Abstract
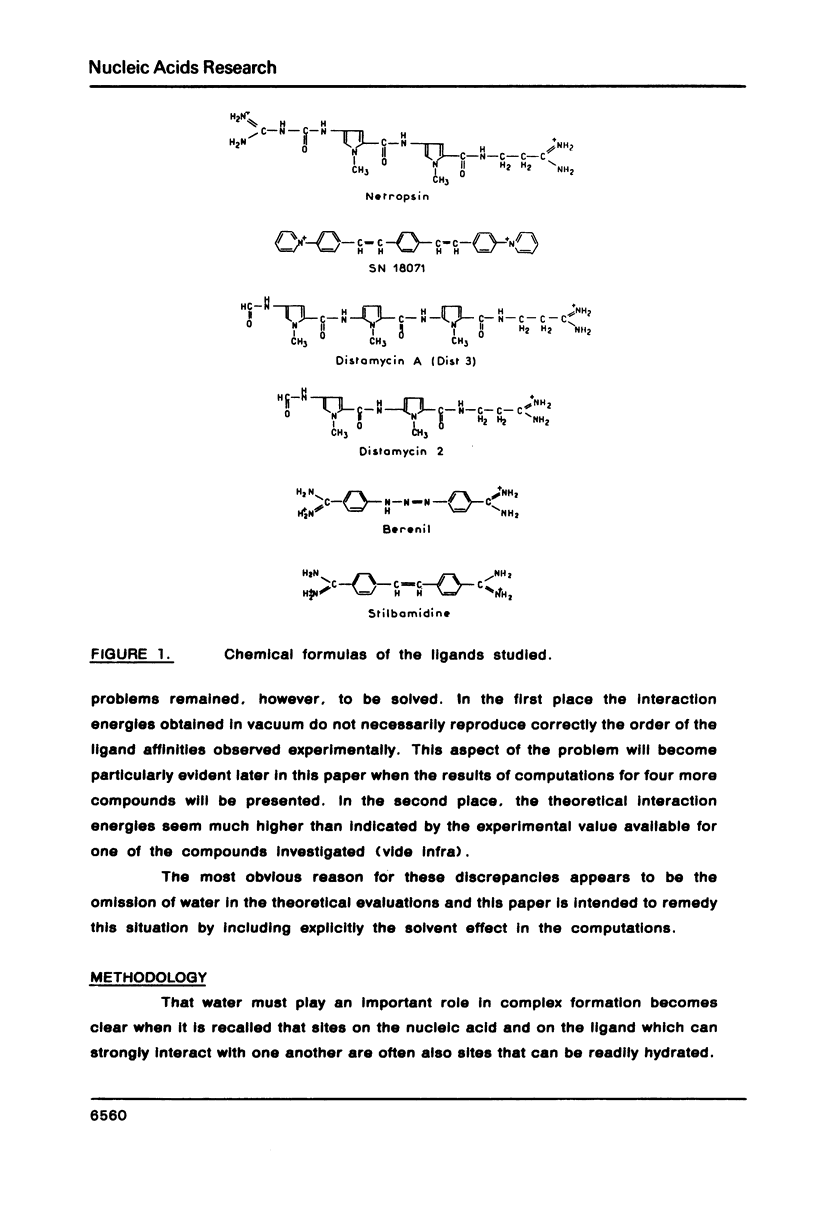
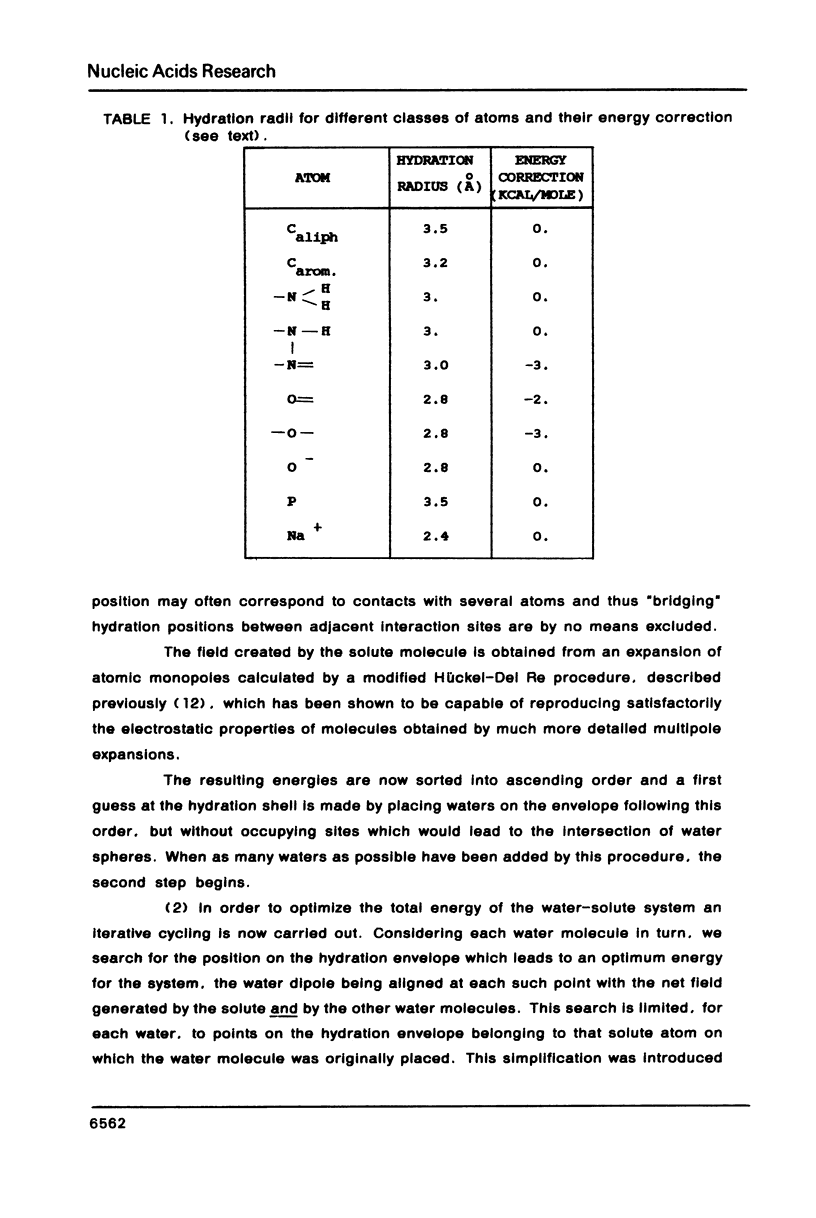
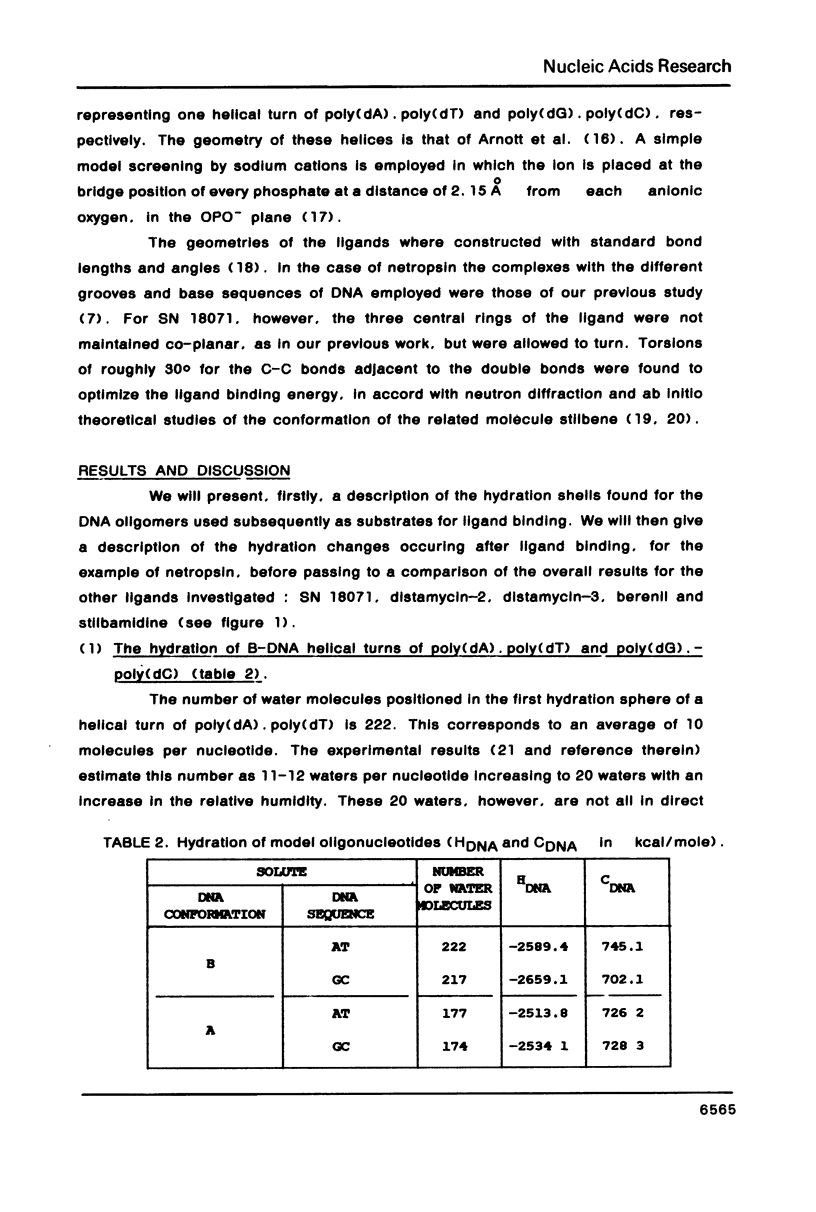
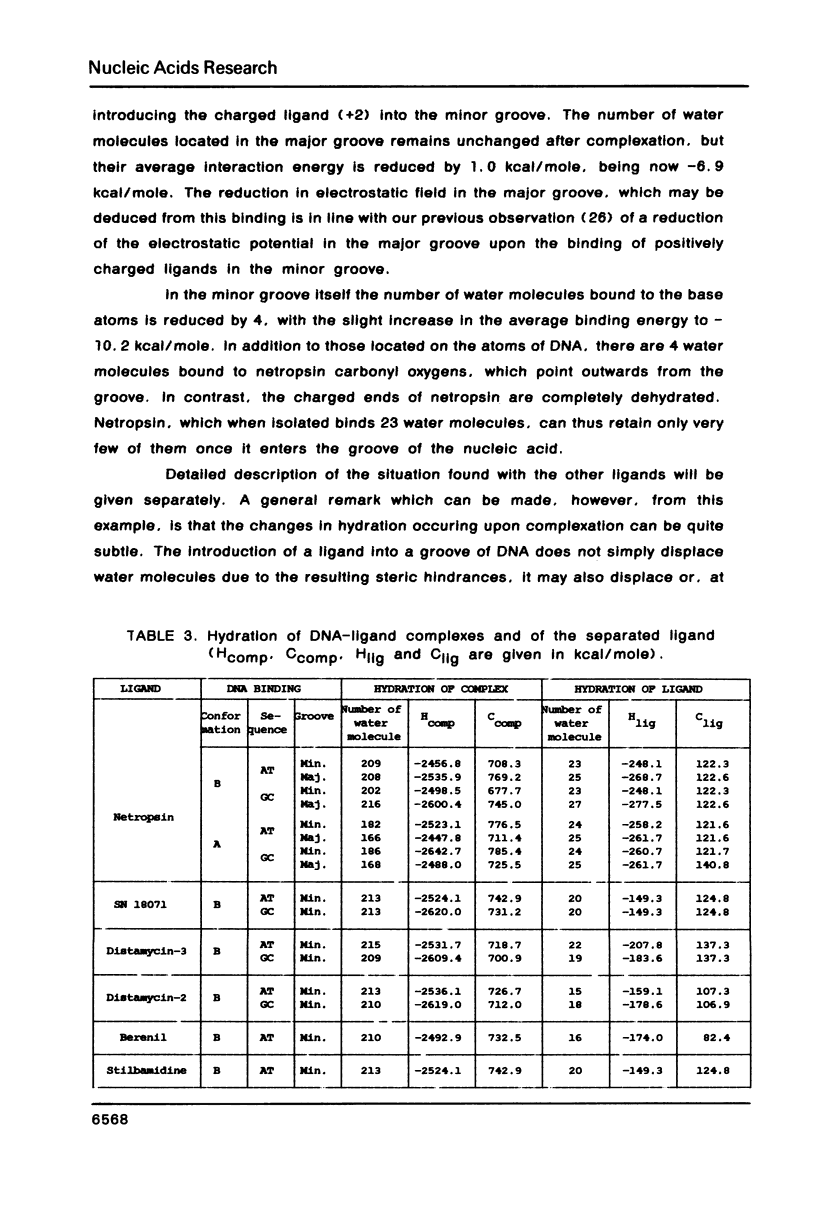
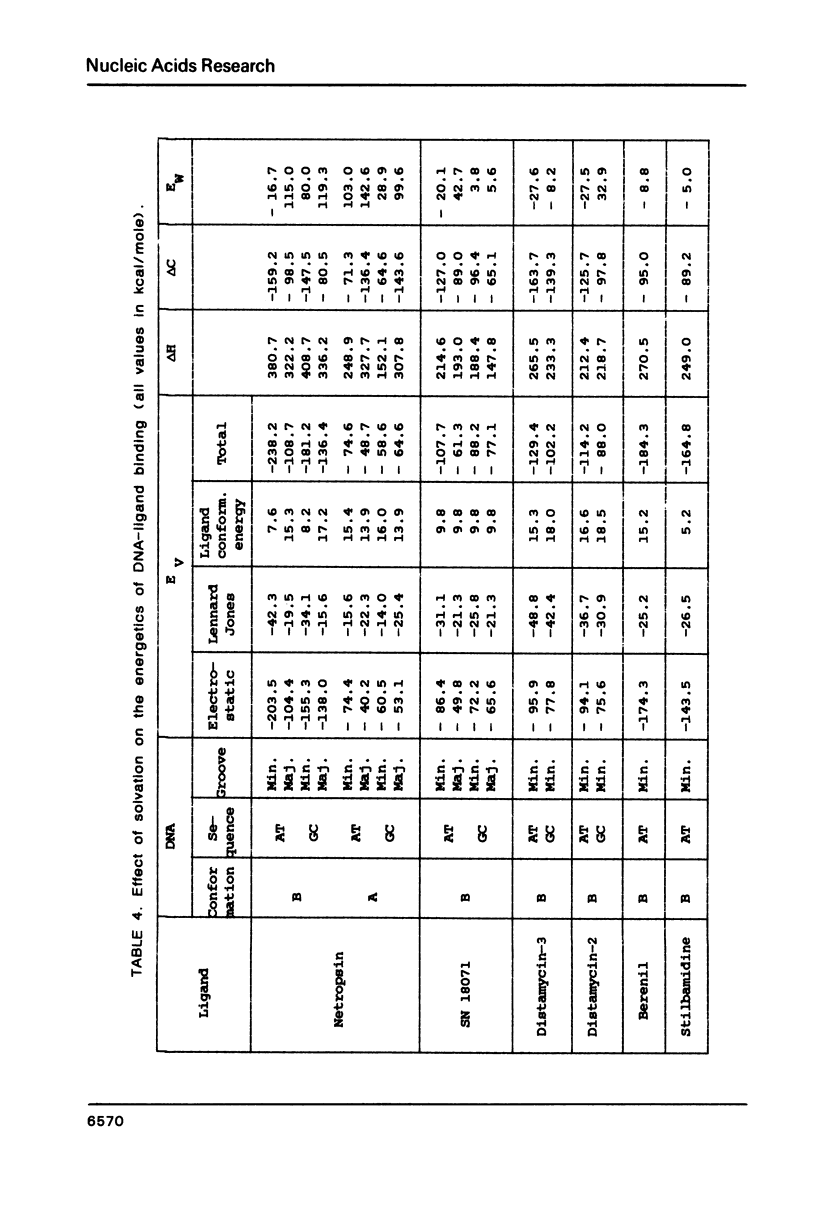
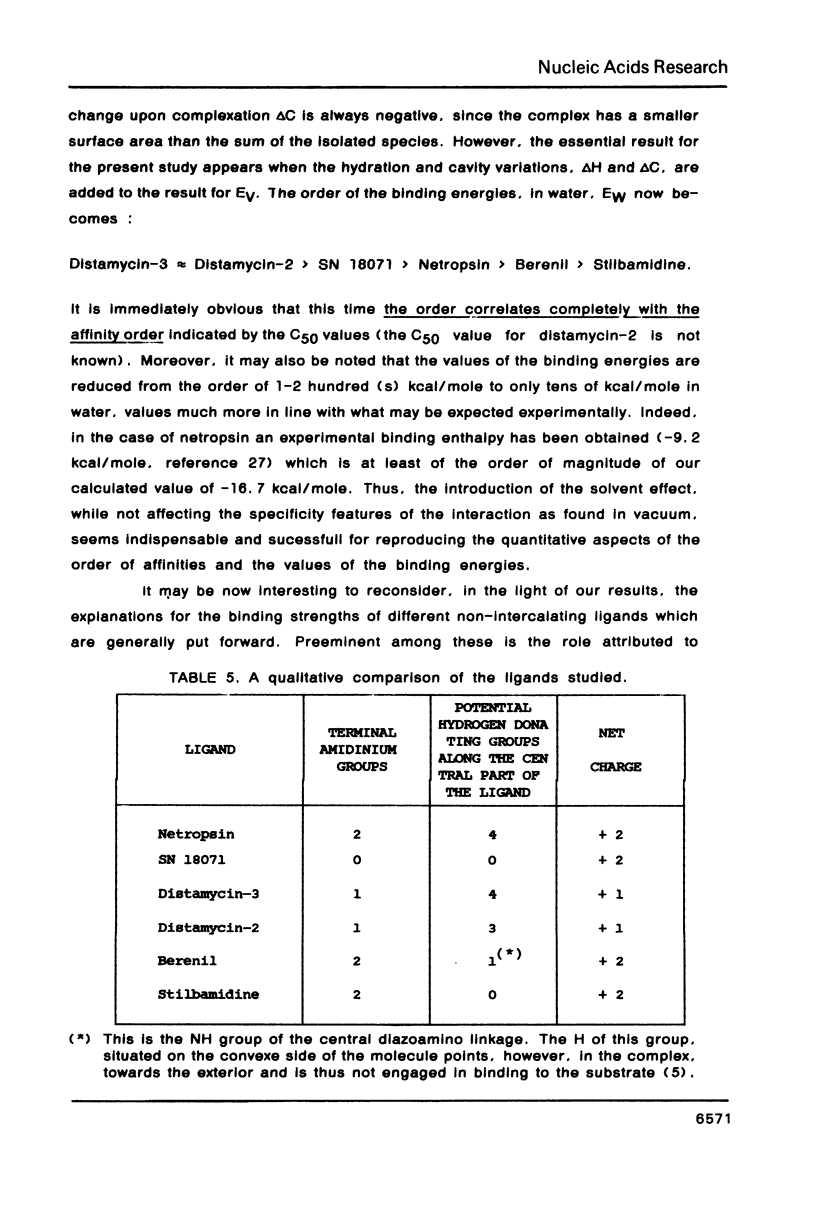
The influence of the solvent on the binding energies to DNA of six non-intercalating antibiotics - netropsin, distamycin-3, distamycin-2, SN 18071, berenil and stilbamidine - is evaluated by combining the effect of the first hydration shell with that of bulk water. The first effect is computed by a methodology based on a spherical/point dipole model of water and limited to electrostatic interaction energies. Hydration shells are obtained which are energy optimized with respect to both water-solute and water-water interactions for the complexes and for the isolated DNA oligomers and ligands. The method allows even very large complexes to be studied in reasonable computation times. The second effect is introduced via a cavity treatment. It is shown that if the vacuum interaction energies already predict correctly the preference of the ligands for the minor groove of AT sequences of B-DNA, the introduction of the solvation effect is indispensable for reproducing the order of affinity of the ligands and for bringing the values of the complexation energies into close agreement with experimental data.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Baguley B. C. Nonintercalative DNA-binding antitumour compounds. Mol Cell Biochem. 1982 Apr 2;43(3):167–181. doi: 10.1007/BF00223008. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Fratini A. V., Drew H. R., Dickerson R. E. Ordered water structure around a B-DNA dodecamer. A quantitative study. J Mol Biol. 1983 Jan 5;163(1):129–146. doi: 10.1016/0022-2836(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Blumenfeld K. S., Breslauer K. J. Calorimetric and spectroscopic investigation of drug-DNA interactions. I. The binding of netropsin to poly d(AT). Nucleic Acids Res. 1983 May 11;11(9):2857–2870. doi: 10.1093/nar/11.9.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska K., Lavery R., Pullman B. Theoretical studies of the selective binding to DNA of two non-intercalating ligands: netropsin and SN 18071. Nucleic Acids Res. 1983 Dec 20;11(24):8825–8839. doi: 10.1093/nar/11.24.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska K., Pullman B. A theoretical evaluation of the effect of netropsin binding on the reactivity of DNA towards alkylating agents. Nucleic Acids Res. 1983 Dec 20;11(24):8841–8845. doi: 10.1093/nar/11.24.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C. Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1975;15(0):285–318. doi: 10.1016/s0079-6603(08)60122-1. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G., Birch-Hirschfeld E., Weiss R., Arcamone F., Guschlbauer W. Chain length-dependent association of distamycin-type oligopeptides with A X T and G X C pairs in polydeoxynucleotide duplexes. Biochim Biophys Acta. 1983 Oct 13;741(1):15–22. doi: 10.1016/0167-4781(83)90004-0. [DOI] [PubMed] [Google Scholar]



