung et al. [6] identified 1,829 node-positive breast cancer patients from the Geneva Cancer Registry. They identified the cutoff points of 0.2 and 0.65, which were validated by a bootstrap procedure. They suggested that the LNR should be considered as an alternative to pN staging because of the stronger statistical power to predict breast cancer-specific survival as well as considering that there is, in practice, wide variation in axillary dissection and node examination in heterogeneous patient populations.
Ratio-based prediction is an emerging issue even in the field of SLNB. A recent Australian study highlights the predictive role of the involved SN ratio that has the largest effect on the odds of finding further axillary nodal involvement [24]. Barranger et al. [25] reported that the ratio of involved SN, primary tumor size and the size of the SN metastasis were independently predictive of non-SN involvement. Using the concept of the SN ratio, the predictors of tumor involvement in the remaining axillary nodes in SN positive breast cancers are being widely investigated. It is expected that more studies will demonstrate the prognostic impact of the SN ratio in the case of SLNB for early stage breast cancer.
Accurate staging information is very important for both patients and clinicians to assess the prognosis and to make informed decisions about breast cancer treatment. Our results are consistent with the findings of recent studies evaluating the relationship between LNR and survival for node positive breast cancer [4-10]. This study may be differentiated from other studies due to the subgroup analyses in each TNM stage and pN stage to evaluate the impact of the LNR. However, the present study has several shortcomings. First, the patient population was identified from the database of the department of radiation oncology and a few patients who had not received postoperative radiotherapy after MRM could not be included to the analysis. Second, it was a retrospective study that the surgical procedures and the methods of pathological examination could not be unified as in the prospective studies. Systematic LNR analysis from multi-institutional randomized patient data with validation in similar independent data sets is needed to define clearly the utility of LNR.
This study supports the hypothesis that, in patients with invasive breast cancer with positive axillary nodes, the ratio between the number of positive nodes and total numbers of dissected nodes is the most powerful predictor of the risk of recurrence and survival. The LNR showed a stronger association with DFS and DSS than did pN staging. The calculation of the LNR is easy and assists in the choice of adjuvant treatment and evaluation of the prognosis in the clinical setting. A future staging system should incorporate the LNR for accurate staging of axillary nodes to overcome the confusion arising from the number of dissected nodes in this era of minimally invasive axillary surgery.
Footnotes
All authors declare no conflicts of interest.
References
- 1.Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17:2334–2340. doi: 10.1200/JCO.1999.17.8.2334. [DOI] [PubMed] [Google Scholar]
- 2.Wilking N, Rutqvist LE, Carstensen J, Mattsson A, Skoog L Stockholm Breast Cancer Study Group. Prognostic significance of axillary nodal status in primary breast cancer in relation to the number of resected nodes. Acta Oncol. 1992;31:29–35. doi: 10.3109/02841869209088261. [DOI] [PubMed] [Google Scholar]
- 3.Schaapveld M, Otter R, de Vries EG, Fidler V, Grond JA, van der Graaf WT, et al. Variability in axillary lymph node dissection for breast cancer. J Surg Oncol. 2004;87:4–12. doi: 10.1002/jso.20061. [DOI] [PubMed] [Google Scholar]
- 4.Voordeckers M, Vinh-Hung V, Van de Steene J, Lamote J, Storme G. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol. 2004;70:225–230. doi: 10.1016/j.radonc.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–2916. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 6.Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- 7.van der Wal BC, Butzelaar RM, van der Meij S, Boermeester MA. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol. 2002;28:481–489. doi: 10.1053/ejso.2002.1239. [DOI] [PubMed] [Google Scholar]
- 8.Kuru B. Prognostic significance of total number of nodes removed, negative nodes removed, and ratio of positive nodes to removed nodes in node positive breast carcinoma. Eur J Surg Oncol. 2006;32:1082–1088. doi: 10.1016/j.ejso.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Lale Atahan I, Yildiz F, Ozyigit G, Sari S, Gurkaynak M, Selek U, et al. Percent positive axillary lymph node metastasis predicts survival in patients with non-metastatic breast cancer. Acta Oncol. 2008;47:232–238. doi: 10.1080/02841860701678761. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Lim HI, Lee SK, Choi JH, Kim WW, Choe JH, et al. The impact of the ratio of positive nodes to removed nodes on recurrence and overall survival in node positive breast cancer patients. J Breast Cancer. 2008;11:194–200. [Google Scholar]
- 11.Grills IS, Kestin LL, Goldstein N, Mitchell C, Martinez A, Ingold J, et al. Risk factors for regional nodal failure after breast-conserving therapy: regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys. 2003;56:658–670. doi: 10.1016/s0360-3016(03)00017-8. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. pp. 223–236. [Google Scholar]
- 13.Veronesi U, Rilke F, Luini A, Sacchini V, Galimberti V, Campa T, et al. Distribution of axillary node metastases by level of invasion. An analysis of 539 cases. Cancer. 1987;59:682–687. doi: 10.1002/1097-0142(19870215)59:4<682::aid-cncr2820590403>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Kiricuta CI, Tausch J. A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer. 1992;69:2496–2501. doi: 10.1002/1097-0142(19920515)69:10<2496::aid-cncr2820691018>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Kuru B, Bozgul M. The impact of axillary lymph nodes removed in staging of node-positive breast carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:1328–1334. doi: 10.1016/j.ijrobp.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol. 1999;17:143–149. doi: 10.1200/JCO.1999.17.1.143. [DOI] [PubMed] [Google Scholar]
- 17.Forrest AP, Everington D, McDonald CC, Steele RJ, Chetty U, Stewart HJ. The Edinburgh randomized trial of axillary sampling or clearance after mastectomy. Br J Surg. 1995;82:1504–1508. doi: 10.1002/bjs.1800821118. [DOI] [PubMed] [Google Scholar]
- 18.Lambah A, Dixon JM, Prescott RJ, Jack W, Forrest AP, Rodger A, et al. Randomised study of axillary clearance versus four node sampling. Eur J Cancer. 2001;37(Suppl 5):2. [Google Scholar]
- 19.Chetty U, Jack W, Prescott RJ, Tyler C, Rodger A. Management of the axilla in operable breast cancer treated by breast conservation: a randomized clinical trial. Edinburgh Breast Unit. Br J Surg. 2000;87:163–169. doi: 10.1046/j.1365-2168.2000.01345.x. [DOI] [PubMed] [Google Scholar]
- 20.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–339. doi: 10.1016/0960-7404(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 21.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg. 2008;206:239–246. doi: 10.1016/j.jamcollsurg.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 22.De Ridder M, Vinh-Hung V, Van Nieuwenhove Y, Hoorens A, Sermeus A, Storme G. Prognostic value of the lymph node ratio in node positive colon cancer. Gut. 2006;55:1681. doi: 10.1136/gut.2006.104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JK, Kapp DS, Cheung MK, Osann K, Shin JY, Cohn D, et al. The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients. Br J Cancer. 2007;97:605–611. doi: 10.1038/sj.bjc.6603898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farshid G, Pradhan M, Kollias J, Gill PG. A decision aid for predicting non-sentinel node involvement in women with breast cancer and at least one positive sentinel node. Breast. 2004;13:494–501. doi: 10.1016/j.breast.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91:113–119. doi: 10.1007/s10549-004-5781-z. [DOI] [PubMed] [Google Scholar]