Abstract
Acute stress impairs the retrieval of hippocampus-dependent memory, and this effect is mimicked by exogenous administration of stress-responsive glucocorticoid hormones. It has been proposed that glucocorticoids affect memory by promoting the release and/or blocking the reuptake of norepinephrine (NE), a stress-responsive neurotransmitter. It has also been proposed that this enhanced NE signaling impairs memory retrieval by stimulating β1-adrenergic receptors and elevating levels of cAMP. In contrast, other evidence indicates that NE, β1, and cAMP signaling is transiently required for the retrieval of hippocampus-dependent memory. To resolve this discrepancy, wild-type rats and mice with and without gene-targeted mutations were stressed or treated with glucocorticoids and/or adrenergic receptor drugs before testing memory for inhibitory avoidance or fear conditioning. Here we report that glucocorticoids do not require NE to impair retrieval. However, stress- and glucocorticoid-induced impairments of retrieval depend on the activation of β2 (but not β1)-adrenergic receptors. Offering an explanation for the opposing functions of these two receptors, the impairing effects of stress, glucocorticoids and β2 agonists on retrieval are blocked by pertussis toxin, which inactivates signaling by Gi/o-coupled receptors. In hippocampal slices, β2 signaling decreases cAMP levels and greatly reduces the increase in cAMP mediated by β1 signaling. Finally, augmenting cAMP signaling in the hippocampus prevents the impairment of retrieval by systemic β2 agonists or glucocorticoids. These results demonstrate that the β2 receptor can be a critical effector of acute stress, and that β1 and β2 receptors can have quite distinct roles in CNS signaling and cognition.
Introduction
Stress and glucocorticoids can have pronounced effects on cognition (Rodrigues et al., 2009; Roozendaal et al., 2009; Wolf, 2009). When administered shortly before testing, they impair the retrieval of hippocampus-dependent memory (de Quervain et al., 1998, 2000; Kuhlmann et al., 2005). Glucocorticoids activate mineralocorticoid (MR) and glucocorticoid (GR) receptors that are prevalent in the hippocampus (Reul and de Kloet, 1985; Morimoto et al., 1996). Glucocorticoids preferentially occupy MR basally; however, glucocorticoids rise significantly following a stressor, causing substantial activation of GR (Reul and de Kloet, 1985). Infusion of a GR-selective agonist into the dorsal hippocampus (DH) 60 min before testing impairs retrieval (Roozendaal et al., 2003), and viral expression of a transdominant GR in the dentate gyrus prevents retrieval impairment by corticosterone (Cort) (Ferguson and Sapolsky, 2008).
Cort likely affects memory retrieval through a non-genomic, membrane-delimited mechanism. Intracerebroventricular administration of a protein synthesis inhibitor does not alter Cort-induced impairment of retrieval (Sajadi et al., 2006), and DH infusion of protein-conjugated Cort, which selectively activates membrane-associated receptors (Zheng et al., 1996), mimics the effects of stress on retrieval (Chauveau et al., 2010). Results from some studies suggest that the impairment of retrieval by Cort depends on signaling by the adrenergic neurotransmitters norepinephrine and epinephrine (NE/E). The nonselective β-adrenergic receptor antagonist propranolol prevents glucocorticoid-induced impairment of retrieval in humans, and DH infusion of the β1-selective receptor antagonist atenolol blocks the impairing effects of a GR agonist in rats, while the β1-selective agonist xamoterol mimics the effects of a GR agonist (Roozendaal et al., 2004a,b; de Quervain et al., 2007).
That β1 signaling might be responsible for the impairing effects of glucocorticoids on retrieval is at odds with the recently described requirement for β1 signaling in hippocampus-dependent memory retrieval. Targeted disruption of the dopamine β-hydroxylase gene (Dbh−/−) in mice results in complete NE/E deficiency and retrieval deficits in spatial navigation and contextual fear (Thomas and Palmiter, 1997; Murchison et al., 2004). The β1-selective agonist xamoterol rescues retrieval in Dbh−/− mice, while the β1-selective antagonist betaxolol produces retrieval deficits in wild-type mice and rats (Murchison et al., 2004). Further, mice with targeted disruption of the gene for the β1 receptor (β1 KO) display deficits in contextual fear memory. These effects are mimicked by infusion of the β1-selective antagonist atenolol into the DH.
The goal of this study was to resolve the conflicting observations that β1 signaling is required for hippocampus-dependent memory retrieval and is responsible for the impairment of retrieval by glucocorticoids. We hypothesized that the β2-adrenergic receptor may mediate the impairing effect, and that the intracellular signaling activated by this receptor opposes the intracellular signaling activated by β1 receptors. Animals were tested using Pavlovian and instrumental fear conditioning. Pharmacologic reagents were administered to wild-type rats and mice, as well as to mice genetically modified to lack either NE/E, β1- or β2-adrenergic receptors. Additional experiments examined the effects of stress on retrieval.
Materials and Methods
Subjects.
Wild-type, Dbh+/−, Dbh−/−, β1 KO, and β2 KO mice were on a hybrid 129/Sv × C57BL/6 background (Thomas et al., 1995; Rohrer et al., 1996; Chruscinski et al., 1999). β3 KO, β1/β3 double KO, and β1/β2/β3 triple KO mice were on a hybrid FVB/N × 129/Sv × C57BL/6 × DBA/2 background (Bachman et al., 2002). Mice were generated by mating either heterozygotes or homozygotes, and genotype was determined by PCR. Because NE is required for fetal survival (Thomas et al., 1995), dams for the Dbh line were treated with a NE precursor as previously described (Ouyang et al., 2004). No significant differences in results were found by sex or parental genotype, so data were combined. Female Fischer 344 rats (Harlan) were 3–4 weeks old upon arrival. Animals were maintained on ad libitum food and water and a 12 h light/dark cycle, with lights on beginning at 7:00 A.M. Animals were housed in relatively small, quiet rooms for 3–4 weeks before studies began to minimize the stress associated with caretaking and colony management during the light phase. Mice were 3–6 months old and rats were 7–11 weeks old when tested. Young female Fischer rats were used because their relatively small size for rats was well accommodated by our conditioning apparatus. In all experiments, animals were tested only once. Studies were performed during the light phase, with most experiments taking place between 8:00 A.M. and 3:00 P.M. Studies were in accordance with NIH guidelines and had the approval of the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Pavlovian fear conditioning.
Adjacent to the training room, mice were placed in pairs into opaque plastic holding buckets (∼12 cm diameter) with bedding and covers for 30–60 min before being manipulated further. Animals were given a 3 min handling session each day for 2 d in the training room. Saline was injected at the end of handling each day. On the following day, training consisted of placing the animal in the conditioning apparatus (ENV-010MC with ENV-414S, Med Associates) for 2 min, after which an 84 dB, 4.5 kHz tone was activated for 30 s. Two seconds before the end of the tone, a 2 s, 1 mA footshock was delivered. The animal was returned to its home cage 30 s after shock and the apparatus was cleaned with Versa-Clean (Fisher). Contextual fear was tested for 5 min in the conditioning apparatus in the absence of the tone. Cued fear was tested in a separate cohort using a Plexiglas cylinder (21 cm diameter, 24 cm tall) with green wire grid floor and vertical green and white wall stripes 240° around. The cylinder was located in a different area of the training room, and was cleaned with lemon-scented Ajax after training each animal. After 2 min of recording baseline activity, the training tone was turned on for 3 min. Percentage freezing was estimated by scoring the presence or absence of nonrespiratory movement every 5 s. Tests were conducted 1 d after training except where indicated.
Instrumental fear conditioning.
Animals were handled as described above, except that rats were placed singly in holding buckets due to size constraints. Training consisted of placing an animal in the lighted chamber of the apparatus used for Pavlovian conditioning and timing its latency to fully enter (except for the tail) the dark chamber. Once the animal entered the dark chamber, the retractable partition separating the two chambers was lowered and a footshock was delivered for 2 s (0.30–0.35 mA for mice, 0.75 mA for rats). After 15 s the animal was returned to its home cage. Animals that did not enter the dark chamber after 100 s during conditioning were excluded (<4% of mice, independent of genotype). Testing was identical to training except that no shock was delivered and the partition remained up. Latencies to enter the dark chamber were recorded. If an animal did not enter the dark chamber within 10 min, it was returned to its cage and assigned a latency of 10 min. Tests were conducted 1 d after training except where indicated.
Restraint stress.
Restraint was chosen as a stressor because it was used in a previous study examining the effects of stress on the retrieval of inhibitory avoidance memory (Rashidy-Pour et al., 2004). Mice were handled as described above, except that they were placed individually into holding buckets because pilot studies indicated that this provided more consistent results. Mice were restrained by being placed into ventilated 50 ml plastic centrifuge tubes (2.8 cm diameter, Fisher) for 2 min (unless noted otherwise), 30 min before testing retrieval.
Drugs.
Fluticasone, betaxolol HCl, ICI 118,551 HCl, procaterol HCl, zinterol HCl (all from Tocris Bioscience), corticosterone (Sigma), and Org 34850 (Organon) were administered subcutaneously 30 min before testing for standard conditions, or 45 min before testing when animals were to be restrained 30 min before testing. Except for Cort, fluticasone, and Org 34850, drugs were dissolved in 0.9% saline [procaterol and zinterol also contained 0.1 mg/ml ascorbic acid, pH 7.4 (Sigma), to protect from oxidation] for systemic injection. Cort and fluticasone were dissolved in dimethylformamide (DMF) and diluted into saline (0.025–0.3% DMF), while Org 34850 was dissolved in 100% dimethyl sulfoxide (DMSO). Vehicle was either saline, saline containing 0.1 mg/ml ascorbic acid, saline containing 0.3% DMF, or 100% DMSO. Injection volumes were 10 μl/g except for Org 34850 and DMSO, which were 2 μl/g. For DH infusion, drugs were dissolved in the vehicles indicated above. Cort was diluted into saline so that final DMF was 0.1–1%. In additional DH experiments, Sp-8-Br-cAMPS (Biolog) and pertussis toxin (PTx, Tocris Bioscience) were dissolved in 0.9% saline. For cAMP levels, (±)-isoproterenol HCl (Sigma) was dissolved in artificial CSF (aCSF) containing 0.1 mg/ml ascorbic acid.
Dorsal hippocampus infusion.
A double guide cannula (C235 system, Plastics One) was implanted under pentobarbital anesthesia (72.5 mg/kg, i.p.) using a stereotax (SAS75/EM40M, Cartesian Research). The guide was placed 1.7 mm posterior to bregma and 1.5 mm bilateral for DH infusions. The guide projected 1.5 mm below the base and the dummy cannula extended 0.5 mm below the guide. One week after surgery, bilateral infusions were made into conscious mice while gently holding the nape of the neck. The dual injection cannula extended 0.9 mm below the guide. Infusions were 0.4 μl/min and the injection cannulae were left in place for 30 s before the mouse was returned to its home cage. Infusion volumes were 1 μl/side except for Cort, which was 0.5 μl/side. Because studies indicate that the effects of PTx are best evaluated 3 d after infusion (Goh and Pennefather, 1989; Stratton et al., 1989), PTx was infused 3 d before testing. To minimize potential effects of PTx on conditioning, training was administered the day after PTx infusion, and testing was performed 2 d after training. A pilot study indicated that 1 and 10 ng of PTx (comparable to or lower than what has been used by others) were similarly effective. Infusion location was assessed by infusing 1 μl of 1% methylene blue after behavioral testing. Methylene blue was observed in the center of the DH in all cases, with spread reaching each hippocampal subfield but not outside the DH, except for some in the cannula track, as previously shown (Murchison et al., 2004). Thus, all animals were included in the current study unless blockage of the injection cannula was observed immediately after drug infusion.
Corticosterone levels.
Mice were handled for 2 d as described above. Between 8:00 A.M. and 11:00 A.M. on the third day, some mice were either left untreated or received an injection of vehicle or Cort 30 min before retro-orbital blood collection using heparin-coated capillary tubes. Other mice were subjected to 2 min of restraint stress 30 min before collection. These mice were pretreated with either vehicle, 1 mg/kg ICI or 15–30 mg/kg Org 34850 15 min before restraint. Blood was placed on ice and then spun at 2000 × g at 4°C for 5 min and plasma was stored at −80°C. Cort was measured in duplicate by 125I-radioimmunoassay (MP Biomedicals) according to instructions.
cAMP levels.
Mice were decapitated without anesthesia and the brain was rapidly removed and chilled in ice-cold aCSF containing sucrose as described previously (Murchison et al., 2004). Brain slices transverse to the DH were cut at 400 μm and DH slices were surgically isolated and allowed to recover for at least 1 h at 37°C in oxygenated regular aCSF. Slices were then transferred to a second chamber containing isoproterenol (and 0.1 μm ICI when selective stimulation of β1 receptors in β3 KO slices was studied) in oxygenated aCSF at 37°C for 2 min, after which they were immediately placed on ice in 0.15 ml of 6% TCA with ∼4000 cpm of [3H]cAMP (adenosine 3′,5′-cyclic phosphate, ammonium salt, [2,8-3H]; PerkinElmer) and then homogenized using a Sonic Dismembrator 100 (Fisher). Pilot experiments indicated that 5 min incubations provided results similar to those for 2 min incubations, while 1 min incubations provided similar results or did not affect cAMP. Pilot incubations indicated that 0.01 μm isoproterenol did not affect cAMP levels. Extracts were centrifuged at 2500 × g at 4°C for 15min, and the pellet was stored at −80°C for performing Bradford assay to determine total protein. Supernatants were extracted four times with 0.6 ml of water-saturated ether, evaporated, reconstituted in 1 ml of assay buffer and stored at −20°C. Recovery was assessed by measuring the amount of [3H]cAMP in each sample, which ranged from 60 to 80%. To determine cAMP levels, a 125I-radioimmunoassay (PerkinElmer) was used using 100 μl of undiluted sample in duplicate according to instructions for the nonacetylated procedure. On the basis of the Bradford assay results, percentage recoveries and standard curves, a cAMP level (pmol/mg protein) was calculated for each slice. For each genotype, the mean for the slices incubated in aCSF alone was normalized to 100%. Actual values were (in pmol/mg protein): 102 ± 11 for β1/β2/β3 triple KO, 95 ± 15 for β1/β3 double KO, 88 ± 7 for β3 KO, and 109 ± 18 for β3 KO with 0.1 μm ICI.
Statistics.
Data were analyzed with Statistica 9.1 (StatSoft) using one- or two-way ANOVA with α = 0.05. The Bartlett χ2 test was used to analyze homogeneity of variances. Duncan's range test was used for post hoc analysis. Data are presented as mean ± SE. For all figures, *p < 0.05, ∧p < 0.01, and #p < 0.001. Comparisons marked as significant are to the reference group except where indicated by lines.
Results
Corticosterone and a GR agonist impair hippocampus-dependent memory retrieval
Most previous animal studies examined the impact of stress or glucocorticoids on the retrieval of instrumental fear memory (inhibitory avoidance) or spatial reference memory (Morris water maze). For comparison to previous studies (Roozendaal et al., 2004a,b), inhibitory avoidance was used in the current study. In addition, we asked whether the retrieval of Pavlovian contextual fear memory, like spatial reference memory, would be sensitive to glucocorticoids. An advantage of studying contextual fear memory is that one can also test cued fear memory that, unlike contextual fear memory, does not depend on the DH (Kim and Fanselow, 1992; Phillips and LeDoux, 1992). In cases where a manipulation affects contextual but not cued fear, non-mnemonic effects of the manipulation on fear or freezing behavior can be ruled out. Unless noted otherwise, animals were trained and then tested 1 d later with either stress or systemic injection administered 30 min before testing or DH infusion 15 min before testing.
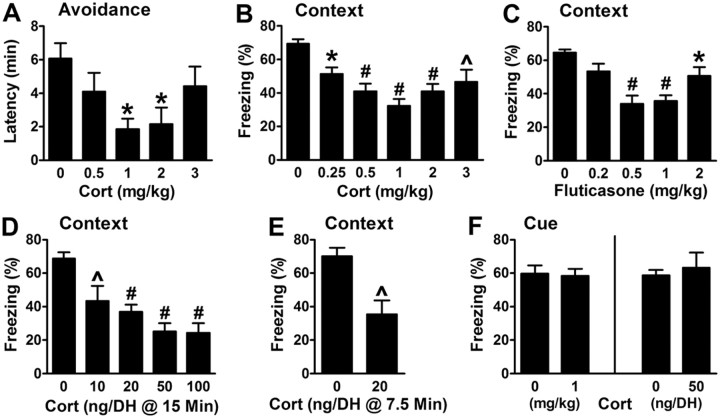
When Cort was administered subcutaneously 30 min before testing retrieval 1 d after training, mice exhibited lower avoidance latencies and reduced contextual freezing (Fig. 1A,B). In both, deficits followed a U-shaped dose–response, with 1 mg/kg Cort producing maximum impairment. To test whether Cort might act through a GR-like mechanism, the highly selective GR agonist fluticasone (Johnson, 1995) was administered subcutaneously before testing contextual fear. Like Cort, fluticasone impaired retrieval in a U-shaped manner (Fig. 1C). This observation extends previous results showing that a GR-selective agonist impairs memory retrieval for inhibitory avoidance and spatial navigation (Roozendaal et al., 2003).
Figure 1.
Corticosterone and a GR agonist impair hippocampus-dependent emotional memory retrieval. A, B, Systemic corticosterone (Cort) disrupts retrieval of inhibitory avoidance and contextual fear. F(4,50) = 3.3 and p = 0.018 for the main effect of dose on avoidance (9–18 per group), and F(5,26) = 7.7 and p = 0.0001 for the main effect of dose on freezing (5–7 per group). For all figures: systemic injection occurred 30 min before testing 1 d after training unless stated otherwise, and *p < 0.05, ∧p < 0.01, #p < 0.001 for post hoc comparisons. C, Fluticasone, a GR agonist, mimics the impairment of contextual fear by cort. F(4,21) = 8.7 and p = 0.0003 for the main effect of dose (5–6 per group). D, Infusion of cort into the DH impairs contextual fear. F(4,21) = 10.6 and p < 0.0001 for the main effect of dose (5–6 per group). For all figures, DH infusions occurred 15 min before testing 1 d after training unless stated otherwise. E, cort impairment of contextual fear is rapid, suggesting a non-genomic mechanism. cort was infused 7.5 min before testing 1 d after training. F(1,8) = 3.54 and p = 0.008 (5 per group). F, Neither subcutaneous injection nor DH infusion of cort affects cued fear (5–8 per group, p > 0.6 for both).
To corroborate the finding that glucocorticoids act in the hippocampus to impair retrieval, bilateral cannulae targeting the DH were implanted and mice were conditioned 1 week later. One day after training, either vehicle or Cort was infused bilaterally into the DH 15 min before testing. There was a dose-dependent decrease in contextual freezing that, at 50 ng, was similar to the magnitude observed after systemic treatment (Fig. 1D). In support of a rapid, non-genomic mechanism of action, DH infusion of 20 ng of Cort 7.5 min before testing contextual fear produced the same degree of impairment as 20 ng infused 15 min before testing (Fig. 1E).
Finally, to determine whether glucocorticoid impairment of contextual fear was specific to hippocampus-dependent memory retrieval, Cort was administered before testing cued fear. Neither systemic nor DH Cort impaired cued fear expression, indicating that these treatments do not act in a nonspecific manner to alter fear, freezing or hippocampus-independent memory retrieval (Fig. 1F).
Impairment of retrieval by glucocorticoids depends on β2 signaling but not NE/E
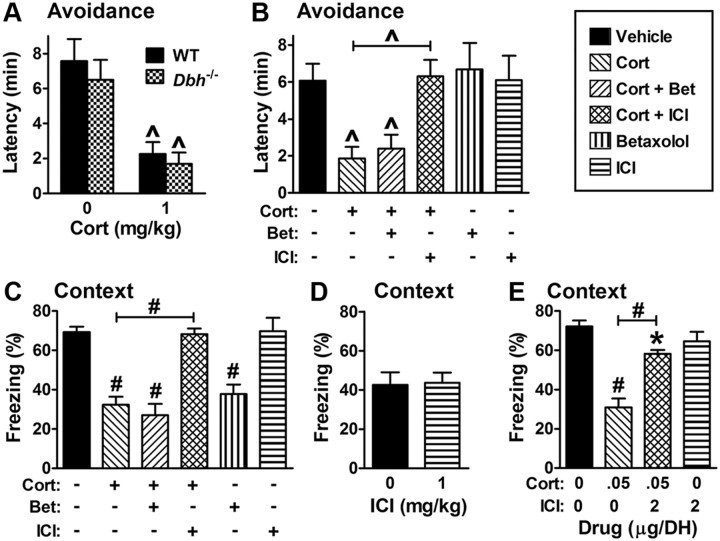
Several studies have suggested that glucocorticoid-induced impairment of memory retrieval may depend upon the release of NE in the CNS (Roozendaal et al., 1999, 2004a,b). To test this possibility, 1 mg/kg cort was administered systemically 30 min before testing retrieval to wild-type and dopamine β-hydroxylase knock-out (Dbh−/−) mice, which completely lack NE/E (Thomas et al., 1998). Instrumental fear memory retrieval was assessed because it is normal in Dbh−/− mice (Thomas and Palmiter, 1997), unlike the retrieval of contextual fear that depends on NE/E. Importantly, the degree of cort-induced impairment displayed in Dbh−/− mice was similar to that seen in control mice, suggesting that NE/E are not required for the impairing effects of exogenous glucocorticoids on retrieval (Fig. 2A).
Figure 2.
Corticosterone impairment of memory retrieval depends on β2 and not β1 signaling or NE/E. A, cort impairs inhibitory avoidance to a similar extent in control and NE/E-deficient Dbh−/− mice. The main effect of treatment is significant (F(1,28) = 25 and p < 0.0001; 7–19 per group), but the main effect of genotype and the interaction are not. B, C, Coinjection of cort with the β2 antagonist ICI 118,551 (ICI) prevents cort impairment of inhibitory avoidance and contextual fear, while coinjection of cort with the β1 antagonist betaxolol (Bet) does not. F(5,53) = 4.5 and p = 0.002 for the main effect of treatment on latency (6–18 per group), and F(5,29) = 16.6 and p < 0.0001 for the main effect of treatment on freezing (5–7 per group). D, ICI does not enhance low levels of contextual fear in the absence of cort (5 per group, p = 0.9). Animals were trained with lower shock intensity (0.35 mA) to approximate cort-impaired freezing. E, Coinfusion of ICI into the DH prevents disruption of contextual fear by cort. F(3,13) = 23 and p < 0.0001 for the main effect of treatment (5 per group).
Previous results using the β1-selective antagonist atenolol and the β1-selective agonist xamoterol suggest that disruption of memory retrieval by glucocorticoids depends on β1 signaling (Roozendaal et al., 2004b). Those findings are in direct contrast to observations indicating that β1 signaling is necessary for the retrieval of an intermediate phase of contextual and spatial memory (Murchison et al., 2004). One potential resolution for this discrepancy is that atenolol and xamoterol affect receptors in addition to β1 at higher doses, and these other receptors may be responsible for the effects of these drugs on glucocorticoid-induced retrieval impairment. A candidate for this other receptor is the β2-adrenergic receptor, which is closely related to the β1 receptor and is also relatively abundant in the CNS.
To distinguish between effects mediated by β1 and β2 receptors, we initially administered either the β1 antagonist betaxolol, the β2 antagonist ICI 118,551 (ICI), or a combination of 1 mg/kg cort and the β1 or β2 antagonist to wild-type mice shortly before testing instrumental or contextual fear. Studies using rat brain tissue indicate that betaxolol and ICI are among the most selective for discriminating between β1 and β2 receptors (Tsuchihashi et al., 1990). Betaxolol was used at 1 mg/kg because this is the lowest dose that mimics the impairment of contextual memory retrieval observed in β1 KO mice (Murchison et al., 2004). ICI was used at 1 mg/kg based on results from the current study (see Fig. 3C). While having no effect on retention latencies for instrumental fear, the β1 antagonist betaxolol impaired contextual fear as expected, indicating that the dose used was sufficient to block β1 receptors (Fig. 2B,C). At the same dose, the β2 antagonist ICI did not alter memory retrieval in either test. Notably, coinjection of the β1 antagonist with cort did not prevent cort-induced impairment of retrieval in either paradigm. In contrast, coadministration of the β2 antagonist with cort completely mitigated cort-induced disruption of memory retrieval in both paradigms.
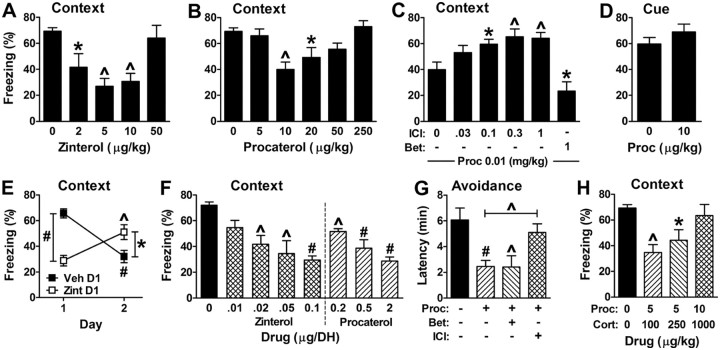
Figure 3.
Direct stimulation of β2 receptors disrupts memory retrieval. A, B, G, The β2 agonists zinterol and procaterol disrupt contextual fear (5–6 per group) and inhibitory avoidance (7–22 per group) retrieval. F(4,20) = 6.4 and p = 0.0017, and F(5,26) = 6 and p = 0.0008 for the main effect of treatment on contextual fear for zinterol and procaterol, respectively. F(3,50) = 3.6 and p = 0.02 for the main effect of treatment on inhibitory avoidance. C, G, The impairment of contextual fear (5–6 per group) and inhibitory avoidance retrieval by procaterol (Proc, 10 μg/kg for G) is blocked by coadministration of a β2 antagonist (ICI, 1 mg/kg for G) but not a β1 antagonist (Bet, 1 mg/kg). F(5,29) = 8.3 and p < 0.0001 for the main effect of treatment on contextual fear. D, Procaterol does not affect retrieval of cued fear (5–8 per group, p = 0.27). E, Retrieval of hippocampus-dependent memory is impaired by zinterol. Vehicle (Veh) or zinterol (Zint, 5 μg/kg) was injected 30 min before testing 1 d after training. As expected, zinterol impaired contextual fear memory relative to vehicle. The mice were tested again the next day without injection. Mice treated with vehicle on Day 1 exhibited significantly lower freezing on Day 2 compared with Day 1, consistent with the extinction of contextual fear. Mice treated with zinterol on Day 1 exhibited significantly higher freezing on Day 2 compared with Day 1, and compared with vehicle-treated mice on Day 2, indicating that extinction was minimal. Because it is not necessary to express contextual fear to obtain extinction (Ouyang and Thomas, 2005), the results suggest that zinterol impairs retrieval rather than the expression of contextual fear. There were 5 mice for Veh and 10 mice for Zint. F(1,26) = 25.4 and p < 0.0001 for the interaction of treatment and day. The main effects of treatment and day were not significant. F, Infusion of the β2 agonist procaterol or zinterol into the DH impairs contextual fear retrieval. F(7,35) = 5.9 and p = 0.0002 for the main effect of treatment (5 per group). H, When coadministered, cort and procaterol impair contextual fear retrieval at considerably lower doses than when either is given individually. F(3,18) = 4.6 and p = 0.014 for the main effect of treatment (5–7 per group).
To determine whether the β2 antagonist ICI nonspecifically elevates freezing when freezing is relatively low, wild-type mice were conditioned using a less intense shock (0.35 mA) and saline or 1 mg/kg ICI was administered 30 min before testing. Contextual fear did not differ between groups, indicating that ICI does not enhance fear or freezing per se (Fig. 2D). Finally, because cort acts in the DH to impair retrieval, we predicted that ICI, when coinfused with cort into the DH, would also block the impairment of retrieval. Indeed, when infused 15 min before testing contextual fear, ICI alone did not affect retrieval, but it substantially reduced the disruption of retrieval by cort (Fig. 2E).
Direct stimulation of β2 receptors impairs memory retrieval
Given that a β2 antagonist blocks the effect of cort, we asked whether selectively stimulating β2 receptors with standard agonists would disrupt memory retrieval. Toward this goal, the β2 agonist zinterol or procaterol was administered systemically to wild-type mice before testing contextual fear. Zinterol and procaterol are among the most potent and selective agonists for β2 relative to β1 receptors (Meunier and Labrie, 1982; Waelbroeck et al., 1983; Beer et al., 1988). Like cort, each β2 agonist reduced freezing with a U-shaped dose–response, with 5–10 μg/kg producing maximum impairment (Fig. 3A,B). Further, the disruption of memory retrieval by procaterol was blocked by the β2 antagonist ICI but not the β1 antagonist betaxolol (Fig. 3C). Like cort, the β2 agonist procaterol did not affect cued fear, indicating that the effect of procaterol is on contextual memory rather than fear or freezing per se (Fig. 3D).
Although motivation and performance effects were excluded above, it remained possible that stimulating β2 receptors affects contextual fear memory by impairing either retrieval itself or expression of the retrieved memory. To distinguish between these possibilities, extinction of contextual fear memory was examined. If β2 stimulation impairs retrieval 1 d after training, then testing memory again the next day (second day after training) should demonstrate normal contextual fear without extinction. If β2 stimulation impairs expression but not retrieval, then it is predicted that testing memory again on the second day will demonstrate reduced (extinguished) contextual fear. In performing similar studies examining the role of NE in retrieval and extinction, we demonstrated that it is necessary to retrieve contextual fear memory for extinction to occur, but it is not necessary to express contextual fear memory for extinction to occur (Ouyang and Thomas, 2005).
When vehicle was administered shortly before testing on the day after training, freezing was high, as expected (Fig. 3E). When these mice were tested again the next day (no injection), freezing was significantly lower. In contrast, when the β2 agonist zinterol was administered shortly before testing on the day after training, freezing was low, as expected. When these mice were tested again the next day (no injection), freezing was significantly higher compared with the first day of testing and to the vehicle-injected mice on the second day of testing. The results are consistent with the idea that zinterol impairs memory retrieval rather than memory expression.
Because systemic β2 agonists impaired the retrieval of contextual but not cued fear memory, a likely site of action for these drugs is the DH. Indeed, we found that contextual retrieval was impaired when β2 agonists were infused directly into the DH 15 min before testing (Fig. 3F). To extend and confirm the specificity of the β2 agonist effect for instrumental fear memory, the β2 agonist procaterol alone or combined with a β1 or β2 antagonist was administered systemically shortly before testing. We observed an impairment of retrieval by procaterol, and this effect was blocked by the β2 antagonist ICI but not the β1 antagonist betaxolol (Fig. 3G). Finally, we asked whether cort and a β2 agonist might interact to affect retrieval. At low doses that did not affect retrieval when given alone, combined cort and procaterol treatment significantly impaired contextual retrieval (Fig. 3H). In contrast, at doses that maximally affected retrieval when given alone, combined cort and procaterol treatment had no effect on retrieval. In other words, when the drugs were combined, a U-shaped dose–response was still observed, but with a leftward shift in potency.
Genetic and interspecies corroboration that β2 signaling mediates impairment of retrieval
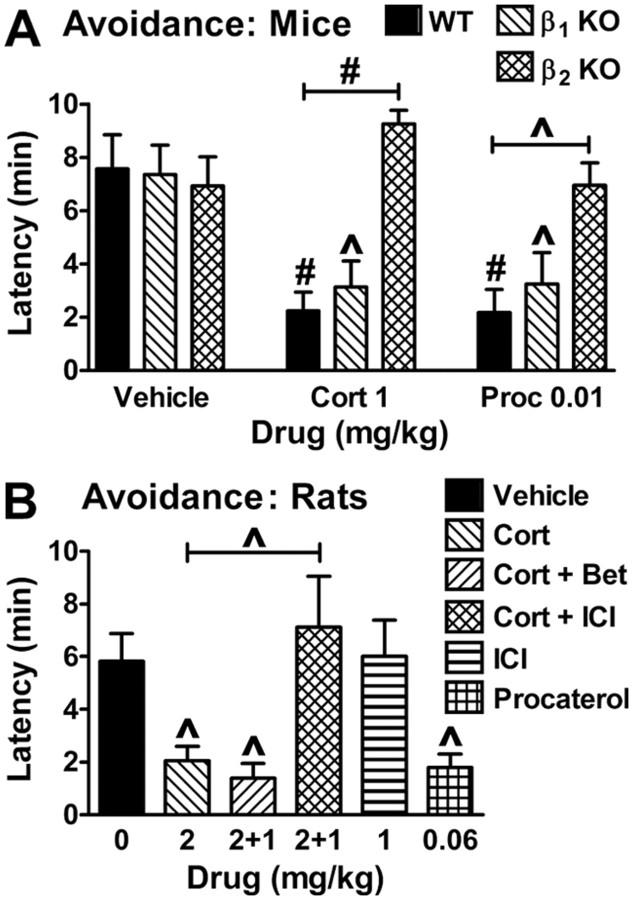
To corroborate the pharmacologic data, the effects of cort and procaterol in mice lacking either β1-or β2-adrenergic receptors were examined. Instrumental fear was examined because β1 KO mice exhibit impaired contextual memory retrieval in the absence of treatment (Murchison et al., 2004). Compared with wild-type mice, both KO lines displayed normal conditioned avoidance, allowing the examination of drug effects (Fig. 4A). cort and the β2 agonist procaterol impaired avoidance similarly in wild-type and β1 KO mice, indicating that β1 signaling is not required for the disruption of retrieval. In contrast, neither cort nor procaterol impaired avoidance in β2 KOs, indicating that β2 receptors are necessary for impairment.
Figure 4.
Genetic and interspecies corroboration that β2 signaling mediates impairment of retrieval. A, cort or procaterol (Proc) disrupts retrieval of inhibitory avoidance in β1 KO but not β2 KO mice. F(2,73) = 8 and p = 0.0008 for the main effect of treatment; F(2,73) = 11.7 and p < 0.0001 for the main effect of genotype; and F(4,71) = 4.5 and p = 0.003 for the interaction of treatment and genotype (7–10 per group). B, cort (2 mg/kg) or procaterol (0.06 mg/kg) impair retrieval of inhibitory avoidance in rats. cort-induced disruption of retrieval is prevented by coadministration of the β2 antagonist ICI (1 mg/kg) but not the β1 antagonist betaxolol (Bet, 1 mg/kg). F(5,37) = 5.6 and p = 0.0006 for the main effect of treatment (5–10 per group).
While our studies using mice were driven in part by the existence of genetic models, most other animal studies investigating the impairment of memory retrieval by cort have used rats. To determine whether our results implicating β2 receptors in the impairment of retrieval might be species-specific, rats were treated with the same pharmacologic reagents that were used in mice. In rats, systemic injections of cort or the β2 agonist procaterol disrupted conditioned avoidance 1 d after training (Fig. 4B). While similar doses of cort (2 mg/kg) reduced retention latencies equally in mice and rats, somewhat higher doses of procaterol were needed in rats to produce the same degree of impairment. Differences in the pharmacologic properties of procaterol between species might account for this. In rats, the effect of cort was blocked by coadministration of a β2 but not a β1 antagonist, indicating that glucocorticoid disruption of retrieval via β2 signaling is shared across species.
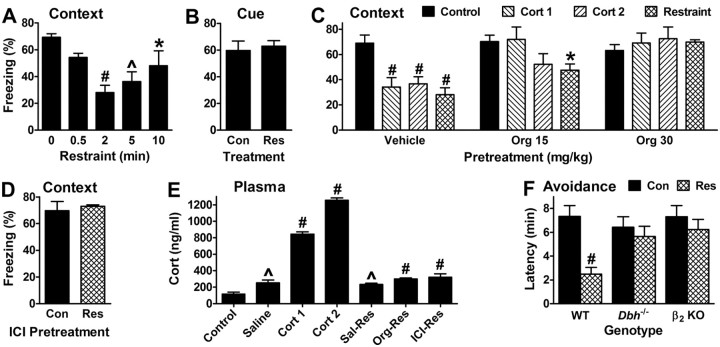
Impairment of memory retrieval by stress requires NE/E and β2 signaling
Like cort, acute stress disrupts hippocampus-dependent memory retrieval in rats (de Quervain et al., 1998). To verify and extend these findings, we subjected wild-type mice to inescapable restraint of various durations 30 min before testing contextual fear. Restraint stress reduced freezing in a U-shaped manner, and at durations of 2 and 5 min, impaired retrieval to an extent similar to that for treatment with cort or a β2 agonist (Fig. 5A). Cued fear was not affected by 2 min restraint, indicating that restraint does not alter fear or freezing per se (Fig. 5B). To test whether restraint impairs retrieval through a mechanism consistent with GR activation, mice were pretreated with the GR antagonist Org 34850 (Org) 15 min before restraint or cort, which were administered 30 min before testing retrieval. At 15 mg/kg, Org completely blocked the effect of treatment with 1 mg/kg cort, and partially blocked the effects of 2 mg/kg cort and restraint (Fig. 5C). At 30 mg/kg, Org fully blocked both cort- and restraint-induced retrieval deficits. That relatively high systemic doses of Org are required for antagonism is consistent with the limited CNS penetration of Org (Bachmann et al., 2003). To test whether stress-induced retrieval deficits depend on β2 signaling, a 1 mg/kg dose of the β2 antagonist ICI was administered 15 min before restraint. ICI completely blocked the effects of restraint on retrieval (Fig. 5D). Further, the elevated levels of plasma cort observed 30 min following restraint were not reduced by pretreatment with ICI or Org, indicating that these compounds do not impair the release of cort (Fig. 5E).
Figure 5.
Impairment of memory retrieval by stress depends on NE/E and β2 signaling. A, Inescapable restraint stress 30 min before testing disrupts contextual fear retrieval, with maximal reduction at intermediate durations. F(4,20) = 5.6 and p = 0.003 for the main effect of duration (5–7 per group). B, Restraint does not affect cued fear (5 per group, p = 0.7). Controls (Con) were injected with vehicle 30 min before testing. C, Pretreatment with the GR antagonist, Org 34850 (Org, 30 mg/kg), 15 min before cort or 2 min restraint stress prevents disruption of contextual fear retrieval. F(2,58) = 17.9 and p < 0.0001 for the main effect of pretreatment; F(3,57) = 4.7 and p = 0.006 for the main effect of treatment; and F(6,54) = 3.7 and p = 0.004 for the interaction of pretreatment and treatment (5–9 per group). D, Pretreatment with the β2 antagonist ICI 15 min before 2 min restraint stress prevents disruption of contextual fear retrieval (5–9 per group, p = 0.65). E, Plasma cort levels rise significantly 30 min after the systemic injection of saline or cort (1 or 2 mg/kg) or restraint (Res). Pretreatment with either Org (30 mg/kg) or ICI (1 mg/kg) 15 min before 2 min restraint stress does not prevent the rise in cort. F(6,46) = 221 and p < 0.0001 for the main effect of treatment (7–8 per group). F, Restraint stress impairs inhibitory avoidance retrieval in WT but not Dbh−/− or β2 KO mice. Mice were either injected with saline or subjected to 2 min restraint 30 min before testing. F(1,89) = 7.9 and p = 0.006 for the main effect of restraint, and F(2,88) = 3.4 and p = 0.038 for the interaction of restraint and genotype (12–23 per group); the main effect of genotype was not significant. The avoidance of restrained Dbh−/− or β2 KO mice was not significantly different from that of their respective controls (p > 0.5 for both).
Interestingly, plasma levels of cort following cort administration or stress did not correspond well with the impairment of retrieval. For example, restraint doubled cort over basal levels, and this level was comparable to that following injection of saline (Fig. 5E). Yet restraint impaired retrieval while saline injection did not. Further, injection of cort at doses that impair retrieval resulted in plasma levels that were 3- to 4-fold greater than those for restraint. Restraint stress activates stress-response systems in addition to that for glucocorticoids, which led us to test whether NE/E might contribute to the impairing effects of restraint on retrieval, even though the impairing effects of exogenous cort are independent of NE/E. Restraint significantly impaired retrieval of instrumental fear in wild-type mice (Fig. 5F). In contrast, restraint did not impair retrieval in Dbh−/− mice. Further supporting of a role for β2 receptors, restraint also did not impair retrieval in β2 KO mice.
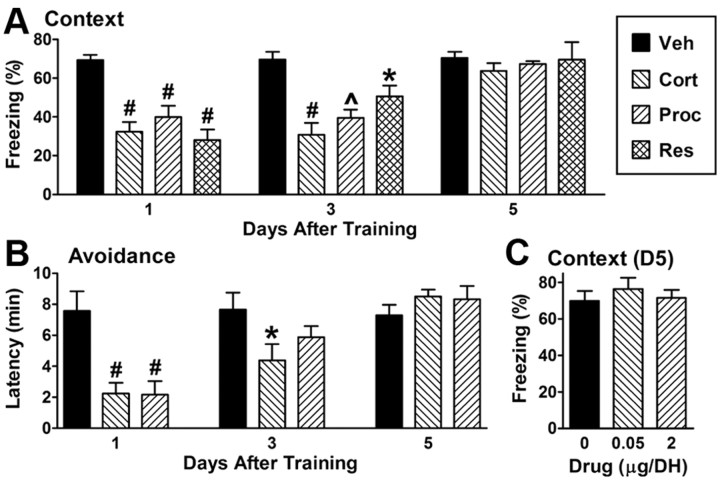
Corticosterone, β2 signaling and stress impair retrieval in a time-limited manner
NE, β1, cAMP and PKA signaling all have time-limited roles in hippocampus-dependent emotional memory retrieval (Murchison et al., 2004; Ouyang et al., 2008). For example, NE/E-deficient mice display deficits in contextual fear 2 h-4 d but not at 1 h or 5 or more days after training, and mice and rats treated with a β antagonist show compromised contextual fear and spatial navigation over a similar time course. Therefore, we determined whether glucocorticoids, β2 signaling and stress might also have time-limited effects on retrieval. In vehicle-injected mice, contextual and instrumental fear did not differ between days 1, 3 and 5 after training (Fig. 6A,B). However, the effects of cort, procaterol and restraint stress were all time-sensitive, being present on days 1 and 3 after training but absent on day 5. DH infusion of cort or procaterol 5 d after training also had no effect on retrieval (Fig. 6C).
Figure 6.
Corticosterone, β2 signaling and stress inhibit hippocampus-dependent memory retrieval in a time-limited manner. A, Relative to vehicle (Veh) injection, cort (1 mg/kg), procaterol (Proc, 10 μg/kg) or restraint (Res, 2 min) impairs contextual fear retrieval at 1 and 3 but not 5 d after training (separate groups of animals for each time point). F(2,70) = 22 and p < 0.0001 for the main effect of day; F(3,69) = 13.1 and p < 0.0001 for the main effect of treatment; and F(6,66) = 3.2 and p = 0.008 for the interaction of day and treatment (5–12 per group). B, cort (1 mg/kg) or procaterol (10 μg/kg) impairs inhibitory avoidance retrieval at 1 d, partially impairs retrieval at 3 d, and does not impair retrieval at 5 d after training (separate groups of animals for each time point). F(2,85) = 13.4 and p < 0.0001 for the main effect of day; F(2,85) = 5.7 and p = 0.007 for the main effect of treatment; and F(4,83) = 4.1 and p = 0.007 for the interaction of day and treatment (7–20 per group). C, Neither infusion of cort nor procaterol into the DH affects contextual fear retrieval 5 d after training. F(2,12) = 0.38 and p = 0.7 (5 per group).
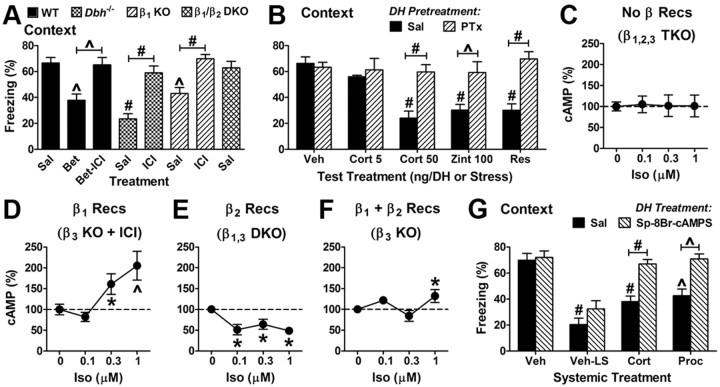
By coupling to Gi/o proteins, β2 suppresses cAMP and opposes the role of β1 in retrieval
To obtain more direct evidence that β1 and β2 receptors oppose each other in retrieval, we asked whether interfering with β2 signaling would rescue retrieval when β1 signaling was blocked or absent. As expected, retrieval was impaired when wild-type mice were treated with a 1 mg/kg dose of the β1 antagonist betaxolol shortly before testing contextual fear 1 d after training (Fig. 7A). However, when a 1 mg/kg dose of the β2 antagonist ICI was combined with betaxolol, no effect on retrieval was observed, i.e., the β2 antagonist reversed the deficit caused by the β1 antagonist. Similarly, 1 mg/kg ICI reversed the retrieval deficits present in Dbh−/− and β1 KO mice. Last, β1/β2 double KO mice exhibited normal contextual fear, providing additional genetic support for the pharmacologic data.
Figure 7.
β2 signaling opposes β1 signaling in retrieval and in cAMP production via Gi/o. A, The impairment of contextual fear retrieval by betaxolol (Bet, 1 mg/kg, 30 min before testing 1 d after training) is prevented by concurrent administration of ICI (1 mg/kg). The deficits in contextual fear retrieval exhibited by Dbh−/− and β1 KO mice 1 d after training are reversed by treatment with ICI 30 min before testing. Finally, β1/β2 double KO (DKO) mice do not exhibit a deficit in contextual fear. F(7,68) = 7.1 and p < 0.0001 for the main effect of group (5–16 per group). B, Infusion of PTx (10 ng) into the DH 1 d before training prevents the impairment of contextual fear retrieval by cort (50 ng), zinterol (100 ng), or restraint (2 min). Restraint was administered 30 min and cort and zinterol were infused 15 min before testing, which was 2 d after training. F(1,48) = 35 and p < 0.0001 for the main effect of pretreatment; F(4,45) = 5.6 and p = 0.001 for the main effect of treatment; and F(4,45) = 5.6 and p = 0.001 for the interaction of pretreatment and treatment (5 per group). C–F, Hippocampal slices were incubated in aCSF with various concentrations of the nonselective β receptor agonist Iso. C, Iso was without effect on cAMP in slices from β1/β2/β3 triple KO (TKO) mice. F(3,21) = 0.01 and p = 1.0 for the main effect of concentration (5–10 per group). D, Selective stimulation of β1 receptors by Iso at 0.3 and 1 μm increased cAMP in slices from β3 KO mice treated with the β2 antagonist ICI. F(3,34) = 7.2 and p = 0.0008 for the main effect of concentration (7–15 per group). E, Selective stimulation of β2 receptors by Iso decreased cAMP in slices from β1/β3 DKO mice. F(3,48) = 5.2 and p = 0.001 for the main effect of concentration (8–35 per group). F, Combined stimulation of β1 and β2 receptors by Iso at 1 μm modestly elevated cAMP levels in slices from β3 KO mice. F(3,38) = 4.1 and p = 0.013 for the main effect of concentration (8–17 per group). G, DH infusion of the cAMP agonist Sp-8-Br-cAMPS (2 μg) prevents retrieval impairment induced by systemic cort (1 mg/kg) or procaterol (10 μg/kg). Veh is systemic injection of vehicle 30 min before testing mice trained with normal shock intensity (1 mA), while Veh-LS is systemic injection of vehicle 30 min before testing mice trained with low shock intensity (0.3 mA). F(3,52) = 26 and p < 0.0001 for the main effect of systemic treatment; F(1,54) = 21 and p < 0.0001 for the main effect of DH treatment; and F(3,52) = 2.9 and p = 0.043 for the interaction of systemic and DH treatment (5–10 per group).
Next, we sought to determine the mechanism by which β2 signaling impairs retrieval. The canonical view is that β-adrenergic receptors couple to and signal via the adenylyl cyclase-stimulatory G-protein Gs. For example, it is through Gs and cAMP that β receptors are currently proposed to enhance synaptic plasticity and memory consolidation (Sara, 2009; Tully and Bolshakov, 2010). However, β2 receptors can signal through Gi/o in the heart (Xiao et al., 1995; Daaka et al., 1997; Devic et al., 2001). Because cAMP signaling is necessary and sufficient for contextual memory retrieval mediated by NE and β1 receptors (Ouyang et al., 2008), we asked whether β2 signaling might oppose β1 signaling in retrieval by activating Gi/o and inhibiting adenylyl cyclase.
To test this idea, PTx was used because it uncouples Gi/o proteins from their receptors by ADP-ribosylation of Gi/o. Because PTx can take several days to be fully effective in the hippocampus in vivo (Goh and Pennefather, 1989; Stratton et al., 1989), we infused PTx into the DH 1 d before fear conditioning to minimize any potential effects on acquisition and consolidation. Mice were then tested for retrieval 2 d after conditioning. Pretreatment with 10 ng of PTx did not affect retrieval in mice infused with vehicle 15 min before testing (Fig. 7B). Strikingly, however, PTx pretreatment completely prevented the impairment of retrieval by 50 ng/DH cort, 100 ng/DH of the β2 agonist zinterol, and 2 min restraint stress. A lower dose of cort (5 ng/DH) was also examined to determine whether PTx pretreatment might shift the dose–response relationship to the left (i.e., more sensitive to cort), but no evidence for this was observed.
Given the results with PTx, we then asked what effect selectively stimulating either β1 or β2 receptors would have on cAMP levels in the DH. To avoid the potential confounds of anesthesia and decapitation on cAMP levels following DH infusion, as well as to more rigorously control the concentration and duration of agonist exposure, DH slices were used. Slices were incubated in aCSF with or without the nonselective β agonist isoproterenol (Iso). To selectively stimulate β1 receptors, slices from β3 KO mice were treated with Iso plus the β2 antagonist ICI. To selectively stimulate β2 receptors, slices from β1/β3 double KO mice were treated with Iso. To stimulate both β1 and β2 receptors, slices from β3 KO mice were treated with Iso. As a control, slices from β1/β2/β3 triple KO mice were treated with Iso. Iso at 0.1–1 μm had no effect on cAMP in the triple KO slices (Fig. 7C). As expected, Iso at 0.3–1 μm increased cAMP when β1 receptors were selectively stimulated (Fig. 7D). In contrast, Iso at 0.1–1 μm caused cAMP to decrease when β2 receptors were selectively stimulated (Fig. 7E). Finally, when β1 and β2 receptors were stimulated simultaneously, only a modest increase in cAMP was observed at 1 μm Iso (Fig. 7F). Together, the results indicate that β1 and β2 receptors functionally oppose one another with respect to cAMP levels in the DH.
Based on the cAMP results, we asked whether augmenting cAMP signaling would prevent retrieval impairment induced by agonists of GR or β2. The cAMP analog Sp-8-Br-cAMPS, at a dose (2 μg/DH) that rescues retrieval in Dbh−/− mice (Ouyang et al., 2008), was infused into the DH immediately before systemic injection of 1 mg/kg cort or a 10 μg/kg dose of the β2 agonist procaterol 30 min before testing. Mice treated with cort or procaterol and infused with the cAMP analog demonstrated significantly improved memory retrieval relative to similarly treated mice infused with saline (Fig. 7G). In contrast, mice trained with the same or a lower shock intensity did not exhibit significantly enhanced freezing when infused with Sp-8-Br-cAMPS relative to saline.
Discussion
The current study examines the roles of NE/E and β-adrenergic signaling in mediating the effects of cort and stress on DH-dependent memory retrieval. We found that cort impairs retrieval of inhibitory avoidance in the absence of NE/E (Fig. 2A), suggesting that NE/E may not be required for this effect of cort. This idea is contrary to the hypothesis that glucocorticoids impair retrieval by enhancing the release of NE (Roozendaal et al., 1999, 2004a,b). In contrast, we found that stress does not impair retrieval of inhibitory avoidance in the absence of NE/E (Fig. 5F), suggesting that NE/E contribute to this effect of stress. The reduced effect of stress in Dbh−/− mice suggests that compensation for the absence of NE/E is an unlikely explanation for the normal response to cort in these mice.
Relevant to the above, high plasma levels of cort were required to maximally impair retrieval when cort was administered before testing (Fig. 5E). In contrast, cort levels following stress were only moderately elevated, and were similar to those observed following saline injection, which does not impair retrieval. We hypothesized that stress-response systems in addition to glucocorticoids are engaged following restraint, and that these other factors synergize with glucocorticoids to impair retrieval. The absence of the stress effect on retrieval in Dbh−/− mice (Fig. 5F) suggests that NE/E are among these factors. Our data indicate that cort and a β2 agonist have considerably more potent effects on retrieval when combined (Fig. 3H), suggesting that NE/E and glucocorticoids released during stress may act in concert to impair retrieval at hormone levels that would be innocuous individually. Consistent with the above, extracellular levels of NE in the DH are significantly elevated 30 min after restraint, whereas they remain unchanged 30 min after saline injection (Abercrombie et al., 1988; Vahabzadeh and Fillenz, 1994).
Although we confirmed that glucocorticoids and NE/E contribute to the impairment of retrieval by stress, the mechanism by which these factors impair retrieval is unclear. Pharmacologic evidence suggested that glucocorticoids and NE/E impair retrieval by activating β1 receptors (Roozendaal et al., 2004b), despite β1 signaling being required for retrieval (Murchison et al., 2004). In the current study, we found that glucocorticoids impair retrieval in the presence of a β1 antagonist (mice and rats) and in β1 KO mice (Figs. 2, 4), and that low doses of β2 receptor agonists impair retrieval in mice and rats (Figs. 3, 4). Finally, the impairing effects of cort, β2 agonists and stress on retrieval were greatly reduced in the presence of a β2 antagonist (mice and rats) and in β2 KO mice (Figs. 2–).
The data implicating β1 receptors in the impairment of retrieval were based in part on the use of the β1-selective agonist xamoterol (Roozendaal et al., 2004b). In that study, doses of 3 and 10 mg/kg were used to demonstrate retrieval impairment. In contrast, xamoterol fully rescues retrieval in Dbh−/− mice at 1 mg/kg. We hypothesized that higher doses of xamoterol activate β2 (as well as β1) receptors, and that activation of β2 receptors impairs retrieval. In support of this, we found that the impairment of retrieval by 6 mg/kg xamoterol is blocked by a β2 antagonist and β2 KO mice, while impairment persists in the presence of a β1 antagonist and in β1 KO mice (Schutsky et al., 2011).
Classically, β-adrenergic receptors couple to the adenylyl cyclase-stimulating G-protein Gs (Sara, 2009; Tully and Bolshakov, 2010). Given that β1 receptors and cAMP signaling in the hippocampus are required for retrieval (Murchison et al., 2004; Ouyang et al., 2008), it was not apparent how β2 receptors would impair retrieval. One possibility is that these two receptor subtypes are expressed on different cells or in distinct subcellular compartments, where increases in cAMP would have opposite effects on retrieval. However, a second possibility was suggested by studies examining cardiac β2 receptors, which can couple to the PTx-sensitive, adenylyl cyclase-inhibiting G-protein Gi (Xiao et al., 1995; Daaka et al., 1997; Devic et al., 2001).
In support of a similar mechanism occurring in the brain, uncoupling Gi/o proteins from their receptors with PTx blocked the impairing effects of cort, a β2 agonist and stress on retrieval (Fig. 7B). Further, cAMP levels in DH slices were increased by selectively stimulating β1 receptors, but were decreased by selectively stimulating β2 receptors (Figs. 7D,E). When β1 and β2 receptors were stimulated simultaneously, only a modest increase in cAMP was observed (Fig. 7F). Thus, β2 receptors can couple to Gi/o in the hippocampus, cause decreases in cAMP levels and oppose the signaling and cognitive actions of β1 receptors. These mechanisms may help explain the recent observations that stimulating β1 or β2 receptors in the prefrontal cortex impairs or enhances working memory, respectively (Ramos et al., 2005, 2008).
Our results are consistent with the finding that cAMP accumulation in DH slices by isoproterenol is inhibited by a β1 but not a β2 antagonist (Fowler and O'Donnell, 1988), and that a β1- but not a β2-selective agonist increases cAMP in cortical neurons (Zhang et al., 2005). Importantly, these and our findings were generated in the absence of a phosphodiesterase inhibitor. In the presence of a phosphodiesterase inhibitor, a condition used in many studies examining cAMP signaling, a β2-selective agonist increases cAMP (Zhang et al., 2005). Finally, our finding that infusion of an analog that mimics cAMP signaling prevents the impairment of retrieval induced by cort or a β2 agonist suggests that these manipulations suppress DH cAMP in vivo (Fig. 7G).
Results from many studies suggest that important interactions underlie the cognitive effects of cort and NE (Hurlemann, 2008; Joëls and Baram, 2009; Roozendaal et al., 2009). Our results suggest that this occurs at least in part at the level of β2-adrenergic signaling, which appears to be downstream of glucocorticoid signaling because a β2 antagonist blocks the impairing effect of cort (Figs. 2, 4). It is interesting to consider how glucocorticoid signaling might activate β2 receptors, given that the effect of cort on retrieval does not require NE/E (Fig. 2A). One possibility is that either cort or GR interact directly with the β2 receptor to cause activation. Another possibility is that cort and GR signal through an intermediary that leads to the activation of β2 signaling. In each of these cases, the β2 receptor would be a key effector for the rapid, non-genomic effects of glucocorticoids on retrieval.
Agonists of GR and β2 receptors tested here exhibited U-shaped dose–response curves for retrieval impairment when given systemically (Figs. 1, 3). These findings suggest that moderate levels of stress can affect memory, but higher levels of stress might have less of an effect. Indeed, we found that 10 min of restraint impaired retrieval less than 2 or 5 min of restraint (Fig. 5A). The reason why impairment dissipates with higher doses is not clear. It is possible that higher levels of stimulation result in more rapid desensitization of β2 signaling, or that the recruitment of additional signaling pathways by β2 receptors mitigates signaling induced by lower levels of stimulation.
In addition to the level of stress being an important determinant of whether effects on memory retrieval are observed, our data demonstrate that the duration between acquisition and retrieval is an important factor (Fig. 6). The time course for the effects of cort, β2 signaling and stress on retrieval is essentially identical to that for the requirement of NE, β1, cAMP and PKA signaling in retrieval (Murchison et al., 2004; Ouyang et al., 2008), i.e., an intermediate phase of memory (∼2 h to 4 d) is susceptible. For comparison, other studies using rodents or humans that report GR agonist or stress effects on retrieval have examined memory 1–3 d after acquisition.
A potentially important aspect of β-adrenergic receptor physiology is that NE is approximately an order of magnitude less potent in activating β2 relative to β1 receptors (Zhang et al., 2004). As a result, it seems likely that moderately arousing conditions would facilitate retrieval through the release of NE in the DH to activate β1 receptors, while strongly arousing conditions typical of “stress” would impair retrieval through the release of glucocorticoids and additional NE that together would activate β2 receptors. Because strongly arousing conditions enhance memory consolidation, this could temporarily facilitate a shift away from processes favoring retrieval toward those favoring consolidation of new information.
Finally, our findings are likely relevant to situations in which stress negatively impacts human cognition. Examinations and public speaking often result in anticipatory arousal and stress that can interfere with cognitive performance. These tasks usually depend on the retrieval of recently learned or reviewed information that is likely susceptible to the impairing effects of stress. Interestingly, the nonselective β antagonist propranolol is reported to enhance cognitive flexibility and performance on examinations (Brewer, 1972; Faigel, 1991; Alexander et al., 2007). Our findings suggest that the effect of propranolol is due to its blockade of the β2 receptor, and that a selective β2 antagonist would be similarly efficacious.
Footnotes
This work was supported by NIH Grant 5R01MH063352 and Department of Defense Grant PT075099 to S.A.T., and NIH Grant 5T32GM008076 to R. Pittman (principal investigator). We thank Dainippon Sumitomo Pharma (Osaka, Japan) for their generous gift of l-threo-3,4-dihyroxyphenylserine, Organon (Newhouse, UK) for Org 34850, Biolog (Bremen, Germany) for Sp-8-Br-cAMP, and B. Kobilka and B. Lowell for providing stock for the β receptor KO mouse lines.
The authors declare no competing financial interests.
References
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Linthorst AC, Holsboer F, Reul JM. Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology. 2003;28:1056–1067. doi: 10.1038/sj.npp.1300158. [DOI] [PubMed] [Google Scholar]
- Beer M, Richardson A, Poat J, Iversen LL, Stahl SM. In vitro selectivity of agonists and antagonists for beta 1- and beta 2-adrenoceptor subtypes in rat brain. Biochem Pharmacol. 1988;37:1145–1151. doi: 10.1016/0006-2952(88)90523-0. [DOI] [PubMed] [Google Scholar]
- Brewer C. Beneficial effect of beta-adrenergic blockade on “exam. nerves.”. Lancet. 1972;2:435. doi: 10.1016/s0140-6736(72)91840-5. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Tronche C, Piérard C, Liscia P, Drouet I, Coutan M, Béracochéa D. Rapid stress-induced corticosterone rise in the hippocampus reverses serial memory retrieval pattern. Hippocampus. 2010;20:196–207. doi: 10.1002/hipo.20605. [DOI] [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am J Psychiatry. 2007;164:967–969. doi: 10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- Faigel HC. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin Pediatr (Phila) 1991;30:441–445. doi: 10.1177/000992289103000706. [DOI] [PubMed] [Google Scholar]
- Ferguson D, Sapolsky R. Overexpression of mineralocorticoid and transdominant glucocorticoid receptor blocks the impairing effects of glucocorticoids on memory. Hippocampus. 2008;18:1103–1111. doi: 10.1002/hipo.20467. [DOI] [PubMed] [Google Scholar]
- Fowler JC, O'Donnell JM. Antagonism of the responses to isoproterenol in the rat hippocampal slice with subtype-selective antagonists. Eur J Pharmacol. 1988;153:105–110. doi: 10.1016/0014-2999(88)90593-6. [DOI] [PubMed] [Google Scholar]
- Goh JW, Pennefather PS. A pertussis toxin-sensitive G protein in hippocampal long-term potentiation. Science. 1989;244:980–983. doi: 10.1126/science.2543072. [DOI] [PubMed] [Google Scholar]
- Hurlemann R. Noradrenergic-glucocorticoid mechanisms in emotion-induced amnesia: from adaptation to disease. Psychopharmacology (Berl) 2008;197:13–23. doi: 10.1007/s00213-007-1002-x. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. The anti-inflammatory profile of fluticasone propionate. Allergy. 1995;50:11–14. doi: 10.1111/j.1398-9995.1995.tb02735.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier H, Labrie F. Specificity of the beta 2-adrenergic receptor stimulating cyclic AMP accumulation in the intermediate lobe of rat pituitary gland. Eur J Pharmacol. 1982;81:411–420. doi: 10.1016/0014-2999(82)90106-6. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci U S A. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci U S A. 2008;105:11993–11997. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Colgan LA, Nou E, Arnsten AF. Beta2 adrenergic agonist, clenbuterol, enhances working memory performance in aging animals. Neurobiol Aging. 2008;29:1060–1069. doi: 10.1016/j.neurobiolaging.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Goñi-Allo B, Aguirre N. Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5-HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology. 2005;30:2180–2191. doi: 10.1038/sj.npp.1300735. [DOI] [PubMed] [Google Scholar]
- Rashidy-Pour A, Sadeghi H, Taherain AA, Vafaei AA, Fathollahi Y. The effects of acute restraint stress and dexamethasone on retrieval of long-term memory in rats: an interaction with opiate system. Behav Brain Res. 2004;154:193–198. doi: 10.1016/j.bbr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci U S A. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur J Neurosci. 1999;11:1317–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proc Natl Acad Sci U S A. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004a;81:150–154. doi: 10.1016/j.nlm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004b;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sajadi AA, Samaei SA, Rashidy-Pour A. Intra-hippocampal microinjections of anisomycin did not block glucocorticoid-induced impairment of memory retrieval in rats: an evidence for non-genomic effects of glucocorticoids. Behav Brain Res. 2006;173:158–162. doi: 10.1016/j.bbr.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schutsky KS, Ouyang M, Thomas SA. Xamoterol impairs hippocampus-dependent emotional memory retrieval via Gi/o-coupled beta2-adrenergic signaling. Learn Mem. 2011;18:598–604. doi: 10.1101/lm.2302811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton KR, Cole AJ, Pritchett J, Eccles CU, Worley PF, Baraban JM. Intrahippocampal injection of pertussis toxin blocks adenosine suppression of synaptic responses. Brain Res. 1989;494:359–364. doi: 10.1016/0006-8993(89)90604-5. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Disruption of the dopamine beta-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav Neurosci. 1997;111:579–589. doi: 10.1037//0735-7044.111.3.579. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi H, Nakashima Y, Kinami J, Nagatomo T. Characteristics of 125I-iodocyanopindolol binding to beta-adrenergic and serotonin-1B receptors of rat brain: selectivity of beta-adrenergic agents. Jpn J Pharmacol. 1990;52:195–200. doi: 10.1254/jjp.52.195. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahabzadeh A, Fillenz M. Comparison of stress-induced changes in noradrenergic and serotonergic neurons in the rat hippocampus using microdialysis. Eur J Neurosci. 1994;6:1205–1212. doi: 10.1111/j.1460-9568.1994.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M, Taton G, Delhaye M, Chatelain P, Camus JC, Pochet R, Leclerc JL, De Smet JM, Robberecht P, Christophe J. The human heart beta-adrenergic receptors. II. Coupling of beta 2-adrenergic receptors with the adenylate cyclase system. Mol Pharmacol. 1983;24:174–182. [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Ji X, Lakatta EG. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Mishler K, Roerig SC, O'Donnell JM. Interaction between the antidepressant-like behavioral effects of beta adrenergic agonists and the cyclic AMP PDE inhibitor rolipram in rats. Psychopharmacology (Berl) 2005;182:104–115. doi: 10.1007/s00213-005-0055-y. [DOI] [PubMed] [Google Scholar]
- Zhang WP, Ouyang M, Thomas SA. Potency of catecholamines and other l-tyrosine derivatives at the cloned mouse adrenergic receptors. Neuropharmacology. 2004;47:438–449. doi: 10.1016/j.neuropharm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ali A, Ramirez VD. Steroids conjugated to bovine serum albumin as tools to demonstrate specific steroid neuronal membrane binding sites. J Psychiatry Neurosci. 1996;21:187–197. [PMC free article] [PubMed] [Google Scholar]