Abstract
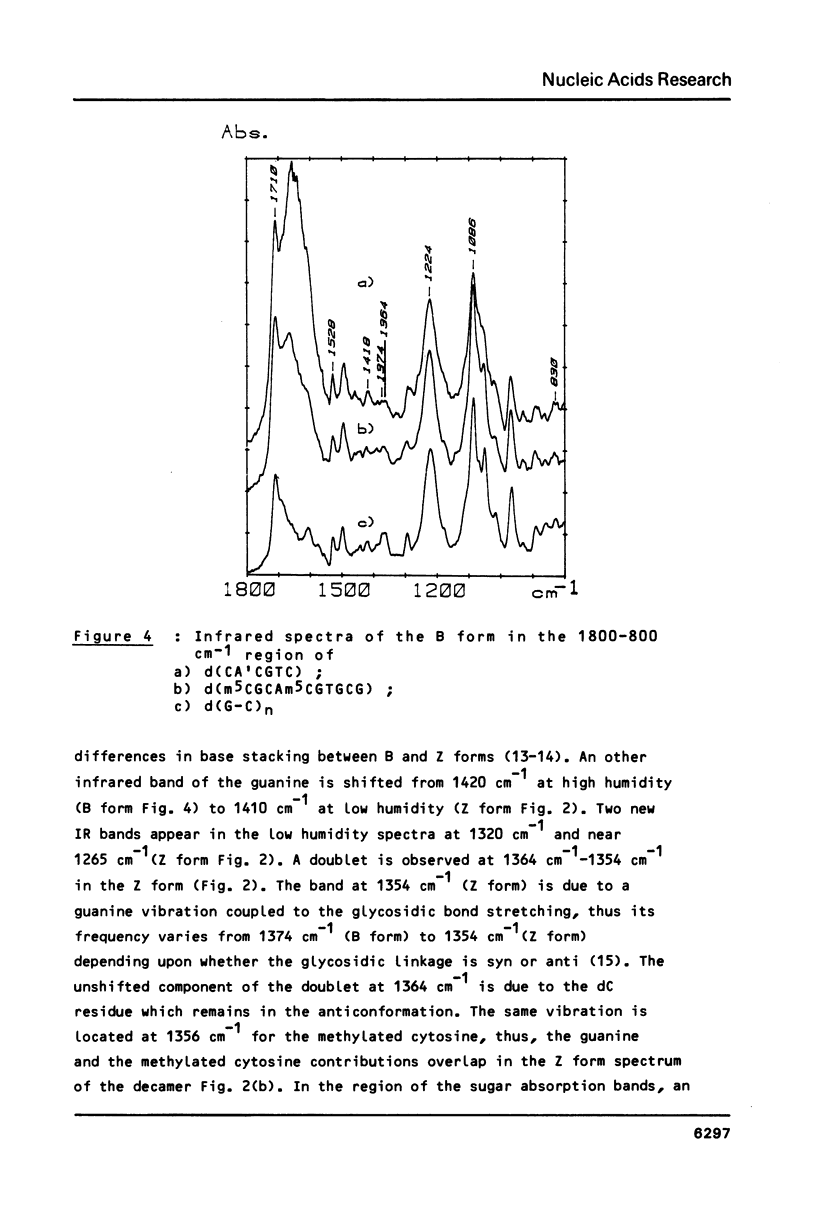
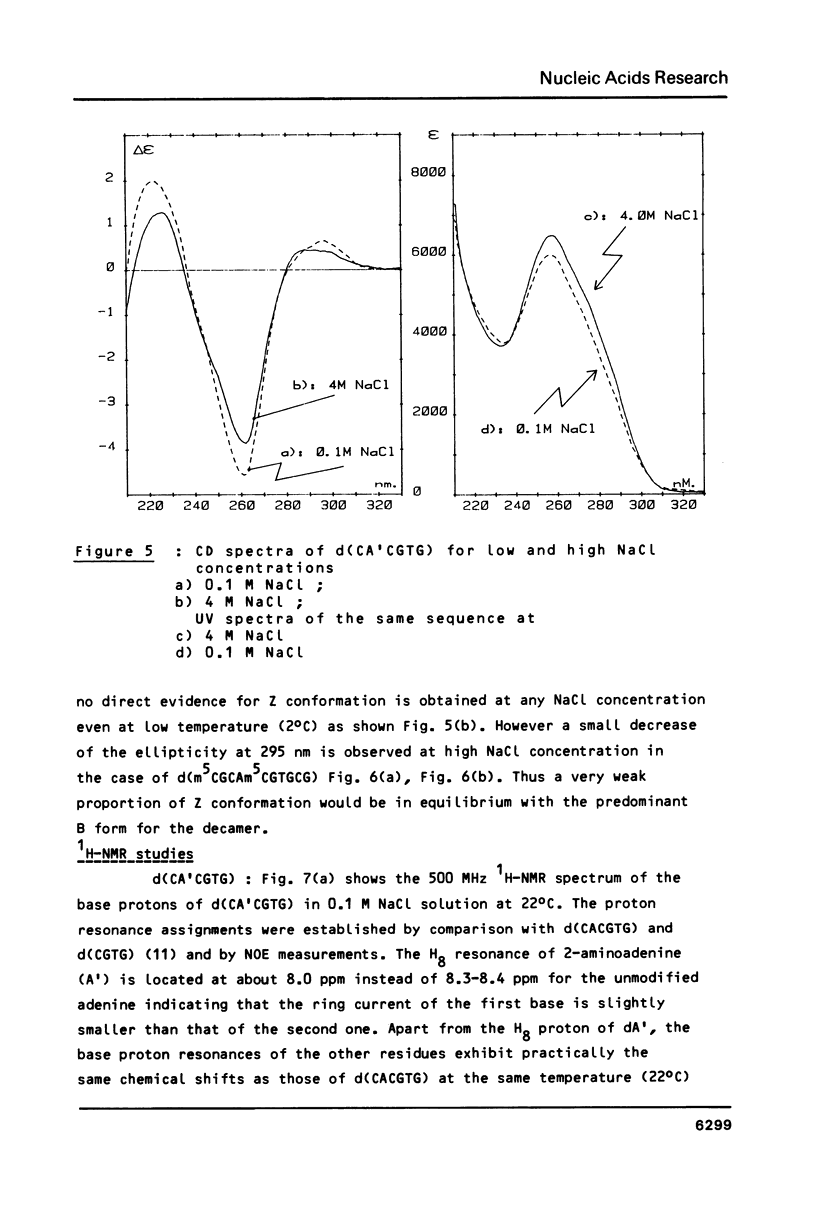
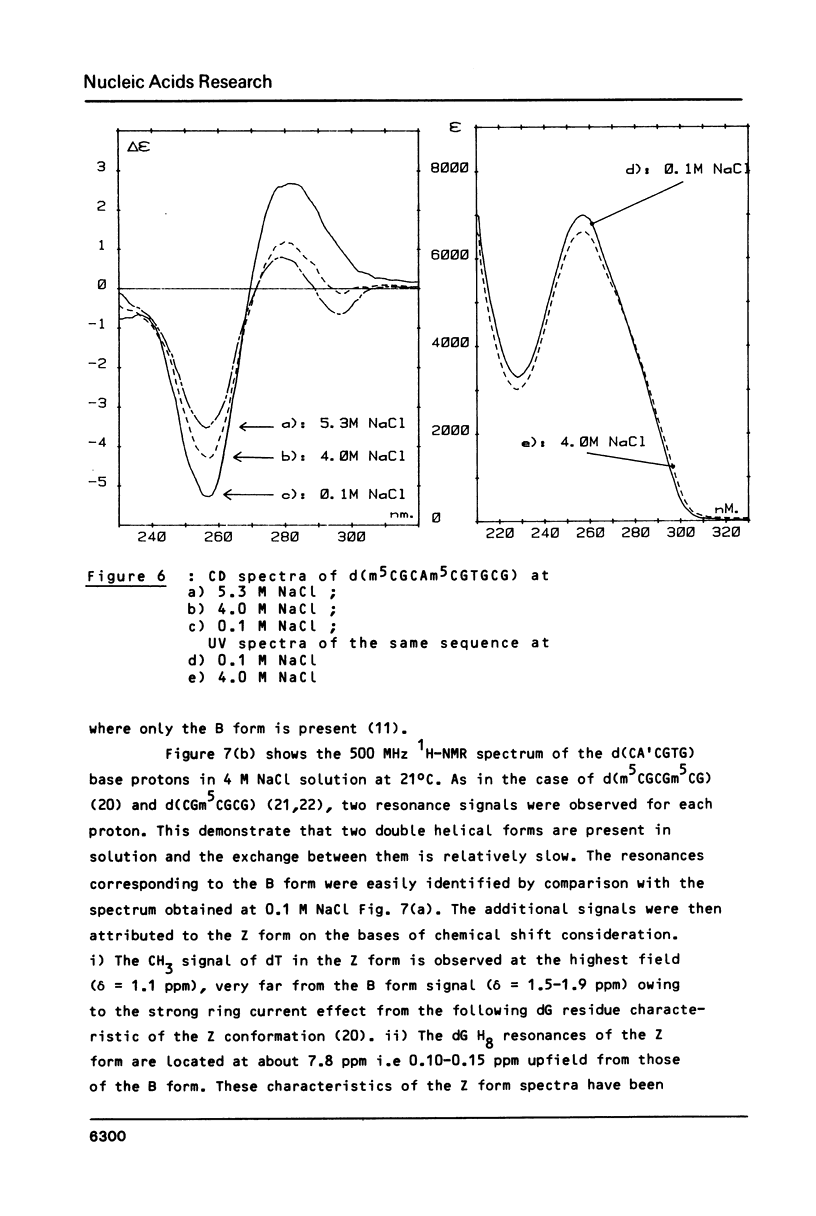
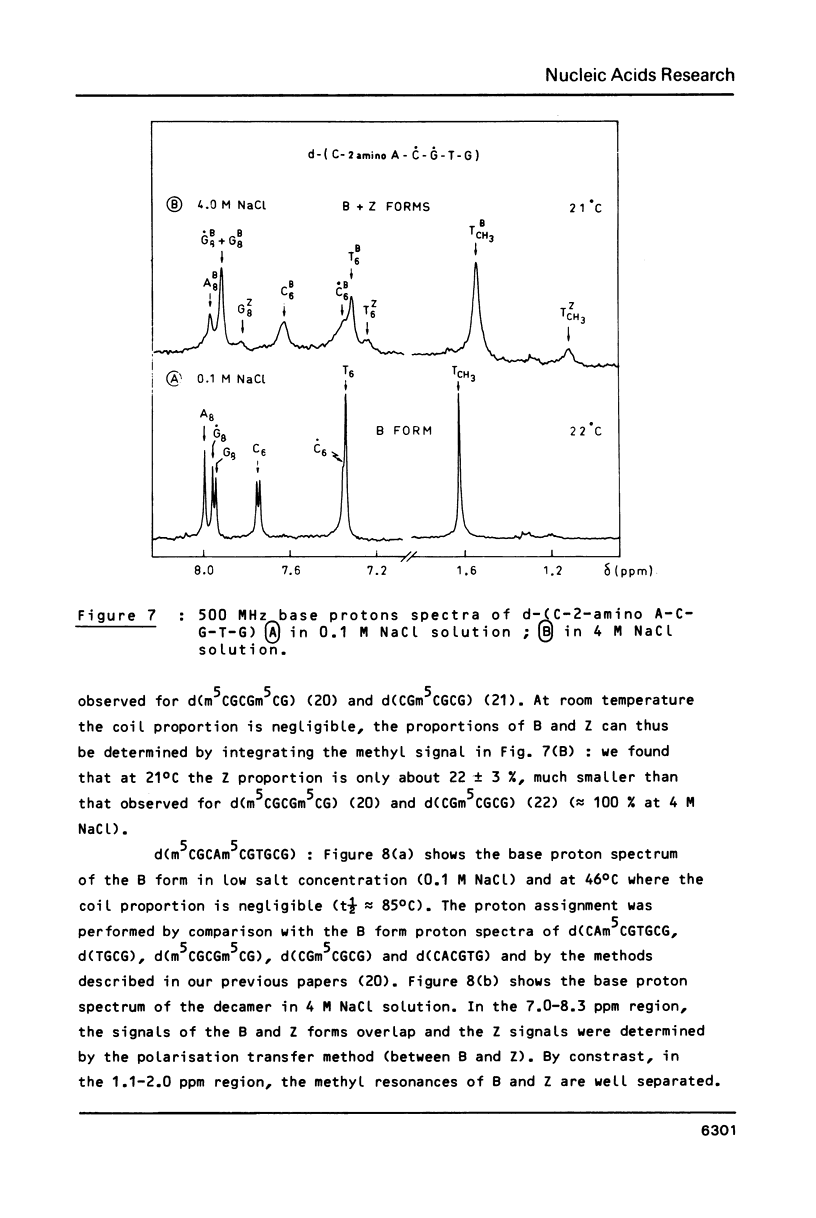
The sequences CA'CGTG (where A' = 2-aminodeoxyadenosine) and m5CGCAm5CGTGCG are prepared and studied by IR, CD and 1H-NMR. Infrared spectra demonstrate the capacity of the modified hexamer and decamer to adopt a Z conformation. The influence of the NH2 substitution on the adenine or of the methylated terminal part of the decamer acting with the increase of the DNA concentration stabilizes the Z conformation at room temperature in low humidity films. Very weak proportion of Z conformation is detected in UV dilute solutions. In more concentrated NMR solutions, the Z proportion induced by high salt content is only 20-25%. The effects of the concentration and of the covalent modification of the bases are discussed.
Full text
PDF

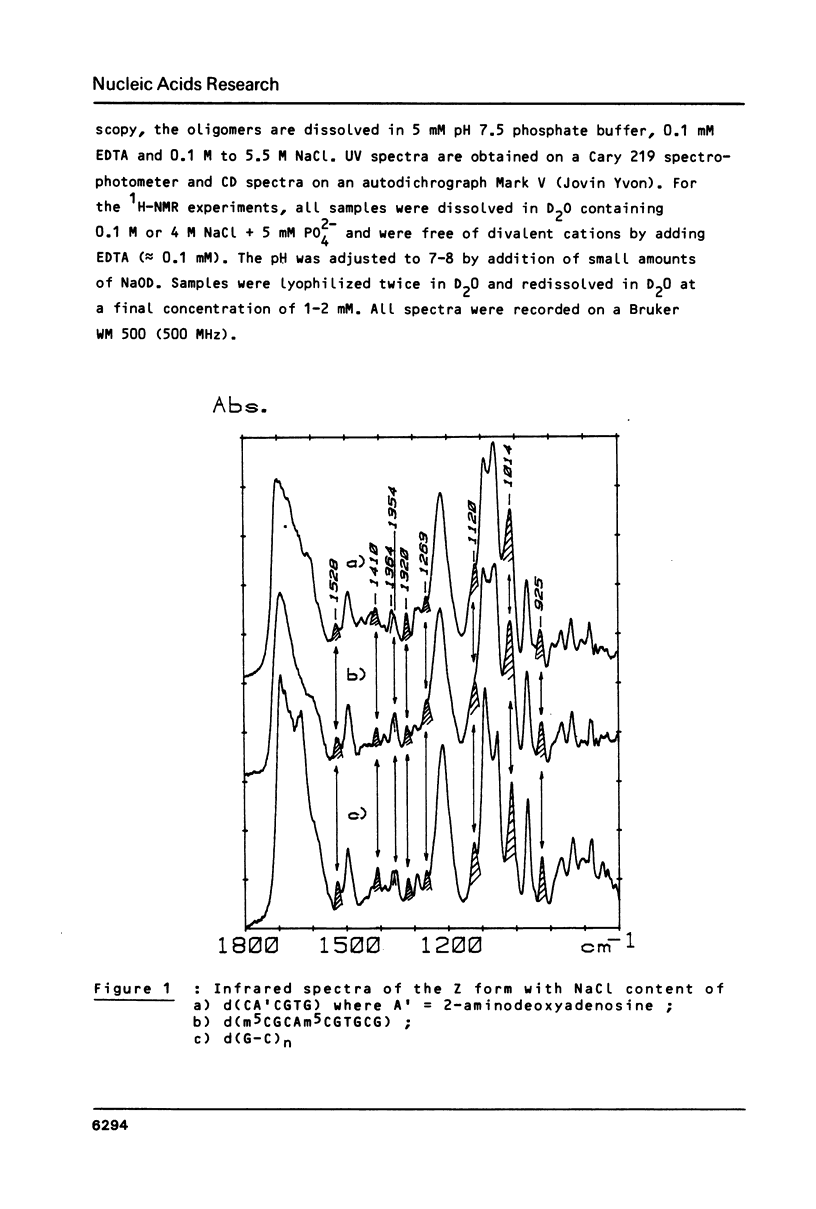
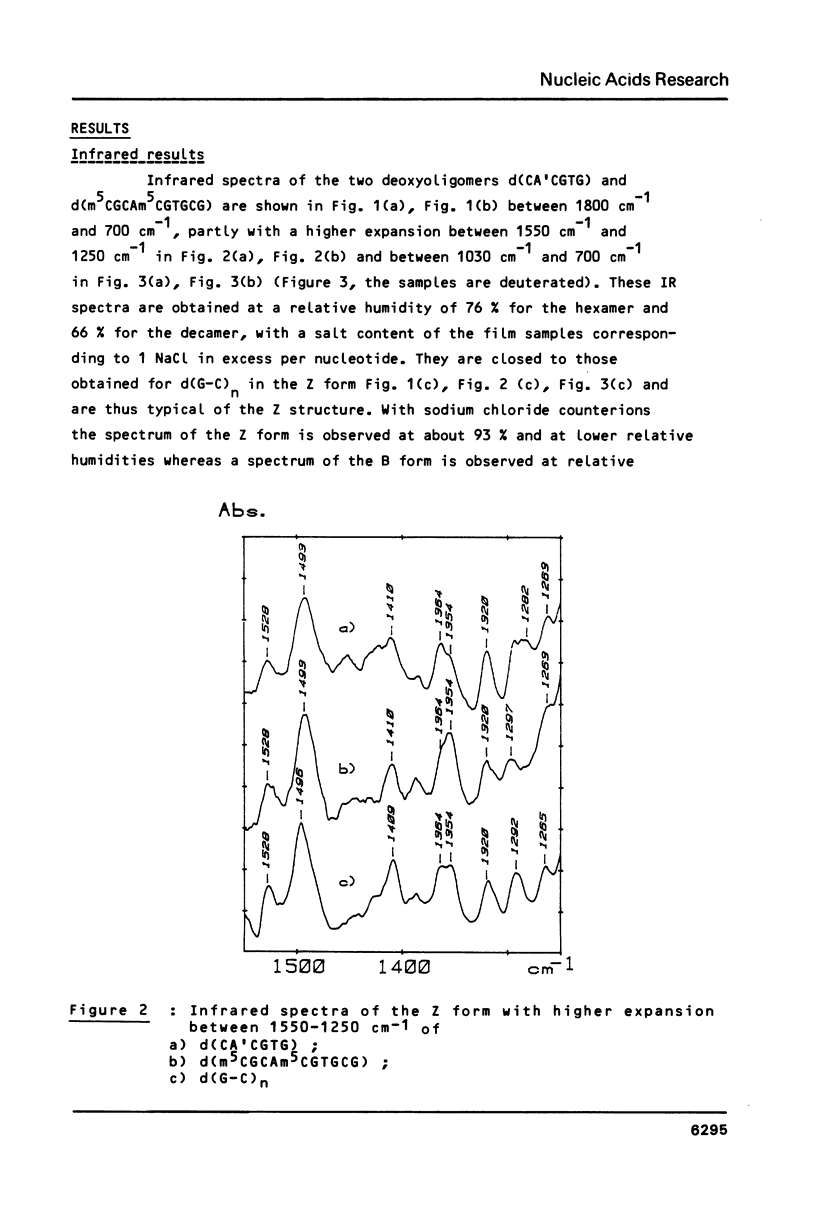
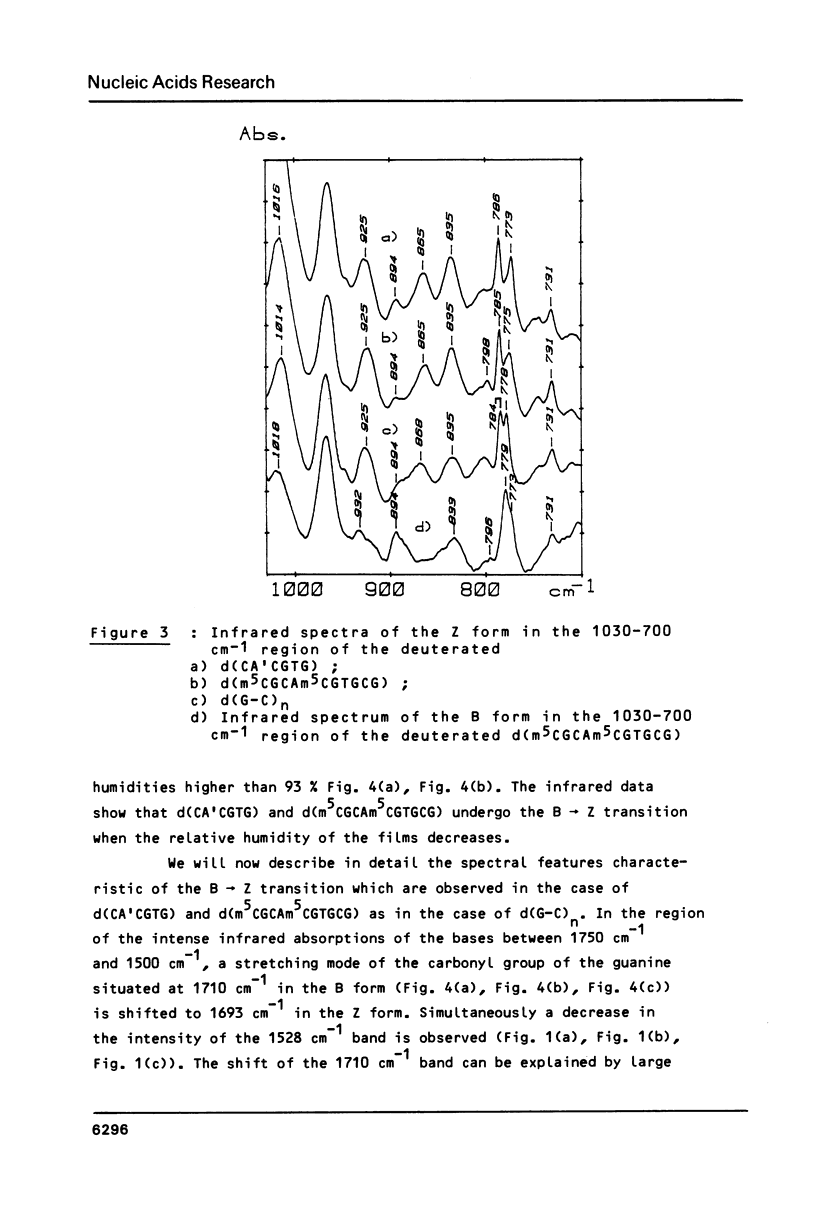




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles J. A., Neumann J. M., Taboury J., Langlois d'Estaintot B., Huynh-Dinh T., Igolen J., Tran-Dinh S. B,Z conformations and mechanism of the Z-B-coil transitions of the self-complementary deoxy-hexanucleotide d(C-G-m5C-G-C-G) by 1H-NMR and CD spectroscopy. J Biomol Struct Dyn. 1984 Jun;1(6):1347–1371. doi: 10.1080/07391102.1984.10507525. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R., Conner B. N., Wing R. M., Fratini A. V., Kopka M. L. The anatomy of A-, B-, and Z-DNA. Science. 1982 Apr 30;216(4545):475–485. doi: 10.1126/science.7071593. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Conformation and dynamics in a Z-DNA tetramer. J Mol Biol. 1981 Nov 15;152(4):723–736. doi: 10.1016/0022-2836(81)90124-8. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Fujii S., Wang A. H., van der Marel G., van Boom J. H., Rich A. Molecular structure of (m5 dC-dG)3: the role of the methyl group on 5-methyl cytosine in stabilizing Z-DNA. Nucleic Acids Res. 1982 Dec 11;10(23):7879–7892. doi: 10.1093/nar/10.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. The influence of the purine 2-amino group on DNA conformation and stability. Synthesis and conformational analysis of d[T(2-aminoA)]3. Nucleic Acids Res. 1982 Jul 24;10(14):4351–4361. doi: 10.1093/nar/10.14.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B., Tran-Dinh S., Neumann J. M., Huynh-Dinh T., Igolen J. Proton NMR study of the B----Z transition of d(CGm5CG)2 and d(CGm5CGCG)2: theory and experiment. Nucleic Acids Res. 1984 Apr 11;12(7):3271–3281. doi: 10.1093/nar/12.7.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B., Thuong N. T., Pouyet J., Ptak M., Leng M. Spectroscopic studies of (m5dC-dG)3: thermal stability of B- and Z-forms. Nucleic Acids Res. 1983 Jul 11;11(13):4453–4466. doi: 10.1093/nar/11.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. B., Chen C. W., Cohen J. S., Miles H. T. Poly(d2NH2A-dT): effect of 2-amino substituent on the B to Z transition. Biochem Biophys Res Commun. 1984 Feb 14;118(3):848–853. doi: 10.1016/0006-291x(84)91472-4. [DOI] [PubMed] [Google Scholar]
- Kirnos M. D., Khudyakov I. Y., Alexandrushkina N. I., Vanyushin B. F. 2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature. 1977 Nov 24;270(5635):369–370. doi: 10.1038/270369a0. [DOI] [PubMed] [Google Scholar]
- McIntosh L. P., Grieger I., Eckstein F., Zarling D. A., van de Sande J. H., Jovin T. M. Left-handed helical conformation of poly[d(A-m5C).d(G-T)]. Nature. 1983 Jul 7;304(5921):83–86. doi: 10.1038/304083a0. [DOI] [PubMed] [Google Scholar]
- Pilet J., Leng M. Comparison of poly(dG-dC).poly(dG-dC) conformations in oriented films and in solution. Proc Natl Acad Sci U S A. 1982 Jan;79(1):26–30. doi: 10.1073/pnas.79.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Ubasawa A. Nature of side-reactions in oligonucleotide synthesis involving arenesulphonyl derivatives of 3-nitro-1,2,4-triazole and related condensing agents. Nucleic Acids Symp Ser. 1980;(7):5–21. [PubMed] [Google Scholar]
- Taillandier E., Taboury J., Liquier J., Sautière P., Couppez M. Structural transitions in DNAs and nucleohistones studied by I.R. spectroscopy. Biochimie. 1981 Nov-Dec;63(11-12):895–898. doi: 10.1016/s0300-9084(82)80282-4. [DOI] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Dinh S., Neumann J. M., Taboury J., Huynh-Dinh T., Renous S., Genissel B., Igolen J. DNA fragment conformations. 1H-NMR comparative studies of helix-coil transition and conformation of d(C-A-C-G-T-G) and d(G-T-G-C-A-C). Influence of helix formation on proton chemical shifts. Eur J Biochem. 1983 Jul 1;133(3):579–589. doi: 10.1111/j.1432-1033.1983.tb07502.x. [DOI] [PubMed] [Google Scholar]
- Tran-Dinh S., Taboury J., Neumann J. M., Huynh-Dinh T., Genissel B., Langlois d'Estaintot B., Igolen J. 1H NMR and circular dichroism studies of the B and Z conformations of the self-complementary deoxyhexanucleotide d(m5C-G-C-G-m5-C-G): mechanism of the Z-B-coil transitions. Biochemistry. 1984 Mar 27;23(7):1362–1371. doi: 10.1021/bi00302a005. [DOI] [PubMed] [Google Scholar]
- Vincent-Fiquet O., Leflon P., Plaquet R., Biserte G. Caractérisation de l'activité L-thréonine désaminasique du foie de cobaye induite par un régime hyperprotidique. Biochimie. 1984 Jan;66(1):43–48. doi: 10.1016/0300-9084(84)90190-1. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]



