Abstract
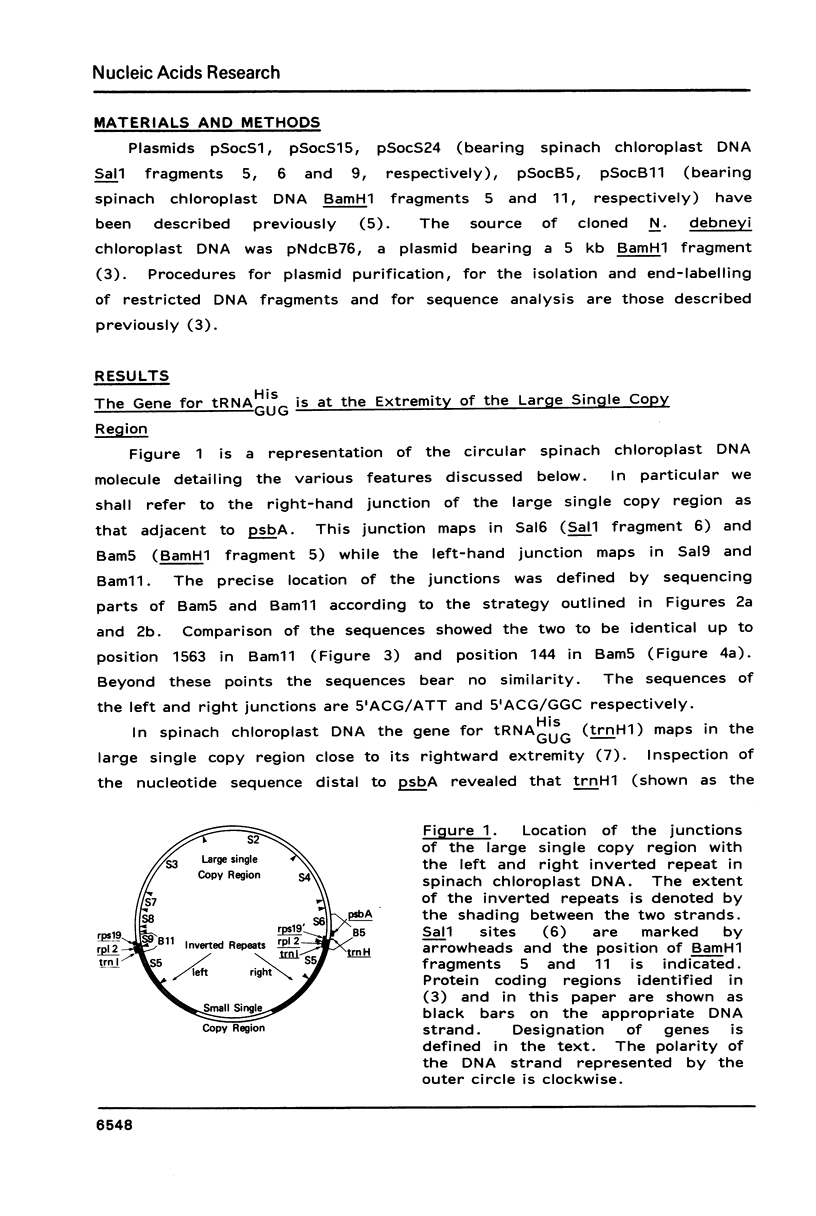
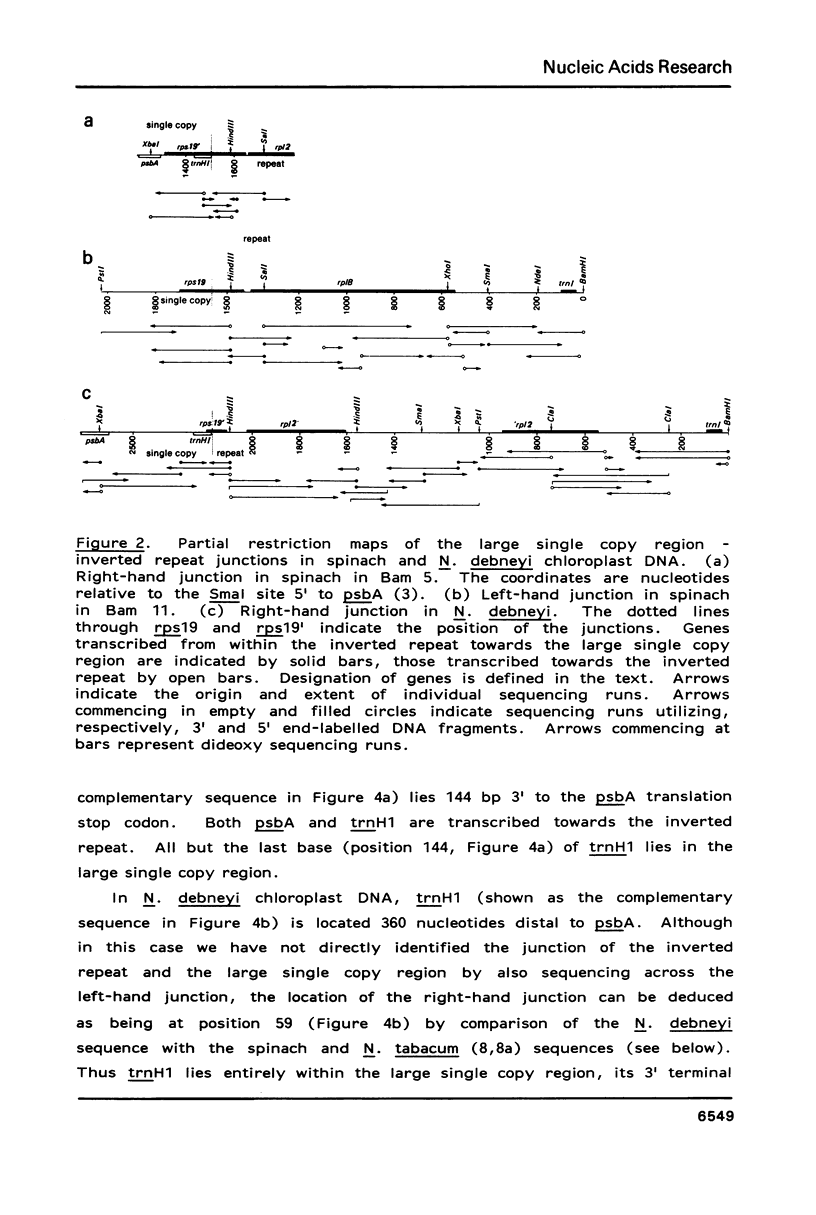
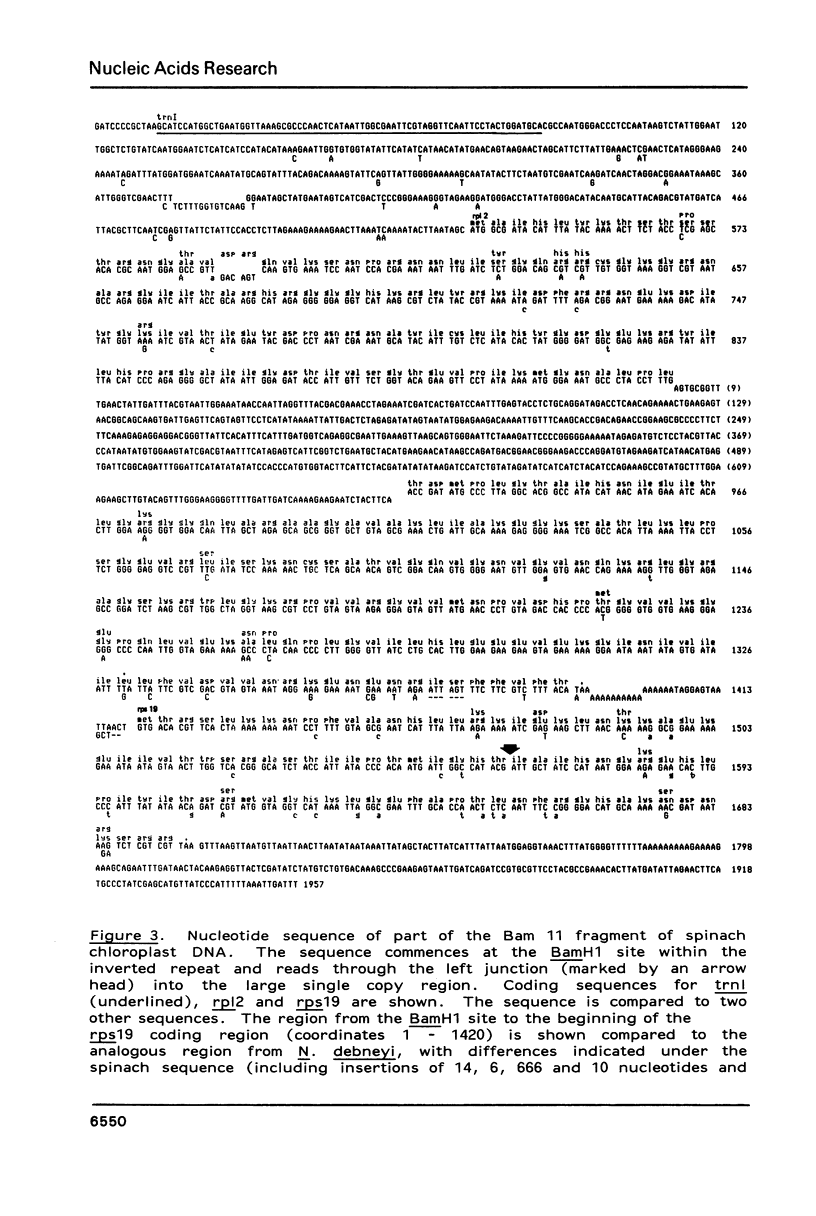
This work describes the organization, at the nucleotide sequence level, of genes flanking the junctions of the large single copy regions and the inverted repeats of Spinacia oleracea (spinach) and Nicotiana debneyi chloroplast DNAs. In both genomes, trnH1, the gene for tRNA-His(GUG) is located at the extremity of the large single copy region 3' to psbA, the gene for the 35 kd Photosystem 2 protein. Both psbA and trnH1 are transcribed towards the inverted repeat. In spinach, the first 48 codons of rps19, the gene for the chloroplast ribosomal protein S19, lie in the inverted repeat and the last 44 codons lie in the large single copy region at the end opposite to that carrying trnH1. The gene for a protein homologous to the E. coli ribosomal protein L2, rp12, is in the inverted repeat immediately 5' to rps19 and, like rps19, is transcribed towards the large single copy region. In N. debneyi, but not in spinach, rp12 is interrupted by a 666 bp insertion. The gene for tRNA-lle(CAT), trnl1, is located in the inverted repeats of spinach and N. debneyi, 5' to rp12 and is transcribed in the same direction as rp12.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorne A. M., Eneas-Filho J., Heizmann P., Mache R. Comparison of ribosomal proteins of chloroplast from spinach and of E. coli. Mol Gen Genet. 1984;193(1):129–134. doi: 10.1007/BF00327425. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Speirs J., Bohnert H. J. Spinach chloroplast mRNA for a 32 000 dalton polypeptide: size and localization on the physical map of the chloroplast DNA. Biochim Biophys Acta. 1980 Dec 11;610(2):297–310. doi: 10.1016/0005-2787(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Herrmann R. G., Whitfeld P. R., Bottomley W. Construction of a SalI/PstI restriction map of spinach chloroplast DNA using low-gelling-temperature-agarose electrophoresis. Gene. 1980 Jan;8(2):179–191. doi: 10.1016/0378-1119(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Kashdan M. A., Dudock B. S. The gene for a spinach chloroplast isoleucine tRNA has a methionine anticodon. J Biol Chem. 1982 Oct 10;257(19):11191–11194. [PubMed] [Google Scholar]
- Koller B., Delius H. Intervening sequences in chloroplast genomes. Cell. 1984 Mar;36(3):613–622. doi: 10.1016/0092-8674(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Koller B., Gingrich J. C., Stiegler G. L., Farley M. A., Delius H., Hallick R. B. Nine introns with conserved boundary sequences in the Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. Cell. 1984 Feb;36(2):545–553. doi: 10.1016/0092-8674(84)90247-2. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Archer R., Zengel J. M. Transcription of the S10 ribosomal protein operon is regulated by an attenuator in the leader. Cell. 1983 May;33(1):241–248. doi: 10.1016/0092-8674(83)90353-7. [DOI] [PubMed] [Google Scholar]
- Montandon P. E., Stutz E. Nucleotide sequence of a Euglena gracilis chloroplast genome region coding for the elongation factor Tu; evidence for a spliced mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5877–5892. doi: 10.1093/nar/11.17.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. J., Richardson C. B., Gillham N. W., Boynton J. E. Sites of synthesis of chloroplast ribosomal proteins in Chlamydomonas. J Cell Biol. 1983 May;96(5):1451–1463. doi: 10.1083/jcb.96.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Z., Jolly S. O., Steinmetz A. A., Bogorad L. Overlapping divergent genes in the maize chloroplast chromosome and in vitro transcription of the gene for tRNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3423–3427. doi: 10.1073/pnas.78.6.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann A., Stutz E. Nucleotide sequence of soybean chloroplast DNA regions which contain the psb A and trn H genes and cover the ends of the large single copy region and one end of the inverted repeats. Nucleic Acids Res. 1983 Oct 25;11(20):7157–7167. doi: 10.1093/nar/11.20.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G. L., Matthews H. M., Bingham S. E., Hallick R. B. The gene for the large subunit of ribulose-1,5-bisphosphate carboxylase in Euglena gracilis chloroplast DNA: location, polarity, cloning, and evidence for an intervening sequence. Nucleic Acids Res. 1982 Jun 11;10(11):3427–3444. doi: 10.1093/nar/10.11.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R., Steinmetz A., Bogorad L. Maize chloroplast DNA encodes a protein sequence homologous to the bacterial ribosome assembly protein S4. Nucleic Acids Res. 1983 Aug 11;11(15):5277–5286. doi: 10.1093/nar/11.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Sugiura M. A putative gene of tobacco chloroplast coding for ribosomal protein similar to E. coli ribosomal protein S19. Nucleic Acids Res. 1983 Mar 25;11(6):1913–1918. doi: 10.1093/nar/11.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi M., Wittmann H. G. Primary structure of protein S19 from the small ribosomal subunit of Escherichia coli. FEBS Lett. 1978 Apr 15;88(2):227–230. doi: 10.1016/0014-5793(78)80180-x. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]



