Abstract
In the title compound, C12H13N5O2, a Schiff-base-derived chelate ligand, the non-aromatic heterocycle and its substituents essentially occupy one common plane (r.m.s. of fitted non-H atoms = 0.0503 Å). The N=C bond is E-configured. Intracyclic angles in the pyridine moiety cover the range 117.6 (2)–124.1 (2)°. Intra- and intermolecular N—H⋯N and N—H⋯O hydrogen bonds are observed in the crystal structure, as are intra- and intermolecular C—H⋯O contacts which, in total, connect the molecules into a three-dimensional network. The shortest ring-centroid-to-ring-centroid distance of 3.5831 (14) Å is between the two different types of six-membered rings.
Related literature
For the crystal structures of two polymorphs of 6-amino-1,3-dimethyl-5-[(E-2-(methylsulfanyl)benzylideneamino]pyrimidine-2,4(1H,3H)-dione, see: Booysen et al. (2011a
▶,b
▶). For graph-set analysis of hydrogen bonds, see: Etter et al. (1990 ▶); Bernstein et al. (1995 ▶). For puckering analysis, see: Cremer & Pople (1975 ▶). For general information about the chelate effect in coordination chemistry, see: Gade (1998 ▶).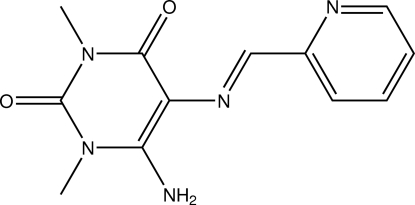
Experimental
Crystal data
C12H13N5O2
M r = 259.27
Orthorhombic,
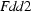
a = 26.5036 (8) Å
b = 28.9987 (14) Å
c = 6.2193 (1) Å
V = 4780.0 (3) Å3
Z = 16
Mo Kα radiation
μ = 0.10 mm−1
T = 200 K
0.27 × 0.14 × 0.06 mm
Data collection
Bruker APEXII CCD diffractometer
11316 measured reflections
1620 independent reflections
1171 reflections with I > 2σ(I)
R int = 0.049
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.079
S = 0.94
1620 reflections
182 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.17 e Å−3
Δρmin = −0.19 e Å−3
Data collection: APEX2 (Bruker, 2010 ▶); cell refinement: SAINT (Bruker, 2010 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811031618/lh5299sup1.cif
Supplementary material file. DOI: 10.1107/S1600536811031618/lh5299Isup2.cdx
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811031618/lh5299Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811031618/lh5299Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H71⋯N5i | 0.93 (3) | 2.08 (3) | 2.928 (3) | 151 (3) |
| N4—H72⋯N3 | 0.84 (2) | 2.25 (2) | 2.661 (3) | 110.2 (18) |
| N4—H72⋯O2ii | 0.84 (2) | 2.54 (2) | 3.108 (3) | 125.8 (19) |
| C7—H7⋯O2 | 0.95 | 2.17 | 2.847 (3) | 127 |
| C11—H11⋯O1iii | 0.95 | 2.54 | 3.313 (3) | 138 |
Symmetry codes: (i) 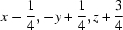 ; (ii)
; (ii) 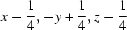 ; (iii)
; (iii)  .
.
Acknowledgments
The authors thank Mr David Neale-Shutte for helpful discussions.
supplementary crystallographic information
Comment
Chelate ligands have found widespread use in coordination chemistry due to the enhanced thermodynamic stability of resultant coordination compounds in relation to coordination compounds exclusively applying comparable monodentate ligands (Gade 1998). Combining different sets of donor atoms in one chelate ligand molecule, a probe for testing and accomodating metal centers of different Lewis acidities is at hand. To enable comparative studies with envisioned coordination compounds, we determined the crystal structure of the title compound. Two crystal structures of 6-amino-1,3-dimethyl-5-[(E-2- (methylsulfanyl)benzylideneamino]pyrimidine-2,4(1H,3H)-dione are apparent in the literature (Booysen et al., 2011a; Booysen et al., 2011b).
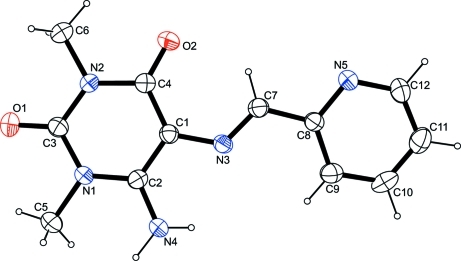
The molecule is a Schiff-base composed of a pyridyl moiety and a 6-amino-1,3- dimethylpyrimidine-2,4(1H,3H)-dione moiety. The C=N double-bond is (E)-configured. A conformation analysis of the non-aromatic six-membered ring (Cremer & Pople, 1975) fails due to the low puckering amplitude (τ = 2.9 °; r.m.s. of fitted non-hydrogen atoms – including the exocyclic substituents – = 0.0503 Å). Intracyclic angles in the pyridyl moiety cover a range from 117.6 (2)–124.1 (2) ° with the smallest angle found on the nitrogen atom and the largest angle found on the unsubstituted carbon atom in ortho position to the nitrogen atom. The least-squares planes defined by the respective atoms of the six-membered heterocycles intersect at an angle of 8.11 (12) °. The amino group is almost planar. The plane defined by its atoms and the least-squares plane defined by the atoms of its six-membered carrier ring enclose an angle of 9.56(2.54) ° (Fig. 1).
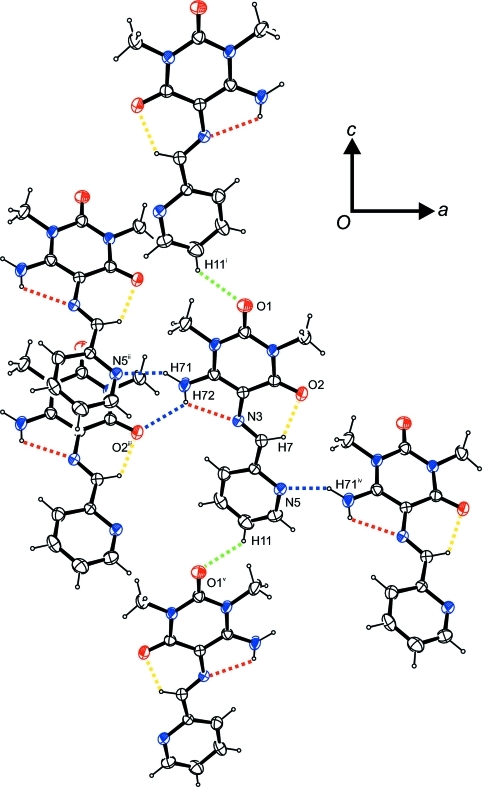
In the crystal structure, hydrogen bonds of N–H···N type as well as N–H···O type are observed. These are intra- as well as intermolecular and, in the case of N–H···N hydrogen bonds, involve only the nitrogen atom of the aromatic system and the Schiff base's double bonded nitrogen atom as acceptor. The intramolecular hydrogen bond shows bifurcation involving an oxygen atom. Apart from these classical hydrogen bonds, intra- as well as intermolecular C–H···O contacts can be observed whose range falls by more than 0.5 Å (in the former case) and by almost 0.2 Å (in the latter case) below the sum of van-der-Waals radii of the atoms participating. While the intramolecular C–H···O contact is apparent between the vinylic hydrogen atom and the neighbouring oxygen atom, the intermolecular C–H···O contacts are supported by the C–H group in para position to the Schiff-base substituent on the aromatic system and the keto group's oxygen atom that is not involved in the intramolecular C–H···O contacts. In terms of graph-set analysis (Etter et al., 1990; Bernstein et al., 1995), the descriptor for the classical hydrogen bonds is S(5)C11(6)C11(8) on the unitary level while a description of the C–H···O contacts necessitates a S(6)C11(12) descriptor on the same level. In total, the molecules are connected to a three-dimensional network. The shortest intercentroid distance between two centers of gravity was measured at 3.5831 (14) Å while the shortest intercentroid distance between two aromatic systems was found at 5.2956 (14) Å. (Fig. 2).
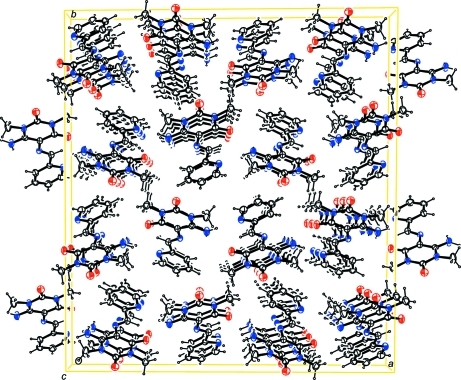
The packing of the title compound in the crystal is shown in Figure 3.
Experimental
Equimolar amounts of picolinaldehyde (1.00 g, 9.36 mmol) and 5,6-diamino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1.59 g) in anhydrous methanol (50 cm3) were refluxed for 3 h. The reaction mixture was allowed to cool to room temperature. An orange precipitate was isolated, which was recrystallized from anhydrous acetonitrile to give orange crystals. The crystals were filtered and dried under vacuum.
Refinement
Carbon-bound H atoms were placed in calculated positions (C—H 0.95 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2Ueq(C). The H atoms of the methyl groups were allowed to rotate with a fixed angle around the C—C bond to best fit the experimental electron density (HFIX 137 in the SHELX program suite (Sheldrick, 2008)), with U(H) set to 1.5Ueq(C). Both nitrogen-bound H atoms were located in a difference Fourier map and refined freely.
Figures
Fig. 1.
The molecular structure of the title compound, with anisotropic displacement ellipsoids (drawn at 50% probability level).
Fig. 2.
Intermolecular contacts, viewed along [0 - 1 0]. Blue dashed lines indicate clasical intermolecular hydrogen bonds, red dashed lines indicate classical intramolecular hydrogen bonds, green dashed lines intermolecular C–H···O contacts and yellow dashed lines intramolecular C–H···O contacts. Symmetry operators: i -x + 1/4, y - 1/4, z + 7/4; iix - 1/4, -y + 1/4, z + 3/4; iiix - 1/4, -y + 1/4, z - 1/4; ivx + 1/4, -y + 1/4, z - 3/4; v -x + 1/4, y + 1/4, z - 7/4.
Fig. 3.
Molecular packing of the title compound, viewed along [0 0 - 1] (anisotropic displacement ellipsoids drawn at 50% probability level).
Crystal data
| C12H13N5O2 | F(000) = 2176 |
| Mr = 259.27 | Dx = 1.441 Mg m−3 |
| Orthorhombic, Fdd2 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: F 2 -2d | Cell parameters from 2610 reflections |
| a = 26.5036 (8) Å | θ = 2.8–25.2° |
| b = 28.9987 (14) Å | µ = 0.10 mm−1 |
| c = 6.2193 (1) Å | T = 200 K |
| V = 4780.0 (3) Å3 | Platelet, red |
| Z = 16 | 0.27 × 0.14 × 0.06 mm |
Data collection
| Bruker APEXII CCD diffractometer | 1171 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.049 |
| graphite | θmax = 28.3°, θmin = 2.1° |
| φ and ω scans | h = −35→32 |
| 11316 measured reflections | k = −38→38 |
| 1620 independent reflections | l = −8→8 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.079 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.94 | w = 1/[σ2(Fo2) + (0.0422P)2] where P = (Fo2 + 2Fc2)/3 |
| 1620 reflections | (Δ/σ)max < 0.001 |
| 182 parameters | Δρmax = 0.17 e Å−3 |
| 1 restraint | Δρmin = −0.19 e Å−3 |
Special details
| Refinement. Due to the absence of a strong anomalous scatterer, the Flack parameter is meaningless. Thus, Friedel opposites (1312 pairs) have been merged and the item was removed from the CIF. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.15951 (6) | 0.02666 (6) | 0.6362 (3) | 0.0368 (5) | |
| O2 | 0.24640 (6) | 0.08960 (6) | 0.0779 (2) | 0.0332 (4) | |
| N1 | 0.11534 (7) | 0.06900 (7) | 0.3925 (3) | 0.0284 (5) | |
| N2 | 0.20212 (7) | 0.05684 (7) | 0.3521 (3) | 0.0267 (5) | |
| N3 | 0.15107 (7) | 0.12779 (6) | −0.0955 (3) | 0.0246 (4) | |
| N4 | 0.06933 (8) | 0.10885 (8) | 0.1366 (4) | 0.0347 (5) | |
| H71 | 0.0405 (11) | 0.0991 (9) | 0.207 (5) | 0.056 (9)* | |
| H72 | 0.0709 (8) | 0.1246 (8) | 0.023 (4) | 0.021 (7)* | |
| N5 | 0.21612 (7) | 0.17411 (7) | −0.5455 (3) | 0.0290 (5) | |
| C1 | 0.15777 (9) | 0.10106 (8) | 0.0864 (4) | 0.0247 (5) | |
| C2 | 0.11379 (9) | 0.09303 (8) | 0.2032 (4) | 0.0250 (5) | |
| C3 | 0.15934 (9) | 0.04947 (8) | 0.4708 (4) | 0.0269 (6) | |
| C4 | 0.20500 (8) | 0.08359 (8) | 0.1627 (4) | 0.0245 (5) | |
| C5 | 0.07022 (10) | 0.06475 (12) | 0.5270 (5) | 0.0497 (8) | |
| H5A | 0.0523 | 0.0943 | 0.5301 | 0.075* | |
| H5B | 0.0801 | 0.0562 | 0.6735 | 0.075* | |
| H5C | 0.0480 | 0.0409 | 0.4675 | 0.075* | |
| C6 | 0.24849 (9) | 0.03686 (9) | 0.4392 (4) | 0.0360 (6) | |
| H6A | 0.2572 | 0.0524 | 0.5741 | 0.054* | |
| H6B | 0.2760 | 0.0409 | 0.3354 | 0.054* | |
| H6C | 0.2433 | 0.0039 | 0.4665 | 0.054* | |
| C7 | 0.18654 (9) | 0.13865 (8) | −0.2257 (4) | 0.0274 (6) | |
| H7 | 0.2197 | 0.1272 | −0.2021 | 0.033* | |
| C8 | 0.17658 (9) | 0.16845 (8) | −0.4101 (3) | 0.0242 (5) | |
| C9 | 0.13029 (9) | 0.18992 (9) | −0.4462 (4) | 0.0321 (6) | |
| H9 | 0.1032 | 0.1861 | −0.3479 | 0.039* | |
| C10 | 0.12463 (10) | 0.21673 (8) | −0.6268 (4) | 0.0366 (6) | |
| H10 | 0.0935 | 0.2319 | −0.6536 | 0.044* | |
| C11 | 0.16425 (10) | 0.22156 (9) | −0.7690 (4) | 0.0393 (7) | |
| H11 | 0.1607 | 0.2392 | −0.8968 | 0.047* | |
| C12 | 0.20894 (10) | 0.20008 (9) | −0.7199 (4) | 0.0362 (7) | |
| H12 | 0.2365 | 0.2039 | −0.8161 | 0.043* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0386 (10) | 0.0401 (10) | 0.0316 (10) | 0.0016 (8) | 0.0004 (8) | 0.0116 (10) |
| O2 | 0.0224 (9) | 0.0446 (11) | 0.0328 (9) | 0.0016 (8) | 0.0037 (7) | 0.0052 (9) |
| N1 | 0.0219 (11) | 0.0349 (12) | 0.0284 (10) | 0.0010 (8) | 0.0044 (9) | 0.0071 (10) |
| N2 | 0.0239 (10) | 0.0303 (11) | 0.0261 (10) | 0.0031 (8) | −0.0012 (8) | 0.0042 (9) |
| N3 | 0.0214 (10) | 0.0288 (11) | 0.0237 (10) | −0.0008 (8) | 0.0020 (9) | −0.0003 (10) |
| N4 | 0.0223 (12) | 0.0480 (14) | 0.0340 (12) | −0.0001 (10) | 0.0035 (10) | 0.0164 (12) |
| N5 | 0.0261 (11) | 0.0345 (12) | 0.0263 (10) | −0.0002 (9) | 0.0031 (8) | 0.0030 (10) |
| C1 | 0.0237 (12) | 0.0276 (13) | 0.0229 (11) | −0.0003 (9) | 0.0002 (10) | −0.0009 (11) |
| C2 | 0.0239 (13) | 0.0261 (12) | 0.0251 (12) | 0.0020 (9) | −0.0016 (10) | 0.0002 (11) |
| C3 | 0.0253 (14) | 0.0273 (13) | 0.0281 (13) | −0.0017 (10) | 0.0004 (10) | 0.0013 (12) |
| C4 | 0.0239 (13) | 0.0249 (12) | 0.0248 (12) | 0.0003 (9) | −0.0003 (10) | −0.0029 (11) |
| C5 | 0.0290 (15) | 0.078 (2) | 0.0423 (16) | 0.0069 (15) | 0.0112 (13) | 0.0267 (16) |
| C6 | 0.0270 (13) | 0.0416 (15) | 0.0393 (15) | 0.0055 (11) | −0.0034 (11) | 0.0085 (13) |
| C7 | 0.0237 (13) | 0.0321 (14) | 0.0264 (12) | 0.0014 (10) | 0.0002 (11) | 0.0000 (11) |
| C8 | 0.0224 (11) | 0.0272 (13) | 0.0230 (11) | −0.0021 (10) | 0.0004 (10) | −0.0026 (10) |
| C9 | 0.0264 (14) | 0.0350 (14) | 0.0349 (14) | 0.0036 (11) | 0.0006 (11) | −0.0011 (12) |
| C10 | 0.0311 (14) | 0.0306 (14) | 0.0480 (17) | 0.0049 (11) | −0.0141 (13) | −0.0018 (14) |
| C11 | 0.0456 (18) | 0.0349 (14) | 0.0373 (15) | −0.0021 (12) | −0.0090 (13) | 0.0100 (13) |
| C12 | 0.0395 (16) | 0.0392 (16) | 0.0298 (14) | −0.0032 (13) | 0.0050 (12) | 0.0073 (12) |
Geometric parameters (Å, °)
| O1—C3 | 1.223 (3) | C5—H5A | 0.9800 |
| O2—C4 | 1.230 (3) | C5—H5B | 0.9800 |
| N1—C2 | 1.368 (3) | C5—H5C | 0.9800 |
| N1—C3 | 1.385 (3) | C6—H6A | 0.9800 |
| N1—C5 | 1.465 (3) | C6—H6B | 0.9800 |
| N2—C3 | 1.370 (3) | C6—H6C | 0.9800 |
| N2—C4 | 1.413 (3) | C7—C8 | 1.460 (3) |
| N2—C6 | 1.463 (3) | C7—H7 | 0.9500 |
| N3—C7 | 1.280 (3) | C8—C9 | 1.394 (3) |
| N3—C1 | 1.383 (3) | C9—C10 | 1.374 (4) |
| N4—C2 | 1.330 (3) | C9—H9 | 0.9500 |
| N4—H71 | 0.93 (3) | C10—C11 | 1.380 (4) |
| N4—H72 | 0.84 (2) | C10—H10 | 0.9500 |
| N5—C12 | 1.334 (3) | C11—C12 | 1.373 (4) |
| N5—C8 | 1.354 (3) | C11—H11 | 0.9500 |
| C1—C2 | 1.393 (3) | C12—H12 | 0.9500 |
| C1—C4 | 1.431 (3) | ||
| C2—N1—C3 | 122.4 (2) | H5A—C5—H5C | 109.5 |
| C2—N1—C5 | 120.6 (2) | H5B—C5—H5C | 109.5 |
| C3—N1—C5 | 116.9 (2) | N2—C6—H6A | 109.5 |
| C3—N2—C4 | 125.46 (19) | N2—C6—H6B | 109.5 |
| C3—N2—C6 | 115.73 (19) | H6A—C6—H6B | 109.5 |
| C4—N2—C6 | 118.74 (19) | N2—C6—H6C | 109.5 |
| C7—N3—C1 | 124.1 (2) | H6A—C6—H6C | 109.5 |
| C2—N4—H71 | 118.6 (18) | H6B—C6—H6C | 109.5 |
| C2—N4—H72 | 113.8 (16) | N3—C7—C8 | 120.6 (2) |
| H71—N4—H72 | 127 (2) | N3—C7—H7 | 119.7 |
| C12—N5—C8 | 117.6 (2) | C8—C7—H7 | 119.7 |
| N3—C1—C2 | 114.4 (2) | N5—C8—C9 | 121.8 (2) |
| N3—C1—C4 | 125.6 (2) | N5—C8—C7 | 114.8 (2) |
| C2—C1—C4 | 120.0 (2) | C9—C8—C7 | 123.4 (2) |
| N4—C2—N1 | 118.0 (2) | C10—C9—C8 | 118.7 (2) |
| N4—C2—C1 | 121.4 (2) | C10—C9—H9 | 120.6 |
| N1—C2—C1 | 120.6 (2) | C8—C9—H9 | 120.6 |
| O1—C3—N2 | 122.3 (2) | C9—C10—C11 | 119.9 (2) |
| O1—C3—N1 | 121.4 (2) | C9—C10—H10 | 120.1 |
| N2—C3—N1 | 116.3 (2) | C11—C10—H10 | 120.1 |
| O2—C4—N2 | 118.9 (2) | C12—C11—C10 | 117.9 (3) |
| O2—C4—C1 | 126.0 (2) | C12—C11—H11 | 121.0 |
| N2—C4—C1 | 115.1 (2) | C10—C11—H11 | 121.0 |
| N1—C5—H5A | 109.5 | N5—C12—C11 | 124.1 (2) |
| N1—C5—H5B | 109.5 | N5—C12—H12 | 118.0 |
| H5A—C5—H5B | 109.5 | C11—C12—H12 | 118.0 |
| N1—C5—H5C | 109.5 | ||
| C7—N3—C1—C2 | −179.2 (2) | C6—N2—C4—O2 | −0.6 (3) |
| C7—N3—C1—C4 | 3.4 (4) | C3—N2—C4—C1 | −4.6 (3) |
| C3—N1—C2—N4 | 177.2 (2) | C6—N2—C4—C1 | 178.8 (2) |
| C5—N1—C2—N4 | −5.7 (3) | N3—C1—C4—O2 | −0.3 (4) |
| C3—N1—C2—C1 | −3.6 (3) | C2—C1—C4—O2 | −177.6 (2) |
| C5—N1—C2—C1 | 173.4 (2) | N3—C1—C4—N2 | −179.7 (2) |
| N3—C1—C2—N4 | 2.3 (3) | C2—C1—C4—N2 | 3.0 (3) |
| C4—C1—C2—N4 | 179.9 (2) | C1—N3—C7—C8 | −177.8 (2) |
| N3—C1—C2—N1 | −176.8 (2) | C12—N5—C8—C9 | −2.0 (3) |
| C4—C1—C2—N1 | 0.8 (3) | C12—N5—C8—C7 | 178.4 (2) |
| C4—N2—C3—O1 | −178.5 (2) | N3—C7—C8—N5 | −174.6 (2) |
| C6—N2—C3—O1 | −1.8 (3) | N3—C7—C8—C9 | 5.8 (3) |
| C4—N2—C3—N1 | 2.1 (3) | N5—C8—C9—C10 | 1.4 (4) |
| C6—N2—C3—N1 | 178.7 (2) | C7—C8—C9—C10 | −179.0 (2) |
| C2—N1—C3—O1 | −177.2 (2) | C8—C9—C10—C11 | 0.6 (4) |
| C5—N1—C3—O1 | 5.6 (4) | C9—C10—C11—C12 | −1.9 (4) |
| C2—N1—C3—N2 | 2.2 (3) | C8—N5—C12—C11 | 0.6 (4) |
| C5—N1—C3—N2 | −174.9 (2) | C10—C11—C12—N5 | 1.4 (4) |
| C3—N2—C4—O2 | 176.0 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H71···N5i | 0.93 (3) | 2.08 (3) | 2.928 (3) | 151 (3) |
| N4—H72···N3 | 0.84 (2) | 2.25 (2) | 2.661 (3) | 110.2 (18) |
| N4—H72···O2ii | 0.84 (2) | 2.54 (2) | 3.108 (3) | 125.8 (19) |
| C7—H7···O2 | 0.95 | 2.17 | 2.847 (3) | 127. |
| C11—H11···O1iii | 0.95 | 2.54 | 3.313 (3) | 138. |
Symmetry codes: (i) x−1/4, −y+1/4, z+3/4; (ii) x−1/4, −y+1/4, z−1/4; (iii) −x+1/4, y+1/4, z−7/4.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5299).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Booysen, I., Muhammed, I., Soares, A., Gerber, T., Hosten, E. & Betz, R. (2011a). Acta Cryst. E67, o1592. [DOI] [PMC free article] [PubMed]
- Booysen, I., Muhammed, I., Soares, A., Gerber, T., Hosten, E. & Betz, R. (2011b). Acta Cryst. E67, o2025–o2026. [DOI] [PMC free article] [PubMed]
- Bruker (2010). APEX2 and SAINT Bruker AXS Inc., Madison, USA.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Gade, L. H. (1998). Koordinationschemie, 1. Auflage. Weinheim: Wiley–VCH.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811031618/lh5299sup1.cif
Supplementary material file. DOI: 10.1107/S1600536811031618/lh5299Isup2.cdx
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811031618/lh5299Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811031618/lh5299Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report