Abstract
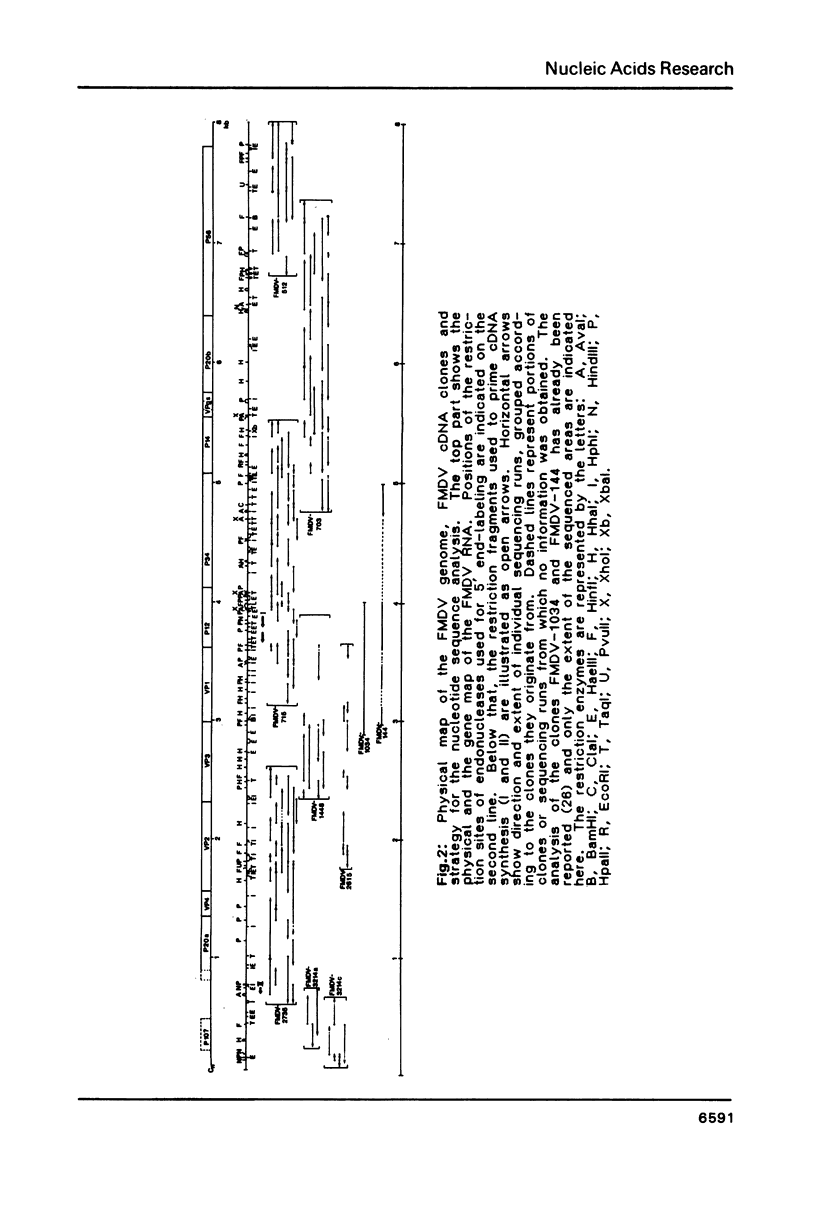
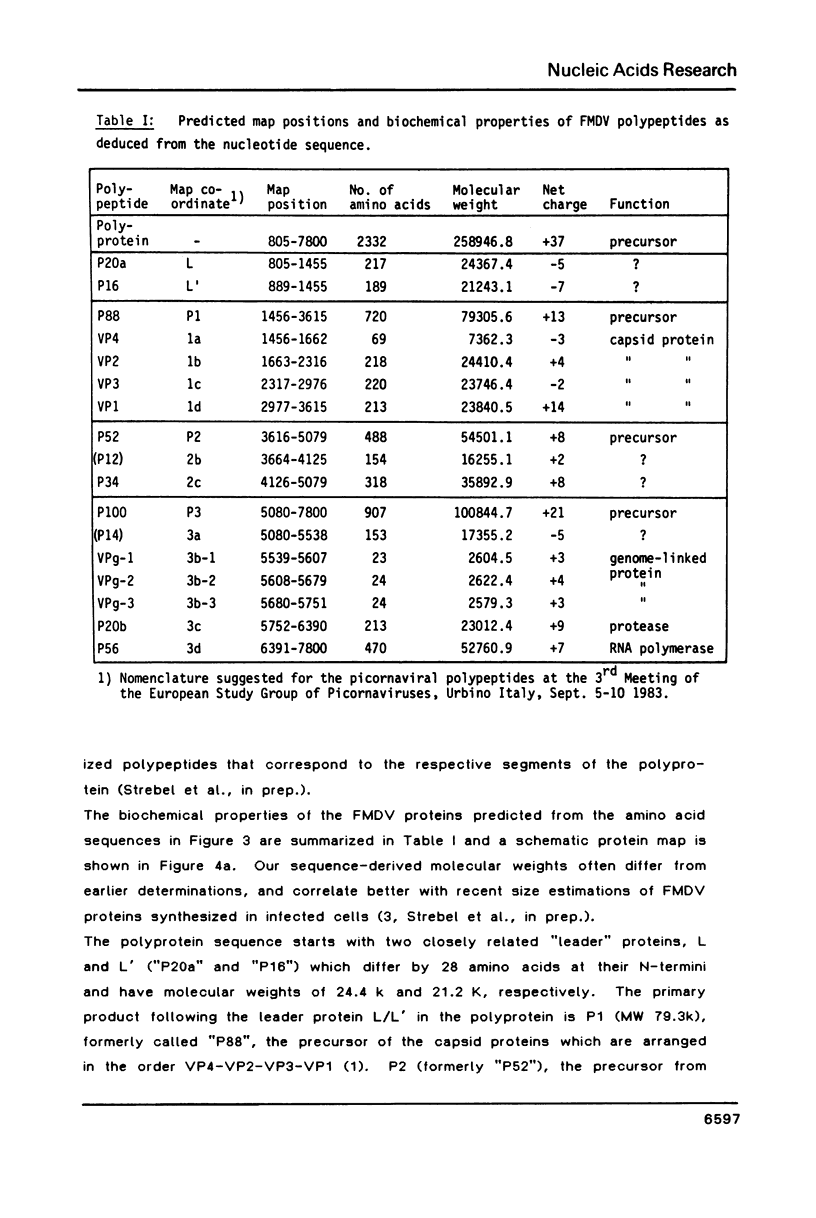
A continuous 7802 nucleotide sequence spanning the 94% of foot and mouth disease virus RNA between the 5'-proximal poly(C) tract and the 3'-terminal poly(A) was obtained from cloned cDNA, and the total size of the RNA genome was corrected to 8450 nucleotides. A long open reading frame was identified within this sequence starting about 1300 bases from the 5' end of the RNA genome and extending to a termination codon 92 bases from its polyadenylated 3' end. The protein sequence of 2332 amino acids deduced from this coding sequence was correlated with the 260 K FMDV polyprotein. Its processing sites and twelve mature viral proteins were inferred from protein data, available for some proteins, a predicted cleavage specificity of an FMDV encoded protease for Glu/Gly(Thr, Ser) linkages, and homologies to related proteins from poliovirus. In addition, a short unlinked reading frame of 92 codons has been identified by sequence homology to the polyprotein initiation signal and by in vitro translation studies.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Feil G., Strohmaier K. The molecular basis of the antigenic variation of foot-and-mouth disease virus. EMBO J. 1983;2(4):555–559. doi: 10.1002/j.1460-2075.1983.tb01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Forss S., Strebel K., Cattaneo R., Feil G. Structure of the FMDV translation initiation site and of the structural proteins. Nucleic Acids Res. 1983 Nov 25;11(22):7873–7885. doi: 10.1093/nar/11.22.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Rowlands D. J., Clarke B. E. The complete nucleotide sequence of the RNA coding for the primary translation product of foot and mouth disease virus. Nucleic Acids Res. 1984 Mar 12;12(5):2461–2472. doi: 10.1093/nar/12.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Forss S., Schaller H. A tandem repeat gene in a picornavirus. Nucleic Acids Res. 1982 Oct 25;10(20):6441–6450. doi: 10.1093/nar/10.20.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Ansorge W. Improvements of DNA sequencing gels. Anal Biochem. 1981 Aug;115(2):450–457. doi: 10.1016/0003-2697(81)90031-2. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., Robson K. H., Brown F. A study of the level of nucleotide sequence conservation between the RNAs of two types serotypes of foot-and-mouth disease virus. J Gen Virol. 1980 Oct;50(2):403–418. doi: 10.1099/0022-1317-50-2-403. [DOI] [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz C., Forss S., Küpper H., Strohmaier K., Schaller H. Nucleotide sequence and corresponding amino acid sequence of the gene for the major antigen of foot and mouth disease virus. Nucleic Acids Res. 1981 Apr 24;9(8):1919–1931. doi: 10.1093/nar/9.8.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H., Keller W., Kurz C., Forss S., Schaller H., Franze R., Strohmaier K., Marquardt O., Zaslavsky V. G., Hofschneider P. H. Cloning of cDNA of major antigen of foot and mouth disease virus and expression in E. coli. Nature. 1981 Feb 12;289(5798):555–559. doi: 10.1038/289555a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Osterburg G., Glatting K. H., Sommer R. Computer programs for the analysis and the management of DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):207–216. doi: 10.1093/nar/10.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. In vitro synthesis and assembly of picornaviral capsid intermediate structures. J Virol. 1982 Dec;44(3):900–906. doi: 10.1128/jvi.44.3.900-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Kirby E. M., Janda M. R., Drake N. L., Duke G. M., Potratz K. F., Collett M. S. The nucleotide and deduced amino acid sequences of the encephalomyocarditis viral polyprotein coding region. Nucleic Acids Res. 1984 Mar 26;12(6):2969–2985. doi: 10.1093/nar/12.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock S. L., McIver C. M., Monahan J. J. Transformation of E. coli using homopolymer-linked plasmid chimeras. Biochim Biophys Acta. 1981 Sep 28;655(2):243–250. doi: 10.1016/0005-2787(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Robertson B. H., Morgan D. O., Moore D. M., Grubman M. J., Card J., Fischer T., Weddell G., Dowbenko D., Yansura D. Identification of amino acid and nucleotide sequence of the foot-and-mouth disease virus RNA polymerase. Virology. 1983 Apr 30;126(2):614–623. doi: 10.1016/s0042-6822(83)80017-8. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Black D. N., Rowlands D. J., Harris T. J., Brown F. Location of the initiation site for protein synthesis on foot-and-mouth disease virus RNA by in vitro translation of defined fragments of the RNA. J Virol. 1980 Jan;33(1):59–68. doi: 10.1128/jvi.33.1.59-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangar D. V. The replication of picornaviruses. J Gen Virol. 1979 Oct;45(1):1–13. doi: 10.1099/0022-1317-45-1-1. [DOI] [PubMed] [Google Scholar]
- Strohmaier K. The N-terminal sequence of three coat proteins of foot-and-mouth disease virus. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1640–1645. doi: 10.1016/0006-291x(78)91191-9. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]


