Abstract
We analyzed the pathogenesis-related generation of H2O2 using the microscopic detection of 3,3-diaminobenzidine polymerization in near-isogenic barley (Hordeum vulgare L.) lines carrying different powdery mildew (Blumeria graminis f.sp. hordei) resistance genes, and in a line expressing chemically activated resistance after treatment with 2,6-dichloroisonicotinic acid (DCINA). Hypersensitive cell death in Mla12 and Mlg genotypes or after chemical activation by DCINA was associated with H2O2 accumulation throughout attacked cells. Formation of cell wall appositions (papillae) mediated in Mlg and mlo5 genotypes and in DCINA-activated plants was paralleled by H2O2 accumulation in effective papillae and in cytosolic vesicles of up to 2 μm in diameter near the papillae. H2O2 was not detected in ineffective papillae of cells that had been successfully penetrated by the fungus. These findings support the hypothesis that H2O2 may play a substantial role in plant defense against the powdery mildew fungus. We did not detect any accumulation of salicylic acid in primary leaves after inoculation of the different barley genotypes, indicating that these defense responses neither relied on nor provoked salicylic acid accumulation in barley.
The role of H2O2 and SA in the defense responses of plants against parasites is controversial. Chen et al. (1993) has argued that SA acts via inhibition of a catalase that subsequently results in accumulation of H2O2, which may involve cross-linking reactions leading to cell wall toughening (Bradley et al., 1992; Brisson et al., 1994) and/or signaling that results in defense gene activation (Chen et al., 1993; Wu et al., 1997; Chamnongpol et al., 1998). The hypersensitive response, a plant defense reaction that restricts biotrophic and other pathogens (Stakman, 1915), seems to be dependent on the availability of SA (Levine et al., 1994; Shirasu et al., 1997; Tenhaken and Rübel, 1997) and H2O2 (Levine et al., 1994; Thordal-Christensen et al., 1997). Bi et al. (1995) and Neuenschwander et al. (1995) have presented data suggesting that systemic acquired resistance does not rely on H2O2 accumulation as a downstream event of elevation of SA content. Moreover, van Wees et al. (1997) and Vidal et al. (1998) have provided evidence for SA-independent biological induction of resistance.
We have chosen the interaction of the powdery mildew fungus (Blumeria graminis f.sp. hordei) with barley (Hordeum vulgare L.) to study the role of reactive oxygen species and SA in constitutively expressed and chemically induced resistance. The developmental stages of powdery mildew fungus during its interaction with its host are well defined, and fungal spores show a synchronized growth upon leaf inoculation. After contact of the spore with the cutin layer of a barley leaf, the following fungal structures differentiate within the first 24 hai (Ellingboe, 1972; Aist and Bushnell, 1991): (a) the primary germ tube (1–2 h), (b) the appressorial germ tube with a mature appressorium (10–12 h), and (c) the haustorium initial (16–24 h), which invaginates the epidermal plasma membrane. Formation of aerial mycelium and sporulation are late differentiation events that occur 5 to 7 d post inoculation. The establishment of a mature haustorium represents a key step for successful fungal reproduction, because it is the only organ that serves to feed the pathogen. Quantitative cytological recordings of incompatible interactions have revealed putative host-cell-defense responses conferring arrest of fungal development at distinct stages (Kita et al., 1981; Koga et al., 1988, 1990). Two of these host-cell responses are easily detected: a subcellular, highly localized cell wall reinforcement at sites of attempted penetration (effective papilla) and an active, rapid death of attacked epidermal cells (HR), which can be visualized by whole-cell autofluorescence under UV excitation (Aist and Israel, 1986; Koga et al., 1990; Görg et al., 1993).
In the present study, we used BghA6, which triggers defense responses in NILs bearing the powdery mildew resistance genes mlo5, Mlg, and Mla12 (Hückelhoven and Kogel, 1998). These genes govern fungal arrest at different stages of the interaction: (a) at the penetration stage within papillae, leaving the attacked cell alive (mlo); (b) within papillae of cells that subsequently undergo HR (Mlg); and (c) by HR after penetration of the epidermal host cell (Mla12). A previous publication compared the PR generation of the superoxide anion (O2⋅−) in these NILs (Hückelhoven and Kogel, 1998; Kogel and Hückelhoven, 1999). An O2⋅− burst was seen at the interaction sites in epidermal cells that had been penetrated by the fungus (compatible interaction and Mla12-mediated resistance), but not at interaction sites showing penetration resistance (Mlg- and mlo5-mediated resistance). These data suggest that the trigger for O2⋅− generation in epidermal cells attacked by powdery mildew fungus was the contact of the parasite with the host plasma membrane after host-cell wall penetration, and that O2⋅− accumulation was not required or sufficient for HR elicitation.
We show here that H2O2, in contrast to O2⋅−, is closely associated with HR and the formation of effective papillae, and that these defense responses are not linked to SA accumulation in barley.
MATERIALS AND METHODS
Plants, Pathogens, and Inoculation
The barley (Hordeum vulgare L.) cv Pallas and the mlo5-, Mlg-, and Mla12-backcross lines in cv Pallas were obtained from Lisa Munk (Royal Veterinary and Agricultural University, Copenhagen, Denmark). Their generation was described previously (Kølster et al., 1986). Plants were grown in a growth chamber at 16°C, 60% RH, and a photoperiod of 16 h (100 μE). Inoculation was with 20 conidia mm−2 from Blumeria graminis DC: Fr. f.sp. hordei, race A6 (BghA6) (Wiberg, 1974) on the 7th d after germination.
Chemical Induction
DCINA (Novartis AG, Basel, Switzerland), formulated as a 25% (w/w) active ingredient with a wettable powder carrier (Métraux et al., 1991), was applied to 4-d-old barley seedlings as a soil drench. The final concentration of the compound used was 8 mg L−1 soil volume. Controls were treated with wettable powder.
Histochemical Detection of H2O2 at Interaction Sites
Detection of H2O2 was performed by an endogenous peroxidase-dependent in situ histochemical staining procedure using DAB, as described by Thordal-Christensen et al. (1997). Seven-day-old primary leaves were cut and placed in a solution of 1 mg mL−1 DAB for 8 h and subsequently in a clearance solution (0.15% TCA [w/v] in ethyl-alcohol:chloroform [4:1, v/v]) for 24 h. The storage of leaf segments, the staining of fungal structures, and the microscopy were done as described by Hückelhoven and Kogel (1998). Because defense responses of barley epidermal leaf cells vary significantly depending on the cell type, evaluation of cellular interaction phenotypes and H2O2 accumulation was restricted to short epidermal cells (A and B cells showing reactions strongly dependent on the genotype) of the adaxial epidermis (Koga et al., 1990). If more than one cellular structure stained with DAB at the same interaction site, we counted the most intensive coloring for statistical evaluation. The autofluorescence of cells undergoing cell death was partly covered by DAB staining, but it was still bright enough to be taken as a reliable measure of HR.
Extraction of SA and HPLC
Eight primary leaves were harvested by freezing in liquid nitrogen and then stored at −80°C. Leaves were homogenized in liquid nitrogen for 3 min, and two portions of 0.3 to 0.5 g fresh weight of frozen material were placed into 2-mL microcentrifuge tubes. Chloroform:methanol (1:2, v/v; 1.3 mL) was added to the samples according to the lipid-extraction method of Bligh and Dyer (1959) before storing the samples for 24 h at −18°C. According to Meuwly and Métraux (1993), the Bligh/Dyer solution contained 300 ng 2-methoxybenzoic acid mL−1 as an internal standard. The internal standard was added after confirming that endogenous levels of 2-methoxybenzoic acid did not change after inoculation. After shaking the samples at 200 rpm on a horizontal shaker for 30 min, 400 μL of distilled water was added for separation of solvent phases. Samples were centrifuged at 20,000g for 20 min at 4°C. The methanol-water phases were placed in another microcentrifuge tube and shaken again with 350 μL of CHCl3. After a second centrifugation (5 min), the methanol-water phase was concentrated in a vacuum centrifuge.
Residues were resuspended in 400 μL of methanol:water (1:1, v/v), centrifuged for 10 min at 20,000g, and placed in HPLC vials for detection of free SA. For hydrolysis of SA-glycosides the concentrated samples were resuspended in 500 μL of 1 m HCl, incubated at 70°C overnight, concentrated again, and resuspended for HPLC in 500 μL of methanol:water (1:1, v:v). The SA content in the extracts was corrected according to the amount of the internal standard. HPLC was performed with a reverse-phase column (LiChroCart 250-4, LiChroSphere C18, 5 μm, Merck, Darmstadt, Germany) linked to a fluorescence photometer (SFM 25, Kontron, Neufarn, Germany) equipped with a 12-μL flow cell. Excitation and emission wavelengths were 298 and 400 nm, respectively. Separation was at 40°C using a flow rate of 1 mL min−1. Elution was carried out with 0.05 m TCA (pH 2.6):methanol (80:20, v/v) for 1 min, followed by a linear 10-min gradient to TCA:methanol (70:30, v/v), isocratic TCA:methanol (70:30, v/v) for 20 min, 5 min washing with methanol (100%), followed by a return to the first step solvent, and equilibration for 10 min before the next run.
RESULTS
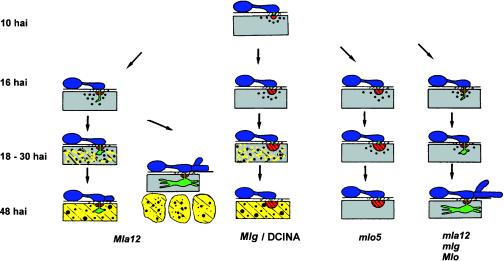
Histochemical localization of H2O2 using DAB in barley primary leaves attacked by BghA6 showed an accumulation of this active oxygen species in cell wall appositions, as well as in cells undergoing HR, which is in agreement with the results of Thordal-Christensen et al. (1997). To determine the spatial and temporal profile of H2O2 generation in these prominent defense reactions, we carried out a comparative kinetic analysis of H2O2 formation at early developmental stages of the interaction of BghA6 with NILs bearing the resistance genes Mla12, Mlg, and mlo5. These genes mediate various highly defined defense phenotypes, including the timing and frequency of HR and effective papillae in response to fungal penetration attempts. The quantitatively predominant plant responses governed by these resistance genes were compared with the susceptible phenotype in Figure 1. Figure 1 also displays the defense phenotype mediated by induction of barley with the chemical DCINA. A more detailed quantitative cytological analysis of the interaction phenotypes indicates that the development of BghA6 on the NILs or DCINA-treated cv Pallas was basically the same as had been observed in earlier studies (Table I; see also Hückelhoven and Kogel, 1998; Kogel and Hückelhoven, 1999).
Figure 1.
Scheme of predominant interaction phenotypes in barley mediated by the powdery mildew resistance genes Mla12, Mlg, and mlo5, or by the resistance-inducing compound DCINA after inoculation with BghA6. Beginning at 16 hai, differences in fungal development are evident on the four NILs. Although the fungus penetrated the epidermal cells of susceptible and Mla12-resistant barley, effective papillae did impede penetration in the Mlg- and the mlo5-mediated response. Mla12 mediated HR in penetrated epidermal cells by 24 to 40 hai. If the fungus was able to establish a differentiated haustorium and branched elongated secondary hyphae, fungal development was arrested by spreading HR of mesophyll cells subjacent to the attacked epidermal cell beginning at 36 hai. The Mlg gene governed effective papilla formation and HR of attacked but noninvaded epidermal cells by 18 to 24 hai. The same phenotype is seen in barley treated with DCINA. The recessive mlo5 gene mediated effective papilla formation, and attacked cells stayed alive. In the compatible interaction, cell wall penetration was followed by formation of a haustorium and elongated secondary hyphae.
Table I.
Resistance responses of cv Pallas NILs and cv Pallas chemically induced by DCINA upon inoculation with BghA6
| Reaction | Interaction Sites
|
||||
|---|---|---|---|---|---|
| Pallasa | BCPMla12 | BCPMlg | BCPmlo5 | DCINA-Induced | |
| h | % | ||||
| Haustorium/elongated secondary hypha | |||||
| 22 | 58 | 62 | 2 | 0 | 29 |
| 30 | 65 | 54 | 1 | 0 | 35 |
| 48 | 68 | 5 | 1 | 0 | 26 |
| Effective papillab | |||||
| 22 | 32 | 21 | 58 | 99 | 40 |
| 30 | 17 | 18 | 23 | 99 | 22 |
| 48 | 22 | 10 | 21 | 98 | 32 |
| HR (epidermis)c | |||||
| 22 | 10 | 17 | 40 | 1 | 31 |
| 30 | 18 | 28 | 76 | 1 | 43 |
| 48 | 10 | 64 | 78 | 2 | 42 |
| HR (mesophyll) | |||||
| 22 | 0 | 0 | 0 | 0 | 0 |
| 30 | 0 | 0 | 0 | 0 | 0 |
| 48 | 0 | 21 | 0 | 0 | 0 |
Interaction phenotypes were microscopically analyzed at 22, 30, and 48 h after inoculation. The data represent each 100 interaction sites per leaf. Each of three independent experiments gave very similar results.
Results shown are from a plant treated with wettable powder as a control for plants treated with DCINA. Wettable powder did not affect the interaction (not shown).
Effective papilla in dead cells were evaluated as HR (epidermis).
In BCPMla12, HR occurred after penetration, whereas in all other NILs, dead cells did not contain a haustorium.
H2O2 in Compatible Interaction
Upon inoculation of the recurrent parent cv Pallas with BghA6, H2O2 accumulated in the epidermal cell wall appositions subjacent to the primary germ tube within 10 hai, as indicated by reddish-brown staining due to DAB polymerization. A low frequency of interaction sites with H2O2 in papillae subjacent to the appressorial germ tube was detected by 20 hai. H2O2 was detected within papillae not penetrated by the fungus (effective papillae, Fig. 2A). Epidermal cells that successfully repelled fungal attack regularly contained brownish vesicles around the papilla, suggesting that the vesicles targeted to the plasma membrane to deliver cell wall material for papilla toughening contained H2O2. Within 22 hai, epidermal cells penetrated by the fungus often showed a local brownish staining of the anticlinal cell wall at approximately 30% of all interaction sites, but ineffective papillae were not stained (shown at 24 hai in Fig. 2B). Local staining of anticlinal cell walls disappeared within 48 hai, when the fungus had developed branched, elongated secondary hyphae. The HR of the attacked epidermal cells, seen at low frequencies (Table I), was always associated with H2O2 accumulation in the entire cell wall or the whole cell, beginning 18 hai. Cells undergoing HR and showing whole-cell H2O2 accumulation were not invaded by the fungus; successful penetration was not followed by cell death in this compatible interaction. In the mesophyll tissue, H2O2 was detected at sites next to epidermal cells undergoing HR. In some incidences, chloroplasts of these cells and of cells in the vicinity of leaf vessels accumulated H2O2 (Fig. 2C), although these cells did not die.
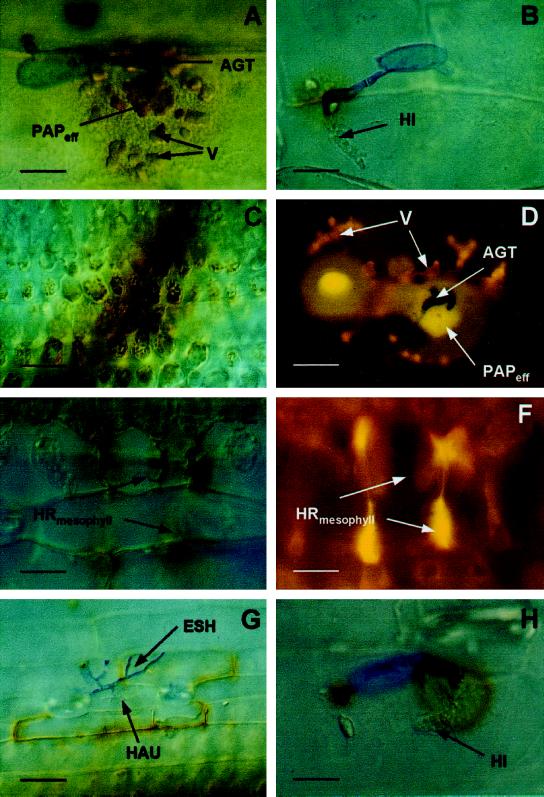
Figure 2.
Microscopic, subcellular localization of H2O2 at interaction sites in barley after inoculation with BghA6. Seven-day-old barley primary leaves of NILs were inoculated with 20 conidia mm−2 of BghA6. At different time points after inoculation, leaves were cut off, placed in a solution of 1 mg mL−1 DAB, and collected for microscopic analysis 8 h later (at the indicated time points). A, Effective papilla (PAPeff) in formation on cv Pallas (22 hai). Papilla and vesicles (V) with DAB polymers are seen beneath the appressorial germ tube (AGT). Scale bar = 8 μm. B, Successful penetration of cv Pallas (24 hai). The fungus had formed a haustorial initial (HI). Whereas the body of the ineffective papilla shows only a faint staining, the anticlinal cell wall close to the penetration site shows typical brownish DAB polymers. Scale bar = 10 μm. C, DAB staining of chloroplasts in healthy mesophyll cells neighboring a transverse vessel in cv Pallas primary leaves. Scale bar = 40 μm. D, BCPmlo5 (30 hai). Autofluorescence under UV-light excitation of effective papilla (PAPeff) halos and vesicles beneath primary and AGTs is indicative of the accumulation of phenolic compounds at a repulsed penetration attempt. Scale bar = 10 μm. E, BCPMla12 (48 hai): mesophyll HR subjacent to a living penetrated epidermal cell (out of focus). Dead cells show DAB staining. Scale bar = 20 μm. F, Epifluorescence microscopy of the interaction site shown in E. The cells in the center have collapsed. Accumulation of polyphenols is indicated by the yellow autofluorescence. Scale bar = 20 μm. G, BCPMla12 (48 hai): epidermal cells surrounding the penetrated, living cell show positive DAB staining, (Legend continues on facing page.)
H2O2 in Resistance Mediated by the mlo5 Gene
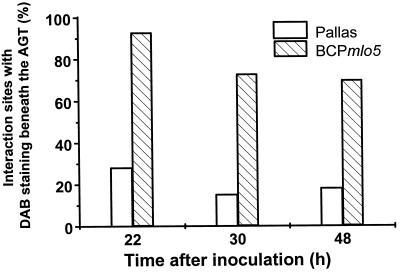
The recessive mlo5 gene mediates penetration resistance against BghA6 in BCPmlo5 due to the formation of effective papillae in approximately 98% of the interaction sites (Table I). The frequency of HR was even lower than in the compatible interaction (<2%). As in all other NILs, unsuccessful penetration was associated with strong H2O2 accumulation in papillae and the surrounding vesicles. Thirty hours after inoculation, vesicles staining positively for H2O2 reached a diameter of 2 μm. A yellow autofluorescence under UV light excitation suggested that these vesicles contained phenolic compounds in addition to H2O2 and peroxidase (Fig. 2D). At the same time, no dark-brown papillae or vesicles were seen in association with ineffective papillae in cv Pallas. The numbers of interaction sites with a stained papilla or staining in the surrounding vesicles 22 to 48 hai are shown in Figure 3. Compared with cv Pallas (genotype Mlo), BCPmlo5 showed very high rates of stained papillae, especially at 22 hai (i.e. 14–22 hai, see legend of Fig. 3), a time point that is critical for penetration resistance. In an independent experiment, BCPmlo5 showed frequencies of DAB staining in papilla clearly higher than in cv Pallas 18 hai (i.e. 10–18 hai).
Figure 3.
Incidence of interaction sites with H2O2 in papillae of attacked cells of the NILs cv Pallas (Mlo) and BCPmlo5 after inoculation with BghA6. At 14, 22, and 40 hai, leaves were cut off and placed in a solution of 1 mg mL−1 DAB. After 8 h (at the indicated time points), leaf segments were analyzed for DAB staining subjacent to appressorial germ tubes (AGT). Each column represents 100 interaction sites per leaf. Three independent experiments gave very similar results.
H2O2 in Resistance Mediated by the Mla12 Gene
Resistance mediated by the Mla12 gene in BCPMla12 against BghA6 was characterized by a high frequency of interaction sites with HR of penetrated epidermal cells at 22 to 40 hai (50% of interaction sites), resulting in fungal arrest. In 30% of the interaction sites, the fungus succeeded in establishing a compatible single-cell interaction, as indicated by the presence of a fully differentiated haustorium inside the epidermal cell and the formation of branched, elongated, secondary hyphae on the leaf surface. In the latter case, fungal growth was effectively arrested beginning 36 hai by mesophyll cell death just subjacent to attacked epidermal cells (depicted at 48 hai in Fig. 1 and Table I).
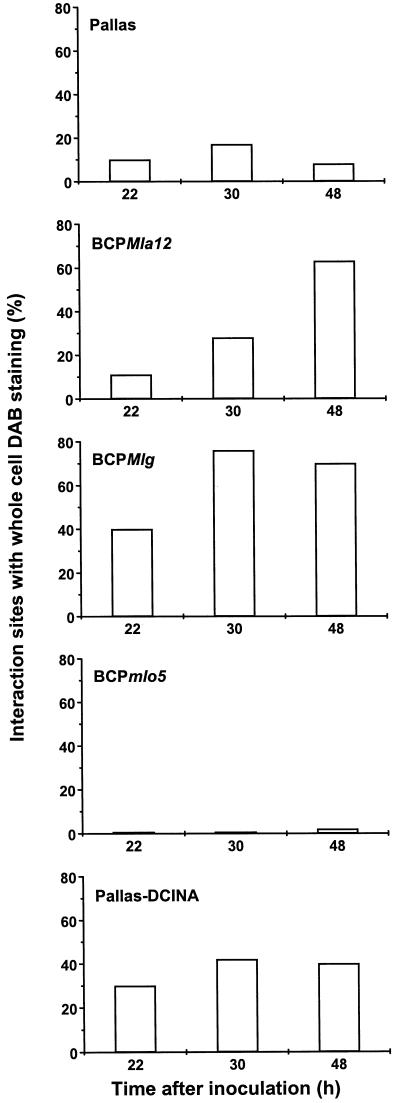
The frequency of interaction sites with local staining of anticlinal walls of penetrated cells (Fig. 2B) reached a level of 30% by 22 hai, indicating that there was no difference in H2O2 formation at this stage of fungal development between compatible and Mla12-incompatible interactions. Later, H2O2 accumulated in penetrated cells undergoing HR (Fig. 4; Table I).
Figure 4.
Incidence of interaction sites with whole-cell DAB staining in NILs bearing the resistance genes Mla12, Mlg, and mlo5, and in cv Pallas expressing chemically induced resistance after inoculation with BghA6. At the indicated time points, leaf segments were microscopically analyzed for positive H2O2 staining. Each column represents 100 interaction sites per leaf. Three independent experiments gave very similar results whereas the attacked cell remained essentially free of dye. ESH, Elongated secondary hyphae; HAU, haustorium. Scale bar = 25 μm. H, BCPMla12 (22 hai): the fungus has penetrated the cell and formed a haustorial initial (HI). A brownish halo (in the focus of the cell wall and plasma membrane) is seen around the penetration site, although the body of the ineffective papilla is only weakly stained. Scale bar = 10 μm. All bright-field microscopic pictures were taken using differential interference contrast.
Mesophyll cell death that was exclusively detected in BCPMla12 with a frequency of 20% of the interaction sites (within 48 hai, see Table I) was closely associated with H2O2 accumulation in all of the cells carrying out HR (Fig. 2, E and F). H2O2 generation and HR in the mesophyll tissue subjacent to interaction sites was a result of a compatible single-cell interaction in the epidermal layer, leading to successful haustorium formation. By 48 hai, epidermal cells next to cells that had been successfully penetrated by the fungus accumulated H2O2 in cell walls, whereas cells containing a haustorium remained essentially free of H2O2 (Fig. 2G).
In addition, approximately 10% of all of the interaction sites with successful fungal penetration (cv Pallas and BCPMla12) showed accumulation of H2O2 in the cell wall or in the plasma membrane area around the penetration site within 22 hai (seen as a brownish ring in Fig. 2H).
H2O2 in Resistance Mediated by the Mlg Gene
The resistance response in BCPMlg against BghA6 was characterized by both the formation of effective papillae and the HR of the attacked epidermal cells. In contrast to the defense phenotype governed by the Mla12 gene, penetration by the fungus and, consequently, contact of the infection peg and haustorium initial with the host plasma membrane were not required for HR elicitation (Fig. 1).
The frequency of interaction sites with attacked cells undergoing HR reached a level of 76% by 30 hai (Table I). The number of interaction sites with H2O2 accumulation in whole cells was strongly correlated with the number of HRs and, compared with cv Pallas, enhanced in all of the evaluated periods (Fig. 4).
H2O2 in Resistance Mediated by the Chemical DCINA
Previous work on the mechanism of chemically induced resistance in barley showed that the microscopically defined defense response induced by DCINA was a phenocopy of the response mediated by the Mlg gene (Kogel et al., 1994; Kogel and Hückelhoven, 1999) (Fig. 1; Table I).
Soil-drench treatment of cv Pallas seedlings with 8 ppm DCINA enhanced the frequency of interaction sites with HR in attacked epidermal cells compared with control plants (Table I). Whole-cell H2O2 accumulation was seen in the cells undergoing HR (Fig. 4). The penetration attempt of the fungus was unsuccessful, and effective papillae were associated with a compact brownish DAB staining (not shown). The pattern of H2O2 generation was similar to that found in BCPMlg (Fig. 4).
Determination of SA in Compatible and Incompatible Interactions of NILs with BghA6
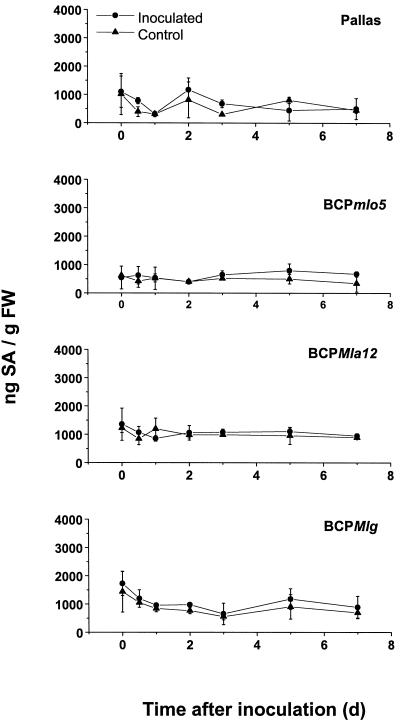
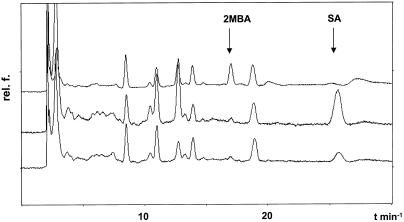
The contents of SA were kinetically analyzed in all of the NILs covering all interaction-relevant time points (Fig. 5). The amount of SA did not differ significantly between Pallas and the corresponding NILs. Upon inoculation with BghA6, basic levels of total SA did not change in the NILs at any time (0–7 d after inoculation; Fig. 5). This time range covers development of papillae, epidermal HR, multicell mesophyll HR, as well as macroscopically visible necrotic leaf spots (the latter exclusively on BCPMla12). In the same kinetic studies, levels of free SA amounted to one-third of the total SA content and it also did not change after inoculation (data not shown). Each experiment included a positive control from TMV-inoculated tobacco (Nicotiana tabacum) cv Xanthi (NN). The SA was separated from other compounds by a chromatographic baseline separation using fluorescence detection. SA was detected at 25.2 min, and the internal standard 2-methoxybenzoic acid at 17.1 min after injection (Fig. 6).
Figure 5.
Endogenous levels of total SA in NILs at different time points after inoculation with BghA6. Seven-day-old primary leaves of the barley lines were inoculated with 20 conidia mm−2. At the indicated time points, leaves were cut off and levels of total SA were determined by HPLC and fluorescence detection. Each point represents the average of duplicates or triplicates of five leaves. Error bars show the sd of the triplicates. Repetition of the experiments led to similar results. FW, Fresh weight.
Figure 6.
Exemplary HPLC chromatograms of three analog leaf extracts of BCPmlo5 from independent experiments (different plant batches). Noninoculated samples were taken on the 8th d after seed germination for determination of total SA. For unknown reasons, extracts showed differing contents of SA, although the contents of other compounds did not differ. The middle and the lower chromatogram show low contents of endogenous 2-methoxybenzoic acid (2MBA). The upper line represents an extract containing 2MBA as an internal standard. rel. f., Relative fluorescence.
Basal levels of SA differed significantly between independent experiments (Fig. 6) but not within an experiment (Fig. 5). Figure 6 shows three representative HPLC traces from independent experiments with SA contents amounting to 101, 1122, and 513 ng g−1 fresh weight. The results with the highest contents of SA are shown in Figure 5.
DISCUSSION
H2O2 Accumulation Is Associated with Barley Defense Phenotypes
In the present work, we have provided evidence for a host genotype-specific production of H2O2 at interaction sites on barley after inoculation with the powdery mildew fungus.
A detailed, cell-type-specific investigation revealed a PR H2O2 burst in association with the most prominent barley defense responses toward an attacking pathogen, i.e. the formation of effective papillae and HR. Comparative analysis of NILs bearing the resistance genes Mlg, Mla12, and mlo5 demonstrated that these genes affect the frequency, but not the quality, of single-cell defense phenotypes associated with H2O2 accumulation. This also applies to the mode of action of the chemical DCINA; i.e. the accumulation pattern of H2O2 associated with effective papillae and HR was quantitatively, but not qualitatively, distinguishable from that observed in all interactions of NILs with BghA6, including the susceptible parent cv Pallas.
Aside from its association with HR, H2O2 strongly accumulated in the effective papillae that prevented the attacking pathogen from penetration into the epidermal host cells (Figs. 2, A and D, and 3). All of the papillae were encircled by vesicles delivering material for cell wall toughening at the site of fungal attack (McKeen and Rimmer, 1973; Bushnell and Berquist, 1975). In our experiments, these vesicles did not only stain positively for H2O2, but also fluoresced under UV excitation, indicating the presence of phenolic material. Because DAB polymerization depends on the presence of H2O2 and peroxidase (Thordal-Christensen et al., 1997), our data suggest that the vesicles targeted to effective papillae contained, in addition to H2O2, peroxidase, and probably partially polymerized phenolics. Both the papilla-associated H2O2 accumulation and the papilla-directed vesicle transport coincided with the arrest of fungal development (Fig. 3; Table I). The absence of H2O2 in papillae at early interaction stages correlated with their inefficiency (Figs. 2B and 3; Table I). The unsuccessful penetration attempts by the pathogen may be explained by papilla toughening through cross-linking reactions driven by H2O2. Consistently, resistance of papilla-bound phenolics to saponification was found in mlo-resistant barley 2 h earlier than in susceptible plants (von Röpenack et al., 1998). H2O2 may also have exerted direct toxic effects on the fungus. Thus, successful penetration may have depended on the ability of the fungus to prevent local H2O2 accumulation. Garre et al. (1998) have recently suggested that catalases secreted by Claviceps purpurea during infection of rye may be suppressors of plant defense.
The PR spatial and temporal patterns of H2O2 accumulation were slightly different from those of O2⋅− in the same NILs (Hückelhoven and Kogel, 1998; Kogel and Hückelhoven, 1999). O2⋅− was localized in a subcellular region where host plasma membrane and fungal haustorium came into contact (genotypes cv Pallas and BCPMla12), but not in association with effective papillae (genotypes BCPMlg and BCPmlo5). Thus, O2⋅− generation in attacked cells was elicited by the cellular contact of host and pathogen, whereas this was not a prerequisite for H2O2 accumulation.
The fact that O2⋅− and H2O2 accumulation could be spatially distinguished in the same defense phenotype also suggests that H2O2 in the cell walls and cytosolic vesicles of attacked epidermal cells did not depend upon preceding O2⋅− generation driven by an NADPH oxidase (Mehdy, 1994) but might have been the product of alternative H2O2-generating sources. Candidate enzymes for PR H2O2 production in the epidermis of barley are peroxidase (Gross et al., 1977; Kerby and Somerville, 1992; Kogel et al., 1994; Scott-Craig et al., 1995), which was inhibited in the O2⋅− assay (Hückelhoven and Kogel, 1998), oxalate oxidase (Zhou et al., 1998), and an oxalate-oxidase-like protein. Wei et al. (1998) have recently shown the latter to be involved in papilla resistance to powdery mildew fungus. PR H2O2 accumulation in the mesophyll tissue was seen especially in BCPMla12 (Fig. 2E), and it was accompanied by mesophyll cell death occurring exclusively in this line. In contrast, a strong infection-related and partly light-dependent O2⋅− burst was found in both BCPMlg and BCPMla12 (Hückelhoven and Kogel, 1998). The O2⋅− burst was essentially associated with chloroplasts, whereas H2O2 was rarely observed in these organelles. Therefore, PR chloroplastic O2⋅− generation did not result in detectable amounts of H2O2 at interaction sites. These data indicate that O2⋅− and H2O2 in the mesophyll tissue were not generated by the same source. We therefore speculate that the H2O2 in the mesophyll was generated by an oxalate oxidase recently localized in this tissue of Mla-resistant barley (Zhou et al., 1998).
A transient accumulation of H2O2 in cv Pallas and BCPMla12 was also seen at the sites of successful penetration near the plasma membranes around papillae (Fig. 2H). Because O2⋅− was detected at the same subcellular region of these NILs (Hückelhoven and Kogel, 1998), H2O2 at this site may have stemmed from O2⋅− that was generated in response to the cellular contact of host and pathogen. This local H2O2 burst was not sufficient to trigger HR. Xu and Heath (1998) recently reported that a Ca2+ influx in cowpea cells attacked by the cowpea rust fungus seemed to be elicited by penetration.
Differences in the pattern of H2O2 accumulation between BCPMla12 and cv Pallas were detected when epidermal and mesophyll cells of BCPMla12 carried out HR with a higher frequency from 30 and 36 hai onward (Figs. 2E and 4). The mesophyll tissue was not directly attacked by the fungus, implying that a signal spreading from the fungus or the attacked cell was essential for this response.
Because H2O2 generation was rarely seen before plant responses became microscopically visible, we suggest that active oxygen species generation in the early stages of incompatible interactions may change the cellular redox state before it becomes detectable by histochemical methods. This interpretation is supported by data from Vanacker et al. (1998), who reported that foliar and apoplastic glutathione contents were increased in resistant barley after inoculation with powdery mildew fungus, although apoplastic glutathione was reduced in susceptible barley. The same study showed that enhanced foliar catalase activity was associated with susceptibility. Our data show that haustorium-invaded cells, even on Mla12-resistant plants (compatible single-cell interaction), were essentially free of H2O2, whereas cells encircling the penetrated cell showed an H2O2 burst (Fig. 2G). This may indicate that single-cell compatibility was the cause or the consequence of enhanced antioxidative status of the host cell. Because haustorium establishment on the susceptible line cv Pallas was never followed by HR and/or strong H2O2 accumulation, we suggest that this was the key event for the achievement of the antioxidative state in the compatible interaction of barley and powdery mildew fungus.
SA Accumulation Is Not Associated with Defense Responses
A newly developed protocol for SA extraction and its separation by HPLC allowed rapid, cheap, and reliable determination of the SA content in barley leaves. Many reports suggest that SA is involved in HR (Levine et al., 1994; Shirasu et al., 1997; Tenhaken and Rübel, 1997). However, in our experiments, SA did not accumulate during the interaction of the NILs with the powdery mildew fungus. This result is supported by the fact that activity of an SA-sensitive salicylate-2-O-glucosyltransferase was not induced during HR and papilla formation after inoculation of BCPMlg with BghA6 (R. Biermann and K.-H. Kogel, unpublished data). Therefore, in barley, SA accumulation was not required for H2O2 accumulation, formation of effective papillae, HR, or the development of necrotic spots (nor did these plant responses elevate the SA content). Pathogen-induced necrotic spots on BCPMla12 covered approximately 10% of the total leaf area at 7 d after inoculation. Thus, it can be excluded that a potential, highly localized PR accumulation of SA was masked by the basal SA content, because the whole leaf, not just the attacked epidermal layer, responded to inoculation. Nevertheless, our data do not exclude that basal SA concentrations were required for the defense responses to occur.
Basal levels of SA differed significantly between independent experiments, but not within the same kinetic analysis. We avoided analytical mistakes by comparing chromatographs of analog samples, clearly demonstrating that the basal content of SA, but not of the other detected compounds, differed from experiment to experiment (Fig. 6). This may explain the greatly differing data for basal SA levels in barley: Vallélian-Bindschedler et al. (1998) found levels of <150 ng g−1 fresh weight, and Raskin et al. (1990) found levels of 2130 ng g−1 fresh weight.
It is well known that PR gene transcripts accumulate upon inoculation of barley (including the NILs) with powdery mildew fungus (Freialdenhoven et al., 1994; Kogel et al., 1994; Gregersen et al., 1997). Therefore, PR-gene activation is not dependent on elevated SA concentrations regardless of the introgressed resistance genes. This agrees with a study by Vallélian-Bindschedler et al. (1998), who showed that PR gene expression in barley after inoculation with B. graminis f.sp. hordei and B. graminis f.sp. tritici was independent of the accumulation of SA. Furthermore, it was shown that biological induction of resistance in dicotyledonous plants did not necessarily depend on SA accumulation (van Wees et al., 1997; Vidal et al., 1998).
Evidence for an SA-sensitive defense pathway comes from experiments with the synthetic SA derivative 3,5-dichlorosalicylic acid, which induced resistance to powdery mildew fungus in cv Pallas (Kogel et al., 1995); and such evidence also comes from experiments with barley mlo-double mutants (genotype mlo ror1), which are highly sensitive to chemical inducers and show enhanced resistance after treatment with SA (B. Jarosch, P. Schulze-Lefert, and K-H. Kogel, unpublished data). Therefore, as in dicotyledonous plants, there may be SA-dependent as well as SA-independent pathways leading to pathogen resistance in barley.
ACKNOWLEDGMENTS
The authors are grateful to Drs. Balázs Barna and Ruth Schiffer for fruitful discussions.
Abbreviations:
- DAB
3,3-diaminobenzidine
- DCINA
2,6-dichloroisonicotinic acid
- hai
hour(s) after inoculation
- HR
hypersensitive cell death response
- NIL
near-isogenic backcross line
- PR
pathogenesis-related
- SA
salicylic acid
Footnotes
The work was supported by the Bundesministerium für Bildung, Wissenschaft Forschung und Technologie (Bonn, Germany), and by the Deutscher Akademischer Austausch Dienst, Bonn, Germany.
LITERATURE CITED
- Aist JR, Bushnell WR (1991) Invasion of plant hosts by powdery mildew fungi and cellular mechanism of resistance. In GT Cole, HC Hoch, eds, The Fungal Spore and Disease Initiation in Plants and Animals. Plenum Press, New York, pp 321–345
- Aist JR, Israel HW. Autofluorescent and ultraviolet-absorbing components in cell walls and papillae of barley coleoptiles and their relationship to disease resistance. Can J Bot. 1986;64:266–272. [Google Scholar]
- Bi J-M, Kenton P, Mur L, Darby R, Draper J. Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J. 1995;8:235–246. doi: 10.1046/j.1365-313x.1995.08020235.x. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell WA, Berquist SE. Aggregation of host cytoplasm and the formation of papillae and haustoria in powdery mildew of barley. Phytopathology. 1975;65:310–318. [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, van Montagu M, Inzé D, van Camp W. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci. 1998;95:5818–5823. doi: 10.1073/pnas.95.10.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant acquired resistance by salicylic acid. Science. 1993;262:1883–1885. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Ellingboe AH. Genetics and physiology of primary infection by Erysiphe graminis. Phytopathology. 1972;62:401–406. [Google Scholar]
- Freialdenhoven A, Scherag B, Hollricher K, Collinge DB, Thordal-Christensen H, Schulze-Lefert P. Nar-1 and Nar-2, two loci required for Mla12-specified race-specific resistance to powdery mildew in barley. Plant Cell. 1994;6:983–994. doi: 10.1105/tpc.6.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garre V, Tenberge KB, Eising H. Secretion of a fungal extracellular catalase by Claviceps purpurea during infection of rye: putative role in pathogenicity and suppression of host response. Phytopathology. 1998;88:744–753. doi: 10.1094/PHYTO.1998.88.8.744. [DOI] [PubMed] [Google Scholar]
- Görg R, Hollricher K, Schulze-Lefert P. Functional analysis and RFLP-mediated mapping of the Mlg resistance locus in barley. Plant J. 1993;3:857–866. [Google Scholar]
- Gregersen PL, Thordal-Christensen H, Förster H, Collinge DB. Differential gene transcript accumulation in barley leaf epidermis and mesophyll in response to attack by Blumeria (syn. Erysiphe graminis f.sp. hordei) Physiol Mol Plant Pathol. 1997;51:85–97. [Google Scholar]
- Gross GG, Janse C, Elstner EF. Involvement of malate, monophenols, and the superoxide radical in hydrogen peroxide formation by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.) Planta. 1977;136:271–276. doi: 10.1007/BF00385995. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R, Kogel KH. Tissue-specific superoxide generation at interaction sites in resistant and susceptible near-isogenic barley lines attacked by the powdery mildew fungus (Erysiphe graminis f.sp. hordei) Mol Plant Microbe Interact. 1998;11:292–300. [Google Scholar]
- Kerby K, Somerville S. Purification of an infection-related, extracellular peroxidase from barley. Plant Physiol. 1992;100:397–402. doi: 10.1104/pp.100.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita N, Toyoda H, Shishiyama J. Chronological analysis of cytological responses in powdery-mildewed barley leaves. Can J Bot. 1981;59:1761–1768. [Google Scholar]
- Koga H, Bushnell WR, Zeyen RJ. Can J Bot. 1990;68:2344–2352. [Google Scholar]
- Koga H, Zeyen RJ, Bushnell WR, Ahlstrand GG. Hypersensitive cell death, autofluorescence, and insoluble silicon accumulation in barley leaf epidermal cells under attack by Erysiphe graminis f. sp. hordei. Physiol Mol Plant Pathol. 1988;32:395–409. [Google Scholar]
- Kogel KH, Beckhove U, Dreschers J, Münch S, Rommé Y. Acquired resistance in barley: the resistance mechanism induced by 2,6-dichloroisonicotinic acid is a phenocopy of a genetically based mechanism governing race-specific powdery mildew resistance. Plant Physiol. 1994;106:1269–1277. doi: 10.1104/pp.106.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel KH, Hückelhoven R. Superoxide generation in chemically activated resistance of barley in response to inoculation with the powdery mildew fungus. J Phytopathol. 1999;147:1–4. [Google Scholar]
- Kogel KH, Ortel B, Jarosch B, Atzorn R, Schiffer R, Wasternack C. Resistance against the powdery mildew fungus (Erysiphe graminis f.sp. hordei) is not associated with enhanced levels of endogenous jasmonates. Eur J Plant Pathol. 1995;101:319–332. [Google Scholar]
- Kølster P, Munk L, Stølen O, Løhde J. Near-isogenic barley lines with genes for resistance to powdery mildew. Crop Sci. 1986;26:903–907. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- McKeen WE, Rimmer SR. Initial penetration process in powdery mildew infection of susceptible barley leaves. Phytopathology. 1973;63:1049–1053. [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J-P, Ahl Goy P, Staub T, Speich J, Steinemann A, Ryals J, Ward E. Induced resistance in cucumber in response to 2,6-dichloroisonicotinic acid and pathogens. In: Hennecke H, Verma DPS, editors. Advances in Molecular Genetics of Plant-Microbe Interactions, Vol 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 432–439. [Google Scholar]
- Meuwly P, Métraux J-P. Ortho-anisic acid as internal standard for the simultaneous quantification of SA and its putative biosynthetic precursors in cucumber leaves. Anal Biochem. 1993;214:500–505. doi: 10.1006/abio.1993.1529. [DOI] [PubMed] [Google Scholar]
- Neuenschwander U, Vernooij B, Friedrich L, Uknes S, Kessmann H, Ryals J. Is hydrogen peroxide a second messenger of salicyclic acid in systemic acquired resistance? Plant J. 1995;8:227–233. [Google Scholar]
- Raskin I, Skubatz H, Tang W, Meeuse BJD. Salicylic acid levels in thermogenic and non-thermogeneic plants. Ann Bot. 1990;66:369–373. [Google Scholar]
- Scott-Craig JS, Kerby KB, Stein BD, Somerville SC. Expression of an extracellular peroxidase that is induced in barley (Hordeum vulgare) by the powdery mildew pathogen (Erysiphe graminis f.sp. hordei) Physiol Mol Plant Pathol. 1995;47:407–418. [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar K, Dixon RA, Lamb C. salicylic acid potentiates an antagonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakman EC. Relation between Puccinia graminis and plants highly resistant to its attack. J Agric Res. 1915;4:193–200. [Google Scholar]
- Tenhaken R, Rübel C. Salicylic acid is needed in hypersensitive cell death but does not act as a catalase inhibitor. Plant Physiol. 1997;115:291–298. doi: 10.1104/pp.115.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Vallélian-Bindschedler L, Métraux J-P, Schweizer P. Salicylic acid accumulation in barley is pathogen specific but not required for defense-gene activation. Mol Plant Microbe Interact. 1998;11:702–705. [Google Scholar]
- Vanacker H, Carver TLW, Foyer C. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998;117:1103–1114. doi: 10.1104/pp.117.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees SCM, Pieterse CMJ, Trijssenaar A, van′t Westende YAM, Hartog F, van Loon LC (1997) Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol Plant Microbe Interact 10: 716–724 [DOI] [PubMed]
- Vidal S, Eriksson ARB, Montesano M, Denecke J, Palva ET. Cell wall-degrading enzymes from Erwinia carotovora cooperate in the salicylic acid-independent induction of a plant defense response. Mol Plant Microbe Interact. 1998;11:23–32. [Google Scholar]
- von Röpenack E, Parr A, Schulze-Lefert P. Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J Biol Chem. 1998;273:9013–9022. doi: 10.1074/jbc.273.15.9013. [DOI] [PubMed] [Google Scholar]
- Wei Y, Zhang Z, Andersen CH, Schmelzer E, Gregersen PL, Collinge DB, Smedegaard-Petersen V, Thordal-Christensen H. An epidermis/papilla-specific oxalate oxidase-like protein in the defense response of barley attacked by the powdery mildew fungus. Plant Mol Biol. 1998;36:101–112. doi: 10.1023/a:1005955119326. [DOI] [PubMed] [Google Scholar]
- Wiberg A. Genetical studies of spontaneous sources of resistance to powdery mildew in barley. Hereditas. 1974;77:89–148. doi: 10.1111/j.1601-5223.1974.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Wu G, Shortt BJ, Lawrence EB, León J, Fitzsimmons KC, Levine EB, Raskin I, Shah DM. Activation of host defense mechanisms by elevated production of H2O2 in transgenic plants. Plant Physiol. 1997;115:427–435. doi: 10.1104/pp.115.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Heath MC. Role of calcium in signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus. Plant Cell. 1998;10:585–597. doi: 10.1105/tpc.10.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Zhang Z, Gregersen PL, Mikkelsen JD, de Neergaard E, Collinge DB, Thordal-Christensen H. Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiol. 1998;117:33–41. doi: 10.1104/pp.117.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]