Abstract
Background
The role of preoperative gabapentin in postoperative pain management is not clear, particularly in patients receiving regional blockade. Patients undergoing thoracotomy benefit from epidural analgesia but still may experience significant postoperative pain. We examined the effect of preoperative gabapentin in thoracotomy patients.
Methods
Adults undergoing elective thoracotomy were enrolled in this prospective, randomized, double-blinded, placebo-controlled study, and randomly assigned to receive 600 mg gabapentin or active placebo (12.5 mg diphenhydramine) orally within 2 hours preoperatively. Standardized management included thoracic epidural infusion, intravenous patient-controlled opioid analgesia, acetaminophen and ketorolac. Pain scores, opioid use and side effects were recorded for 48 hours. Pain was also assessed at 3 months.
Results
One hundred twenty patients (63 placebo and 57 gabapentin) were studied. Pain scores did not significantly differ at any time point (p=0.53). Parenteral and oral opioid consumption was not significantly different between groups on postoperative day 1 or 2 (p>0.05 in both cases). The frequency of side effects such as nausea and vomiting or respiratory depression was not significantly different between groups, but gabapentin was associated with decreased frequency of pruritus requiring nalbuphine (14% gabapentin vs. 43% control group, p<0.001). The frequency of patients experiencing pain at 3 months post-thoracotomy was also comparable between groups (70% gabapentin vs. 66% placebo group, p=0.72).
Conclusions
A single preoperative oral dose of gabapentin (600 mg) did not reduce pain scores or opioid consumption following elective thoracotomy, and did not confer any analgesic benefit in the setting of effective multimodal analgesia that included thoracic epidural infusion.
Keywords: Pain, Postoperative, post-thoracotomy pain, Preanesthetic Medication, Acute Pain Service, Patient-Controlled Epidural Analgesia, gabapentin
Introduction
Patients undergoing thoracotomy may experience significant postoperative pain.1 Previous studies provide substantial support for the use of thoracic epidural infusion of local anesthetics and/or opioids.2, 3 Management by an inpatient pain service also increases the quality of perioperative analgesia in thoracotomy patients.4, 5 Unfortunately, severe pain, particularly with cough or movement, is still common.2
Adjuvant drugs such as gabapentin may be beneficial in postoperative pain management. Recent literature demonstrates that preoperative administration of a single dose of gabapentin (600–1800 mg) decreases postoperative pain scores and opioid consumption.6, 7 However, it is not clear whether preoperative gabapentin is beneficial in older patients or in conjunction with regional blockade.8, 9 To date, a single, small (n=51) study evaluated the effect of 1200 mg gabapentin on post-thoracotomy shoulder pain in patients receiving thoracic epidural infusions of bupivacaine and fentanyl.9 Gabapentin demonstrated no analgesic benefit or opioid-sparing effect. However, several aspects of the study design limit the generalizability of its results. Acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDS) were not utilized, although both have demonstrated benefit in decreasing post-thoracotomy shoulder pain.10–12 Importantly, sedation scores at 4 hours postoperatively were increased in the gabapentin group, and a 74 year-old patient in the gabapentin group experienced postoperative respiratory arrest that required tracheal intubation and ventilation. Indeed, typical side effects of gabapentin (e.g., sedation and dizziness) might be more pronounced in older thoracotomy patients and confounded by fluid restriction and/or epidural infusion-related hypotension. Clearly, the unique characteristics of the thoracotomy population dictate a careful study design when evaluating sedating medications such as gabapentin, and also call for adequately-powered, multimodal studies that utilize the full range of medications with proven benefit including acetaminophen and/or NSAIDS.
We hypothesized that preoperative administration of gabapentin would decrease the severity and incidence of overall acute postoperative pain in patients undergoing thoracotomy with epidural analgesia and a multimodal analgesic regimen managed by an inpatient pain service that includes oral acetaminophen, intravenous ketorolac, and patient-controlled intravenous fentanyl. Our hypothesis was that a single preoperative dose of oral gabapentin (600 mg) would decrease postoperative pain scores at rest (primary outcome) and with coughing for the first 48 hours post-thoracotomy compared with active placebo. Secondary outcome analyses included postoperative opioid consumption, side effects and the frequency of post-thoracotomy pain syndrome at three months.
Methods
This randomized, placebo-controlled, double-blinded study was approved by the Institutional Review Board and registered in ClinicalTrials.gov (NCT00588159). Patients were recruited from the Mayo Clinic, in Rochester, Minnesota, and written informed consent was obtained at their preoperative visit. Inclusion criteria were patients aged 45–75 years undergoing elective thoracotomy. Exclusion criteria included planned chest wall resection (unplanned chest wall resections were included because these study patients had already been randomized), cardiovascular surgery, and/or gastroesophageal surgery; current enrollment in another post-thoracotomy analgesic research protocol; pre-existing pain syndromes; daily opioid therapy in excess of 20 mg of oral morphine equivalents; current gabapentin or pregabalin therapy; allergy to any study medication; coagulation or infectious issues that would preclude epidural catheter placement; severe psychological disorders or inability to understand the study protocol; prisoners or other institutionalized individuals; and severe hepatic, renal or cardiovascular disorders. Women who could become pregnant (premenopausal or lacking surgical sterilization) were not included in this study.
A randomized, double-blinded, placebo-controlled study design was employed. Operations were performed by any of 6 board certified general thoracic surgeons at our institution. Using a block randomization schedule stratified by surgeon (size =2), patients were allocated to receive either 600 mg of gabapentin or active placebo (diphenhydramine 12.5 mg) orally within 2 hours preoperatively. Active placebo was utilized to assist patient blinding, as a mild amount of sedation is consistent with the expected acute effects of 600 mg oral gabapentin. The placebo or the gabapentin treatment was provided in an identical fashion. Placebo capsules of identical shape and size to commercially available gabapentin were manufactured by the hospital pharmacy. All members of the surgical, nursing, and acute pain service teams were blinded to group assignment. Preoperatively, a thoracic epidural catheter at the T4–8 level was placed using standard technique. An epidural infusion of 0.075% bupivacaine and 10 mcg/mL hydromorphone was delivered at 6 mL/hr. General anesthesia was based on inhaled agents after intravenous induction at the attending anesthesiologist’s discretion. Intravenous (IV) opioids were administered according to a standardized protocol.
In the post-anesthesia care unit (PACU), the Richmond Agitation Sedation Scale (RASS)13 score was obtained if the numerical rating pain score (NRS; 0–10) could not be collected. IV fentanyl was given for NRS ≥ 4 as needed, 25 mcg IV every 2 minutes up to a maximum 200 mcg in the PACU. If additional analgesia was required, 15 mg of IV ketorolac was given once for NRS ≥ 4.
During the first 48 hours post-operatively, acetaminophen (650 mg orally every 6 hours) or IV ketorolac (15 mg every 6 hours) was administered depending on pain severity. An IV fentanyl patient-controlled analgesic (PCA) was initiated for rescue (NRS ≥ 4). Adjustments to the epidural infusion (mixture and rate) were performed by the inpatient pain service team depending on analgesic effect and side effect profile, according to a standardized protocol. The epidural infusion could be temporarily stopped due to hypotension, excessive sedation, or hypoventilation, and then restarted at 4 ml/hr. For moderate or severe pain, the epidural infusion could be increased to 8 ml/hr. Initial IV PCA fentanyl settings were 10 mcg every 10 minutes with a 200 mcg 4 hour lockout. If a patient remained in moderate or severe pain despite utilizing 4 or more PCA boluses of 10 mcg fentanyl in one hour, the PCA settings could be increased to 20 mcg IV every 10 minutes with a 400 mcg four hour lockout, and subsequently to 25 mcg every 10 minutes with a 600 mcg 4 hour lockout and a 25 mcg/hr infusion if necessary. At any time, the patient, inpatient pain service or other members of the attending staff could withdraw a patient from the study protocol.
For the first 48 hours following surgery, nursing staff collected NRS pain scores every 4 hours at rest (primary outcome). The inpatient pain service team members collected standardized data on each postoperative morning regarding pain scores at rest and with coughing (using the NRS scale), side effects such as nausea or vomiting, pruritus, sedation, dizziness, respiratory depression, and medication use. Nausea or vomiting was queried by asking, “Do you have feelings of nausea or vomiting at this time?” and classified using the following grading scale: mild: no antiemetic medications needed or given; moderate: antiemetic medications needed and/or given; and severe: antiemetic medications given but not beneficial. Pruritus was queried by asking, “Do you itch?” and the pruritus grading scale was the same as that of nausea and vomiting except for utilizing antipruritic medications instead of antiemetic medications. Sedation was graded by nursing staff every 4 hours using the following sedation scale: 0=awake; 1=drowsy, dozes intermittently, easily arousable; 2=sedated, sleeps, but can be aroused; 3=difficult to arouse. Dizziness was queried by asking, “Do you feel dizzy at this time?” Based on current practice at our institution, postoperative day 0 (POD0) was defined as the day of surgery, postoperative day 1 (POD1) as the first day after surgery, and postoperative day 2 (POD2) as the second day following the day of surgery. A study coordinator contacted the patient 3 months postoperatively by mail, or, if no response, by telephone, and asked whether they had experienced any surgical site pain in the last week. All outcome assessors were blinded to group allocation.
Statistical analysis
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Mayo Foundation.14 Demographic, intraoperative, postoperative, and three-month follow-up data from the gabapentin treatment and placebo control groups were summarized and compared between groups. The primary outcome of the study was NRS pain scores at rest in the first 48 hours post-thoracotomy. To account for the repeated assessments, NRS pain scores were compared between groups using generalized estimating equations (GEE). To supplement the repeated measures analysis of overall pain scores, the assessment of pain with coughing on the mornings of POD1 and POD2 was compared using the two-sample t-test. Secondary analyses compared groups with respect to total oral and IV opioid equivalent use15 and ketorolac use for 0–24 hours and 24–48 hours. Additional secondary endpoints included pain scores at three-month follow-up. For all secondary and exploratory analyses, continuous variables were compared between groups using the two-sample t-test (or rank sum test) and categorical variables were compared using the chi-square test or Fisher’s exact test. All analyses were performed using SAS version 9.1 (SAS Institute Inc.; Cary, NC). The level of statistical significance for all tests was p<0.05.
Power Calculation
Based on preliminary data from a retrospective review of 56 thoracotomy patients’ records, the mean pain rating at rest was 3.3 and the standard deviation was 2.2 units. Using a standard deviation of postoperative pain scores of 2.0 units, we determined that an effective sample-size of N=120 (60 per group) would be required for the current study to provide statistical power (two-tailed, α=0.05) of 80% to detect a mean difference between groups of 1.0 on the 11 point (0–10) NRS.
Results
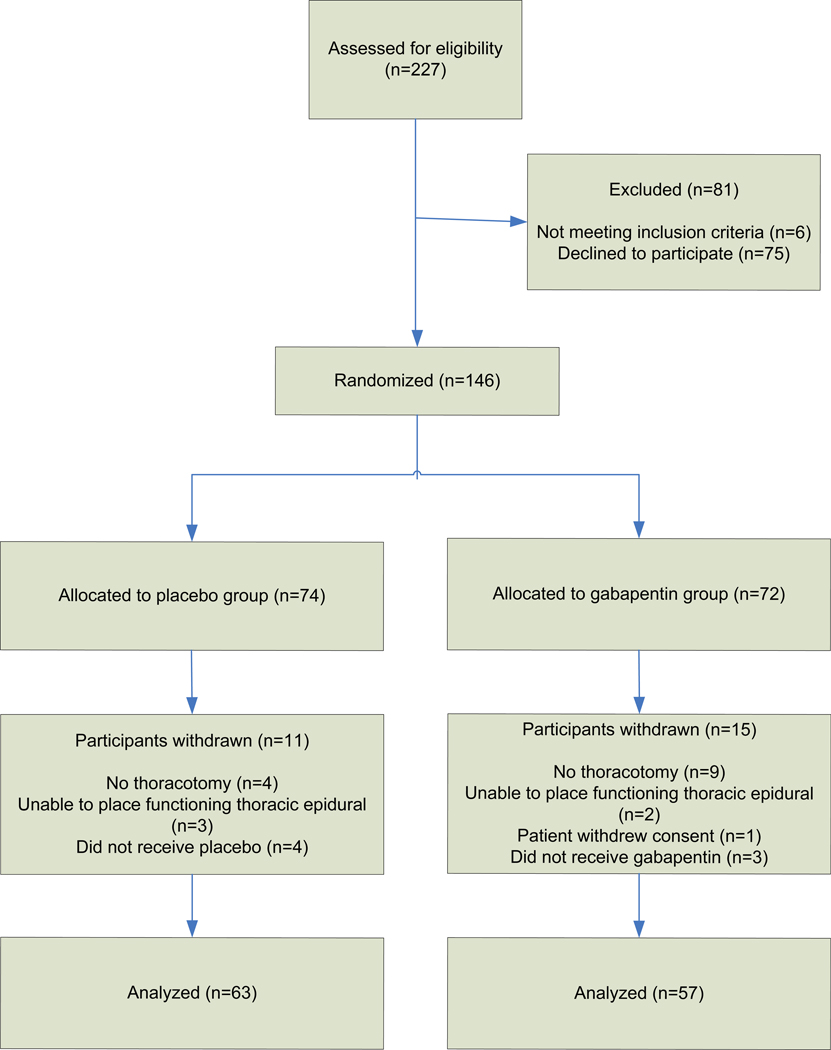
Two hundred twenty-seven patients were evaluated for enrollment in the study (Figure 1, CONSORT [Consolidated Standards of Reporting Trials]16 flow diagram). Six patients did not meet inclusion criteria, and 75 declined to participate. The remaining 146 patients were randomized on the day of surgery. After randomization, 26 patients were subsequently excluded. One patient withdrew consent before taking the study medication, 7 patients were excluded because the study medication was not administered due to logistical constraints (4 in the control group, 3 in the gabapentin group), 13 patients were excluded because they did not undergo thoracotomy (4 in the control group, 9 in the gabapentin group), and 5 patients were excluded because they did not receive a functioning thoracic epidural (3 in the control group, 2 in the gabapentin group). The decision to exclude subjects from the analysis was made without knowledge of treatment assignment. There were no differences in patient exclusion characteristics between the control and gabapentin groups. Thus, data were analyzed for 63 control group patients and 57 gabapentin group patients.
Figure 1.
CONSORT16 diagram
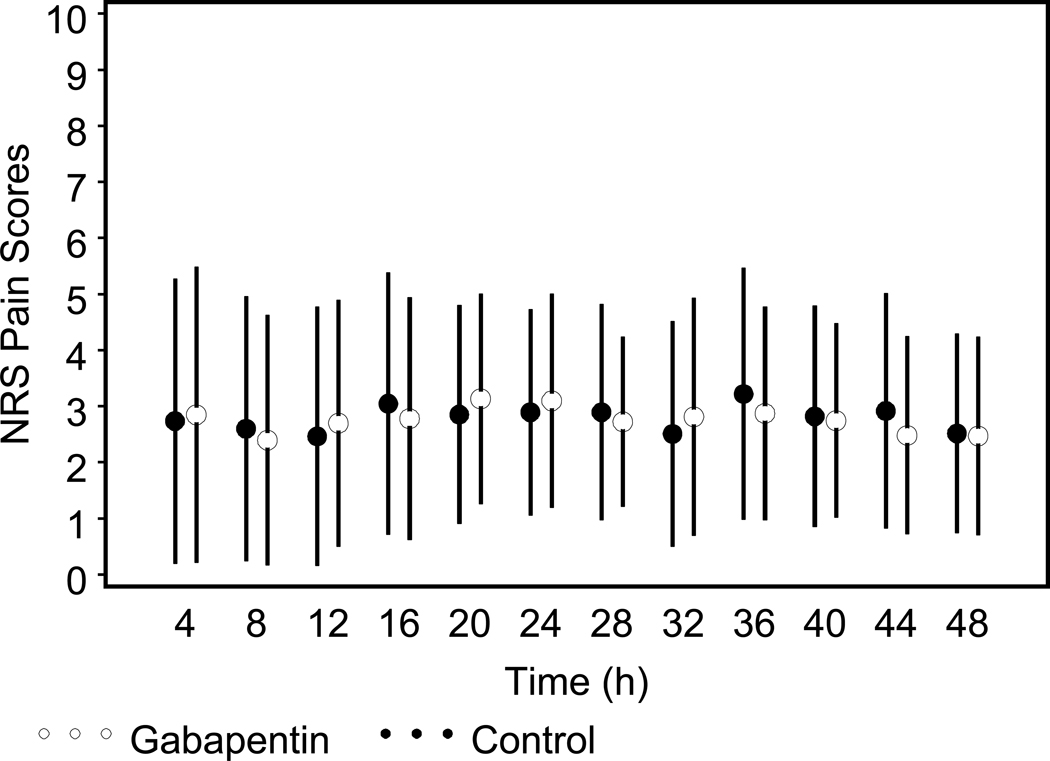
Baseline patient and surgical characteristics did not differ across groups (Tables 1 and 2). Postoperative pain scores over the first 48 hours were low and did not differ significantly between treatment groups (p=0.98 from repeated measures GEE analysis). On postoperative day 1 (POD1), mean NRS pain score (0–10) at rest was 2.9 ± 1.8 in the control vs. 3.1 ± 1.9 in the gabapentin group (p=0.53) (Figure 2). On POD2, mean NRS pain score at rest was 2.5 ± 1.8 in the control vs. 2.5 ± 1.8 in the gabapentin group (p=0.92). On POD1, mean pain scores with coughing were 5.2 ± 2.9 in the gabapentin group vs. 5.0 ± 2.6 in the control group (p=0.74). On POD2, mean pain scores with coughing were 5.0 ± 2.2 vs. 5.1 ± 2.5 in the gabapentin and control groups, respectively (p=0.78). There were no significant differences between surgeons regarding pain scores at rest or with coughing.
Table 1.
Patient Characteristics
| Characteristic | Gabapentin (N=57) |
Control (N=63) |
||
|---|---|---|---|---|
| Age (years, mean ± SD) | 64.4 | ± 7.4 | 64.3 | ± 6.8 |
| BMI (kg/m2, mean ± SD) | 28.1 | ± 4.8 | 28.4 | ± 4.3 |
| Gender | ||||
| . Male (n, %) | 32 | (56%) | 30 | (48%) |
| . Female (n, %) | 25 | (44%) | 33 | (52%) |
| ASA Physical Status (n, %) | ||||
| . 1 | 0 | (0%) | 0 | (0%) |
| . 2 | 24 | (42%) | 22 | (35%) |
| . 3 | 33 | (58%) | 41 | (65%) |
| . 4 | 0 | (0%) | 0 | (0%) |
Table 2.
Procedural Characteristics
| Characteristic | Gabapentin (N=57) |
Control (N=63) |
||
|---|---|---|---|---|
| Type of surgery (n, %) | ||||
| . Lobectomy | 32 | (56%) | 37 | (59%) |
| . Bilobectomy | 4 | (7%) | 2 | (3%) |
| . Wedge resection | 8 | (14%) | 16 | (25%) |
| . Segmentectomy | 4 | (7%) | 3 | (5%) |
| . Pneumonectomy | 2 | (4%) | 3 | (5%) |
| . Other | 7 | (12%) | 2 | (3%) |
| Chest wall resection (n, %) | ||||
| . No | 56 | (98%) | 60 | (95%) |
| . Yes | 1 | (2%) | 3 | (5%) |
| Duration of anesthesia (hours, mean ± SD) | 4.6 | ±1.4 | 4.6 | ±1.3 |
Figure 2.
Mean (± 1 SD) pain scores over the first 48 hours postoperatively for gabapentin and control groups.
Analgesic use did not differ between groups at any time point after surgery up to POD3 (Table 3). On POD1, 109 (91%) patients had an epidural infusion of 0.075% bupivacaine and 10 mcg/mL hydromorphone and 6 (5%) patients had an epidural infusion of 10 mcg/mL hydromorphone, with no difference between groups (p=0.56). On POD2, 101 (84%) patients had an epidural infusion of bupivacaine and hydromorphone and 10 (8%) patients had an epidural infusion of hydromorphone only, again with no difference between groups (p=0.15). Epidural infusion rates on POD1 were not significantly different between groups (mean 5.2 ± 2.3 mL/hr for the gabapentin group vs. 6.0 ± 1.5 mL/hr for control, p=0.08). On POD2, epidural infusion rates were significantly lower in the gabapentin group compared to control (mean, 4.9 ± 2.8 mL/hr for gabapentin vs. 6.0 ± 1.9 mL/hr for control, p=0.02). The average duration that epidural catheters remained indwelling was not significantly different between groups (60 ± 20 hours vs. 62 ± 13 hours in the gabapentin and control groups; respectively; p=0.54).
Table 3.
Analgesic Use
| Characteristic | Gabapentin (N=57) |
Control (N=63) |
P-value* | ||
|---|---|---|---|---|---|
| OR opioid equivalent ** | 0.144 | ||||
| mean ± SD | 91.8 | ± 33.5 | 83.5 | ± 29.1 | |
| median, Q1–Q3 | 82.5 | 75–105 | 82.5 | 68–98 | |
| PACU opioid equivalent ** | 0.265 | ||||
| mean ± SD | 12.9 | ± 19.0 | 19.0 | ± 24.9 | |
| median, Q1–Q3 | 0.0 | 0–28 | 10.5 | 0–30 | |
| PACU ketorolac (mg) | 0.970 | ||||
| mean ± SD | 8.3 | ± 7.5 | 8.5 | ± 8.0 | |
| median, Q1–Q3 | 15.0 | 0–15 | 15.0 | 0–15 | |
| Day 1 opioid equivalent ** | 0.340 | ||||
| mean ± SD | 111.9 | ± 116.9 | 118.1 | ± 94.7 | |
| median, Q1–Q3 | 70.5 | 30–158 | 99.6 | 32–183 | |
| Day 1 ketorolac (mg) | 0.365 | ||||
| mean ± SD | 10.0 | ± 15.4 | 11.2 | ± 13.7 | |
| median, Q1–Q3 | 0.0 | 0–15 | 0.0 | 0–15 | |
| Day 2 opioid equivalent ** | 0.735 | ||||
| mean ± SD | 114.4 | ± 106.8 | 121.6 | ± 110.3 | |
| median, Q1–Q3 | 90 | 44–181 | 99 | 33–164 | |
| Day 2 ketorolac (mg) | 0.243 | ||||
| mean ± SD | 15.0 | ± 17.7 | 11.7 | ± 16.5 | |
| median, Q1–Q3 | 15.0 | 0–30 | 0.0 | 0–15 | |
P-values are from rank-sum tests.
OR, operating room; PACU, postanesthesia care unit.
Opioid equivalents were calculated as oral morphine equivalents (mg).15
Duration of stay in the PACU did not differ significantly between groups, but a trend was noted toward longer PACU stays in the gabapentin group (2.3 ± 1.0 hours) compared to controls (2.0 ± 0.8 hours, p=0.054). Naloxone administration for respiratory depression or excessive sedation did not differ significantly between groups (2 [3.5%] patients in the gabapentin group received naloxone in the PACU vs. no patients in the control group; Fisher’s exact test: p=0.22). No patient received naloxone after PACU discharge.
The frequency of adverse side effects was not different between groups, with the exception of pruritus requiring treatment with nalbuphine (14% gabapentin group vs. 43% control group, p<0.001; Table 4). There was no significant difference in nausea, vomiting, or use of antiemetics on POD1 or POD2 between groups. On POD1, 25% of patients in the gabapentin group had nausea or vomiting compared with 18% of control patients. On POD2, 20% of gabapentin patients had nausea or vomiting vs. 13% of controls. Accordingly, there was no significant difference in antiemetic administration (ondansetron or promethazine) between groups. There was no significant difference in sedation on POD1 or POD2; mild sedation was noted in 18% of gabapentin patients vs. 15% of controls on POD1, and in 10% of gabapentin patients vs. 11% of controls on POD2. Dizziness did not differ significantly between groups on POD1 (16% in both groups), or POD2 (16% of gabapentin group vs. 11% of control group).
Table 4.
Additional Side Effects
| Characteristic | Gabapentin (N=57) |
Control (N=63) |
P-value* | ||
|---|---|---|---|---|---|
| Admission to intensive care unit (n, %) | 0.85 | ||||
| . No | 53 | (93%) | 58 | (92%) | |
| . Yes | 4 | (7%) | 5 | (8%) | |
| Respiratory depression (n, %) | |||||
| . No | 57 | (100%) | 63 | (100%) | |
| . Yes | 0 | (0%) | 0 | (0%) | |
| Nalbuphine use in first 48 hours (n, %) | <0.001 | ||||
| . No | 49 | (86%) | 36 | (57%) | |
| . Yes | 8 | (14%) | 27 | (43%) | |
| Antiemetics in first 48 hours (n, %) | 0.97 | ||||
| . No | 46 | (81%) | 51 | (81%) | |
| . Yes | 11 | (19%) | 12 | (19%) | |
P-values are from chi-square tests.
The frequency of patients experiencing pain at 3 months post-thoracotomy did not differ significantly between groups (70% in the gabapentin vs. 66% in the control group, P=0.72).
Discussion
The most important finding in this study, and the primary outcome, is that a single preoperative dose of 600 mg of oral gabapentin did not decrease acute postoperative pain scores in the setting of multimodal analgesia. There were also no significant differences between control and gabapentin groups in parenteral or oral opioid consumption, acetaminophen or ketorolac administration. The generally older age and greater co-existing morbidity of patients undergoing thoracotomy requires specific evaluation of the therapeutic role of any new analgesic adjuvant added to multimodal regimens. While it may be challenging to demonstrate an additional analgesic benefit of a systemic agent in the setting of perioperative neuraxial analgesia, others have been successful in demonstrating improved analgesia and/or reduced side effects with combination regimens.17 Of note, this is the first adequately-powered multimodal study to evaluate preoperative gabapentin in thoracotomy patients.9
Both patient and surgical characteristics may contribute to the varying efficacy of gabapentin administration in reducing pain scores or opioid-related side effects across surgeries and anesthetic techniques.18 Although some studies reported a decrease in acute pain scores with gabapentin in the setting of regional analgesia or local anesthetic infiltration,19–21 this beneficial effect of gabapentin was not found in other studies8, 9, 22–25 including ours. In the present study, gabapentin administration also did not decrease the frequency of nausea or vomiting, as might be expected if opioid consumption was decreased by gabapentin. In this regard, our results are also consistent with findings in abdominal hysterectomy patients,26, lumbar laminectomy and discectomy patients,23 and a recent meta-analysis.27 Our study population included a small fraction of patients (N=4; 3%) who underwent unplanned chest wall resection in the course of their thoracotomy; there is a paucity of data regarding differences in analgesic requirements in this subgroup of patients. Regardless, if these patients had been excluded, none of the results would have changed significantly. It is crucial to evaluate whether gabapentin or other anti-neuropathic pain medications show greater effects in surgical populations with a higher incidence and/or severity of postoperative pain (e.g., thoracotomy).
Post-thoracotomy pain syndrome, defined as pain that recurs or persists along a thoracotomy scar at least two months following the surgical procedure,28 is unfortunately common. Recent prospective studies have reported six month and one year residual pain rates of 62% 29 and 21%, respectively.30 Chronic post-thoracotomy pain is usually mild or moderate, but in 5% of patients the pain is severe and disabling.31 The 68% overall frequency of chronic post-thoracotomy pain at three months observed in our study is consistent with these previous studies, and was not decreased by a single preoperative dose of 600 mg of oral gabapentin. This contrasts with recent prospective studies that determined that one dose of 1200 mg of oral gabapentin decreased neuropathic pain at 6 months in patients undergoing hysterectomy, inguinal herniorrhaphy or thyroidectomy.21, 22, 32 These studies however did not use multimodal analgesic regimens that included regional anesthetic techniques. Unfortunately, there is a high frequency of post-thoracotomy pain despite excellent perioperative analgesia.
Perioperative gabapentin dose-response data are lacking in thoracotomy patients, but our selection of 600 mg was based on previous studies in a surgical setting. The optimal preemptive dose of gabapentin for postoperative pain relief after single-level lumbar diskectomy and its effect on fentanyl consumption was determined to be 600 mg.33 A larger median effective analgesic ED50 dose of 21.7 mg/kg was determined in an up-down dose finding study in patients undergoing lumbar spinal fusion.34 However, these were relative young patients (mean age: 41 years33 and 51 years34). We expected and found that patients undergoing thoracotomy would be older than in many previous gabapentin studies (mean age 64 years in our study). Therefore, the dose of gabapentin chosen (a single 600 mg dose preoperatively) was selected to strike a balance between its potential anti-hyperalgesic effect and age-related side effects that may affect patient comfort and safety. In contrast, a previous study on thoracotomy patients that utilized 1200 mg of oral gabapentin preoperatively resulted in respiratory arrest in one patient that required tracheal reintubation and mechanical ventilation. Sedation scores in the gabapentin group in that study were also higher at 4 hours postoperatively than in the placebo group.9
Of note, in our study, gabapentin administration was associated with a decreased incidence of pruritus postoperatively on both POD1 and 2, and resulted in decreased nalbuphine administration. A systematic review of randomized controlled trials of perioperative gabapentin noted that six studies have reported on the incidence of postoperative pruritus, and combined data showed that gabapentin was associated with less pruritus compared with control.35 One possible mechanism is that decreased opioid requirements in gabapentin groups contribute to decreased opioid-related side effects, such as pruritus. In our study, IV and oral opioid consumption did not vary significantly between groups, but epidural infusion rates did differ significantly between the groups on POD2 (not on POD1). The starting epidural infusion was standardized in our protocol, and adjustments to the epidural infusion (mixture and rate) were performed by the inpatient pain service team depending on analgesic effect and side effect profile. It is likely that by POD2 the optimal epidural infusion for each patient had been achieved. A reduced overall epidural hydromorphone dose may have contributed to decreased pruritus in the gabapentin group, resulting in less nalbuphine administration. Reduced epidural infusion rates reflected intervention by the acute pain service in minimizing sedation or respiratory depression, i.e., dose-limiting side effects of gabapentin. Interestingly, a previous study on gabapentin in thoracotomy patients also demonstrated a trend toward decreased epidural infusion volume administered in the gabapentin group compared to the placebo group (P=0.06), but without a significant difference in pruritus scores.9
Increased sedation and/or prolonged PACU duration with gabapentin has been shown in some studies.6, 9, 36 In the present study, PACU duration did not differ significantly between groups, but a trend was present toward prolonged PACU stay (18 min) in the gabapentin group. This prolongation was similar to that observed in patients undergoing lower extremity surgery at our medical center, also utilizing a multimodal analgesic protocol that included 600 mg of gabapentin and peripheral nerve blockade.8 A similar finding was also seen in a recent thyroidectomy study utilizing 1200 mg of gabapentin.22 The actual reason(s) for prolonged PACU stay cannot be ascertained in any of these studies. Of note, after discharge from the PACU, no significant difference in sedation was noted as measured using a validated sedation scale, and no patients received naloxone for sedation or respiratory depression.
In the present study, a single dose of gabapentin was administered preoperatively. Adding gabapentin to a multimodal analgesic regimen that includes regional anesthesia may have limited our ability to demonstrate an impact on acute and/or chronic pain scores. Recent studies in a variety of surgical patient populations have used longer courses (1–10 days) of gabapentin administration with variable results.26, 37–41 Unfortunately, information on adverse effects of gabapentin (e.g., sedation, dizziness) was not consistently and/or prospectively collected. Thoracotomy patients may be particularly susceptible to the adverse effects of gabapentin because of several factors including their generally advanced age, coexisting morbid conditions and perioperative fluid management. Thus, future studies should address whether longer administration of either low doses of gabapentin or even escalating regimens will prove beneficial to thoracotomy patients beyond the immediate perioperative period, with an acceptable side effect profile.
In summary, a preoperative oral dose of gabapentin 600 mg did not reduce pain scores or parenteral or oral opioid consumption in the first 48 hours following elective thoracotomy. This dose did not confer any analgesic benefit in the setting of effective multimodal analgesia. The frequency of pain at 3 months post-thoracotomy was 68% overall, highlighting the importance of continued study of analgesic therapies or surgical improvements that may impact this long-term outcome.
Acknowledgements
The authors wish to acknowledge contributions from the following individuals: Anita E. Baumgartner, R.N., Steven R. Holets, R.R.T., Gregory A. Wilson, R.R.T., Shonie L. Buenvenida, R.N., James D. Hannon, M.D., Beth A. Elliott, M.D., Daryl J. Kor, M.D., Y.S. Prakash, M.D., Ph.D., Elizabeth A. Miller, R.N., Susan A. Vale, R.N., Roberta J. Stephenson, R.N., Beverly J. O Byrne, R.N., Susan J. Utesch, R.N., Laurie A. Meade, R.N., and Lavonne M. Liedl, R.R.T. as well as Mayo Clinic Department of Anesthesiology residents, Mayo Clinic Anesthesia Clinical Research Unit (ACRU), Mayo Clinic Division of Thoracic Surgery residents, fellows, and physician assistants, Mayo Clinic Department of Nursing, and secretarial assistance by Marcia J. Crane and Pamela J. Fenske.
Funding: Supported by the Mayo Foundation for Education and Research (ClinicalTrials.gov number NCT00588159). The project described was also supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
References
- 1.Tan N, Agnew NM, Scawn ND, Pennefather SH, Chester M, Russell GN. Suprascapular nerve block for ipsilateral shoulder pain after thoracotomy with thoracic epidural analgesia: a double-blind comparison of 0.5% bupivacaine and 0.9% saline. Anesthesia & Analgesia. 2002;94:199–202. doi: 10.1097/00000539-200201000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Della Rocca G, Coccia C, Pompei L, Costa MG, Pierconti F, Di Marco P, et al. Post-thoracotomy analgesia: epidural vs intravenous morphine continuous infusion. Minerva Anestesiologica. 2002;68:681–693. [PubMed] [Google Scholar]
- 3.Mendola C, Ferrante D, Oldani E, Cammarota G, Cecci G, Vaschetto R, et al. Thoracic epidural analgesia in post-thoracotomy patients: comparison of three different concentrations of levobupivacaine and sufentanil. Br. J. Anaesth. 2009;102:418–423. doi: 10.1093/bja/aep004. [DOI] [PubMed] [Google Scholar]
- 4.Cassivi SD, Allen MS, Vanderwaerdt GD, Ewoldt LL, Cordes ME, Wigle DA, et al. Patient-centered quality indicators for pulmonary resection. Annals of Thoracic Surgery. 2008;86:927–932. doi: 10.1016/j.athoracsur.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Popping DM, Zahn PK, Van Aken HK, Dasch B, Boche R, Pogatzki-Zahn EM. Effectiveness and safety of postoperative pain management: a survey of 18 925 consecutive patients between 1998 and 2006 (2nd revision): a database analysis of prospectively raised data. British Journal of Anaesthesia. 2008;101:832–840. doi: 10.1093/bja/aen300. [DOI] [PubMed] [Google Scholar]
- 6.Pandey CK, Priye S, Singh S, Singh U, Singh RB, Singh PK. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Canadian Journal of Anaesthesia. 2004;51:358–363. doi: 10.1007/BF03018240. [DOI] [PubMed] [Google Scholar]
- 7.Rorarius MG, Mennander S, Suominen P, Rintala S, Puura A, Pirhonen R, et al. Gabapentin for the prevention of postoperative pain after vaginal hysterectomy. Pain. 2004;110:175–181. doi: 10.1016/j.pain.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich CC, Kinney MA, Pulido JN, Hoehn SL, Torsher LC, Frie ED, et al. Preoperative gabapentin in patients undergoing primary total knee arthroplasty. Acute Pain. 2009;11:57–63. [Google Scholar]
- 9.Huot M-P, Chouinard P, Girard F, Ruel M, Lafontaine ER, Ferraro P. Gabapentin does not reduce post-thoracotomy shoulder pain: a randomized, double-blind placebo-controlled study. Canadian Journal of Anaesthesia. 2008;55:337–343. doi: 10.1007/BF03021488. [DOI] [PubMed] [Google Scholar]
- 10.Barak M, Ziser A, Katz Y. Thoracic epidural local anesthetics are ineffective in alleviating post-thoracotomy ipsilateral shoulder pain. Journal of Cardiothoracic and Vascular Anesthesia. 2004;18:458–460. doi: 10.1053/j.jvca.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Burgess FWPHDMD, Anderson DMMD, colonna DMD, Sborov MJMD, Cavanangh DGMD. Ipsilateral Shoulder Pain Thoracic Surgery. Anesthesiology. 1993;78:365–367. doi: 10.1097/00000542-199302000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Mac TB, Girard F, Chouinard P, Boudreault D, Lafontaine ER, Ruel M, et al. Acetaminophen Decreases Early Post-Thoracotomy Ipsilateral Shoulder Pain in Patients With Thoracic Epidural Analgesia: A Double-Blind Placebo-Controlled Study. Journal of Cardiothoracic and Vascular Anesthesia. 2005;19:475–478. doi: 10.1053/j.jvca.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: Validity and Reliability in Adult Intensive Care Unit Patients. Am. J. Respir. Crit. Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanks GW, Conno Fd, Cherny N, Hanna M, Kalso E, McQuay HJ, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84:587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D for the CG. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Annals of Internal Medicine. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 17.Grass JA, Sakima NT, Valley M, Fischer K, Jackson C, Walsh P, et al. Assessment of Ketorolac as an Adjuvant to Fentanyl Patient-controlled Epidural Analgesia after Radical Retropubic Prostatectomy. Anesthesiology. 1993;78:642–648. [PubMed] [Google Scholar]
- 18.Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD005452. CD005452. [DOI] [PubMed] [Google Scholar]
- 19.Fassoulaki A, Melemeni A, Stamatakis E, Petropoulos G, Sarantopoulos C. A combination of gabapentin and local anaesthetics attenuates acute and late pain after abdominal hysterectomy. European Journal of Anaesthesiology. 2007;24:521–528. doi: 10.1017/S0265021506002134. [DOI] [PubMed] [Google Scholar]
- 20.Turan A, Kaya G, Karamanlioglu B, Pamukcu Z, Apfel CC. Effect of oral gabapentin on postoperative epidural analgesia. British Journal of Anaesthesia. 2006;96:242–246. doi: 10.1093/bja/aei294. [DOI] [PubMed] [Google Scholar]
- 21.Sen H, Sizlan A, Yanarates O, Senol MG, Inangil G, Sucullu I, et al. The effects of gabapentin on acute and chronic pain after inguinal herniorrhaphy. European Journal of Anaesthesiology. 2009;26:772–776. doi: 10.1097/EJA.0b013e32832ad2fa. [DOI] [PubMed] [Google Scholar]
- 22.Brogly N, Wattier JM, Andrieu G, Peres D, Robin E, Kipnis E, et al. Gabapentin attenuates late but not early postoperative pain after thyroidectomy with superficial cervical plexus block. Anesth Analg. 2008;107:1720–1725. doi: 10.1213/ane.0b013e318185cf73. [DOI] [PubMed] [Google Scholar]
- 23.Radhakrishnan M, Bithal PK, Chaturvedi A. Effect of preemptive gabapentin on postoperative pain relief and morphine consumption following lumbar laminectomy and discectomy: a randomized, double-blinded, placebo-controlled study.[see comment] Journal of Neurosurgical Anesthesiology. 2005;17:125–128. doi: 10.1097/01.ana.0000167147.90544.ab. [DOI] [PubMed] [Google Scholar]
- 24.Clarke H, Pereira S, Kennedy D, Andrion J, Mitsakakis N, Gollish J, et al. Adding gabapentin to a multimodal regimen does not reduce acute pain, opioid consumption or chronic pain after total hip arthroplasty. Acta Anaesthesiologica Scandinavica. 2009;53:1073–1083. doi: 10.1111/j.1399-6576.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 25.Adam F, Menigaux C, Sessler DI, Chauvin M. A single preoperative dose of gabapentin (800 milligrams) does not augment postoperative analgesia in patients given interscalene brachial plexus blocks for arthroscopic shoulder surgery. Anesthesia & Analgesia. 2006;103:1278–1282. doi: 10.1213/01.ane.0000237300.78508.f1. [DOI] [PubMed] [Google Scholar]
- 26.Dierking G, Duedahl TH, Rasmussen ML, Fomsgaard JS, Moiniche S, Romsing J, et al. Effects of gabapentin on postoperative morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind trial. Acta Anaesthesiologica Scandinavica. 2004;48:322–327. doi: 10.1111/j.0001-5172.2004.0329.x. [DOI] [PubMed] [Google Scholar]
- 27.Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Canadian Journal of Anaesthesia. 2006;53:461–469. doi: 10.1007/BF03022618. [DOI] [PubMed] [Google Scholar]
- 28.Merskey H, Bogduk N. Post-thoracotomy Pain Syndrome. In: Merskey H, Bogduk N, editors. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Second ed. Vol. Seattle: IASP Press; 1994. pp. 143–144. [Google Scholar]
- 29.Senturk M, Ozcan PE, Talu GK, Kiyan E, Camci E, Ozyalcin S, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesthesia & Analgesia. 2002;94:11–15. doi: 10.1213/00000539-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Ochroch EA, Gottschalk A, Augostides J, Carson KA, Kent L, Malayaman N, et al. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology. 2002;97:1234–1244. doi: 10.1097/00000542-200211000-00029. [DOI] [PubMed] [Google Scholar]
- 31.Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. European Journal of Cardio-Thoracic Surgery. 2000;18:711–716. doi: 10.1016/s1010-7940(00)00569-8. [DOI] [PubMed] [Google Scholar]
- 32.Sen H, Sizlan A, Yanarates O, Emirkadi H, Ozkan S, Dagli G, et al. A comparison of gabapentin and ketamine in acute and chronic pain after hysterectomy. Anesthesia & Analgesia. 2009;109:1645–1650. doi: 10.1213/ANE.0b013e3181b65ea0. [DOI] [PubMed] [Google Scholar]
- 33.Pandey CK, Navkar DV, Giri PJ, Raza M, Behari S, Singh RB, et al. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized, double-blind, placebo-controlled study. Journal of Neurosurgical Anesthesiology. 2005;17:65–68. doi: 10.1097/01.ana.0000151407.62650.51. [DOI] [PubMed] [Google Scholar]
- 34.Van Elstraete AC, Tirault M, Lebrun T, Sandefo I, Bernard J-C, Polin B, et al. The median effective dose of preemptive gabapentin on postoperative morphine consumption after posterior lumbar spinal fusion. Anesthesia & Analgesia. 2008;106:305–308. doi: 10.1213/01.ane.0000297417.05690.31. [DOI] [PubMed] [Google Scholar]
- 35.Ho K-Y, Gan TJ, Habib AS. Gabapentin and postoperative pain - a systematic review of randomized controlled trials. Pain. 2006;126:91–101. doi: 10.1016/j.pain.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Ture H, Sayin M, Karlikaya G, Bingol CA, Aykac B, Ture U. The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcraniotomy pain: a prospective randomized study. Anesthesia & Analgesia. 2009;109:1625–1631. doi: 10.1213/ane.0b013e3181b0f18b. [DOI] [PubMed] [Google Scholar]
- 37.Fassoulaki A, Patris K, Sarantopoulos C, Hogan Q. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesthesia & Analgesia. 2002;95:985–991. doi: 10.1097/00000539-200210000-00036. [DOI] [PubMed] [Google Scholar]
- 38.Fassoulaki A, Stamatakis E, Petropoulos G, Siafaka I, Hassiakos D, Sarantopoulos C. Gabapentin attenuates late but not acute pain after abdominal hysterectomy. European Journal of Anaesthesiology. 2006;23:136–141. doi: 10.1017/S0265021505002048. [DOI] [PubMed] [Google Scholar]
- 39.Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesthesia & Analgesia. 2005;101:1427–1432. doi: 10.1213/01.ANE.0000180200.11626.8E. [DOI] [PubMed] [Google Scholar]
- 40.Turan A, White PF, Karamanlioglu B, Memis D, Tasdogan M, Pamukcu Z, et al. Gabapentin: an alternative to the cyclooxygenase-2 inhibitors for perioperative pain management. Anesthesia & Analgesia. 2006;102:175–181. doi: 10.1213/01.ane.0000184824.43411.63. [DOI] [PubMed] [Google Scholar]
- 41.Gilron I, Orr E, Tu D, O'Neill JP, Zamora JE, Bell AC. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy.[see comment] Pain. 2005;113:191–200. doi: 10.1016/j.pain.2004.10.008. [DOI] [PubMed] [Google Scholar]