Abstract
Experiments were performed to confirm that the aldimine bond formation is a spontaneous reaction, because attempts to find an enzyme catalyzing the last decisive step in betaxanthin biosynthesis, the aldimine formation, failed. Feeding different amino acids to betalain-forming hairy root cultures of yellow beet (Beta vulgaris L. subsp. vulgaris “Golden Beet”) showed that all amino acids (S- and R-forms) led to the corresponding betaxanthins. We observed neither an amino acid specificity nor a stereoselectivity in this process. In addition, increasing the endogenous phenylalanine (Phe) level by feeding the Phe ammonia-lyase inhibitor 2-aminoindan 2-phosphonic acid yielded the Phe-derived betaxanthin. Feeding amino acids or 2-aminoindan 2-phosphonic acid to hypocotyls of fodder beet (B. vulgaris L. subsp. vulgaris “Altamo”) plants led to the same results. Furthermore, feeding cyclo-3-(3,4-dihydroxyphenyl)-alanine (cyclo-Dopa) to these hypocotyls resulted in betanidin formation, indicating that the decisive step in betacyanin formation proceeds spontaneously. Finally, feeding betalamic acid to broad bean (Vicia faba L.) seedlings, which are known to accumulate high levels of Dopa but do not synthesize betaxanthins, resulted in the formation of dopaxanthin. These results indicate that the condensation of betalamic acid with amino acids (possibly including cyclo-Dopa or amines) in planta is a spontaneous, not an enzyme-catalyzed reaction.
Betalains, i.e. the red-violet betacyanins and yellow betaxanthins, are water-soluble pigments of chemotaxonomic significance occurring in certain members of the plant order Caryophyllales and some higher fungi (Steglich and Strack, 1990). While betacyanins are formed in a condensation reaction of BA and cyclo-Dopa (or cyclo-Dopa 5-O-glucoside), the yellow betaxanthins are products of BA with other amino acids and amines. Feeding Dopa to cotyledons of different Amaranthus species stimulates amaranthin biosynthesis and leads to the formation of some betaxanthins, but not dopaxanthin (French et al., 1974; Guidici de Nicola et al., 1975; Bianco-Colomas, 1980). Induction of betaxanthin (mainly vulgaxanthin I) and BA formation has also been observed in some cases after Dopa feeding to betalain-forming inflorescences and petals of different plants (Rink and Böhm, 1985, 1991). Cross-breeding of differently colored lines of large-flowered purslane (Portulaca grandiflora Hook.) suggested the involvement of three genes in the control of betalain biosynthesis (Adachi et al., 1985), disregarding a gene responsible specifically for betaxanthin formation. Later, a hypothetical model was proposed that included transport of BA into the vacuole, where under acidic conditions condensation between BA and amino acids or amines proceeds spontaneously (Trezzini, 1990; Trezzini and Zryd, 1990). This model has been corroborated by Böhm et al. (1991) and Hempel and Böhm (1997) by feeding amino acids to seedlings and hairy root cultures of yellow beet (Beta vulgaris L. subsp. vulgaris “Golden Beet”) (nomenclature of cultivated beets according to Lange et al., 1998), which resulted in the formation of the corresponding betaxanthins.
The present study was undertaken to confirm the spontaneous character of the last decisive step in betaxanthin biosynthesis. To demonstrate the amino acid specificity and the stereoselectivity of this reaction, nearly all proteinogenic amino acids, including some (R)-forms were fed to hairy root cultures of yellow beet. In addition, the effect of increasing the endogenous level of Phe on betaxanthin formation was studied by treating the hairy root cultures with AIP, a potent inhibitor of PAL activity. Spontaneous aldimine formation in planta was further confirmed by feeding experiments with young fodder beet plants and plants that do not belong to betalain-forming taxa, i.e. broad bean (Vicia faba L.) and pea (Pisum sativum L.).
MATERIALS AND METHODS
Plant Material
Hairy root cultures from yellow beet (Beta vulgaris L. subsp. vulgaris “Golden Beet” [GH] Garden Beet Group; formerly Beta vulgaris L. var. lutea, lines 5A and 7) were grown at a light intensity of 65 μmol m−2 s−1 at 25°C under a photoperiod of 16 h of light/8 h of dark on a shaker (120 rpm). Subcultivation was carried out on d 7 by transferring root tips (approximately 1 cm, 0.3 g fresh weight) into 30 mL of modified 2,4-D-free B5 medium (Gamborg et al., 1968) containing 30 g L−1 Suc, 18.6 mg L−1 Na2EDTA, and 13.8 mg L−1 FeSO4·7H2O in 100-mL Erlenmeyer flasks.
A hairy root culture was also established from red beet (Beta vulgaris L. subsp. vulgaris “Egyptian Flatround” [Garden Beet Group]) by Dr. I. Kuzovkina (K.A. Timiryasev Institute of Plant Physiology, Russian Academy of Sciences, Moscow) grown in the dark at 25°C on a shaker (120 rpm). Subcultivation was carried out on d 14 by transferring root tips (approximately 1 cm, 0.3 g fresh weight) into 30 mL of modified hormone- and Gly-free, one-half-strength Murashige and Skoog medium (Murashige and Skoog, 1962) containing 20 g L−1 Suc, 660 mg L−1 CaCl2·2H2O, 80 mg L−1 myo-inositol, 0.1 mg L−1 pyridoxine·HCl, and 0.1 mg L−1 thiamine·2HCl in 100-mL Erlenmeyer flasks.
Suspension cultures from red beet plants were grown at a light intensity of 65 μmol m−2 s−1 at 25°C under a photoperiod of 16 h of light/8 h of dark on a shaker (120 rpm). Subcultivation was carried out on d 14 by transferring cells into 30 mL of modified hormone-free Murashige and Skoog medium containing 30 g L−1 Suc, 21.1 mg L−1 Na2EDTA, and 15.7 mg L−1 FeSO4·7H2O in 250-mL Erlenmeyer flasks.
Suspension cultures from Dorotheanthus bellidiformis (Burm. f.) N.E.Br. were grown at a light intensity of 65 μmol m−2 s−1 at 25°C under a photoperiod of 16 h of light/8 h of dark on a shaker (120 rpm). Subcultivation was carried out on d 5 by transferring cells into 30-mL Linsmaier and Skoog medium (Linsmaier and Skoog, 1965) containing 20 g L−1 Suc, 0.5 mg L−1 pyridoxine·HCl, 0.1 mg L−1 thiamine·2HCl, 0.2 mg L−1 kinetin, and 0.5 mg L−1 nicotinic acid in 250-mL Erlenmeyer flasks.
Fodder beet (Beta vulgaris L. subsp. vulgaris “Altamo” [FH] Fodder Beet Group), broad bean (Vicia faba L. “Fribo”), and pea (Pisum sativum L. “Belinda”) were grown from seeds in a greenhouse and cultivated in soil under natural-daylight conditions.
Partial Syntheses of Diastereoisomeric Betaxanthins
Method 1: BA from Lyophilized Red Beet Juice
Lyophilized red beet juice (10 g) (Roth, Karlsruhe, Germany) was dissolved under stirring in 20 mL of water. After centrifugation for 10 min at 15,000g the supernatant was partitioned three times with 30 mL of EtOAc to remove soluble material. Aqueous NH3 solution (25%) was added to the ice-cooled solution, pH 4.8, to reach pH 11.3. After hydrolysis for 30 min at room temperature, 5 n HCl was slowly added under ice-cooling to reach pH 2.0. The mixture was immediately partitioned three times with 50 mL of EtOAc. The combined solvent fractions were concentrated in vacuo to 3 mL and then re-extracted with 3 mL of water. The yellow aqueous phase was added (100 μL each) to Eppendorf vials containing different (S)-amino acids and amines (dopamine and tyramine) (25 μmol in 100 μL of water), vortexed for 1 min, and centrifuged for 5 min at 15,000g. The supernatants were analyzed by HPLC using solvent systems 1 and 2. The yield was 0.8 to 6.0 nmol, depending on the amino acids or amine used.
Method 2: BA from Betanin:Isobetanin
Aqueous NH3 solution (25%) was added to a solution of betanin:isobetanin (2:1; 3 μmol in 2 mL of water) to reach pH 11.3 for hydrolysis at room temperature (30 min). After acidification to pH 2.0 with 5 n HCl under ice-cooling, the mixture was immediately partitioned three times with 5 mL of EtOAc. After evaporation of the solvent under reduced pressure the residue was solved in 3 mL of water and processed as described in method 1. This method yielded 0.2 to 0.8 nmol, or 0.6% to 2.4%, depending on the amino acids or amine used.
Method 3: Direct Addition of Hydrolyzed Betanin/Isobetanin to Amino Acids
Betanin:isobetanin (9:1; 800 nmol in 2 mL of water) was hydrolyzed as described in method 2 and added without acidification in 100-μL aliquots to 25 μmol (S)- and (R)-amino acids in 100 μL of water. After vortexing (1 min), the reaction mixtures were reduced to dryness in a concentrator (model 5301, Eppendorf), resuspended in 200 μL of water, and centrifuged for 5 min at 15,000g. The supernatants were analyzed as described in method 1. In this case the yield was 0.5 to 17.6 nmol, or 2% to 77%, depending on the amino acid or amine used.
Isolation and Identification of the Major Betalains from Hairy Root Culture
Hairy root culture material (line 5A; 20 g) was frozen in liquid N2, homogenized in a mortar, and extracted with 60 mL of 80% aqueous methanol containing 50 mm ascorbate. The extract was concentrated to 5 mL and applied on a Dowex 1 × 8 (formiate form), 50- to 100-mesh column (250 × 30 mm i.d.). The elution was performed with water (500 mL) and with a stepwise formic acid gradient (0.5, 1.0, 2.0, 3.5, and 7.0 n). The fractions (2.0 and 3.5 n) containing the main betaxanthin were concentrated and purified by semiprep HPLC (solvent system 3). Identification was performed by ESI-MS (positive ion mode and positive daughter ion scan).
For the preparation of 2-descarboxybetanidin, a suggested betacyanin constituent of hairy root culture, 100 μL of mushroom tyrosinase (1 mg mL−1, Sigma) was added to 1 mL of dopamine (25 mm in 0.02 m KPi buffer, pH 6.8) and shaken for 10 min at room temperature. The reaction mixture was treated with 1 mL of ascorbate (0.2 m). After 5 min, methanol (2 mL) was added, the mixture was centrifuged for 5 min at 15,000g, and the supernatant was concentrated to 0.5 mL from which a 100-μL aliquot was purified with a semiprep HPLC (solvent system 3). Fifty microliters of BA (25 nmol) was added to 50 μL of the 2-descarboxy-cyclo-Dopa fraction. The 2-descarboxybetanidin formed was analyzed by HPLC (solvent system 2; Rt = 32.8 min, λmax = 536 nm), and used for co-injection experiments with hairy root extracts.
Feeding Experiments
Amino acids (Gly, [S]-Ala, [S]-Ser, [S]-Thr, [S]-Leu, [S]-Ile, [S]-Val, [S]-Gln, [S]-Asn, [S]-Glu, [S]-Asp, [S]-Lys, [S]-Arg, [S]-Orn, [S]-Met, [S]-Trp, [S]-Phe, [S]-His, [S]-Pro, [S]-Hyp, and [S]-4-thiaprolin) were dissolved in water and fed by sterile filtration to hairy root cultures of yellow beet at d 7 after subcultivation (final amino acid concentration, 2 mm). Cultures with water added served as a control. After 24 h the hairy roots were harvested, extracted, and analyzed by HPLC. The competition experiments (addition of [S]-Phe and [R]-Phe separately and combined) and the feeding of (R)-amino acids were performed in the same way. In saturation experiments (S)-Ala, (R)-Ala, and (S)-Thr were fed at d 7 under the same conditions with increasing final concentrations (2, 5, 10, 20, and 50 mm). In addition to the (NH4)2SO4 concentration (1 mm) of the nutrition solution, (NH4)2SO4 was fed at d 7 under the same conditions to reach final concentrations of 3, 6, 11, 21, and 51 mm. Furthermore, 5 mm (S)-Leu was fed from d 4 to 8, and the hairy roots were harvested 24 h after each application. To study the competition in the uptake of (R)-Phe in the presence of (S)-Phe (final concentration: 2 mm each) at d 7, 20 μL (0.74 MBq) of 3H-labeled (S)-Phe ([S]-[2,6-3H2]Phe, specific activity 200 TBq mmol−1, TRK 552, Amersham) and 50 μL (0.185 MBq) of 14C-labeled (R)-Phe ([R]-[1-14C]Phe, specific activity: 200 GBq mmol−1, ARC 1116, Biotrend, Cologne, Germany) were applied. To monitor uptake and for the calculation of the 3H-to-4C ratio in the nutrition solution, two 50 μL-aliquots were used 0, 1, 2, 4, 8, 12, and 24 h after addition for analysis by liquid scintillation counting (Ultima Gold XR, Packard Instruments, Meriden, CT) for 2 min with LS 6000 TA (Beckman).
AIP dissolved in water (2 mL of 0.25 mm) was fed from d 4 to 7 to a hairy root culture, which was harvested at d 8. To compare the capability in betaxanthin formation of different cell cultures (S)-Phe was fed under similar conditions to hairy root and suspension cultures of red beet and to suspension cultures of D. bellidiformis (final concentration, 2 mm), which were harvested after 24 h.
(S)-Phe and (R)-Phe (10 mL of 10 mm) were fed separately to ten 23-d-old de-rooted fodder beet plants via the hypocotyls, which were harvested after 48 h. AIP (5 mL of 50 μm in 0.1 m KPi buffer, pH 7.0, three plants for 24 h) was fed to the same plant material (5 weeks old). Controls were treated with 5 mL of 0.1 m KPi-buffer, pH 7.0. cyclo-Dopa (2 mL of 2 mm containing 80 mm ascorbate, five plants) was fed to de-rooted fodder beet plants (23-d-old) via the hypocotyls and extracts were made after 1.5 and 24 h for HPLC analysis. Controls were treated with 80 mm ascorbate.
BA (1.4 mL each, 0.285 mm in 0.1 m KPi buffer, pH 6.8, two plants) was fed to de-rooted 14-d-old broad bean and pea plants via the hypocotyls, which were extracted after 24 h for HPLC analysis. Controls were treated with 0.1 m KPi buffer, pH 6.8. All feeding experiments were performed in duplicate, and mean values were obtained. Controls (water, buffer, or ascorbate, instead of amino acids) were included.
Quantification of Betalains
After harvesting hairy roots, suspension-cultured cells, or hypocotyls, the material was washed briefly with distilled water, blotted dry between filter paper, frozen in liquid N2, and homogenized in a mortar. The betalains were extracted with 80% aqueous methanol containing 50 mm ascorbate at a tissue:solvent ratio of 1 g 3 mL−1. After centrifugation at 15,000g for 10 min at 4°C, the supernatants were removed. Two aliquots of 20 μL were diluted to 1 mL with water and the absorbance was measured at 475 nm (for betaxanthins) and 540 nm (for betacyanins) with a photometer (Shimadzu, Columbia, MD). For quantification of the compounds, the mean molar extinction coefficient for betaxanthins (48 × 106 cm2 mol−1; Girod and Zryd, 1991b) and for betanin (62 × 106 cm2 mol−1; Wyler et al., 1959) were used. After HPLC analysis of the extracts the peak areas of individual compounds were compared with those of standard compounds. The HPLC separations were performed (solvent system 1) using an autosampler (20-μL injections). BA was quantified by HPLC using a purified standard (molar extinction coefficient, 25 × 106 cm2 mol−1; Girod and Zryd, 1991b).
Enzymic cyclo-Dopa Preparation
Mushroom tyrosinase (800 μL, 1 mg mL−1, Sigma) was added to 10 mL of Dopa (10 mm in 0.02 m KPi buffer, pH 6.8) and shaken for 8 min at room temperature. The reaction mixture was then treated with 10 mL of 0.2 m ascorbate. Proteins were precipitated by addition of 20 mL of methanol after 5 min, and the mixture was centrifuged at 15,000g for 5 min. The supernatant was concentrated to 6 mL and purified by preparative HPLC. The yield was 40 μmol (40%), and the λmax was 286 nm (HPLC-PDA) (the λmax was 285 nm in 20% HCl [Wyler and Chiovini, 1968]).
Spontaneous Condensation of cyclo-Dopa with BA as a Function of pH
Ten microliters of BA (5 nmol) in water was added to a mixture of 80 μL of citrate/phosphate buffer (0.1 m citrate, 0.2 m NaPi buffer, 10 mm ascorbate, pH range 3.0–6.5) and 10 μL of cyclo-Dopa (40 nmol, 160 mm ascorbate) in a 100-μL cuvette (final ascorbate concentration, 25 mm). The increase in A540 was measured in intervals of 30 s for 10 min with a photometer (Beckman) at room temperature. The actual pH in the reaction mixture was measured directly after the experiment. The same assay at pH 6.0 was used to test the effect of protein extracts from a hairy root culture of yellow beet on betanidin formation by replacing 20 μL of the buffer with 20 μL of the protein extract.
Preparation of Protein Extracts
Protein extracts from the hairy root culture of yellow beets were prepared according to the methods (including [NH4]2SO4 precipitation) of Steiner et al. (1996), De-Eknamkul et al. (1997), and Terradas and Wyler (1991) (60% acetone precipitation).
Photometric Assay for Vulgaxanthin I Formation
Ten microliters of 100 mm (S)-Gln in water (final [S]-Gln concentration, 10 mm) was added to a mixture of 80 μL of citrate/phosphate buffer (0.1 m citrate, 0.2 m NaPi-buffer, and 10 mm ascorbate, pH 6.0) and 10 μL of BA (5 nmol) in water in a 100 μL-cuvette. The extinction at 475 nm was measured in intervals of 30 s for 10 min with a photometer (Beckman) at room temperature. The actual pH in the reaction mixture was measured directly after the experiment. The same assay was used to test the effect of protein extracts from hairy roots on the vulgaxanthin I formation by replacing 20 μL of the buffer with 20 μL of the protein extract.
HPLC Assay for Vulgaxanthin I Formation
A mixture of 50 μL 0.1 m KPi-buffer, pH 6.5, containing 50 mm ascorbate, 20 μL of 10 mm (S)-Gln, and 20 μL of protein extract from hairy roots was preincubated at 30°C for 5 min. The reaction was started by the addition of 10 μL of BA (5 nmol) in water. After 60 min at 30°C, 100 μL of methanol was added, centrifuged at 15,000g for 5 min, and the supernatant (50 μL) was analyzed by HPLC (solvent system 1).
Amino Acid Analyses
The plant material (1 g fresh weight) was frozen in liquid N2 and homogenized in a mortar. The amino acids were extracted with ethanol at a solvent:tissue ratio of 4 mL g−1. After centrifugation at 15,000g for 10 min at 4°C, the supernatants were removed and the pellets were re-extracted with 3 mL of 80% aqueous ethanol. Water (3 mL) was added to the combined supernatants, and the mixture was partitioned with 5 mL of CHCl3 until the CHCl3 fractions were colorless. The aqueous upper phases were concentrated to dryness in a rotary evaporator, dissolved in water, and aliquots were used for amino acid analysis (ABI 420A, Applied Biosystems, Foster City, CA) with the inclusion of (S)-Gln and (S)-Asn in the amino acid standards. For Dopa analysis, extraction was carried out in the presence of ascorbate (100 mm) and the extract was analyzed by HPLC as described previously (Steiner et al., 1996).
HPLC
Analytical and semipreparative HPLC was performed with a system from Waters (Milford, MA). The liquid chromatograph was equipped with a 5-μm Nucleosil C18 column (250 × 4 mm i.d., Macherey-Nagel, Düren, Germany), and the following solvent and gradient systems were used. Solvent system 1: A, 1.5% ortho-phosphoric acid in water; B, 80% acetonitrile in water; linear gradient from 100% A to 70% A in (A plus B) within 40 min. The flow rate was 1 mL min−1. Solvent system 2: A, 50 mm NaH2PO4, and 2.5 mm triethylamine, adjusted to pH 4.2 with H3PO4 and B, 40% acetonitrile in water (buffered-ion pairing system and a step-wise gradient, according to the method of Trezzini and Zryd [1991]). Solvent system 3: A, 1% formic acid in water; B, 80% acetonitrile in water; gradient as in solvent system 1.
Compounds were detected at 405, 475, and 540 nm or by maxplot detection between 220 and 650 nm (PDA). Injection volume was 10 or 20 μL in analytical work and 100 μL in semipreparative work. For preparative HPLC the liquid chromatograph (System Gold, Beckman) was equipped with a 10-μm Nucleosil 100–10 C18 column (250 × 40 mm i.d.; VarioPrep, Macherey-Nagel). The cyclo-Dopa purification was performed isocratically (0.1% acetic acid in water) with a flow rate of 11 mL min−1 and detection at 280 nm. All betalain extracts were analyzed by HPLC (solvent system 1).
ESI-MS
Positive ESI-MS was performed (TSQ 7000, Finnigan, Bremen, Germany; electrospray voltage 4.5 kV, N2 as sheath gas) using a syringe pump (Harvard Apparatus, South Natick, MA) operating at a flow rate of 5 μL min−1.
RESULTS
Diastereoisomeric Betaxanthins Can Easily Be Prepared and Separated by HPLC
Betaxanthin standards necessary for the identification of the metabolites of amino acid feedings to hairy root cultures and plants have been prepared in three different ways. The simplest and most rapid procedure (method 1) is the hydrolysis of commercial lyophilized red beet juice (containing racemic betanin) by aqueous ammonia solution, extraction of the liberated racemic BA after acidification (optimal at pH 1.0–2.0), and its addition to different amino acids directly yielding the diastereoisomeric betaxanthins ([2S/S]- and [2S/R]-forms). Using the solvent system and the stepwise gradient system (Trezzini and Zryd, 1991) (solvent system 2) and an optimized linear gradient system (solvent system 1), 21 of 25 isomer pairs could be separated. However, due to the racemic nature of the starting material, an isomer assignment of the separated peaks was not possible. Starting the partial synthesis with purified betanin:isobetanin mixtures (2:1) (method 2), the betaxanthin isomers were obtained in the same ratio, the larger peaks of the isomer pairs corresponded to the (2S/S)-forms (Table I). With this method some (R)-amino acids were also transformed to the corresponding betaxanthins and analyzed by HPLC (Table II). In addition, a modified version (method 3) of the known synthetic procedure (Trezzini and Zryd, 1991) was used to synthesize betaxanthins in higher amounts; but for use as standards a subsequent purification was necessary.
Table I.
Retention times and HPLC-PDA data of stereoisomeric betaxanthins (derived from Gly, (S)-amino acids, and amines), BA, and betanin
| Amino Acid/Amine | Solvent System
1
|
Solvent System 2
|
||||
|---|---|---|---|---|---|---|
| Rt
|
HPLC-PDAa | Rt
|
HPLC-PDAa | |||
| 2S/S | 2S/R | 2S/S | 2S/R | |||
| min | λmax (nm) | min | λmax (nm) | |||
| His | 8.6 | 8.4 | 472 | 5.3 | 5.4 | 477 |
| Lys | 9.5 | 468 | 5.0 | 5.3b | 475 | |
| Asn | 10.6 | 469 | 4.4 | 4.6 | 472 | |
| Ser | 11.1 | 466 | 3.9 | 474 | ||
| Arg | 11.5 | 468 | 8.0 | 8.3 | 475 | |
| Cystine | 12.0 | 11.9c | 475 | 5.6 | 6.0d | 475 |
| Gln | 12.0 | 468 | 6.0 | 6.4 | 475 | |
| Asp | 12.5 | 12.3 | 467 | 3.4 | 474 | |
| Hyp | 12.6 | 480 | 4.3 | 4.7 | 481 | |
| Gly | 12.7e | 465 | 6.0e | 472 | ||
| Thr | 13.5 | 13.3 | 468 | 6.2 | 6.5 | 475 |
| Citrulline | 14.7 | 14.5 | 467 | 8.6 | 8.8 | 475 |
| Glu | 14.9 | 14.7 | 468 | 7.7 | 8.0 | 475 |
| Ala | 17.4 | 16.7 | 465 | 8.5 | 8.7 | 474 |
| Pro | 19.8 | 19.3 | 477 | 10.5 | 10.0 | 485 |
| −(BA) | 21.8 | 405 | 9.6 | 425 | ||
| Dopa | 22.3 | 22.1 | 470 | 12.4 | 477 | |
| CDGf(betanin: isobetanin) | 22.7 | 24.1 | 538 | 14.0 | 16.3 | 536 |
| Dopamine | 24.7e | 457 | 18.1e | 474 | ||
| Tyr | 25.2 | 24.8 | 469 | 19.4 | 473 | |
| Val | 26.4 | 25.0 | 467 | 12.9 | 12.5 | 475 |
| Met | 26.4 | 25.3 | 468 | 13.3 | 12.7 | 476 |
| Tyramine | 29.3e | 459 | 20.5e | 469 | ||
| Ile | 33.0 | 31.5 | 466 | 16.1 | 15.8 | 475 |
| Leu | 34.0 | 32.7 | 468 | 16.4 | 476 | |
| Phe | 34.5 | 33.3 | 472 | 18.2 | 17.9 | 477 |
| Trp | 37.3 | 36.8 | 472 | 19.8 | 18.1 | 478 |
BA extracted from hydrolyzed betanin:isobetanin mixture (2:1) was added to different (S)-amino acids and amines. After workup (see Methods) the betaxanthins were analyzed by HPLC (solvent systems 1 and 2). When only one Rt is given for an isomer pair, the separation was not achieved.
λmax of the (2S/S)-form.
Additional peak at Rt 8.6 min (λmax 469 nm).
Additional peak at Rt 29.8 min (λmax 474 nm), cystein yielded with BA two compounds, Rt 17.2 and 18.1 min (both λmax 497 nm).
Additional peak at Rt 19.4 min (λmax 474 nm), cystein yielded with BA two compounds, Rt 7.9 and 9.9 min (both λmax 495 nm).
Only S- and R-isomers of the BA moiety.
CDG, cyclo-Dopa 5-O-glucoside.
Table II.
Rt of diastereoisomeric betaxanthins prepared from (S)- and (R)-amino acids
| Amino Acid | Solvent System 1
|
Solvent
System 2
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2S/R | 2S/S | 2R/R | 2R/S | 2S/R | 2S/S | 2R/R | 2R/S | |
| min | ||||||||
| (S)-Glu | 14.7 | 14.9 | – | – | 8.0 | 7.7 | – | – |
| (R)-Glu | – | – | 14.9 | 14.7 | – | – | 7.7 | 8.0 |
| (S)-Val | 25.0 | 26.4 | – | – | 12.5 | 12.9 | – | – |
| (R)-Val | – | – | 26.4 | 25.0 | – | – | 12.9 | 12.5 |
| (S)-Phe | 33.3 | 34.5 | – | – | 17.9 | 18.2 | – | – |
| (R)-Phe | – | – | 34.5 | 33.3 | – | – | 18.2 | 17.9 |
| (S)-Trp | 36.8 | 37.3 | – | – | 18.1 | 19.8 | – | – |
| (R)-Trp | – | – | 37.3 | 36.8 | – | – | 19.8 | 18.1 |
A betanin:isobetanin mixture (9:1) was hydrolyzed by an aqueous NH3 solution (25%) at pH 11.3 for 30 min and added to different (S)- and (R)-amino acids. After workup (see Methods) the betaxanthins were analyzed by HPLC (solvent systems 1 and 2).
Betalain Accumulation Coincides with Rapid Growth of Yellow Beet Hairy Root Cultures
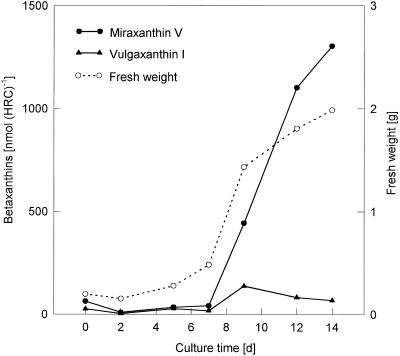
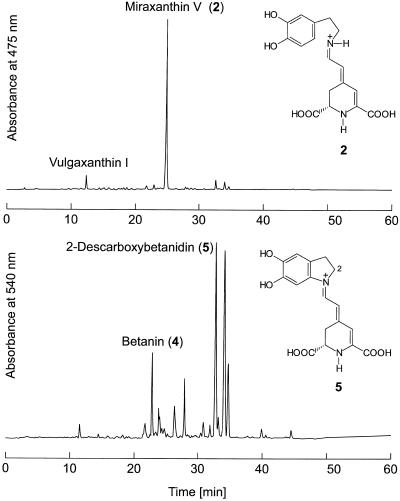
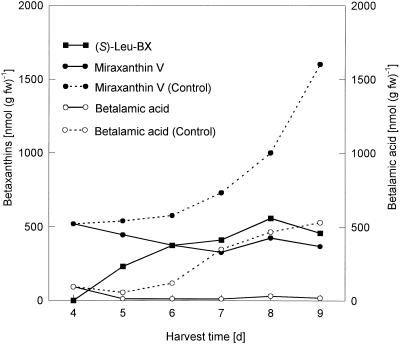
In liquid culture, both hairy root culture lines 5A and 7 showed the most intensive fresh weight increase between d 7 and 9, which was paralleled by a steep increase in miraxanthin content (Fig. 1). HPLC analysis of an extract (Fig. 2) showed that the betalain mixture consisted predominantly of betaxanthins, a major form and a minor form, together with a lower portion (<30%) of different betacyanins. Whereas nothing was known about the identity of the betacyanins of the hairy root culture, the two betaxanthins were recently identified as vulgaxanthin I ([S]-Gln-betaxanthin) and portulacaxanthin II ([S]-Tyr-betaxanthin) (Hempel and Böhm, 1997). The identity of the betaxanthin (λmax = 468 nm) eluting at 12.0 min was confirmed as vulgaxanthin I, which is typical for the genus Beta, but the major betaxanthin (Rt = 24.7 min; λmax = 457 nm) did not match synthetically prepared (S)-Tyr-betaxanthin (Rt = 25.2 min; λmax = 469 nm). Therefore, hairy root material was extracted and the major betaxanthin was purified by conventional anion-exchange chromatography on a Dowex 1 × 8 column (Strack et al., 1993) and by semipreparative HPLC (data not shown). The purified betaxanthin was analyzed by ESI-MS.
Figure 1.
Time course of growth (fresh weight increase) and betaxanthin content (miraxanthin V and vulgaxanthin I) in hairy root culture of yellow beet.
Figure 2.
HPLC elution profiles of betalains in hairy root culture of yellow beets. Top, betaxanthins (A475); Bottom, betacyanins (A540). Full scales are different in top (0.50 absorbance units) and bottom (0.05 absorbance units). Peak numbers correspond to the biosynthetic scheme in Figure 10.
In the positive ion mode a protonated molecular ion ([M+H]+) was observed at m/z 347 (18%). Collision-induced dissociation of the parent ion at m/z 347 yielded an informative daughter-ion spectrum dominated by the successive loss of the carboxyl groups, m/z 303 ([M+H-CO2]+) (18%), m/z 301 ([M+H-HCO2H]+) (16%), m/z 257 ([M+H-CO2-HCO2H]+) (16%), and m/z 255 ([M+H-2HCO2H]+) (17%). Furthermore, prominent ions at m/z 211 (30%) and m/z 137 (100%, base peak) appeared. The ion at m/z 137 represents the deaminated component conjugated to BA. A MS database search for Mr 153 (m/z 137 + NH2) gave two plausible hits: octopamine (1-[4-hydroxyphenyl]-2-amino-1-ethanol) and dopamine. The pair of betaxanthins prepared by condensation of (S/R)-octopamine with BA according to method 1 (see Methods) showed retention characteristics in HPLC (Rt = 22.0 and 22.3 min; λmax = 460 nm) that were clearly different from the isolated endogenous compound. The betaxanthin prepared in the same way starting from dopamine was found to be identical in all respects (Rt, λmax, and MS fragmentation pattern) to the main betaxanthin in hairy root culture.
Betanin was readily identified by co-chromatography (Rt = 22.7 min; λmax = 538 nm) with betanin from a suspension culture of B. vulgaris (Bokern et al., 1991) from the previously unknown betacyanins from hairy root culture. The other major betacyanins showed PDA spectra (λmax = 536–538 nm) comparable to those of betanin, but they were remarkably less polar. As dopamine and the dopamine-derived miraxanthin V were major components of the extract, the occurrence of a dopamine-derived betacyanin was assumed. By tyrosinase-catalyzed formation of 2-descarboxy-cyclo-Dopa from dopamine and its condensation with BA, the synthesis of 2-descarboxy-betanidin was achieved. By comparison and co-chromatography with this synthetic compound, the most prominent betacyanin peak of the hairy root culture extract was identified as 2-descarboxy-betanidin (Rt = 32.8 min; λmax = 536 nm). Furthermore, the hairy root culture extracts contained, in addition to miraxanthin V (1 μmol g−1 fresh weight), a high concentration of the precursors BA (0.3 μmol g−1 fresh weight) and dopamine (15 μmol g−1 fresh weight).
Aldimine Formation in Hairy Root Cultures after Feeding of Amino Acids Shows Neither Amino Acid Specificity Nor Stereoselectivity
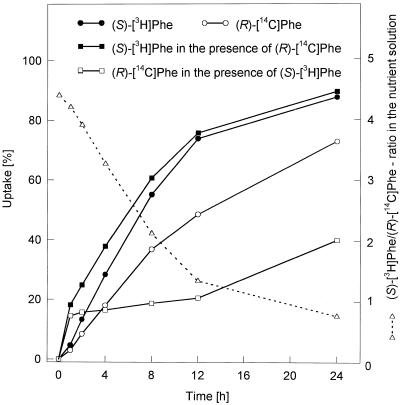
The results of (S)-amino acid feeding to a hairy root culture (Table III) showed that all amino acids were accepted in the formation of the corresponding betaxanthins, but to a different extent. Also, (S)-4-thiaprolin, a synthetic amino acid, led to formation of the respective betaxanthin. In parallel feedings of the (S)- and (R)-isomers of different amino acids, both stereoisomers were incorporated into the corresponding betaxanthins to the same extent (Table IV). Simultaneous application of (S)- and (R)-Phe to hairy root cultures unexpectedly yielded a (S)-Phe-betaxanthin/(R)-Phe-betaxanthin ratio of 10:1. This unexpected result could be clarified by uptake studies using (S)-[2,6-3H2]Phe/(R)-[1-14C]Phe mixtures. The 3H-to-14C ratio of the compounds decreased in the nutrition solution within the feeding time (24 h) from 4.4 to 0.75 (Fig. 3).
Table III.
Feeding of amino acids to hairy root cultures of yellow beet, line 5A
| Amino Acid (2 mm) | Constitutive Betaxanthins
|
|||||
|---|---|---|---|---|---|---|
| Formed Betaxanthins
|
Miraxanthin
V
|
Betalamic acid
|
||||
| nmol/HRC | % Incorporation | nmol/HRC | % Control | nmol/HRC | % Control | |
| d-7 Extract | – | – | 998 | – | 327 | – |
| Control | – | – | 1457 | 100 | 345 | 100 |
| Gly | 98 | 0.15 | 1560 | 107 | 328 | 95 |
| (S)-Ala | 87 | 0.14 | 2477 | 170 | 431 | 124 |
| (S)-Ser | 210 | 0.34 | 2188 | 150 | 229 | 87 |
| (S)-Thr | 497 | 0.80 | 1471 | 101 | 209 | 61 |
| d-7 Extract | – | – | 1015 | – | 311 | – |
| Control | – | – | 2297 | 100 | 621 | 100 |
| (S)-Leu | 946 | 1.53 | 1543 | 67 | 265 | 43 |
| (S)-Ile | 603 | 0.97 | 1373 | 60 | 264 | 43 |
| (S)-Val | 426 | 0.69 | 1857 | 81 | 267 | 43 |
| d-7 Extract | – | – | 761 | – | 290 | – |
| Control | – | – | 1417 | 100 | 372 | 100 |
| (S)-Gln | 197 | 0.32 | 1672 | 118 | 343 | 92 |
| (S)-Asn | 162 | 0.26 | 1520 | 107 | 243 | 65 |
| (S)-Glua | 35 | 0.06 | 2448 | 172 | 329 | 88 |
| (S)-Asp | 28 | 0.05 | 1812 | 128 | 260 | 70 |
| d-7 Extract | – | – | 1289 | – | 312 | – |
| Control | – | – | 2271 | 100 | 319 | 100 |
| (S)-Lys | 128 | 0.21 | 2890 | 127 | 192 | 69 |
| (S)-Arg | 285 | 0.46 | 2184 | 96 | 142 | 45 |
| (S)-Orn | 84 | 0.14 | 1967 | 87 | 86 | 27 |
| (S)-Met | 479 | 0.77 | 1775 | 78 | 98 | 31 |
| (S)-Trp | 245 | 0.40 | 1792 | 79 | 92 | 29 |
| d-7 Extract | – | – | 896 | – | 446 | – |
| Control | – | – | 2958 | 100 | 272 | 100 |
| (S)-Phe | 646 | 1.04 | 1423 | 48 | 109 | 24 |
| (S)-His | 1204 | 1.94 | 2375 | 80 | 96 | 22 |
| (S)-Pro | 300 | 0.84 | 3127 | 106 | 255 | 57 |
| (S)-Hyp | 877 | 1.41 | 2184 | 74 | 93 | 21 |
| Control | – | – | 2326 | 100 | 520 | 100 |
| (S)-ThiaPro | 701 | 1.13 | 1192 | 51 | 465 | 89 |
Amino acids were fed by sterile filtration to hairy root cultures (HRC) (final concentration, 2 mm) at d 7 after subcultivation. After 24 h the hairy roots were harvested and analyzed as detailed in Methods. Values are means of two independent experiments.
(S)-Gln-betaxanthin (vulgaxanthin I) was formed.
Table IV.
Feeding of (S)- and (R)-amino acid pairs to hairy root cultures (HRC) of yellow beet, line 5A
| Amino Acid (2 mm) | Constitutive Betaxanthins
|
|||||
|---|---|---|---|---|---|---|
| Formed Betaxanthins
|
Miraxanthin
V
|
Betalamic acid
|
||||
| nmol/HRC | % Incorporation | nmol/HRC | % Control | nmol/HRC | % Control | |
| d-7 Extract | – | – | 764 | – | 268 | – |
| Control | – | – | 1444 | 100 | 392 | 100 |
| (S)-Ala | 105 | 0.17 | 2691 | 186 | 567 | 145 |
| (R)-Ala | 104 | 0.17 | 1308 | 91 | 189 | 48 |
| (S)-His | 769 | 1.24 | 1082 | 75 | 151 | 39 |
| (R)-His | 295 | 0.48 | 1686 | 117 | 304 | 78 |
| d-7 Extract | – | – | n.d.a | – | n.d. | – |
| Control | – | – | 1754 | 100 | 598 | 100 |
| (S)-Leu | 673 | 1.09 | 1464 | 83 | 232 | 39 |
| (R)-Leu | 744 | 1.20 | 1709 | 97 | 221 | 37 |
| d-7 Extract | – | – | 1141 | – | 71 | – |
| Control | – | – | 2117 | 100 | 57 | 100 |
| (S)-Phe | 566 | 0.90 | 1533 | 72 | 35 | 61 |
| (R)-Phe | 661 | 1.07 | 1698 | 80 | 33 | 58 |
| d-7 Extract | – | – | 342 | – | n.d. | – |
| Controlb | – | – | 976 | 100 | n.d. | n.d. |
| (S)-Valb | 111 | 0.18 | 200 | 20 | n.d. | n.d. |
| (R)-Valb | 115 | 0.19 | 1047 | 107 | n.d. | n.d. |
Amino acids were fed by sterile filtration to HRC (final concentration, 2 mm) at d 7 after subcultivation. After 24 h the hairy roots were harvested and analyzed as detailed in Methods. Each value is the average of duplicate samples.
n.d., Not determined.
Hairy root culture, line 7, was used.
Figure 3.
Time course of uptake (24 h) of tritium-labeled (S)-Phe and 14C-labeled (R)-Phe (alone and combined, in the presence of 2 mm (S)- and (R)-Phe) by hairy root culture of yellow beets (at d 7) and 3H-to-14C ratio in the nutrient solution. Each value is the average of duplicate samples.
Exogenously Applied Amino Acids Compete with the Endogenous Dopamine in Betaxanthin Formation
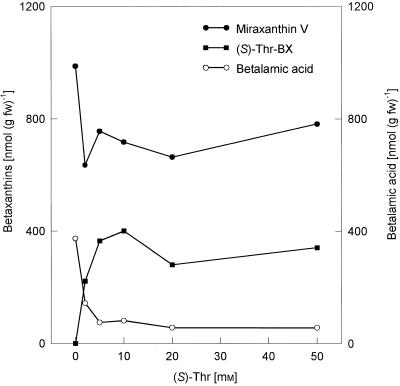
Feeding of (S)-Thr, an amino acid of high solubility, with final concentrations up to 50 mm in the nutrition solution to hairy root culture at d 7, led to an increased (S)-Thr-betaxanthin formation (optimum, 10 mm (S)-Thr), with simultaneous decreased BA and miraxanthin V levels compared with the control (Fig. 4). To suppress the miraxanthin V formation more efficiently, high amounts of (S)-Leu (5 mm) were given daily to the hairy root culture between d 4 and 8, and hairy roots were harvested 24 h after each addition. The strong increase of the miraxanthin V and BA content seen in the controls was totally suppressed, with a simultaneous increase in the (S)-Leu-betaxanthin level (Fig. 5).
Figure 4.
Feeding of (S)-Thr in increasing concentrations (final: 2–50 mm) to hairy root culture of yellow beet (at d 7) for 24 h and betalain analysis by HPLC.
Figure 5.
Repeated daily feeding of (S)-Leu (5 mm) to hairy root culture of yellow beets from d 4 and 8 and betalain analysis by HPLC 24 h after each application. Dotted lines, Control; solid lines, (S)-Leu feeding.
Feeding of (S)-Ala in increasing concentrations (2–50 mm) led, in addition to the formation of (S)-Ala-betaxanthin, to the appearance of an additional betaxanthin, the (S)-Gln-derived vulgaxanthin I. This induction could be mimicked by feeding (NH4)2SO4, but not by increasing concentrations of (R)-Ala.
Endogenously Increased Phe Level Leads to the Formation of (S)-Phe-Betaxanthin
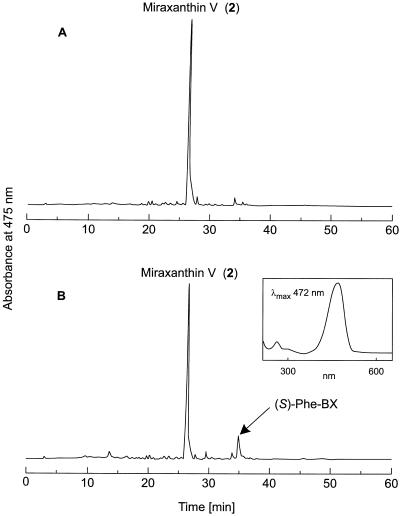
In another experiment, betaxanthin formation was affected indirectly without amino acid feeding. The addition of AIP, a strong inhibitor of PAL (EC 4.3.1.5), to a hairy root culture led to an increase of the endogenous (S)-Phe level and, subsequently, (S)-Phe-betaxanthin, which was missing in the control culture (Fig. 6), was detectable.
Figure 6.
Repeated daily feeding of AIP (16 μm) to hairy root culture of yellow beets between d 4 and 7 and betalain analysis by HPLC at d 8. A, Control; B, AIP feeding. Full scales of A475 = 0.6 absorbance units. The inset in B is the PDA spectrum of the newly formed (S)-Phe-betaxanthin. Peak numbers correspond to the biosynthetic scheme in Figure 10.
Feeding of Amino Acids to Fodder Beet Seedlings Confirms the Hairy Root Culture Results
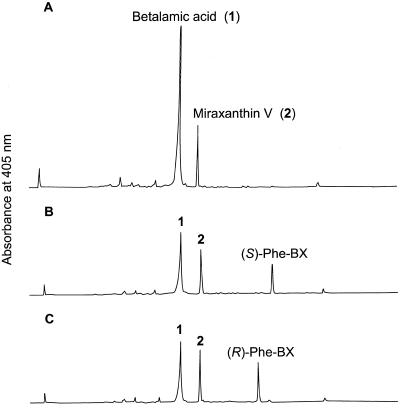
To show that the results of the amino acid feeding experiments with hairy root cultures are transferable to whole plants, amino acids were fed to de-rooted fodder beet plants via the hypocotyls. (S)- and (R)-Phe were taken up and incorporated into the corresponding betaxanthins in the same way as in the hairy root culture (Fig. 7). The application of AIP (50 μm) to the same system for 24 h also led to the increase of the (S)-Phe level and to the formation of (S)-Phe-betaxanthin, although to a smaller extent than in the hairy root culture experiment. As AIP itself is an amino acid and could result in the formation of a derived betaxanthin, the AIP-betaxanthin was synthesized as the standard, but no AIP-betaxanthin was found in the extract after AIP feeding, obviously due to the low concentration applied. Furthermore, feeding of cyclo-Dopa (2 mm) in the presence of ascorbate for stabilization, a red coloration of the hypocotyls was observed after only 60 min. HPLC analysis of the extract proved that betanidin had been formed and was accompanied by low amounts of betanin (Fig. 8).
Figure 7.
Feeding of (S)-Phe and (R)-Phe (10 mL at 10 mm) to 10 23-d-old de-rooted fodder beet plants via the hypocotyls for 48 h and betalain analysis of hypocotyl extracts by HPLC. A, Control; B, (S)-Phe feeding; C, (R)-Phe feeding. Full scales of A405 = 0.24 absorbance units. Peak numbers correspond to the biosynthetic scheme in Figure 10.
Figure 8.
Feeding of cyclo-Dopa to five 23-d-old de-rooted fodder beet plants via hypocotyls for 1.5 h and betalain analysis of hypocotyl extracts by HPLC. A, Control (80 mm ascorbate); B, cyclo-Dopa feeding (2 mm, in the presence of 80 mm ascorbate). Scales of A405 (full scale = 0.12 absorbance units) and A540 (full scale = 0.5 absorbance units) are the same in A and B. Peak numbers correspond to the biosynthetic scheme in Figure 10. 3′, Isobetanidin (the [2S/15R]-isomer of betanidin).
BA Feeding Leads to Betaxanthin Formation in Plants That Do Not Belong to the Caryophyllales
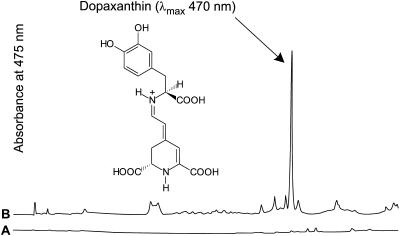
BA isolated from fodder beet hypocotyls and purified by preparative HPLC (data not shown) was fed in phosphate-buffered solution, pH 6.8, for 24 h to 2-week-old de-rooted broad bean and pea seedlings via the hypocotyls. Although the uptake was low, HPLC analysis of the hypocotyl extracts of both plants showed the presence of betaxanthins, identified by their characteristic online UV/Vis spectra. The major betaxanthin from the broad bean experiment (Fig. 9) was readily identified as dopaxanthin (λmax = 470 nm) by comparison with a synthetic standard compound. Amino acid analysis of hypocotyl extracts of broad bean seedlings revealed that Dopa was present at the highest concentration of all amino acids determined (Table V).
Figure 9.
Feeding of BA (1.4 mL of 0.285 mm in 0.1 m KPi buffer, pH 6.8) to two 14-d-old de-rooted broad bean plants via the hypocotyls for 24 h and betalain analysis of hypocotyl extracts by HPLC. A, Control (0.1 m KPi buffer, pH 6.8); B, BA feeding (0.285 mm in 0.1 m KPi buffer, pH 6.8). Scales of A475 (full scale = 0.07 absorbance units) are the same in A and B.
Table V.
Amino acid analysis of extracts from hypocotyls of 14-d-old broad bean plants used in BA-feeding experiments
| (S)-Dopa | (S)-Asp | (S)-Glu | (S)-Asn | (S)-Gln | (S)-His |
|---|---|---|---|---|---|
| μmol g−1 fresh wt | |||||
| 23.0 | 1.1 | 1.6 | 21.5 | 1.0 | 1.4 |
Amino acids were extracted and analyzed as detailed in Methods. Each value is the average of duplicate samples (se < 10%). The concentrations of all other amino acids were <1 μmol g−1 fresh weight.
Protein Extracts Do Not Catalyze the Formation of Vulgaxanthin I and Betanidin
Despite the evidence for spontaneity in the condensation reaction, enzyme extracts were prepared to study the possible catalysis of the condensation reaction. De-Eknamkul et al. (1997) found enzyme-catalyzed condensation of dopamine with the iridoid aldehyde secologanin, including aldimine formation. Protein extracts from hairy root culture prepared according to their procedure and similarly, the other extracts (acetone powder, [NH4]2SO4 precipitation), did not catalyze the formation of vulgaxanthin I or betanidin (measured photometrically or by HPLC). Whereas the spontaneous condensation of cyclo-Dopa with BA could be kinetically measured at 540 nm (Table VI), the monitoring of vulgaxanthin I formation at 475 nm failed. Table VI shows the increasing rates of the spontaneous formation of betanidin with the decreasing pH values. In the physiologically relevant pH range above 6.0, the spontaneous reaction was negligible. This was the prerequisite for all of the enzymatic attempts at betaxanthin formation.
Table VI.
pH dependence of the spontaneous in vitro condensation of cyclo-Dopa with BA as shown by betanidin formation at five different pH values
| pH 6.2 | pH 5.3 | pH 4.5 | pH 3.8 | pH 3.1 |
|---|---|---|---|---|
| nmol min−1 | ||||
| 0.01 | 0.15 | 0.26 | 0.32 | 0.42 |
BA (5 nmol) was added to cyclo-Dopa (40 nmol) in 0.1 m citrate/0.2 m NaPi-buffer (pH 3.0–6.5, containing 10 mm ascorbate). The kinetics of the A540 increase was monitored photometrically for 10 min. The betanidin formation (nmol min−1) was calculated from the linear parts of the progress curves at different pHs. Each value is the average of duplicate samples.
DISCUSSION
General Features of Betalain Biosynthesis
In contrast to the well-characterized genes and enzymes involved in anthocyanin biosynthesis (Heller and Forkmann, 1993), there are only two enzymes known to be involved in the biosynthesis of the basic skeleton of the betalains: tyrosinase, which is responsible for the formation of Dopa and cyclo-Dopa (Mueller et al., 1996; Steiner et al., 1996, 1999) and Dopa dioxygenase, which catalyzes the Dopa extradiol cleavage, leading to the formation of the chromophor BA (Girod and Zryd, 1991a; Terradas and Wyler, 1991; Hinz et al., 1997; Mueller et al., 1997a, 1997b) (for a recent review, see Roberts and Strack, 1999). Two further steps need to be clarified: (a) the possible glucosylation of cyclo-Dopa before its condensation with BA as an alternative to the glucosylation of betanidin (Heuer and Strack, 1992; Heuer et al., 1996; Vogt et al., 1997), and (b) the condensation reaction between BA and amino acids (including cyclo-Dopa) and amines (i.e. aldimine formation). This condensation reaction was studied in betaxanthin and betacyanin biosynthesis in vivo and in vitro to verify the earlier suggestion that this step proceeds nonenzymatically, i.e. spontaneously (Trezzini, 1990; Trezzini and Zryd, 1990; Hempel and Böhm, 1997). If the condensation reaction was catalyzed by an enzyme, amino acid specificity and stereoselectivity for the natural (S)-forms of amino acids should be found.
Partial Syntheses and Separation of Betaxanthins
As a prerequisite for the analysis of the products expected from the amino acid feedings, a simple method for the synthesis of stereoisomeric betaxanthins and their analytical separation had to be elaborated. Only the separation of one isomeric betaxanthin pair ([2S/11S]-indicaxanthin/[2S/11R]-indicaxanthin) was known (Terradas and Wyler, 1991). A method for the extraction of BA from acidified solutions (Döpp et al., 1982) was adapted to the isolation of BA from hydrolyzed betanin:isobetanin mixtures. This BA had to be added to amino acids and amines to get the diastereoisomeric betaxanthins suitable as standards. As the isomer ratio of the betaxanthins obtained was the same as in the starting material (betanin/isobetanin), a significant racemization of the BA under the hydrolysis conditions did not occur. Trezzini and Zryd (1991) used a buffered-ion pairing solvent system and a stepwise gradient (solvent system 2) to separate semisynthetic betaxanthins by HPLC.
Solvent system 2 was used in parallel with an optimized linear gradient (solvent system 1) to separate the obtained betaxanthin isomers (Table I). With solvent system 2 the elution sequence of the betaxanthin isomers was dependent on the polarity of the betaxanthins: 2S/S-isomers eluted earlier than the 2S/R-isomers in the case of polar betaxanthins, whereas later-eluting betaxanthins showed the reverse sequence. In contrast, all isobetaxanthins (2S/R-forms) exhibited shorter Rts than the betaxanthins (2S/S-forms) with solvent system 1. With both solvent systems a separation of BA from its isoform was not possible. Comparing the absorbance maxima of the betaxanthins in the visible range (measured by HPLC-PDA), a small bathochromic shift was detected with the change from solvent system 1 to solvent system 2, due to the differing pH conditions (Table I). Similar pH-dependent alterations of λmax were observed formerly with betacyanins (Huang and von Elbe, 1986).
When some (R)-amino acids were used in parallel with the natural (S)-forms in betaxanthin synthesis, all four possible diastereoisomers (2S/S, 2S/R, 2R/S, 2R/R) were obtained (Table II). The separation experiments revealed that the 2S/S- and 2R/R-isomers had identical Rts and, likewise, the 2S/R- and 2R/S-derivatives could not be separated with our solvent systems. As in feeding experiments, only the endogenous (S)-BA may react with (S)- and (R)-amino acids; the possible metabolites can be easily separated in most cases with our analytical tools. For the preparation of betaxanthins in larger amounts, synthesis method 3 (see Methods), which is a modification of a known procedure (Trezzini and Zryd, 1991), was used and can be recommended due to the increased betaxanthin yields, but a subsequent purification is necessary. When a hydrolyzed betanin/isobetanin sample was divided into two equal parts and further processed according to methods 2 and 3, the betaxanthin yields with method 2 were only 5% of those obtained with method 3, obviously due to the low solubility of BA in EtOAc and its increased instability at low pH (Huang and von Elbe, 1987). Despite the low yields, method 2 is the preferred procedure to synthesize betaxanthin standards because of its simplicity and the purity of the betaxanthins obtained.
Characterization of the Hairy Root Culture from Yellow Beet and Identification of the Major Betalains
During the logarithmic phase of the fresh-weight increase of hairy roots (d 7–9) a parallel rise in the betaxanthin content occurred (Fig. 1). Both hairy root lines (5A and 7) produced a betalain mixture consisting of a minor and a major betaxanthin, together with a low portion (<30%) of different betacyanins (Fig. 2). In contrast to the results of Hempel and Böhm (1997), the major betaxanthin has been identified as dopamine-betaxanthin by ESI-MS, as well as by comparison (Rt 24.7 = min; λmax = 457 nm) and co-chromatography in HPLC with synthetic dopamine-betaxanthin. This betaxanthin, first isolated as miraxanthin V (λmax = 458.5 nm in the presence of HCl) from flowers of Mirabilis jalapa (Piattelli et al., 1965), is already known as a constitutive component of the betaxanthins of callus cultures of B. vulgaris (Girod and Zryd, 1991b). Furthermore, in accordance with previous results (Hempel and Böhm, 1997), the identity of the minor betaxanthin (λmax = 468 nm) eluting at 12 min was confirmed as (S)-Gln-betaxanthin (vulgaxanthin I) by comparison and co-chromatography with the synthetic vulgaxanthin I standard. The occurrence of eight betalains in the roots of the yellow beets from which the hairy root cultures were derived has been reported, including vulgaxanthin I and vulgaxanthin II (Savolainen and Kuusi, 1978).
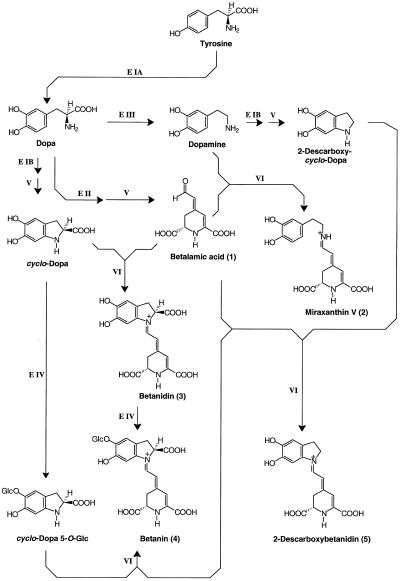
Betanin was readily identified from the different betacyanins occurring at low concentration in hairy root extracts by comparison (Rt = 22.7 min; λmax = 538 nm) and co-chromatography with authentic betanin from suspension culture of B. vulgaris (Bokern et al., 1991). From the high level of dopamine in the hairy root culture, the occurrence of a dopamine-derived betacyanin (2-descarboxy-betanidin) was suggested and verified. The natural occurrence of 2-descarboxy-betanidin was hitherto only once reported as a minor betacyanin constituent in flowers of Carpobrotus acinaciformis (L.) L. Bol. (Aizoaceae) (Piattelli and Impellizzeri, 1970), a xerophilous plant native to South Africa, but not as a pigment component in the genus Beta (Chenopodiaceae) until now. In addition to the betalains identified, the precursors of miraxanthin V, dopamine and BA, also occurred in high concentrations. A scheme illustrating the different steps of betalain biosynthesis in hairy root culture is given in Figure 10.
Figure 10.
Scheme of betalain biosynthesis in hairy root culture. Enzymatically catalyzed steps: E IA, hydroxylating activity of tyrosinase; E IB, oxidizing activity of tyrosinase; E II, Dopa dioxygenase; E III, Dopa decarboxylase; E IV, glucosyltransferase. Spontaneous steps: V, cyclization reactions; and VI, condensation reactions (aldimine formation). Compound numbers of this scheme correspond to peak numbers in Figures 2, 6, 7, and 9.
Feeding of Amino Acids to Hairy Root Culture of Yellow Beets and Other Cell Cultures
The amino acid-feeding experiments were undertaken to investigate the amino acid specificity and the stereoselectivity of the decisive step in betaxanthin biosynthesis. The results of amino acid feeding to hairy root cultures summarized in the Table III show that all amino acids were used in the formation of the corresponding betaxanthins, but to a different extent, and thus no amino acid specificity was detectable. As noted previously by Hempel and Böhm (1997), (S)-Glu did not give the expected vulgaxanthin II, but instead yielded vulgaxanthin I. There is no clear trend in betaxanthin formation concerning the polarity and charge of the fed amino acids. Polar or basic amino acids as (S)-Hyp and (S)-His resulted in equally high incorporation rates as the nonpolar neutral amino acids (S)-Leu and (S)-Phe. Results of (S)-Tyr and tyramine feedings could not be included in Table III. Due to the high tyrosinase activity of the hairy roots, the culture turned rapidly black (melanin formation) after feeding of these compounds.
The different uptake rates of the amino acids and their involvement in the primary metabolism and protein synthesis may have a substantial influence on the intracellular amino acid concentration, which is available for condensation with BA. By comparison of the increases of the miraxanthin V levels found at d 7 with those found on the day of harvest (d 8) in the controls with those of the amino acid-feeding experiments, it is obvious that the amino acids compete for the BA with the endogenous dopamine. This frequently results in a lower miraxanthin V content, and in most cases, in a lower BA content (Table III). Deviations from this trend might be caused by remarkably higher fresh weights of the hairy roots in the fed plants than in the water controls. Additionally, (S)-4-thiaprolin, a synthetic sulfur analog of (S)-Pro, was accepted as a precursor and yielded a high incorporation rate (Table III).
Feeding experiments with (S)- and (R)-amino acids to test the stereoselectivity of the condensation reaction clearly showed that the isomers were similarly accepted (Table IV); therefore, the aldimine bond formation must proceed without any stereoselectivity, which is indicative of a spontaneous process. The apparent stereoselectivity for the (S)-isomer in simultaneous feeding of (S)- and (R)-Phe mixtures was proven to be caused by an inhibited uptake of (R)-Phe in the presence of the (S)-isomer, as shown in a double-labeling experiment (Fig. 3). The decrease of the (S)-[2,6-3H2]Phe/(R)-[1-14C]Phe ratio in the nutrition solution demonstrated a preferential uptake of (S)-Phe in the presence of (R)-Phe, explaining the feeding results.
Saturation experiments (2–50 mm amino acids) were performed to determine whether amino acids applied exogenously in increasing concentrations can compete with the endogenous dopamine in betaxanthin formation. The results (Fig. 4) show that the (S)-Thr-betaxanthin levels increased with increasing (S)-Thr-concentrations (up to 10 mm), with a simultaneous decrease in the BA and miraxanthin V levels compared with water controls. An almost complete stop in miraxanthin V formation was achieved by daily application of (S)-Leu (5 mm) from d 4 to 8 (Fig. 5). Due to the constant high supply of (S)-Leu (final concentration in the hairy roots was found to be 30 mm), the miraxanthin V level did not increase because the accumulating BA was rapidly consumed in the condensation reaction with (S)-Leu and was, therefore, unavailable for condensation with endogenous dopamine.
Increasing concentrations (up to 50 mm) of (S)-Ala, which showed a relatively low rate of incorporation into (S)-Ala-betaxanthin (Table III), also showed an interesting side effect: a concentration-dependent increase of another betaxanthin, the (S)-Gln-derived vulgaxanthin I. This phenomenon can be explained by the action of Ala:2-oxoglutarate aminotransferase (EC 2.6.1.19), which leads to the formation of pyruvate and glutamate. Gln is then formed by ammonia fixation via Gln synthetase (EC 6.3.1.2). To determine whether vulgaxanthin I formation is indeed dependent upon increased ammonia fixation, (NH4)2SO4 was added to the standard nutrition solution, which led to a concentration-dependent accumulation of vulgaxanthin I, with the optimum at 20 mm (NH4)2SO4. In accordance with this hypothesis, (R)-Ala-feeding (2–50 mm) did not result in the formation of vulgaxanthin I, but only in increased (R)-Ala-betaxanthin levels. Thus, it could be shown that the betaxanthin biosynthesis can be regulated in vivo not only by amino acid feeding, but also by substances only indirectly involved in biosynthesis. It can be argued that betaxanthin formation after exogenous amino acid feeding may be a “detoxification” process that does not reflect normal endogenous conditions.
To exclude this argument, we tried to increase the level of a specific amino acid in the hairy roots by other means. For this purpose AIP, a strong inhibitor of PAL (EC 4.3.1.5), was added over 4 d to hairy root cultures. Although the content of phenylpropanes was relatively low in the hairy roots, the AIP treatment increased the (S)-Phe level, and (S)-Phe-betaxanthin (missing in the control culture) was subsequently formed (Fig. 6). Thus, we have shown that by endogenous increase of the concentration of a certain amino acid, the spontaneous formation of the corresponding betaxanthin derived from this amino acid can be induced. When (S)-Phe was fed under the same conditions to suspension cultures of red beets and D. bellidiformis, which form mainly or exclusively betacyanins, respectively, the formation of a betaxanthin derived from (S)-Phe was not observed, whereas in a hairy root culture of red beets (S)-Phe-betaxanthin could be detected after feeding, although in comparably low amounts. This may indicate that the BA formation in these systems is controlled differently than those in mainly betaxanthin-forming cultures, and that BA is possibly channeled immediately into betacyanins.
Feeding Experiments with Fodder Beet Plants
HPLC of hypocotyl extracts from fodder beet plants showed a betaxanthin and BA pattern very similar to that of the hairy root culture of yellow beets. Therefore, we used these plants to prove whether or not the condensation reaction in amino acid feedings proceeds in the same way as in hairy root cultures. (S)- and (R)-Phe were taken up by de-rooted fodder beet plants and were incorporated into the corresponding betaxanthins in the same way and to the same extent as in the hairy root culture without any stereoselectivity (Fig. 7). The application of AIP (50 μm) to the same plant material for 24 h also led to an increase in the (S)-Phe-level and to the formation of (S)-Phe-betaxanthin, although to a smaller extent than in the hairy root culture experiment (data not shown).
To rule out spontaneous condensation as being specific for betaxanthins, cyclo-Dopa, the building block of all betacyanins, was fed to this system. After less than 1 h, a red coloration of the hypocotyls was observed. HPLC analyses proved that betanidin was formed and was accompanied by low amounts of betanin (Fig. 8). From the HPLC insets it can be seen that the betanidin formation proceeded at the expense of the free BA, the concentration of which was clearly higher in the buffer controls than in the fed group. The results of amino acid (including cyclo-Dopa) feedings suggest that condensation in both betaxanthin and betacyanin biosynthesis proceeds according to the same mechanism.
BA Feeding to Broad Bean and Pea Plants
To find additional evidence for the spontaneous character of the condensation reaction, BA was fed to broad bean and pea seedlings, which do not belong to the betalain-synthesizing Caryophyllales. The analyses of both extracts after BA feeding showed the presence of betaxanthins, in contrast to extracts of buffer-treated plants. The major betaxanthin from the broad bean experiment (Fig. 9) was identified as dopaxanthin (λmax = 470 nm) on the basis of the Rt and co-injection with a synthesized standard compound. Amino acid analysis and Dopa determination of hypocotyl extracts by HPLC revealed that the Dopa concentration was higher than that of all other amino acids (Table V). Because the Asn concentration was also high, the preferred formation of dopaxanthin was an unexpected outcome, and may indicate a different localization of different amino acids, leading to a more facilitated access of BA to Dopa than to Asn. The pattern of distribution and concentration of amino acids in the vacuole is similar to that in the cytoplasm, but quite different from that in the chloroplast (Mimura et al., 1990), the site of synthesis of many amino acids in higher plants. Because it is reasonable to assume that broad bean hypocotyls (which do not synthesize betalains) do not have an enzyme catalyzing the condensation reaction, the dopaxanthin formation observed must have resulted from a nonenzymic spontaneous process.
Spontaneous versus Enzymatic Condensation Reactions
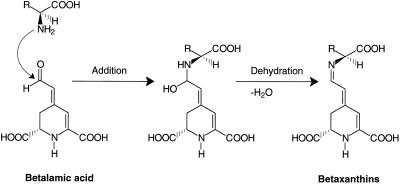
The formation of the aldimine bond in the betaxanthin biosynthesis proceeds in two steps: the nucleophilic addition of the amino group at the aldehyde group leads to an intermediate from which water is eliminated, forming the aldimine bond (Fig. 11). The reaction of an amine with an aldehyde is an enzymatically important catalyzed step in benzylisoquinoline biosynthesis (dopamine with 4-hydroxyphenylacetaldehyde or 3,4-dihydroxyphenylacetaldehyde), which leads, however, to the cyclized intermediates norcoclaurine and norlaudanosoline, respectively (Rueffer and Zenk, 1987). Similarly, the condensation of dopamine with the iridoid aldehyde secologanin was found to be catalyzed by cell-free extracts of Alangium lamarckii (De-Eknamkul et al., 1997). This cyclization proceeded, in contrast to the betaxanthin formation, directly to tetrahydroisoquinoline derivatives (R- and S-form), which spontaneously cyclized further in lactam formation. The first reaction steps can also proceed nonenzymic at pH 5.0 (Itoh et al., 1995).
Figure 11.
Scheme illustrating the formation of the aldimine bond in betaxanthin biosynthesis. In the first step the amino group of amino acids is added to the aldehyde moiety of the BA and the intermediate then eliminates water, resulting in the aldimine bond.
The involvement of nonenzymic steps in the biosynthesis of secondary compounds is a rare but important phenomenon; for example, transformation of neopinone to codeinone (Gollwitzer et al., 1993) in morphine biosynthesis; intramolecular cyclization of γ-methylaminobutyraldehyde to N-methyl pyrrolinium cation, and its coupling with acetoacetic acid giving hygrine (Endo et al., 1988); Michael-type addition of l-kynurenine to N-β-alanyldopamine quinone methide leads to papiliochrome II, a yellow wing pigment of butterflies (Saul and Sugumaran, 1991); hydration at position 6 of protein-bound dopaquinone to form 6-hydroxyDopa (Topa), the precursor of Topayquinone that was identified as an essential co-factor of copper amine oxidase (Mure and Tanizawa, 1997); and late biosynthetic steps in the formation of antibiotics (Mayer and Thiericke, 1993).
Similar to various other extraction methods, protein extraction from hairy roots carried out according to the method of De-Eknamkul et al. (1997) did not show enzymatically catalyzed betaxanthin formation. The development of an enzyme assay was complicated by the fact that BA and amino acids condensed spontaneously under acidic conditions and also under the conditions of the HPLC analysis (1.5% H3PO4). Attempts to pursue the betaxanthin formation photometrically at 475 nm failed. In contrast, the condensation of cyclo-Dopa with BA could be measured photometrically at 540 nm (Table VI) and showed a strong increase in the rate of spontaneous condensation with decreasing pH values. In the physiological pH range above 6.0, however, the spontaneous reaction was negligible. This was the prerequisite for the enzymatic attempts of betaxanthin formation described above.
CONCLUSION
Considering previously published studies and the results of our experiments, we are convinced that the condensation process of the BA with amino acids (including cyclo-Dopa) or amines in plants is a spontaneous rather than an enzyme-catalyzed reaction. This assumption is substantiated by the following lines of evidence: (a) experiments failed to detect protein-catalyzed betaxanthin formation; (b) the formation of betaxanthins after amino acid feeding to a hairy root culture of yellow beets showed neither an amino acid specificity nor a stereoselectivity; (c) the betaxanthin formation was also observed with unnatural precursors ([S]-4-thiaprolin); (d) (S)-Phe-betaxanthin formation was detected after inhibition of PAL by AIP due to an increase of the endogenous (S)-Phe level; (e) the results of betaxanthin formation in hairy root cultures of yellow beets were reproduced with intact plants (fodder beets). Furthermore, the formation of a betacyanin, betanidin, has been demonstrated by feeding cyclo-Dopa to these plants; and (f) application of BA to plants that do not form betaxanthin led to betaxanthin formation.
Finally, two questions remain to be answered: How do betaxanthin-forming plants achieve the accumulation of specific betaxanthin patterns (Steglich and Strack, 1990), most likely irrespectively of the pattern of soluble amino acids/amines in these plants? And in which subcellular compartment is the aldimine formation located? Is there a specific BA transporter in the tonoplast, assuming that the vacuole is the site of this reaction?
ACKNOWLEDGMENTS
We thank Barbara Kolbe (Institut für Pflanzenbiochemie [IPB], Halle, Germany) for skillful technical assistance in plant cell cultivation; Dr. Hartmut Böhm (Deutsches Institut für Ernährungsforschung, Bergholz-Rehbrücke, Germany) for providing hairy root cultures of yellow beets; Dr. Inna Kuzovkina (K.A. Timiryasev Institute of Plant Physiology, Russian Academy of Sciences, Moscow) for establishing a hairy root culture of red beets; Dr. Alfred Baumert (IPB) for (S)-4-thiaprolin synthesis; Dr. Nicolaus Amrhein (Eidgenössische Technische Hochschule Zürich, Switzerland) for providing AIP and proposing the AIP-feeding experiments; Dr. Jürgen Schmidt (IPB) for MS measurements; and Dr. Michael Kiess (Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany) for amino acid analyses. We are also grateful to Christine Kaufmann and Annett Kohlberg (IPB) for the figures and photographs. Finally, we thank Ulrike Steiner (IPB) for valuable discussions and Martina Rauscher (IPB) for critical reading of the manuscript.
Abbreviations:
- AIP
2-aminoindan 2-phosphonic acid
- BA
betalamic acid
- Dopa
3-(3,4-dihydroxyphenyl)-Ala
- ESI-MS
electrospray ionization MS
- EtOAc
ethyl acetate
- PAL
Phe ammonia-lyase
- PDA
photodiode array detection
- Rt
retention time
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (Bonn) and the Fonds der Chemischen Industrie (Frankfurt/M.). N.K. was supported by HSP III of the Kultusministerium des Landes Sachsen-Anhalt (Germany).
LITERATURE CITED
- Adachi T, Nakatsukasa M, Asaka Y, Uta T. Genetic analysis and some properties of flower color mutants found in the progenies of x-ray irradiated Portulaca sp. “Jewel.”. Jpn J Breed. 1985;35:183–192. [Google Scholar]
- Bianco-Colomas J. Qualitative and quantitative aspects of betalains biosynthesis in Amaranthus caudatus L. var. pendula seedlings. Planta. 1980;149:176–180. doi: 10.1007/BF00380880. [DOI] [PubMed] [Google Scholar]
- Böhm H, Hermersdörfer H, Schönfeld A. Betaxanthinbildende hairy-root-kulturen von Beta vulgaris var. lutea. Vorträge für Pflanzenzüchtung. 1991;21:105–108. [Google Scholar]
- Bokern M, Heuer S, Wray V, Witte L, Macek T, Vanek T, Strack D. Ferulic acid conjugates and betacyanins from cell cultures of Beta vulgaris. Phytochemistry. 1991;30:3261–3265. [Google Scholar]
- De-Eknamkul W, Ounaroon A, Tanahashi T, Kutchan TM, Zenk MH. Phytochemistry. 1997;45:477–484. [Google Scholar]
- Döpp H, Maurer S, Sasaki AN, Musso H (1982) Fliegenpilzfarbstoffe, VIII. Die Konstitution der Musca-aurine. Liebigs Ann Chem 254–264
- Endo T, Hamaguchi N, Hashimoto T, Yamada Y. Non-enzymatic synthesis of hygrine from acetoacetic acid and from acetonedicarboxylic acid. FEBS Lett. 1988;234:86–90. [Google Scholar]
- French CJ, Pecket RC, Smith H. Effect of exogenous DOPA and tyrosine on amaranthin synthesis and pigment type in Amaranthus. Phytochemistry. 1974;13:1505–1511. [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Girod P-A, Zryd J-P. Phytochemistry. 1991a;30:169–174. [Google Scholar]
- Girod P-A, Zryd J-P. Secondary metabolism in cultured red beet (Beta vulgaris L.) cells: differential regulation of betaxanthin and betacyanin biosynthesis. Plant Cell Tissue Organ Cult. 1991b;25:1–12. [Google Scholar]
- Giudici de Nicola M, Amico V, Sciuto S, Piattelli M. Light control of amaranthin synthesis in isolated Amaranthus cotyledons. Phytochemistry. 1975;14:479–481. [Google Scholar]
- Gollwitzer J, Lenz R, Hampp N, Zenk MH. The transformation of neopinone to codeinone in morphine biosynthesis proceeds non-enzymatically. Tetrahedron Lett. 1993;34:5703–5706. [Google Scholar]
- Heller W, Forkmann G. Biosynthesis of flavonoids. In: Harborne JB, editor. The Flavonoids. Advances in Research Since 1986. London: Chapman & Hall; 1993. pp. 499–535. [Google Scholar]
- Hempel J, Böhm H. Betaxanthin pattern of hairy roots from Beta vulgaris var. lutea and its alteration by feeding of amino acids. Phytochemistry. 1997;44:847–852. [Google Scholar]
- Heuer S, Strack D. Synthesis of betanin from betanidin and UDP-glucose by a protein preparation from cell suspension cultures of Dorotheanthus bellidiformis (Burm.f.) N.E.Br. Planta. 1992;186:626–628. doi: 10.1007/BF00198045. [DOI] [PubMed] [Google Scholar]
- Heuer S, Vogt T, Böhm H, Strack D. Partial purification and characterization of UDP-glucose:betanidin 5-O- and 6-O-glucosyltransferases from cell suspension cultures of Dorotheanthus bellidiformis (Burm.f.) N.E.Br. Planta. 1996;199:244–250. [Google Scholar]
- Hinz UG, Fivaz J, Girod P-A, Zryd J-P. The gene coding for the DOPA dioxygenase involved in betalain biosynthesis in Amanita muscaria and its regulation. Mol Gen Genet. 1997;256:1–6. doi: 10.1007/s004380050539. [DOI] [PubMed] [Google Scholar]
- Huang AS, von Elbe JH. Stability comparison of two betacyanine pigments: amaranthine and betanine. J Food Sci. 1986;51:670–674. [Google Scholar]
- Huang AS, von Elbe JH. Effect of pH on the degradation and regeneration of betanine. J Food Sci. 1987;52:1689–1693. [Google Scholar]
- Itoh A, Tanahashi T, Nagakura N. J Nat Prod. 1995;58:1228–1239. [Google Scholar]
- Lange W, Brandenburg WA, De Bock TSM (1998) Proposal for a new taxonomical classification of the cultivated forms of beet, Beta vulgaris L. In L Frese, L Panella, HM Srivastava, W Lange, eds, International Beta Genetic Resources Network. A Report on the 4th International Beta Genetic Resources Workshop and World Beta Network Conference, Izmir (Turkey), International Crop Network Series No. 12. IPGRI, Rome, pp 16–22
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. [Google Scholar]
- Mayer M, Thiericke R. A non-enzymic reaction in the late biosynthesis of the decarestrictine family. J Antibiotics. 1993;46:1372–1380. doi: 10.7164/antibiotics.46.1372. [DOI] [PubMed] [Google Scholar]
- Mimura T, Sakano K, Tazawa M. Changes in the subcellular distribution of free amino acids in relation to light conditions in cells of Chara corallina. Bot Acta. 1990;103:42–47. [Google Scholar]
- Mueller LA, Hinz U, Uzé M, Sautter C, Zryd J-P. Biochemical complementation of betalain biosynthetic pathway in Portulaca grandiflora by a fungal 3,4-dihydroxyphenylalanine dioxygenase. Planta. 1997a;203:260–263. [Google Scholar]
- Mueller LA, Hinz U, Zryd J-P. The formation of betalamic acid and muscaflavin by recombinant DOPA-dioxygenase from Amanita. Phytochemistry. 1997b;44:567–569. [Google Scholar]
- Mueller LA, Hinz U, Zryd J-P. Characterization of a tyrosinase from Amanita muscaria involved in betalain biosynthesis. Phytochemistry. 1996;42:1511–1515. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Mure M, Tanizawa K. Chemical and biochemical characteristics of topa quinone. Biosci Biotech Biochem. 1997;61:410–417. [Google Scholar]
- Piattelli M, Impellizzeri G. y. 1970;9:2553–2556. [Google Scholar]
- Piattelli M, Minale L, Nicolaus RA. Pigments of centrospermae. V. Betaxanthins from Mirabilis jalapa L. Phytochemistry. 1965;4:817–823. [Google Scholar]
- Rink E, Böhm H. Changed betaxanthin pattern in violet flowers of Portulaca grandiflora after feeding of DOPA. Phytochemistry. 1985;24:1475–1477. [Google Scholar]
- Rink E, Böhm H. Effect of DOPA feeding on betaxanthins in various species of Centrospermae. Phytochemistry. 1991;30:1109–1112. [Google Scholar]
- Roberts M-J, Strack D. Alkaloids, amines and betalains. In: Wink M, editor. The Role of Plant Secondary Metabolites and TheirUtilisation in Biotechnology, Annual Plant Reviews 1999: Biochemistry, Function and Application of Plant Natural Products. Physiology & Biochemistry of Secondary Metabolism. Sheffield, UK: Academic Press; 1999. , in press. [Google Scholar]
- Rueffer M, Zenk MH. Distant precursors of benzoisoquinoline alkaloids and their enzymatic formation. Z Naturforsch. 1987;42c:319–332. [Google Scholar]
- Saul SJ, Sugumaran M. Quinone methide as a reactive intermediate formed during the biosynthesis of papiliochrome II, a yellow wing pigment of papillionid butterflies. FEBS Lett. 1991;279:145–148. doi: 10.1016/0014-5793(91)80270-d. [DOI] [PubMed] [Google Scholar]
- Savolainen K, Kuusi T. The stability properties of golden beet and red beet pigments: influence of pH, temperature, and some stabilizers. Z Lebensm-Unters-Forsch. 1978;166:19–22. doi: 10.1007/BF01122999. [DOI] [PubMed] [Google Scholar]
- Steglich W, Strack D. Betalains. In: Brossi A, editor. The Alkaloids. Chemistry and Pharmacology, Vol 39. London: Academic Press; 1990. pp. 1–62. [Google Scholar]
- Steiner U, Schliemann W, Böhm H, Strack D (1999) Tyrosinase involved in betalain biosynthesis of higher plants. Planta (in press)
- Steiner U, Schliemann W, Strack D. Assay for tyrosine hydroxylation activity of tyrosinase from betalain-forming plants and cell cultures. Anal Biochem. 1996;238:72–75. doi: 10.1006/abio.1996.0253. [DOI] [PubMed] [Google Scholar]
- Strack D, Steglich W, Wray V. Betalains. In: Dey PM, Harborne JB, Waterman PG, editors. Methods in Plant Biochemistry, Vol 8. Alkaloids and Sulphur Compounds. London: Academic Press; 1993. pp. 421–450. [Google Scholar]
- Terradas F, Wyler H. 2,3- and 4,5-Secodopa, the biosynthetic intermediates generated from l-Dopa by an enzyme system extracted from the fly agaric, Amanita muscaria L., and their spontaneous conversion to muscaflavin and betalamic acid, respectively, and betalains. Helv Chim Acta. 1991;74:124–140. [Google Scholar]
- Trezzini GF (1990) Génétique des bétalaines chez Portulaca grandiflora Hook. PhD thesis. University of Lausanne, Switzerland
- Trezzini GF, Zryd J-P. Portulaca grandiflora: a model system for the study of the biochemistry and genetics of betalain biosynthesis. Acta Hortic. 1990;280:581–585. [Google Scholar]
- Trezzini GF, Zryd J-P. Characterization of some natural and semi-synthetic betaxanthins. Phytochemistry. 1991;30:1901–1903. [Google Scholar]
- Vogt T, Zimmermann E, Grimm R, Meyer M, Strack D. Are the characteristics of betanidin glucosyltransferases from cell-suspension cultures of Dorotheanthus bellidiformis indicative of their phylogenetic relationship with flavonoid glucosyltransferases? Planta. 1997;203:349–361. doi: 10.1007/s004250050201. [DOI] [PubMed] [Google Scholar]
- Wyler H, Chiovini J. Die Synthese von Cyclodopa (Leucodopachrom) Helv Chim Acta. 1968;51:1476–1494. doi: 10.1002/hlca.19680510636. [DOI] [PubMed] [Google Scholar]
- Wyler H, Vincenti G, Mercier M, Sassu G, Dreiding AS. Zur Konstitution des Randenfarbstoffes Betanin. Helv Chim Acta. 1959;42:1696–1698. [Google Scholar]