Abstract
The title compound, C14H20O5S·0.5H2O, crystallizes with two organic molecules and a solvent water molecule in the asymmetric unit. In both molecules, the hexapyranosyl rings adopt a slightly distorted chair conformation (5 C 2) with four substituents in equatorial positions and one substituent in an axial position. The main difference between the organic molecules is the dihedral angle between the phenyl ring and the best plane defined by the O—C1—C2—C3 atoms (r.m.s deviations = 0.003 and 0.043 Å) of the hexapyranosyl rings [47.4 (4) and 86.5 (4)°]. In the asymmetric unit, molecules are linked by two strong O—H⋯O hydrogen bonds. In the crystal, the components are linked by a total of 10 distinct O—H⋯O hydrogen bonds, resulting in the formation of a two-dimensional network parallel to the ab plane.
Related literature
For synthetic methods see: Helferich & Türk (1956 ▶). For pharmacological properties of the title compound, see: De Bruyne et al. (1977 ▶); Choi et al. (2003 ▶). Gutiérrez et al. (2011 ▶). For puckering parameters see: Cremer & Pople (1975 ▶).
Experimental
Crystal data
C14H20O5S·0.5H2O
M r = 309.37
Orthorhombic,
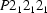
a = 4.8358 (4) Å
b = 14.8218 (16) Å
c = 41.390 (3) Å
V = 2966.6 (5) Å3
Z = 8
Mo Kα radiation
μ = 0.24 mm−1
T = 173 K
0.20 × 0.09 × 0.08 mm
Data collection
Refinement
R[F 2 > 2σ(F 2)] = 0.058
wR(F 2) = 0.117
S = 0.68
5217 reflections
377 parameters
H-atom parameters constrained
Δρmax = 0.28 e Å−3
Δρmin = −0.30 e Å−3
Absolute structure: Flack (1983 ▶), 2269 Friedel pairs
Flack parameter: −0.25 (16)
Data collection: X-AREA (Stoe & Cie, 2001 ▶); cell refinement: X-AREA; data reduction: X-AREA; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL-Plus (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶)’.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811031667/om2454sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811031667/om2454Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O31—H31⋯O1W | 0.84 | 1.92 | 2.759 (7) | 179 |
| O41—H41⋯O41A | 0.84 | 1.95 | 2.779 (7) | 169 |
| O51—H51⋯O1Wi | 0.84 | 1.98 | 2.773 (6) | 156 |
| O61—H61⋯O31Aii | 0.84 | 1.86 | 2.697 (7) | 172 |
| O31A—H31A⋯O61iii | 0.84 | 1.92 | 2.650 (7) | 145 |
| O41A—H41A⋯O41iv | 0.84 | 2.05 | 2.788 (7) | 147 |
| O51A—H51A⋯O61Av | 0.84 | 2.10 | 2.785 (7) | 138 |
| O61A—H61A⋯O31ii | 0.84 | 2.02 | 2.744 (6) | 145 |
| O1W—H1WA⋯O31v | 0.84 | 2.23 | 2.989 (8) | 150 |
| O1W—H1WB⋯O41Av | 0.84 | 2.43 | 3.270 (6) | 180 |
Symmetry codes: (i) 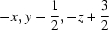 ; (ii)
; (ii) 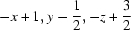 ; (iii)
; (iii) 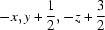 ; (iv)
; (iv) 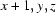 ; (v)
; (v) 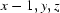 .
.
Acknowledgments
We thank OTKA, the Hungarian Scientific Research Fund (grant Nos. IN-79731 and NK-68578) for financial support. IB thanks the Spanish Research Council (CSIC) for the provision of a free-of-charge license to the Cambridge Structural Database.
supplementary crystallographic information
Comment
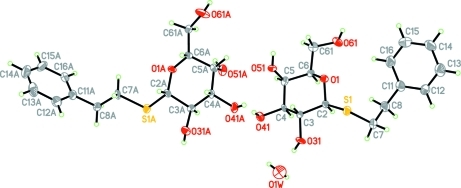
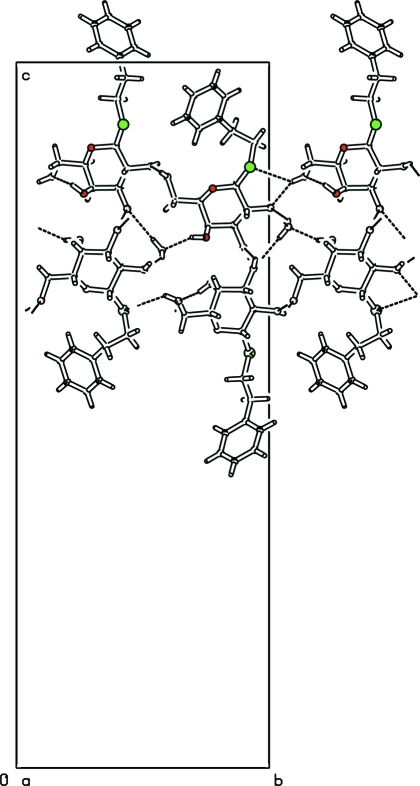
2-Phenylethyl-1-thio-β-D-galactopyranoside is one of the most potent inhibitors of β-galactosidase (EC 3.2.1.23) (De Bruyne,et al., 1977) and a radiologically labeled derivative has also been used for imaging of LacZ gene expression. (Choi et al., 2003). It was recently found to be moderately active in tests against Trypanosoma cruzi, the causal agent of Chagas disease (Gutiérrez et al., 2011). In the title compound, it crystallizes with two organic molecules and a solvent water molecule in the asymmetric unit, Fig. 1. In both molecules the hexapyranosyl rings adopts a slightly distorted chair conformation (5C2) (QT= 0.574 (6) Å, θ= 3.4 (6)°, φ2 = 8(9)°; QT= 0.587 (6) Å, θ= 9.2 (7)°, φ2 = 299 (4)° for both molecules respectively), (Cremer & Pople, 1975) with four substituents in equatorial positions and one substituent in an axial position. The main difference between the organic molecules is the dihedral angle between the phenyl ring and the best plane defined by the atoms O1/C2/C3/C4 and O1A/C2A/C3A/C4A (r.m.s deviation 0.003 Å; 0.043Å respectively), of the hexapyranosyl rings [47.4 (4) and 86.5 (4)°]. The max. deviation for the best planes of the hexapyranosyl rings are: 0.290 (7)Å and 0.050 (6) Å) for molecules A and B respectively. The mean bond distances are: C—O 1.425 (7) Å, Csp3—Csp3 1.524 (9)Å and aromatic C—C 1.386 (11) Å. In the asymmetric unit the three molecules are linked by two strong O— H···O hydrogen bonds and the crystal packing is stabilized by eight O— H···O hydrogen bonding leading to the formation of a two-dimensional network parallel to the ab plane, Fig. 2, Table 1.
Experimental
1-thio-2,3,4,6-tetra-O-acetyl-β-D-galactopyranose(0.364 g,1 mmol) was dissolved in acetonitrile (2 ml) and 1-bromo-2-phenylethane (0.185 g, 1 mmol) and triethyl amine (242µl, 2 mmol) added. The reaction mixture was stirred at RT until disappearence of the starting materials (TLC, 60 min). The solvent was removed under reduced pressure, and the residue purified by column chromatography (EtOAc: hexane - 8: 2) to give 2-phenylethyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-galactopyranoside (1). Syrup, 402 mg (86%). [α]D -22.9(CHCl3,c 0.15), Lit. (Helferich & Türk, 1956). [α]D -19.2 (CHCl3). HR—MS:m/z calcd. for C22H28O9S[M+Na]+: 491.135. Found: 491.138. 1H-NMR (CDCl3, 500 MHz): δ 7.20–7.35 (m,5H, Phenyl-H); 5.44 (br.s, 1H, H-4); 5.26 (t, 1H, H-2, J2,3 9.9 Hz); 5.03 (br.d, 1H, H-3); 4.44 (d, 1H, H-1, J1,2 10.1 Hz); 4.10–4.20 (m,2H, H-6a,b); 3.90 (m, 1H, H-5); 2.92 (m, 1H, S-CH2a); 2.94 (m, 2H, Ph-CH2); 3.00 (m, 1H, S-CH2 b);2.16, 2.06, 2.04,1.99 (s, 4x3H, 4x COCH3);13C-NMR (CDCl3, 125 MHz): δ 130.5 (Phenyl-C); 85.6 (C-1); 76.5(C-5); 73.3 (C-3); 69.0 (C-4); 68.7 (C-2); 62.2 (C-6); 38.2 (Ph-CH2); 33.1 (S-CH2); 22.3 (4xCOCH3). The product (0.300 g, 0.64 mmol) was deacetylated by treatment with catalytic amount of NaOMe in methanol. The reaction mixture was stirred at room temperature until completion (TLC 20 min). After neutralization with a cation exchanger (Amberlyst 15) the solvent was removed under reduced pressure and the title molecule, 2-phenylethyl- 1-thio-β-D-galactopyranoside, was isolated as a white solid (MeOH: EtOAc - 2:8), 185 mg (96.3%). [α]D -22.4 (MeOH, c 0.11). Lit. (Helferich &Türk,1956) [α]D -32.2 (MeOH). HR—MS: m/z calcd. for C14H20O5S [M+Na]+:323.094. Found: 323.094.
1H-NMR(CD3OD, 500 MHz): δ 7.15–7.30 (m, 5H, Phenyl-H); 4.34 (d, 1H, H-1, J1,2 9.6 Hz); 3.90 (dd, 1H,H-4, J4,5 ~ 1 Hz); 3.77(dd, 1H, H-6a, J6a,6 b 11.5 Hz, J5,6a 6.6 Hz); 3.71(dd, 1H, H-6 b, J5,6 b 5.3 Hz); 3.57 (t, 1H, H-2, J2,3 9.6 Hz); 3.53(m, 1H, H-5); 3.46 (1H, dd, H-3, J3,4 3.4 Hz);2.92 (m, 1H, S-CH2a);2.95 (m, 2H, Ph-CH2); 3.02(m, 1H, S-CH2 b); 13C-NMR (CDCl3, 125 MHz): δ 131.3, 131.0, 128.9 (Phenyl-C); 88.4 (C-1); 81.5 (C-5); 77.1 (C-3);72.3 (C-2); 71.3 (C-4); 63.5 (C-6); 38.6 (Ph-CH2); 33.2 (S-CH2). Colourless single crystals suitable for X-ray analysis were obtained by slow evaporation of an aqueous solution.
Refinement
All H atoms could be located by difference Fourier synthesis but were ultimately placed in calculated positions using a riding model with C—H = 0.95 - 1.00 Å and O—H = 0.84 Å with fixed individual displacement parameters [Uiso(H) = 1.2 Ueq(C) or Uiso(H) = 1.5 Ueq(O)].
Figures
Fig. 1.
Perspective view of the asymmetric unit of the title compound, with the atom numbering. Displacement ellipsoids are shown at the 50% probability level.
Fig. 2.
A Packing diagram, viewed down the c axis.
Crystal data
| C14H20O5S·0.5H2O | F(000) = 1320 |
| Mr = 309.37 | Dx = 1.385 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 3425 reflections |
| a = 4.8358 (4) Å | θ = 2.8–25.6° |
| b = 14.8218 (16) Å | µ = 0.24 mm−1 |
| c = 41.390 (3) Å | T = 173 K |
| V = 2966.6 (5) Å3 | Needle, colourless |
| Z = 8 | 0.20 × 0.09 × 0.08 mm |
Data collection
| Stoe IPDS II two-circle diffractometer | 5217 independent reflections |
| Radiation source: fine-focus sealed tube | 2395 reflections with I > 2σ(I) |
| graphite | Rint = 0.169 |
| ω scans | θmax = 25.0°, θmin = 2.8° |
| Absorption correction: multi-scan (MULABS; Spek, 200; Blessing, 1995) | h = −4→5 |
| Tmin = 0.954, Tmax = 0.981 | k = −17→17 |
| 14206 measured reflections | l = −49→47 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.058 | H-atom parameters constrained |
| wR(F2) = 0.117 | w = 1/[σ2(Fo2) + (0.0007P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.68 | (Δ/σ)max = 0.001 |
| 5217 reflections | Δρmax = 0.28 e Å−3 |
| 377 parameters | Δρmin = −0.30 e Å−3 |
| 0 restraints | Absolute structure: Flack (1983), 2269 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: −0.25 (16) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.3816 (4) | 0.92470 (10) | 0.85163 (4) | 0.0217 (4) | |
| O1 | 0.2305 (9) | 0.7771 (2) | 0.82113 (11) | 0.0186 (11) | |
| C2 | 0.1463 (14) | 0.8684 (3) | 0.82448 (14) | 0.0171 (14) | |
| H2 | −0.0432 | 0.8697 | 0.8341 | 0.020* | |
| C3 | 0.1355 (15) | 0.9128 (3) | 0.79134 (14) | 0.0192 (15) | |
| H3 | 0.3253 | 0.9114 | 0.7818 | 0.023* | |
| C4 | −0.0578 (14) | 0.8611 (4) | 0.76924 (14) | 0.0150 (14) | |
| H4 | −0.2469 | 0.8656 | 0.7789 | 0.018* | |
| C5 | 0.0165 (15) | 0.7603 (4) | 0.76836 (16) | 0.0206 (17) | |
| H5 | −0.1313 | 0.7263 | 0.7566 | 0.025* | |
| C6 | 0.0370 (14) | 0.7259 (4) | 0.80286 (15) | 0.0176 (15) | |
| H6 | −0.1488 | 0.7321 | 0.8132 | 0.021* | |
| C7 | 0.1644 (18) | 0.9413 (4) | 0.88681 (16) | 0.0298 (18) | |
| H7A | 0.2706 | 0.9759 | 0.9031 | 0.036* | |
| H7B | 0.0036 | 0.9785 | 0.8804 | 0.036* | |
| C8 | 0.0570 (17) | 0.8546 (4) | 0.90277 (18) | 0.0335 (19) | |
| H8A | −0.0909 | 0.8706 | 0.9183 | 0.040* | |
| H8B | −0.0258 | 0.8156 | 0.8859 | 0.040* | |
| C11 | 0.2787 (17) | 0.8018 (4) | 0.92039 (17) | 0.0302 (19) | |
| C12 | 0.3759 (19) | 0.8276 (4) | 0.95047 (17) | 0.036 (2) | |
| H12 | 0.3035 | 0.8805 | 0.9603 | 0.043* | |
| C13 | 0.5745 (19) | 0.7786 (5) | 0.96658 (19) | 0.040 (2) | |
| H13 | 0.6369 | 0.7982 | 0.9872 | 0.049* | |
| C14 | 0.684 (2) | 0.7011 (4) | 0.9531 (2) | 0.044 (2) | |
| H14 | 0.8212 | 0.6671 | 0.9641 | 0.053* | |
| C15 | 0.588 (2) | 0.6744 (5) | 0.9230 (2) | 0.050 (3) | |
| H15 | 0.6588 | 0.6205 | 0.9137 | 0.060* | |
| C16 | 0.395 (2) | 0.7234 (4) | 0.90633 (19) | 0.042 (2) | |
| H16 | 0.3392 | 0.7048 | 0.8854 | 0.050* | |
| O31 | 0.0486 (12) | 1.0048 (3) | 0.79387 (11) | 0.0352 (14) | |
| H31 | −0.0953 | 1.0262 | 0.7855 | 0.053* | |
| O41 | −0.0739 (10) | 0.8992 (3) | 0.73781 (10) | 0.0234 (11) | |
| H41 | 0.0863 | 0.9052 | 0.7303 | 0.035* | |
| O51 | 0.2726 (10) | 0.7497 (2) | 0.75186 (12) | 0.0239 (12) | |
| H51 | 0.3191 | 0.6951 | 0.7521 | 0.036* | |
| C61 | 0.1245 (16) | 0.6275 (4) | 0.80496 (16) | 0.0254 (16) | |
| H61B | 0.0055 | 0.5902 | 0.7908 | 0.030* | |
| H61C | 0.3182 | 0.6210 | 0.7976 | 0.030* | |
| O61 | 0.0999 (11) | 0.5973 (3) | 0.83768 (11) | 0.0276 (11) | |
| H61 | 0.2498 | 0.5739 | 0.8436 | 0.041* | |
| S1A | 0.1800 (4) | 0.92289 (11) | 0.58806 (4) | 0.0207 (4) | |
| O1A | 0.4361 (10) | 0.7963 (2) | 0.62109 (10) | 0.0185 (11) | |
| C2A | 0.4278 (15) | 0.8930 (4) | 0.61866 (14) | 0.0174 (15) | |
| H2A | 0.6142 | 0.9150 | 0.6118 | 0.021* | |
| C3A | 0.3507 (14) | 0.9383 (3) | 0.65050 (15) | 0.0162 (14) | |
| H3A | 0.1519 | 0.9264 | 0.6556 | 0.019* | |
| C4A | 0.5327 (14) | 0.9033 (4) | 0.67780 (14) | 0.0166 (15) | |
| H4A | 0.7246 | 0.9268 | 0.6747 | 0.020* | |
| C5A | 0.5420 (14) | 0.8010 (4) | 0.67805 (16) | 0.0170 (15) | |
| H5A | 0.6784 | 0.7805 | 0.6947 | 0.020* | |
| C6A | 0.6377 (15) | 0.7696 (3) | 0.64495 (15) | 0.0171 (14) | |
| H6A | 0.8174 | 0.7998 | 0.6398 | 0.021* | |
| C7A | 0.3763 (16) | 0.8986 (4) | 0.55177 (15) | 0.0246 (16) | |
| H7A1 | 0.5560 | 0.9305 | 0.5526 | 0.030* | |
| H7A2 | 0.4135 | 0.8330 | 0.5504 | 0.030* | |
| C8A | 0.2146 (16) | 0.9289 (5) | 0.52203 (15) | 0.0298 (18) | |
| H8A1 | 0.0333 | 0.8979 | 0.5216 | 0.036* | |
| H8A2 | 0.1805 | 0.9946 | 0.5233 | 0.036* | |
| C11A | 0.3715 (16) | 0.9077 (4) | 0.49135 (15) | 0.0265 (16) | |
| C12A | 0.5655 (18) | 0.9652 (5) | 0.47895 (18) | 0.0323 (19) | |
| H12A | 0.6040 | 1.0207 | 0.4896 | 0.039* | |
| C13A | 0.7059 (17) | 0.9425 (5) | 0.45085 (19) | 0.042 (2) | |
| H13A | 0.8432 | 0.9822 | 0.4426 | 0.050* | |
| C14A | 0.650 (2) | 0.8640 (6) | 0.43483 (19) | 0.046 (2) | |
| H14A | 0.7484 | 0.8492 | 0.4157 | 0.055* | |
| C15A | 0.4537 (19) | 0.8077 (5) | 0.44622 (19) | 0.039 (2) | |
| H15A | 0.4146 | 0.7536 | 0.4348 | 0.047* | |
| C16A | 0.3062 (18) | 0.8271 (4) | 0.47450 (17) | 0.034 (2) | |
| H16A | 0.1666 | 0.7874 | 0.4822 | 0.041* | |
| O31A | 0.3948 (11) | 1.0327 (2) | 0.64734 (11) | 0.0213 (10) | |
| H31A | 0.2432 | 1.0585 | 0.6437 | 0.032* | |
| O41A | 0.4250 (10) | 0.9385 (3) | 0.70761 (10) | 0.0241 (11) | |
| H41A | 0.5551 | 0.9456 | 0.7208 | 0.036* | |
| O51A | 0.2770 (10) | 0.7663 (3) | 0.68599 (11) | 0.0261 (12) | |
| H51A | 0.2409 | 0.7220 | 0.6740 | 0.039* | |
| C61A | 0.6753 (15) | 0.6677 (4) | 0.64172 (16) | 0.0196 (15) | |
| H61D | 0.5043 | 0.6362 | 0.6484 | 0.023* | |
| H61E | 0.7153 | 0.6517 | 0.6190 | 0.023* | |
| O61A | 0.8991 (13) | 0.6415 (3) | 0.66183 (13) | 0.0420 (15) | |
| H61A | 0.8688 | 0.5898 | 0.6693 | 0.063* | |
| O1W | −0.4231 (11) | 1.0767 (3) | 0.76658 (13) | 0.0445 (14) | |
| H1WA | −0.5761 | 1.0770 | 0.7762 | 0.067* | |
| H1WB | −0.4623 | 1.0412 | 0.7514 | 0.067* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0215 (10) | 0.0253 (7) | 0.0184 (9) | 0.0002 (8) | −0.0020 (8) | −0.0018 (7) |
| O1 | 0.016 (3) | 0.018 (2) | 0.022 (3) | 0.0012 (18) | −0.004 (2) | 0.0002 (19) |
| C2 | 0.016 (4) | 0.020 (3) | 0.015 (4) | 0.003 (3) | −0.001 (3) | 0.002 (3) |
| C3 | 0.029 (4) | 0.015 (3) | 0.014 (3) | 0.003 (3) | −0.005 (3) | −0.004 (2) |
| C4 | 0.012 (4) | 0.024 (3) | 0.009 (4) | 0.004 (3) | 0.003 (3) | 0.006 (2) |
| C5 | 0.020 (4) | 0.025 (3) | 0.017 (4) | −0.003 (3) | 0.003 (3) | −0.003 (3) |
| C6 | 0.019 (4) | 0.017 (3) | 0.017 (4) | −0.002 (3) | −0.001 (3) | −0.003 (3) |
| C7 | 0.038 (5) | 0.035 (4) | 0.017 (4) | 0.004 (4) | 0.000 (4) | 0.000 (3) |
| C8 | 0.033 (5) | 0.039 (4) | 0.028 (5) | 0.007 (4) | 0.003 (4) | 0.003 (3) |
| C11 | 0.041 (5) | 0.024 (3) | 0.025 (5) | −0.010 (3) | 0.002 (4) | 0.007 (3) |
| C12 | 0.044 (6) | 0.038 (4) | 0.024 (4) | 0.011 (4) | 0.011 (4) | 0.002 (3) |
| C13 | 0.051 (6) | 0.040 (4) | 0.030 (5) | −0.010 (4) | 0.004 (5) | 0.008 (4) |
| C14 | 0.063 (7) | 0.028 (4) | 0.041 (5) | 0.004 (4) | −0.013 (5) | 0.007 (3) |
| C15 | 0.066 (7) | 0.033 (4) | 0.053 (6) | 0.011 (5) | −0.001 (6) | −0.004 (4) |
| C16 | 0.062 (6) | 0.034 (4) | 0.029 (5) | −0.005 (4) | −0.013 (5) | −0.004 (3) |
| O31 | 0.055 (4) | 0.021 (2) | 0.029 (3) | 0.020 (2) | −0.018 (3) | 0.001 (2) |
| O41 | 0.021 (3) | 0.029 (2) | 0.021 (3) | 0.000 (2) | −0.005 (2) | 0.0035 (19) |
| O51 | 0.034 (3) | 0.013 (2) | 0.024 (3) | 0.0057 (19) | 0.002 (3) | 0.0008 (19) |
| C61 | 0.019 (4) | 0.024 (3) | 0.033 (4) | −0.009 (3) | 0.002 (4) | 0.001 (3) |
| O61 | 0.023 (3) | 0.028 (2) | 0.032 (3) | 0.001 (2) | 0.002 (2) | 0.0133 (19) |
| S1A | 0.0180 (9) | 0.0286 (8) | 0.0154 (9) | 0.0032 (7) | −0.0022 (7) | −0.0005 (7) |
| O1A | 0.020 (3) | 0.0161 (19) | 0.020 (3) | 0.0050 (19) | −0.006 (2) | 0.0004 (17) |
| C2A | 0.017 (4) | 0.021 (3) | 0.014 (4) | 0.002 (3) | −0.003 (3) | 0.004 (2) |
| C3A | 0.015 (4) | 0.015 (3) | 0.019 (3) | −0.003 (3) | 0.000 (3) | −0.002 (3) |
| C4A | 0.020 (4) | 0.021 (3) | 0.008 (3) | −0.005 (3) | −0.007 (3) | −0.002 (3) |
| C5A | 0.014 (4) | 0.019 (3) | 0.018 (4) | −0.002 (3) | −0.002 (3) | 0.002 (3) |
| C6A | 0.015 (4) | 0.018 (3) | 0.019 (4) | 0.003 (3) | −0.003 (3) | 0.002 (3) |
| C7A | 0.029 (4) | 0.033 (3) | 0.012 (4) | 0.004 (3) | 0.003 (4) | 0.007 (3) |
| C8A | 0.043 (5) | 0.031 (3) | 0.016 (4) | 0.014 (4) | −0.003 (3) | −0.003 (3) |
| C11A | 0.026 (4) | 0.035 (4) | 0.018 (4) | 0.007 (4) | −0.006 (3) | 0.001 (3) |
| C12A | 0.034 (5) | 0.035 (4) | 0.028 (4) | 0.002 (4) | −0.004 (4) | 0.002 (3) |
| C13A | 0.025 (5) | 0.067 (5) | 0.032 (5) | −0.001 (4) | 0.004 (4) | 0.016 (4) |
| C14A | 0.042 (6) | 0.074 (5) | 0.022 (5) | 0.023 (5) | 0.000 (4) | 0.000 (4) |
| C15A | 0.044 (6) | 0.042 (4) | 0.032 (5) | 0.013 (4) | 0.005 (5) | −0.011 (4) |
| C16A | 0.051 (6) | 0.031 (4) | 0.022 (4) | 0.007 (4) | 0.002 (4) | 0.005 (3) |
| O31A | 0.022 (3) | 0.0197 (19) | 0.023 (3) | −0.002 (2) | −0.008 (3) | 0.002 (2) |
| O41A | 0.032 (3) | 0.027 (2) | 0.014 (2) | −0.005 (2) | −0.003 (2) | −0.0025 (19) |
| O51A | 0.029 (3) | 0.028 (2) | 0.021 (3) | −0.012 (2) | 0.003 (2) | −0.001 (2) |
| C61A | 0.020 (4) | 0.021 (3) | 0.018 (4) | 0.001 (3) | 0.003 (3) | 0.000 (3) |
| O61A | 0.050 (4) | 0.019 (2) | 0.057 (4) | 0.005 (3) | −0.021 (3) | 0.007 (2) |
| O1W | 0.026 (3) | 0.049 (3) | 0.058 (4) | −0.015 (3) | 0.005 (3) | −0.002 (3) |
Geometric parameters (Å, °)
| S1—C2 | 1.804 (7) | S1A—C7A | 1.813 (7) |
| S1—C7 | 1.812 (7) | O1A—C2A | 1.437 (6) |
| O1—C2 | 1.420 (7) | O1A—C6A | 1.443 (8) |
| O1—C6 | 1.422 (7) | C2A—C3A | 1.526 (8) |
| C2—C3 | 1.522 (8) | C2A—H2A | 1.0000 |
| C2—H2 | 1.0000 | C3A—O31A | 1.421 (6) |
| C3—O31 | 1.432 (7) | C3A—C4A | 1.523 (8) |
| C3—C4 | 1.516 (9) | C3A—H3A | 1.0000 |
| C3—H3 | 1.0000 | C4A—O41A | 1.437 (7) |
| C4—O41 | 1.420 (7) | C4A—C5A | 1.517 (8) |
| C4—C5 | 1.537 (8) | C4A—H4A | 1.0000 |
| C4—H4 | 1.0000 | C5A—O51A | 1.419 (8) |
| C5—O51 | 1.423 (8) | C5A—C6A | 1.519 (9) |
| C5—C6 | 1.519 (9) | C5A—H5A | 1.0000 |
| C5—H5 | 1.0000 | C6A—C61A | 1.527 (7) |
| C6—C61 | 1.521 (8) | C6A—H6A | 1.0000 |
| C6—H6 | 1.0000 | C7A—C8A | 1.525 (9) |
| C7—C8 | 1.535 (9) | C7A—H7A1 | 0.9900 |
| C7—H7A | 0.9900 | C7A—H7A2 | 0.9900 |
| C7—H7B | 0.9900 | C8A—C11A | 1.512 (9) |
| C8—C11 | 1.515 (10) | C8A—H8A1 | 0.9900 |
| C8—H8A | 0.9900 | C8A—H8A2 | 0.9900 |
| C8—H8B | 0.9900 | C11A—C12A | 1.368 (11) |
| C11—C12 | 1.385 (10) | C11A—C16A | 1.419 (9) |
| C11—C16 | 1.416 (10) | C12A—C13A | 1.388 (10) |
| C12—C13 | 1.376 (11) | C12A—H12A | 0.9500 |
| C12—H12 | 0.9500 | C13A—C14A | 1.366 (11) |
| C13—C14 | 1.383 (10) | C13A—H13A | 0.9500 |
| C13—H13 | 0.9500 | C14A—C15A | 1.348 (12) |
| C14—C15 | 1.386 (11) | C14A—H14A | 0.9500 |
| C14—H14 | 0.9500 | C15A—C16A | 1.400 (10) |
| C15—C16 | 1.371 (12) | C15A—H15A | 0.9500 |
| C15—H15 | 0.9500 | C16A—H16A | 0.9500 |
| C16—H16 | 0.9500 | O31A—H31A | 0.8400 |
| O31—H31 | 0.8395 | O41A—H41A | 0.8400 |
| O41—H41 | 0.8400 | O51A—H51A | 0.8400 |
| O51—H51 | 0.8400 | C61A—O61A | 1.420 (8) |
| C61—O61 | 1.431 (8) | C61A—H61D | 0.9900 |
| C61—H61B | 0.9900 | C61A—H61E | 0.9900 |
| C61—H61C | 0.9900 | O61A—H61A | 0.8400 |
| O61—H61 | 0.8400 | O1W—H1WA | 0.8394 |
| S1A—C2A | 1.799 (7) | O1W—H1WB | 0.8399 |
| C2—S1—C7 | 101.4 (4) | C2A—O1A—C6A | 109.8 (4) |
| C2—O1—C6 | 111.8 (5) | O1A—C2A—C3A | 112.7 (5) |
| O1—C2—C3 | 109.5 (5) | O1A—C2A—S1A | 108.3 (4) |
| O1—C2—S1 | 108.7 (4) | C3A—C2A—S1A | 109.7 (4) |
| C3—C2—S1 | 112.5 (4) | O1A—C2A—H2A | 108.7 |
| O1—C2—H2 | 108.7 | C3A—C2A—H2A | 108.7 |
| C3—C2—H2 | 108.7 | S1A—C2A—H2A | 108.7 |
| S1—C2—H2 | 108.7 | O31A—C3A—C4A | 108.5 (5) |
| O31—C3—C4 | 110.2 (5) | O31A—C3A—C2A | 108.5 (5) |
| O31—C3—C2 | 110.9 (5) | C4A—C3A—C2A | 110.4 (5) |
| C4—C3—C2 | 110.2 (5) | O31A—C3A—H3A | 109.8 |
| O31—C3—H3 | 108.5 | C4A—C3A—H3A | 109.8 |
| C4—C3—H3 | 108.5 | C2A—C3A—H3A | 109.8 |
| C2—C3—H3 | 108.5 | O41A—C4A—C5A | 111.6 (5) |
| O41—C4—C3 | 112.7 (5) | O41A—C4A—C3A | 107.7 (5) |
| O41—C4—C5 | 112.2 (5) | C5A—C4A—C3A | 111.3 (5) |
| C3—C4—C5 | 111.2 (5) | O41A—C4A—H4A | 108.7 |
| O41—C4—H4 | 106.8 | C5A—C4A—H4A | 108.7 |
| C3—C4—H4 | 106.8 | C3A—C4A—H4A | 108.7 |
| C5—C4—H4 | 106.8 | O51A—C5A—C4A | 109.7 (5) |
| O51—C5—C6 | 110.9 (6) | O51A—C5A—C6A | 111.9 (5) |
| O51—C5—C4 | 108.8 (5) | C4A—C5A—C6A | 108.0 (5) |
| C6—C5—C4 | 108.6 (5) | O51A—C5A—H5A | 109.1 |
| O51—C5—H5 | 109.5 | C4A—C5A—H5A | 109.1 |
| C6—C5—H5 | 109.5 | C6A—C5A—H5A | 109.1 |
| C4—C5—H5 | 109.5 | O1A—C6A—C5A | 109.1 (5) |
| O1—C6—C5 | 111.3 (5) | O1A—C6A—C61A | 106.9 (5) |
| O1—C6—C61 | 107.4 (5) | C5A—C6A—C61A | 114.7 (5) |
| C5—C6—C61 | 113.2 (5) | O1A—C6A—H6A | 108.6 |
| O1—C6—H6 | 108.3 | C5A—C6A—H6A | 108.6 |
| C5—C6—H6 | 108.3 | C61A—C6A—H6A | 108.6 |
| C61—C6—H6 | 108.3 | C8A—C7A—S1A | 110.0 (5) |
| C8—C7—S1 | 115.4 (5) | C8A—C7A—H7A1 | 109.7 |
| C8—C7—H7A | 108.4 | S1A—C7A—H7A1 | 109.7 |
| S1—C7—H7A | 108.4 | C8A—C7A—H7A2 | 109.7 |
| C8—C7—H7B | 108.4 | S1A—C7A—H7A2 | 109.7 |
| S1—C7—H7B | 108.4 | H7A1—C7A—H7A2 | 108.2 |
| H7A—C7—H7B | 107.5 | C11A—C8A—C7A | 111.1 (6) |
| C11—C8—C7 | 113.6 (7) | C11A—C8A—H8A1 | 109.4 |
| C11—C8—H8A | 108.8 | C7A—C8A—H8A1 | 109.4 |
| C7—C8—H8A | 108.8 | C11A—C8A—H8A2 | 109.4 |
| C11—C8—H8B | 108.8 | C7A—C8A—H8A2 | 109.4 |
| C7—C8—H8B | 108.8 | H8A1—C8A—H8A2 | 108.0 |
| H8A—C8—H8B | 107.7 | C12A—C11A—C16A | 119.6 (7) |
| C12—C11—C16 | 117.5 (7) | C12A—C11A—C8A | 122.0 (6) |
| C12—C11—C8 | 122.0 (6) | C16A—C11A—C8A | 118.4 (7) |
| C16—C11—C8 | 120.5 (7) | C11A—C12A—C13A | 119.9 (7) |
| C13—C12—C11 | 121.8 (7) | C11A—C12A—H12A | 120.1 |
| C13—C12—H12 | 119.1 | C13A—C12A—H12A | 120.1 |
| C11—C12—H12 | 119.1 | C14A—C13A—C12A | 121.1 (8) |
| C12—C13—C14 | 120.7 (8) | C14A—C13A—H13A | 119.5 |
| C12—C13—H13 | 119.7 | C12A—C13A—H13A | 119.5 |
| C14—C13—H13 | 119.7 | C15A—C14A—C13A | 119.8 (8) |
| C13—C14—C15 | 118.2 (8) | C15A—C14A—H14A | 120.1 |
| C13—C14—H14 | 120.9 | C13A—C14A—H14A | 120.1 |
| C15—C14—H14 | 120.9 | C14A—C15A—C16A | 121.6 (7) |
| C16—C15—C14 | 121.9 (8) | C14A—C15A—H15A | 119.2 |
| C16—C15—H15 | 119.0 | C16A—C15A—H15A | 119.2 |
| C14—C15—H15 | 119.0 | C15A—C16A—C11A | 118.0 (8) |
| C15—C16—C11 | 119.9 (7) | C15A—C16A—H16A | 121.0 |
| C15—C16—H16 | 120.0 | C11A—C16A—H16A | 121.0 |
| C11—C16—H16 | 120.0 | C3A—O31A—H31A | 109.5 |
| C3—O31—H31 | 125.0 | C4A—O41A—H41A | 109.5 |
| C4—O41—H41 | 109.5 | C5A—O51A—H51A | 109.5 |
| C5—O51—H51 | 109.5 | O61A—C61A—C6A | 108.1 (5) |
| O61—C61—C6 | 109.3 (5) | O61A—C61A—H61D | 110.1 |
| O61—C61—H61B | 109.8 | C6A—C61A—H61D | 110.1 |
| C6—C61—H61B | 109.8 | O61A—C61A—H61E | 110.1 |
| O61—C61—H61C | 109.8 | C6A—C61A—H61E | 110.1 |
| C6—C61—H61C | 109.8 | H61D—C61A—H61E | 108.4 |
| H61B—C61—H61C | 108.3 | C61A—O61A—H61A | 109.5 |
| C61—O61—H61 | 109.5 | H1WA—O1W—H1WB | 99.1 |
| C2A—S1A—C7A | 100.7 (3) | ||
| C6—O1—C2—C3 | −63.2 (7) | C6A—O1A—C2A—C3A | −59.8 (7) |
| C6—O1—C2—S1 | 173.6 (4) | C6A—O1A—C2A—S1A | 178.7 (4) |
| C7—S1—C2—O1 | −109.5 (4) | C7A—S1A—C2A—O1A | −76.8 (5) |
| C7—S1—C2—C3 | 129.0 (5) | C7A—S1A—C2A—C3A | 159.8 (4) |
| O1—C2—C3—O31 | 179.5 (5) | O1A—C2A—C3A—O31A | 169.9 (5) |
| S1—C2—C3—O31 | −59.5 (6) | S1A—C2A—C3A—O31A | −69.4 (6) |
| O1—C2—C3—C4 | 57.2 (7) | O1A—C2A—C3A—C4A | 51.1 (7) |
| S1—C2—C3—C4 | 178.2 (4) | S1A—C2A—C3A—C4A | 171.8 (4) |
| O31—C3—C4—O41 | 57.8 (7) | O31A—C3A—C4A—O41A | 69.2 (6) |
| C2—C3—C4—O41 | −179.5 (5) | C2A—C3A—C4A—O41A | −171.9 (5) |
| O31—C3—C4—C5 | −175.2 (5) | O31A—C3A—C4A—C5A | −168.2 (5) |
| C2—C3—C4—C5 | −52.5 (7) | C2A—C3A—C4A—C5A | −49.3 (7) |
| O41—C4—C5—O51 | 57.6 (7) | O41A—C4A—C5A—O51A | 53.7 (7) |
| C3—C4—C5—O51 | −69.6 (6) | C3A—C4A—C5A—O51A | −66.6 (7) |
| O41—C4—C5—C6 | 178.4 (6) | O41A—C4A—C5A—C6A | 175.9 (5) |
| C3—C4—C5—C6 | 51.3 (7) | C3A—C4A—C5A—C6A | 55.6 (7) |
| C2—O1—C6—C5 | 63.8 (7) | C2A—O1A—C6A—C5A | 66.0 (6) |
| C2—O1—C6—C61 | −171.8 (5) | C2A—O1A—C6A—C61A | −169.4 (5) |
| O51—C5—C6—O1 | 63.5 (6) | O51A—C5A—C6A—O1A | 57.2 (6) |
| C4—C5—C6—O1 | −56.0 (7) | C4A—C5A—C6A—O1A | −63.6 (6) |
| O51—C5—C6—C61 | −57.5 (7) | O51A—C5A—C6A—C61A | −62.7 (7) |
| C4—C5—C6—C61 | −177.1 (5) | C4A—C5A—C6A—C61A | 176.5 (6) |
| C2—S1—C7—C8 | 61.9 (6) | C2A—S1A—C7A—C8A | −174.0 (5) |
| S1—C7—C8—C11 | 71.3 (7) | S1A—C7A—C8A—C11A | −178.9 (5) |
| C7—C8—C11—C12 | 77.3 (9) | C7A—C8A—C11A—C12A | −86.0 (8) |
| C7—C8—C11—C16 | −102.6 (8) | C7A—C8A—C11A—C16A | 96.4 (8) |
| C16—C11—C12—C13 | −1.1 (12) | C16A—C11A—C12A—C13A | −3.0 (11) |
| C8—C11—C12—C13 | 179.0 (8) | C8A—C11A—C12A—C13A | 179.5 (7) |
| C11—C12—C13—C14 | −0.1 (13) | C11A—C12A—C13A—C14A | 1.4 (12) |
| C12—C13—C14—C15 | 0.0 (13) | C12A—C13A—C14A—C15A | 0.5 (12) |
| C13—C14—C15—C16 | 1.4 (14) | C13A—C14A—C15A—C16A | −0.7 (13) |
| C14—C15—C16—C11 | −2.7 (14) | C14A—C15A—C16A—C11A | −0.9 (12) |
| C12—C11—C16—C15 | 2.5 (12) | C12A—C11A—C16A—C15A | 2.7 (11) |
| C8—C11—C16—C15 | −177.7 (8) | C8A—C11A—C16A—C15A | −179.6 (7) |
| O1—C6—C61—O61 | 65.0 (7) | O1A—C6A—C61A—O61A | 172.2 (5) |
| C5—C6—C61—O61 | −171.8 (6) | C5A—C6A—C61A—O61A | −66.6 (7) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O31—H31···O1W | 0.84 | 1.92 | 2.759 (7) | 179. |
| O41—H41···O41A | 0.84 | 1.95 | 2.779 (7) | 169. |
| O51—H51···O1Wi | 0.84 | 1.98 | 2.773 (6) | 156. |
| O61—H61···O31Aii | 0.84 | 1.86 | 2.697 (7) | 172. |
| O31A—H31A···O61iii | 0.84 | 1.92 | 2.650 (7) | 145. |
| O41A—H41A···O41iv | 0.84 | 2.05 | 2.788 (7) | 147. |
| O51A—H51A···O61Av | 0.84 | 2.10 | 2.785 (7) | 138. |
| O61A—H61A···O31ii | 0.84 | 2.02 | 2.744 (6) | 145. |
| O1W—H1WA···O31v | 0.84 | 2.23 | 2.989 (8) | 150. |
| O1W—H1WB···O41Av | 0.84 | 2.43 | 3.270 (6) | 180. |
Symmetry codes: (i) −x, y−1/2, −z+3/2; (ii) −x+1, y−1/2, −z+3/2; (iii) −x, y+1/2, −z+3/2; (iv) x+1, y, z; (v) x−1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: OM2454).
References
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Choi, J. H., Choe, Y. S., Lee, K.-H., Choi, Y., Kim, S. E. & Kim, B.-T. (2003). Carbohydr. Res 338, 29–34. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- De Bruyne, C. K. & Yde, M. (1977). Carbohydr. Res. 56, 153-164. [DOI] [PubMed]
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Gutiérrez, B., Muñoz, C., Osorio, L., Ambati, A. K., Kövér, K. E., Sagua, H., Araya, J. E., Morales, P., Szilágyi, L. & González, J. (2011). Acta Trop. Submitted.
- Helferich, B. & Türk, D. (1956). Chem. Ber. 89, 2215–2219.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (2001). X-AREA Stoe & Cie, Darmstadt, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811031667/om2454sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811031667/om2454Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report