Abstract
In this paper, we describe the design and validation of a bioreactor for the in vitro culture of whole rodent lung tissue. Many current systems only enable large segments of lung tissue to be studied ex vivo for up to a few hours in the laboratory. This limitation restricts the study of pulmonary biology in controlled laboratory settings, and also impacts the ability to reliably culture engineered lung tissues in the laboratory. Therefore, we designed, built and validated a bioreactor intended to provide sufficient nutrient supply and mechanical stimulation to support cell survival and differentiation in cultured lung tissue. We also studied the effects of perfusion and ventilation on pulmonary cell survival and maintenance of cell differentiation state. The final bioreactor design described herein is capable of supporting the culture of whole native lung tissue for up to 1 week in the laboratory, and offers promise in the study of pulmonary biology and the development of engineered lung tissues in the laboratory.
Keywords: bioreactor, lung culture, tissue engineering
Introduction
Studies of pulmonary biology are restricted by the limited availability of in vitro systems for tissue and organ culture. While the in vitro culture of human pulmonary epithelium has been extensively demonstrated from both adult and fetal human lung tissue (17), it has proven more difficult to culture rodent epithelium in the laboratory, as the cells tend to rapidly de-differentiate (27). As a result, rodent studies of pulmonary biology have relied more heavily on in vivo work. Herein, we describe a bioreactor for the in vitro culture of whole lung tissue, which allows the culture of rodent lung tissue for at least a week in the laboratory. This could assist the study of pulmonary biology, lung development, physiology, and pharmacology, and additionally would enable the growth of engineered lung tissue.
The in vitro culture of whole rodent lungs has previously been limited to the study of fetal lung tissues, which do not require active perfusion or ventilation due to their small size and ease of nutrient transfer by simple diffusion (33). Fetal explant cultures can be maintained for up to 2–4 weeks in the laboratory, although tissue development and organ growth are slower than in vivo (15). Some studies have demonstrated the culture of whole adult rodent lungs in vitro. However, the length of culture is typically 1–4hrs, with most groups focusing on very short-term studies lasting 30–60 minutes. Most of these studies utilize a commercially available system from Hugo Sacks Elektronik (Hugstetten, Germany) (13,30,31). However, there is no evidence that this system, or other systems that have been separately developed (22,29), has been utilized or validated for long-term cultures lasting longer than 24 hours. Similarly, the culture of explanted lungs from larger animals is limited, with studies lasting only 2–3 hours (18).
A bioreactor that supports lung tissue long-term can also be useful for lung tissue engineering. Prior attempts at pulmonary tissue engineering have been limited to small tissue sizes that permit nutrient delivery to the growing tissues via diffusion (1,7,8,19,20), although recently described work demonstrates that larger tissues can be developed (9,24). In work by Cortiella and colleagues, engineered lung culture relied solely on diffusion, with no vascular perfusion or airway ventilation (9), while Price and co-workers provided ventilation of developing engineered tissues but no vascular perfusion (24). Additionally, recent work demonstrates that the bioreactor described herein can be used to culture whole engineered rodent lungs by combining appropriate cell sources with vascular perfusion and airway ventilation, and that these engineered tissues can efficiently participate in gas exchange when transplanted into a host (23). The development of a bioreactor capable of in vitro culture of true 3-dimensional segments of lung tissue is an important step in the development of clinically useful engineered lung tissue.
The lung bioreactor in this report enables the perfusion of culture medium through the vasculature, the movement of medium or air in and out of the airways, and the ventilation of the lungs via negative (as well as positive) pressure. In order to validate the bioreactor, we performed experiments using freshly explanted whole rat lungs. The explanted lungs were placed directly into the bioreactor and cultured for up to 1 week. Under optimized bioreactor conditions, we demonstrate the maintenance of cellular phenotype and pulmonary architecture, with minimal cellular apoptosis.
Materials and Methods
Whole lung culture
All animal experimental work was approved by the Yale University Institutional Animal Care and Use Committee. Lungs were harvested from young adult (3 month-old) male Fischer 344 rats. After induction of anesthesia with sodium pentobarbital (Sigma), a transverse incision was made below the costal margin, entering the abdominal cavity. The chest was entered and the lungs perfused via the right ventricle with PBS containing 50 U/ml heparin (Sigma) and 1ug/ml sodium nitroprusside (Fluka). The heart, lungs and trachea were dissected free and removed en bloc from the animal. Cannulae were connected to the trachea and to the pulmonary artery trunk via the right side of the heart. The cannulae were secured with 5-0 prolene suture. The airway was lavaged with 2% amphotericin, penicillin and streptomycin in PBS, followed by two lavages with PBS, and the lung was transferred in sterile fashion to the bioreactor and maintained in an incubator at 37°C and 5% CO2 for the duration of culture. Vascular perfusion and airway ventilation were performed as dictated by the experimental conditions, with culture medium for all experiments being BGJb medium (Invitrogen) with 0.2ug/ml ascorbic acid (Sigma), 100U/ml penicillin (Gibco), and 100ug/ml streptomycin (Gibco).
Bioreactor components and assembly
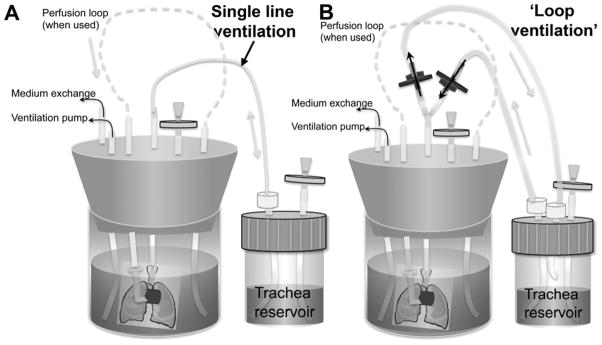
All bioreactor components were obtained from Cole-Parmer (Vernon Hills, IL) unless otherwise noted. A silicone stopper and 500ml glass jar formed the basis of the bioreactor. PharMed tubing (Westlake, OH), sizes L/S 14 and L/S 16, was inserted through the silicone stopper to enable the necessary connections to the lung, including a perfusion loop, tracheal connection, air ventilation, and medium exchange ports. Pressures were monitored using a TruWave pressure transducer (Edwards Lifesciences, Irvine, CA) at the tracheal and pulmonary arterial cannulae. Perfusion was accomplished using a Masterflex L/S variable speed roller pump (Masterflex, Vernon Hills, IL). Ventilation was enabled using a multichannel programmable syringe pump (Cole Parmer), with inhalation and exhalation each performed over 30 seconds (Fig. 1). Dissolved oxygen concentration was measured with a Milwaukee SM600 dissolved oxygen meter.
Figure 1.
Bioreactor schematic diagrams. Lung is contained within main bioreactor, with cannulae in the trachea and pulmonary artery. Pulmonary arterial cannula is connected to a perfusion loop to enable vascular perfusion. Tracheal cannula connects to a separate tracheal reservoir, which can be filled with culture medium to allow ventilation with medium, or can be empty to enable ventilation with air. A: Schematic showing single-line ventilation, wherein medium follows the same path into and out of the lung during ventilation; B: Schematic demonstrating ‘loop’ ventilation, wherein medium follows a different path during inhalation and exhalation, thereby delivering fresh medium to the lung with each breath. One-way valves are indicated in blue, while grey discs indicate air filters.
Histology and immunofluorescence
After the desired culture period, lungs were fixed, paraffin-embedded and sectioned. Routine histology (H&E) was performed, as well as immunofluorescence for aquaporin-5 (type I epithelium; Millipore; 1:1000 dilution), surfactant protein C (type II epithelium; Millipore; 1:1000 dilution), CCSP (Clara cell secretory protein 10; 1:20,000 dilution1), and PECAM-1 (CD-31, marker for endothelium; Santa Cruz; 1:1000 dilution). For immunofluorescence, sections (5μm) were deparaffinized, rehydrated, and incubated in PBS with 0.2% triton-X (buffer) for 15 minutes. Antigen retrieval was performed using 0.02M citric acid in PBS for 20min at 75–85C. Blocking was performed for 1 hour at room temperature in PBS with 1% bovine serum albumin and 0.75% glycine. Primary antibodies were applied overnight at 4°C, and secondary antibodies were applied for 1 hour at 1:500 dilution. Secondary antibodies were obtained from Invitrogen (AlexaFluor 555 or AlexaFluor 488). Images were acquired with a Zeiss Axiovert 200M inverted fluorescent microscope.
Cell proliferation was assessed via staining for proliferating cell nuclear antigen (PCNA) (Zymed, San Francisco, CA), and apoptotic nuclei were detected with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stain (Calbiochem, San Diego, CA). Manufacturer’s instructions were followed for both assays.
Microsphere ventilation assay
During whole lung cultures described in this manuscript, we found that the vasculature was well preserved in lungs that were ventilated but not perfused, implying that ventilation alone was sufficient to maintain the vasculature. In order to determine if ventilation is sufficient to induce movement of medium into the lung vasculature and thereby provide nutrients to the lung, we developed a simple assay using 5μm polystyrene microspheres (SPI Supplies, West Chester PA). Lungs were connected to the bioreactor, as described above, and ventilated but not perfused. The bioreactor chamber was filled with medium containing microspheres (0.1 million per ml). The culture was allowed to proceed, with ventilation only, for 3 hours. The lung was then fixed, paraffin-embedded, sectioned and analyzed using routine histology (H&E) for the presence of microspheres.
Statistics
Experiments were performed in triplicate, with representative images shown. For statistical analysis, one-way ANOVA with Dunnett’s multiple comparison test was used to determine significance; for all comparisons, n=4 measurements were performed.
Results
Bioreactor design requirements
The bioreactor design incorporates key features of the rodent in vivo environment, but is also designed to be flexible, allowing the user to modify several key parameters depending upon the desired conditions.
The bioreactor schematic is shown in Figure 1, with additional diagrams provided in other published work from our laboratory (23). The bioreactor is designed to:
Perfuse medium through the vasculature at the physiological levels of a rodent (for rat, up to 80ml/min).
Continuously ventilate the lungs with air or medium through the trachea with negative pressure ventilation.
Allow for different media to bathe the vascular and airway compartments of the lung.
Allow for gas exchange into the culture medium, while simultaneously meeting the requirements for ventilation.
Maintain perfusion pressures within normal rodent physiological values, with a pulmonary artery pressure of less than 15–30mmHg (16).
Be small and self-contained such that it can fit within the physical confines of a standard tissue culture incubator.
Bioreactor perfusion system
Perfusion to the lung is provided via a roller pump that circulates medium from the main bioreactor into the pulmonary artery, at a user-specified rate (Fig. 1). The pulmonary vein is not connected directly to the perfusion loop but rather venous effluent drains directly into the main bioreactor reservoir. As a result, the pressure in the vasculature is affected by the pressure in the bioreactor, which is modulated to induce ventilatory movements. We note that this linkage is not physiologic, but is a result of the lack of a pulmonary venous catheter. The perfusion pressure is kept below ~30mmHg, the maximum value typically seen in the pulmonary arterial system (16). Typical pressures in the pulmonary artery are given in table 1, where the baseline pressure varies between 10–16mmHg, with 16 mmHg being analogous to the peak pulmonary systolic pressure; pressures are lowered to 0–6mmHg during application of negative pressure to create breathing movements (23). Typical vascular flow rates of culture medium were 2–5 ml/min, which are sufficient to provide adequate nutrient delivery to the tissue, although below the normal physiologic range. Perfusion flow rates were intentially kept low because high flow leads to arterial hypertension in the absence of ventilation, and because in vitro it is not necessary to oxygenate the entire blood volume of the animal, but only to provide nutrient delivery to the explanted tissue.
Table 1.
Vascular and airway pressures in lung bioreactor
| Location | Pressure | |
|---|---|---|
| Trachea | Inhalation | 0 mmHg |
| Exhalation | −3 mmHg | |
| Pulmonary artery | Inhalation | 10–16 mmHg |
| Exhalation | 0–6 mmHg | |
Bioreactor ventilation system
In vivo, breathing is normally accomplished via negative pressure ventilation, with the lung expanding due to a negative pressure created in the thoracic cavity by the diaphragm and rib cage. After inhalation, the breathing muscles relax and the lung passively deflates. In the bioreactor, negative pressure ventilation is effected using a syringe pump to withdraw a volume of air from the main bioreactor, which is kept airtight. This negative pressure within the bioreactor is compensated by medium (or air) flowing into the lungs through the trachea from the separate tracheal reservoir (Fig. 1). For exhalation, the syringe pump reverses direction to push air back into the main bioreactor, causing medium (or air) to flow back into the tracheal reservoir. We used a slow ventilation rate of 1 respiration per minute due to the time needed for the syringe pump to effect a negative pressure in the bioreactor. The bioreactor can also utilize positive pressure ventilation, by connecting the syringe pump directly to the tracheal cannula or tracheal reservoir, which can also enable more rapid ventilation. However, positive pressure ventilation, such as used in mechanical ventilation of surgical patients and in critical care situations, is known to induce pulmonary injury (5,21,28,29). As a result, we utilized negative pressure ventilation for in vitro lung cultures. Dissolved oxygen concentration does not drop significantly over the course of culture, and remains at 6.0–7.0mg/L, due to gas exchange that occurs in the tracheal reservoir during ventilation. These levels of dissolved oxygen correspond to oxygen tensions that are higher than normal physiological levels of 80–100mmHg (6–7mg/L corresponds to a partial pressure of 137–159mmHg).
Effects of ventilation with air versus medium on overall native lung morphology
We evaluated the effects of ventilating lungs cultured in the bioreactor with either culture medium or room air (~20% O2). Lungs were ventilated continuously at 1 breath/min with either air or liquid medium over the 3-day course of culture; the vasculature was not perfused for this set of studies. We hypothesized that ventilation with medium would offer improved cell survival as this would provide improved nutrient delivery, which may be important in the bioreactor as there is no perfused bronchial circulation. However, pulmonary epithelium is frequently cultured in the presence of an air-liquid interface, which has been shown to enable appropriate pulmonary development in fetal rat lungs (15). Therefore, we also examined the hypothesis that ventilation with medium may result in loss of epithelial differentiation state, due to the lack of an air-liquid interface.
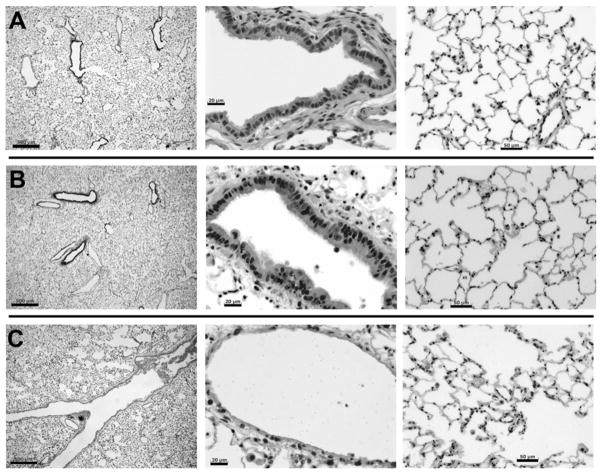
After 3 days of culture, significant differences were noted between lungs ventilated with medium versus air (Fig. 2). Medium-ventilated lungs have similar morphology to native lung; however, air-ventilated lungs show greatly dilated airways, with cell debris evident in the airway. Furthermore, the bronchial and bronchiolar epithelium of air-ventilated lung was completely absent, a finding that was consistent across the entire lung. In addition, dilated peripheral airspaces were evident, as shown in the right panel of Figure 2. This epithelial damage due to ventilation with air may be due to repeated collapsing of airways at exhalation, as in the bioreactor, exhalation is partly passive (due to elastic relaxation of the lung) but also partly active due to repressurization of the bioreactor to reverse the negative pressure of inhalation (4). Overall, it appears that that ventilation with air in the bioreactor causes destruction of the airway epithelium and dilation of peripheral airspaces. In contrast, ventilation with culture medium is sufficient to maintain normal lung morphology and airway structure.
Figure 2.
Native lung and lung cultured for 3 days with either liquid or air ventilation. A: Native lung; B: lung cultured for 3 days with ventilation with culture medium; C: lung cultured for 3 days with ventilation with air. Left panels show overall lung structure, including medium-sized airways, wherein scale bars = 500μm; center panels show small airways, with scale bars = 20μm; right panels show distal alveolar structure, with scale bars = 50μm.
Effect of perfusion on cell survival
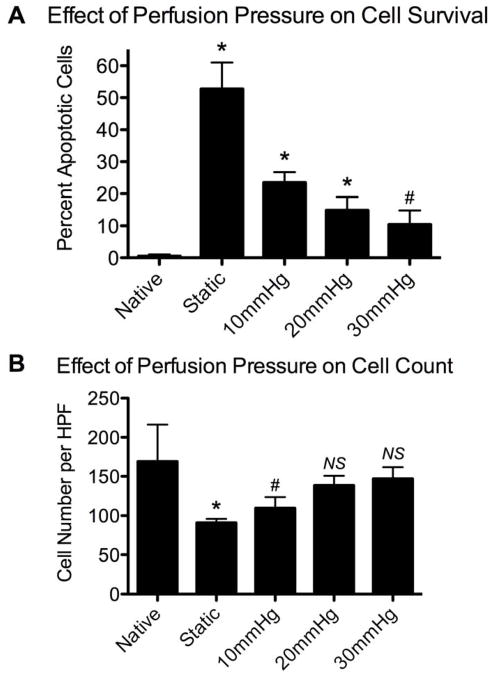
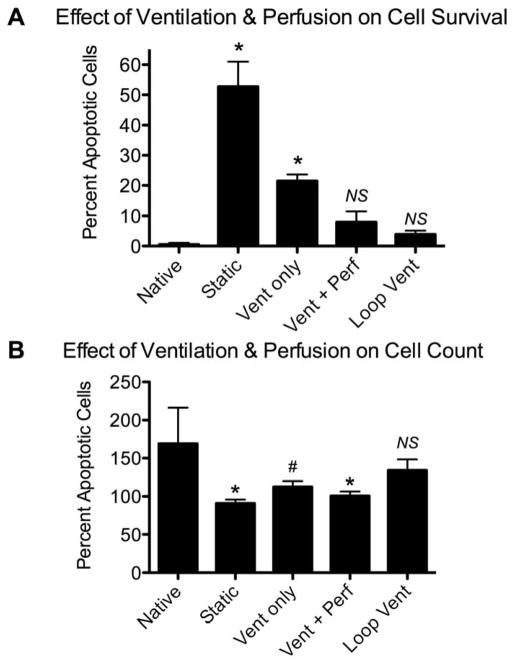
We examined the effect of vascular perfusion pressures on cell survival during 3-day lung cultures in the bioreactor. In the absence of vascular perfusion, cell survival was poor, with 52.8±8.2% of cells undergoing apoptosis and cell density greatly diminished (Fig. 3A, B). Vascular perfusion at 10 or 20 mmHg improved cell survival, but apoptosis rates were still significantly increased over native (23.5±3.2% and 14.9±4.1%, respectively, with p<0.01; Fig. 3A). With vascular perfusion of 30 mmHg, apoptosis was observed in 10.4±4.4% of cells, compared to 0.49±0.56% for native lung, although this difference was not significant (p<0.05; see Fig. 3A). Additionally, with higher perfusion pressures, overall cell density was improved (Fig. 3B). Native lung had significantly more cells per high power field (HPF) than statically cultured lung (169±47 vs. 91±5, p<0.01). With perfusion at 10mmHg, cell density improved to 110±14 cells per HPF (p<0.05 compared to native), while with perfusion at 20mmHg or 30mmHg cell counts were not significantly different from native (139±12 and 147±15; Fig. 3B).
Figure 3.
Effect of vascular perfusion and pressure on cell survival in native and cultured lung tissue. Native lung is compared to lung cultured for 3 days either under static conditions or with vascular perfusion at 10, 20 or 30 mmHg. A: Percentage of apoptotic cells (via TUNEL staining); B: cell number per high power microscopic field. * indicates p<0.01, # indicates p<0.05, and ‘NS’ indicates not significant compared to native.
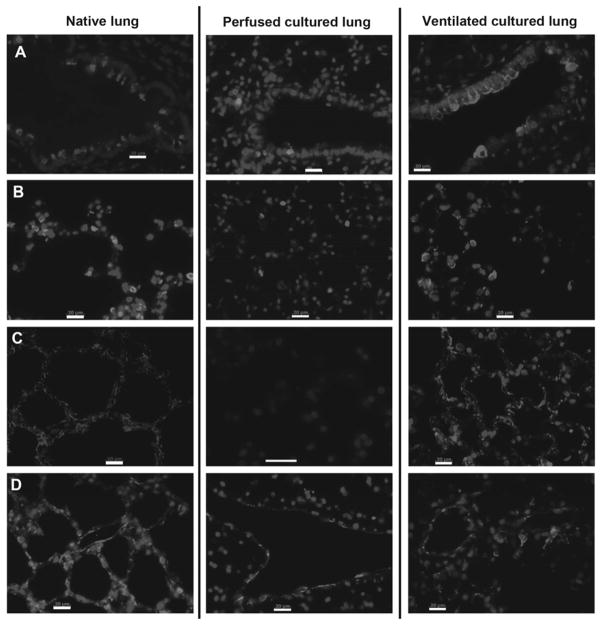
Regardless of perfusion pressure, maintenance of cellular differentiation was poor with vascular perfusion alone (Fig. 4). In the absence of any ventilatory movements and with vascular perfusion alone, substantially lower expression of epithelial markers was noted by immunohistochemical staining after 3 days. Reduced expression of Clara cell secretory protein (CCSP, expressed by Clara cells lining small to medium sized airways) and pro-surfactant protein C (SPC, expressed by type II epithelial cells which are found in the corners of alveoli) was observed in perfused lung tissues. Expression of aquaporin-5 (AQP, normally expressed by the flattened type I epithelium that lines the alveolar surfaces) was completely absent after 3 days of culture in lungs receiving only perfusion (Fig. 4, center panels). Endothelial expression of the marker PECAM-1 (platelet endothelial cell adhesion molecule-1) was observed in the larger vessels of the vasculature, but decreased expression was observed in capillaries (Fig. 4). These experiments demonstrated that perfusion alone was not sufficient to maintain sufficient cell survival or cellular differentiation of the epithelium or the vascular endothelium. These findings are in contrast to the ability of ventilation with culture medium alone to sustain cellular differentiation for up to 7 days, as shown in the right panels of Fig. 4 and discussed in the ensuing sections.
Figure 4.
Cell differentiation in native and cultured lung. Left panels show native lung, center panels are perfused cultured lung (3 days, 30mmHg vascular perfusion), and right panels are ventilated cultured lung (7 days, negative pressure ventilation with culture medium). A: Clara cells are indicated by expression of CCSP (red); B: Type II epithelium is indicated by expression of pro-SPC (red); C: Type I epithelium is stained for AQP-5 (red); and D: Endothelium is stained for PECAM-1 (red). In all panels, nuclei are counterstained blue with DAPI, and scale bars are 20μm.
Effect of medium flow path in the airway compartment on cell survival
Ventilation with medium permitted the maintenance of lung morphology and cell differentiation for both 3-day and 7-day culture periods (Fig. 2, 4). However, without “refreshing” of medium that was being breathed in and out of the airway, we found significantly higher rates of apoptotic cells in ventilated lungs as compared to native lungs after 3 days of culture (Fig. 5). We hypothesized that cell survival was negatively impacted due to insufficient fresh medium entering the trachea over days of ventilation. Hence, we modified the bioreactor such that the medium followed a different path into and out of the lung during ventilation (‘loop’ ventilation), as outlined in Figure 1. This modification greatly increased the volume of fresh medium entering the lungs with each breath.
Figure 5.
Effect of ventilation and perfusion on cell survival in cultured lung tissue. Native lung is compared to lung cultured for 3 days under static conditions, with single-line ventilation (‘Vent only’), with single-line ventilation and vascular perfusion (‘Vent+Perf’) or with ‘loop’ ventilation (‘Loop vent’). A: Percentage of apoptotic cells (via TUNEL staining); B: Cell number per high power microscopic field. * indicates p<0.01, # indicates p<0.05, and ‘NS’ indicates not significant compared to native.
The percent of apoptotic cells was reduced to 3.9±1.2% for ‘loop’ ventilation from 21.5±2.2% for ventilation with a single line (‘vent only’ in Fig. 5; p < 0.01 for both conditions compared to native, wherein 0.49±0.56% of cells were apoptotic). ‘Loop’ ventilation increases the delivery of medium to the lung by reducing the amount of ‘recycled’ medium. Vascular perfusion, together with ventilation, also reduces cell apoptosis, to 7.9±3.6% from 21.5±2.2% for single-line ventilation alone, although cell number is similar at 101±5.7 and 113±7.6 and cells per HPF, respectively. The ‘loop’ ventilation modification led to increased cell density, which was not significantly different from native (134±15 for ‘loop’ ventilation vs. 169±47 cells per HPF for native; Fig. 5B). Thus, ventilation alone enables cell survival of lung tissue in the bioreactor over the course of 3-day culture periods, provided that sufficient fresh medium is delivered to the lung using the ‘loop’ ventilation modification to minimize the amount of recycled medium.
Long-term cultures to validate bioreactor design
In order to more fully validate the bioreactor design, we performed 7-day cultures of native lung. These cultures utilized ventilation with medium using the ‘loop’ modification described above, and without any vascular perfusion. Lungs were evaluated via histology for cell proliferation, apoptosis, and maintenance of cellular differentiation via staining for aquaporin-5 (type I epithelium), surfactant protein C (type II epithelium), Clara cell secretory protein (Clara cells), and PECAM-1 (endothelium) (Fig. 4). Lower magnification images are not distinguishable from those shown in Figure 2 for medium breathing, wherein culture length was 3 days. Importantly, patterns of expression of cellular markers were not substantially different from native lung (Fig. 4, with right-most panels showing 7-day ventilated lungs and left-most panels showing native lung). Clara cells are found lining small to medium airways, as in native lung, with apical expression of CCSP (Fig. 4A). Type II epithelial cells are found distributed throughout alveoli, and expression of SPC is similar to native (Fig. 4B). Type I epithelial cells are noted lining alveolar surfaces, with expression of AQP-5 indistinguishable from native (Fig. 4C). Additionally, endothelial expression of PECAM-1 is noted in both large vessels as well as capillary networks (Fig. 4D), although the expression of PECAM-1 appears somewhat weaker than native. Overall, using medium ventilation with the ‘loop’ modification to maximize fresh medium delivery, the expression of these cellular markers and distribution of cell types in the cultured lung is highly similar to native lung, suggesting that the bioreactor can provide suitable nutrient delivery and physical stimulation to maintain lung survival over one week of in vitro culture.
Ventilation alone enables passive perfusion of the vasculature of the lung
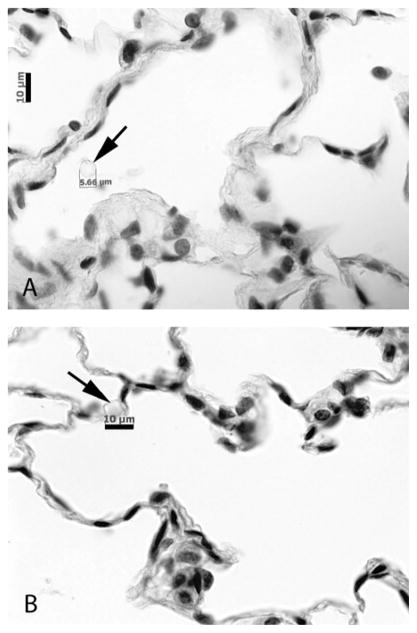
Ventilation alone is sufficient to enable survival and differentiation of the endothelium, even in the absence of active vascular perfusion (Figs. 4,5). Because the vascular cells appeared viable even in the absence of vascular perfusion, we speculated that the physical movements of the lung during ventilation may induce a passive ‘perfusion’ of medium into and out of the lung vasculature. To test this, we ventilated lungs for 3 hours in the bioreactor, which was filled with culture medium containing 5μm microspheres (100,000 microspheres per ml of medium). Under these conditions, the approximate diffusion length for a 5μm particle is 68 μm, which is a measure of the approximate distance a particle would travel due to simple diffusion. Microspheres were found in the vasculature after this period of ventilation (Fig. 6), having migrated distances at least 100 times farther than the diffusion length and indicating that passive vascular perfusion is occurring with ventilation in the bioreactor. We note that microspheres were not found in the airways or alveoli during this experiment.
Figure 6.
Ventilation enables passive perfusion of pulmonary vasculature. Lungs were cultured with ventilation only, in a bioreactor filled with 5μm microspheres. Microspheres are found in both larger vessels (A) and capillary structures (B), indicating that the vasculature is passively perfused due to ventilatory motions. Scale bars are 10μm in both panels.
Discussion
In this paper, we demonstrate the design and validation of a bioreactor for the in vitro culture of whole rodent lungs. The bioreactor is capable of replicating key aspects of the in vivo environment, and demonstrates promise for use in studies of pulmonary biology and in the development of engineered lung tissue.
Vascular perfusion alone was found to be inadequate to maintain cultured lung tissue. Cellular differentiation was lost, and apoptotic rates were high. Improved cell survival with higher perfusion pressures is likely due to improved nutrient delivery to the tissue, as higher pressures could both deliver more medium to the tissue as well as enable the perfusion of vessels that have physiologically vasoconstricted due to the absence of ventilation.
Ventilation with air was found to have clear negative effects on the airway epithelium of cultured lung tissue, with almost complete loss of the airway epithelial lining. These findings were surprising given that pulmonary epithelial cells are frequently cultured at an air-liquid interface, and the air interface has been shown to induce epithelial differentiation (12,14,32). This may be related to the method of exhalation in the bioreactor, which is somewhat different than normal physiology. In vivo, exhalation occurs due to elastic recoil of the lungs, whereas in the bioreactor, passive recoil partly accounts for exhalation, but is also aided by reversal of the negative pressure in the bioreactor. This pressure increase may result in low residual volumes and may cause airways to collapse upon end-exhalation, thereby damaging the airway epithelium with repeated collapsing and reopening. These pressures would also be present during liquid-ventilated cultures, but airways may resist collapse due to the presence of culture medium filling the airways. Additionally, ventilation with air would provide less nutrient support to the cultured tissue than ventilation with medium, which may contribute to the observed loss of airway epithelium and damage to peripheral airspaces.
An air-liquid interface enables the maintenance of epithelial differentiation state in vitro, and isolated pulmonary epithelial cells generally dedifferentiate in the absence of an air liquid interface (10,11,26). However, we observed maintenance of epithelial differentiation markers in lungs ventilated with medium. This may be due to the native lung environment, which remains relatively undisturbed in these experiments but is highly disrupted during cell isolation procedures for in vitro cell culture experiments. This finding, together with the failure of perfused lung cultures to demonstrate this maintenance of cellular differentiation, illustrates the importance of breathing movements in the maintenance of epithelial differentiation. Further studies could examine if minimal breathing movements are sufficient to maintain surfactant production and epithelial differentiation, as well as the effects of “noisy ventilation”. In these studies, we ventilated the native lung tissues at 1 breath/min, with ventilation occurring continuously over the culture period (either 3 or 7 days). The introduction of noise into mechanical ventilation profiles, as well as the use of periodic ventilatory sighs, has been shown to be beneficial to lung function variables over short time periods (2,3,28). The bioreactor we describe here could be used to examine the long-term effects of such ventilatory techniques.
The final bioreactor design, incorporating continuous ventilation of liquid medium through a ventilation ‘loop’ to allow continuous delivery of fresh medium, was validated using 7-day cultures of native lung. These culture conditions allowed the maintenance of cell survival, as well as maintenance of epithelial and endothelial phenotype. Surfactant expression was retained by type II epithelial cells, capillary beds were retained as demonstrated by PECAM staining, and flattened type I epithelium was present that retained expression of aquaporin-5. In addition, the airway epithelium was intact and Clara cells were noted at similar densities to native lung. The ability to culture whole lung tissue in the laboratory for as long as 7 days could enable a wide range of studies of lung physiology that previously were not possible. The bioreactor conditions can allow ventilation and/or perfusion at desired rates, and chemical or growth factors can easily be administered. Lung injury models can be applied to the bioreactor, and lung repair can be assessed under controlled conditions (6,25). Due to the absence of a blood supply and thus potential progenitor cell recruitment from the bone marrow or other sources, lung repair from tissue-resident stem cells or differentiated cells can be assessed. In addition, lung physiology studies that were previously restricted to short time periods, lasting less than a few hours, can be carried out over several days, enabling the study of long-term cell and organ changes to applied physiological conditions.
Although the system we describe has many promising features, it has several limitations and continued bioreactor improvements will allow the system to better mirror normal physiology. Such improvements could include more rapid ventilation rates, ventilation with a constant zero or negative pressure environment (i.e., without positive pressure during exhalation), or the uncoupling of ventilation and perfusion pressures. Adjustment to the ventilatory parameters may reduce epithelial damage during air ventilation, such as monitoring residual volumes, removing positive pressure application during exhalation, or using reduced tidal volumes.
Despite the potential utility of this system, more detailed validation and study of lung behavior in the bioreactor is needed. First, it would be insightful to perform pulmonary function testing on lungs cultured in the bioreactor, including lung mechanics, perfusion-ventilation studies (V/Q testing), and lung volume measurements. Measurement of pulmonary vascular leak would be useful, including the study of whether the addition of exogenous agents or modifications in perfusion or ventilation conditions affects vascular permeability. Detailed mechanical studies would also be important, to determine if lung elasticity or strength is affected by prolonged in vitro culture. Finally, more precise control of ventilation, such as by controlling tracheal pressure during ventilation, would enable more detailed study of ventilatory effects on the cultured lungs (2,3,28).
Acknowledgments
Acknowledgements and Disclosures: Funding for this work was provided by the Yale University Department of Anesthesia, and by NIH HL 098220 (to LEN). THP is supported by NIH T32 GM007171. LEN holds stock in Humacyte, a regenerative medicine company. Humacyte did not fund these studies, and did not affect the design, interpretation, or reporting of any of the experiments herein.
Footnotes
CCSP antibody kindly provided by Barry Stripp, Duke University.
References
- 1.Andrade C, Wong A, Waddell T, Keshavjee S, Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L510–518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- 2.Arold S, Mora R, Lutchen K, Ingenito E, Suki B. Variable tidal volume ventilation improves lung mechanics and gas exchange in a rodent model of acute lung injury. Am J Resp Crit Care Med. 2002;165:366–371. doi: 10.1164/ajrccm.165.3.2010155. [DOI] [PubMed] [Google Scholar]
- 3.Arold S, Suki B, Alencar A, Lutchen K, Ingenito E. Variable ventilation induces endogenous surfactant release in normal guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2003;285:L370–375. doi: 10.1152/ajplung.00036.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bilek AM, Dee KC, Gaver DP. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol. 2003;94:770–783. doi: 10.1152/japplphysiol.00764.2002. [DOI] [PubMed] [Google Scholar]
- 5.Button B, Boucher R. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Resp Physiol Neurobiol. 2008;163:189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. Human umbilical cord blood-derived mesenchymal cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Marsilio E, Goldstein R, Yannas I, Spector M. Formation of lung alveolar-like structures in collagen-glycosaminoglycan scaffolds in vitro. Tissue Eng Part A. 2005;11:1436–1448. doi: 10.1089/ten.2005.11.1436. [DOI] [PubMed] [Google Scholar]
- 8.Cortiella J, Nichols J, Kojima K, Bonassar L, Dargon P, Roy A, Vacant M, Niles JA, Vacanti CA. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng Part A. 2006;12:1213–1225. doi: 10.1089/ten.2006.12.1213. [DOI] [PubMed] [Google Scholar]
- 9.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of Acellular Natural Lung Matrix on Murine Embryonic Stem Cell Differentiation and Tissue Formation. Tissue Eng Part A. 2010;16(8):2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 10.Davidson D, Gray M, Kilanowski F, Tarran R, Randell S, Sheppard D, Argent BE, Dorin JR. Murine epithelial cells: isolation and culture. J Cystic Fibrosis. 2004;3:59–62. doi: 10.1016/j.jcf.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Dobbs L, Pian M, Maglio M, Dumars S, Allen L. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol. 1997;273:L347–354. doi: 10.1152/ajplung.1997.273.2.L347. [DOI] [PubMed] [Google Scholar]
- 12.Gruenert D, Finkbeiner W, Widdicombe J. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- 13.Held H, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharm. 1999;126:1191–1199. doi: 10.1038/sj.bjp.0702394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosokawa T, Betsuyaku T, Nishimura M, Furuyama A, Katagiri K, Mochitate K. Differentiation of tracheal basal cells to ciliated cells and tissue reconstruction on the synthesized basement membrane substratum in vitro. Connect Tissue Res. 2007;48:9–18. doi: 10.1080/03008200601017488. [DOI] [PubMed] [Google Scholar]
- 15.Jaskoll T, Don-Wheeler G, Johnson R, Slavkin H. Embryonic mouse lung morphogenesis and type II cytodifferentiation in serumless, chemically defined medium using prolonged in vitro cultures. Cell Diff. 1988;24:105–117. doi: 10.1016/0045-6039(88)90062-0. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Huang W, Jiang Z, Gregersen H, Fung Y-C. Tissue remodeling of rat pulmonary arteries in recovery from hypoxic hypertension. Proc Natl Acad Sci USA. 2004;101:11488–11493. doi: 10.1073/pnas.0404084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liley H, Ertsey R, Gonzales L, Odom M, Hawgood S, Dobbs L, Ballard PL. Synthesis of surfactant components by cultured type II cells from human lung. Biochim Biophys Acta. 1988;961:86–95. doi: 10.1016/0005-2760(88)90133-6. [DOI] [PubMed] [Google Scholar]
- 18.Linder A, Friedel G, Fritz P, Kivisto KT, McClellan M, Toomes M. The ex-vivo isolated, perfused human lung model: description and potential applications. Thorac Cardiovasc Surg. 1996;44:140–146. doi: 10.1055/s-2007-1012003. [DOI] [PubMed] [Google Scholar]
- 19.Mondrinos M, Koutzaki S, Jiwanmall E, Li M, Dechadarevian J-P, Lelkes P, Finck CM. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng Part A. 2006;12:717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 20.Mondrinos M, Koutzaki S, Poblete H, Crisanti M, Lelkes P, Finck C. In vivo pulmonary tissue engineering: contribution of donor-derived endothelial cells to construct vascularization. Tissue Eng Part A. 2008;14:361–368. doi: 10.1089/tea.2007.0041. [DOI] [PubMed] [Google Scholar]
- 21.Oeckler R, Hubmayr R. Cell wounding and repair in ventilator injured lungs. Resp Physiol Neurobiol. 2008;163:44–53. doi: 10.1016/j.resp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peták F, Albu G, Lele E, Hantos Z, Morel D, Fontao F, Habre W. Lung mechanical and vascular changes during positive- and negative-pressure lung inflations: importance of reference pressures in the pulmonary vasculature. J Appl Physiol. 2009;106:935–942. doi: 10.1152/japplphysiol.00831.2007. [DOI] [PubMed] [Google Scholar]
- 23.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-Engineered Lungs for In Vivo Implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price A, England K, Matson A, Blazar B, Panoskaltsis-Mortari A. Development of a Decellularized Lung Bioreactor System for Bioengineering the Lung: The Matrix Reloaded. Tissue Eng Part A. 2010;16(8):2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prota LF, Lassance RM, Maron-Gutierrez T, Castiglione RC, Garcia CS, Santana MC, Souza-Menezes J, Abreu SC, Samoto V, Santiago MF, Capelozzi VL, Takiya CM, Rocco PR, Morales MM. Bone marrow mononuclear cell therapy led to alveolar-capillary membrane repair improving lung mechanics in endotoxin-induced acute lung injury. Cell Transplant. 2010;19(8):965–971. doi: 10.3727/096368910X506845. [DOI] [PubMed] [Google Scholar]
- 26.Rice W, Conkright J, Na C-L, Ikegami M, Shannon J, Weaver T. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;283:L256–264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- 27.Shannon J, Mason R, Jennings S. Functional differentiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions. Biochim Biophys Acta. 1987;931:143–156. doi: 10.1016/0167-4889(87)90200-x. [DOI] [PubMed] [Google Scholar]
- 28.Spieth P, Carvalho A, Güldner A, Pelosi P, Kirichuk O, Koch T, de Abreu MG. Effects of different levels of pressure support variability in experimental lung injury. Anesthesiology. 2009;110:342–350. doi: 10.1097/ALN.0b013e318194d06e. [DOI] [PubMed] [Google Scholar]
- 29.Sundaresan A, Yuta T, Hann C, Chase J, Shaw G. A minimal model of lung mechanics and model-based markers for optimizing ventilator treatment in ARDS patients. Comp Meth Prog Biomed. 2009;95:166–180. doi: 10.1016/j.cmpb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 30.von Bethmann A, Brasch F, Nüsing R, Vogt K, Volk H, Müller K, Wendel A, Uhlig S. Hyperventilation induces release of cytokines from perfused mouse lung. Am J Resp Crit Care Med. 1998;157:263–272. doi: 10.1164/ajrccm.157.1.9608052. [DOI] [PubMed] [Google Scholar]
- 31.Witzenrath M, Gutbier B, Hocke A, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, Schutte H. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med. 2006;34:1947–1954. doi: 10.1097/01.CCM.0000220496.48295.A9. [DOI] [PubMed] [Google Scholar]
- 32.Wong A, Keating A, Lu W-Y, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zgleszewski S, Cilley R, Krummel T, Chinoy M. Maintenance of fetal murine pulmonary microvasculature in heart-lung en bloc whole organ culture. J Ped Surg. 1997;32:1161–1168. doi: 10.1016/s0022-3468(97)90675-8. [DOI] [PubMed] [Google Scholar]