Abstract
The larynx is the "bottleneck" of the human airway. For this reason, the effects of stenosing laryngeal pathologies on the vital factor respiratory gas exchange are particularly critical.
Internal stabilization is a prerequisite for recovery of the laryngeal respiratory function in severe forms of inspiratory collapse (laryngomalacia). Effective laser surgery techniques have been developed to this end in recent years.
Glottis-dilating surgery in cases of bilateral vocal cord motion impairment is now moving in the direction of endoscopic laser cordotomy or cordectomy, whereas arytenoidectomy and open surgical procedures are now used only rarely due to higher secondary morbidity rates. In individual cases, in particular if functional recovery is expected, temporary laterofixation of a vocal cord using an endoscopic suturing technique can be a helpful approach.
Extensive laryngeal defects can be covered by means of composite grafts with mucosal lining, a supporting skeleton and their own vascularization. Autologous transplantation of the larynx, with its complex surgical and immunological problems, has become a manageable procedure. The problems of post-transplantation reinnervation and risk assessment of immunosuppression-induced recurrence of the tumor are still under consideration.
Reanimation of the bilaterally paralyzed larynx by means of neurorrhaphy (neurosuture), neural grafting and, more recently, functional electrostimulation (pacemaker) represents a challenge for the coming years. In most cases of paralysis of the recurrent laryngeal nerve, a part of the muscles is maintained by synkinetic reinnervation when therapy is carried out, which however also prevents effective vocal cord movement due to simultaneous activity of agonists and antagonists. Modulation of reinnervation by means of electrostimulation and modern genetic therapy approaches justify hopes of better outcomes in the future.
Keywords: larynx, malacia, vocal cord paralysis/surgery, reconstruction, transplantation, reinnervation, pacemaker
Abstract
Der Kehlkopf stellt hinsichtlich des Atemwegsquerschnittes den Flaschenhals des menschlichen Atemweges dar. Aus diesem Grund wirken sich stenosierende Kehlkopferkrankungen in besonderem Maße auf den lebenswichtigen Atemgasaustausch aus.
Die Wiederherstellung der Atemfunktion des Larynx erfordert bei schweren Formen des inspiratorischen Kollapses (Laryngomalazie) die innere Stabilisierung. In den letzten Jahren sind hierzu wirksame Verfahren der laserchirurgischen Intervention entwickelt worden.
Die Glottis erweiternde Chirurgie bei bilateraler Immobilität der Stimmlippen zeigt einen Trend zur endoskopischen laserchirurgischen Chordotomie bzw. Chordektomie, während die Arytänoidektomie und offen chirurgische Verfahren wegen stärkerer Sekundärmorbidität nur noch selten zum Einsatz kommen. Im Einzelfall, insbesondere bei absehbarer Funktionswiederkehr, kann die temporäre Laterofixation einer Stimmlippe durch eine endoskopische Nahttechnik hilfreich sein.
Ausgedehnte Kehlkopfdefekte können mit Composite grafts, die über eine Schleimhautauskleidung, ein Stützskelett und eine eigene Gefäßversorgung verfügen, gedeckt werden. Die autologe Transplantation des Kehlkopfes ist mit ihren komplexen chirurgischen und immunologischen Problemen beherrschbar geworden. Probleme der Reinnervation nach Transplantation und der Abwägung des Risikos eines durch die Immunsuppression induzierten Tumorrezidivs sind noch Gegenstand weiterer Untersuchungen.
Die Reanimation des bilateral gelähmten Kehlkopfes durch Nervennaht, durch Nerventransfer und neuerdings durch funktionelle Elektrostimulation (Pacemaker) stellt die Herausforderung für die nächsten Jahre dar. Bei der überwiegenden Zahl der Rekurrensparesen liegt zum Zeitpunkt der Therapie eine synkinetische Reinnervation vor, die einen Teil der Muskulatur erhält, aber durch gleichzeitige Aktivität von Agonisten und Antagonisten eine effektive Stimmlippenbewegung verhindert. Die Modulation der Reinnervation durch Elektrostimulation und moderne Ansätze der Gentherapie lassen ein zukünftig besseres Outcome erwarten.
1. Respiratory Function of the Larynx
"Breathing is a double blessing:
Drawing air, and its release;
First beset, then comes refreshment;
The wonders of this life are mixed..."
Johann Wolfgang v. Goethe [1] (Fig. 1)
Figure 1. Noura El-Kordy, Breath, Pastell on paper, 50 x 60 cm, 2002.
The normal function of the human larynx not only produces vocalization and affords aspiratory protection, it also ensures the free passage of the respiratory gases [2], [3]. The glottis, as a physiological narrowing of the airway [4], [5], [6], can adapt its opening cross-section dynamically to various respiratory situations. A healthy larynx ensures correctly timed closure of the central airways during coughing, lifting or applying abdominal pressure [7].
Congenital and acquired stenosis of the laryngeal lumen and bilateral glottic motility impairments affect respiratory function profoundly, as do inspiratory collapse states.
The objective of successful laryngeal reconstruction procedures in terms of respiratory function is recovery of sufficient transglottic respiratory gas passage.
The spectrum of reconstructive procedures is broad, including stabilization of collapsed segments and dilation or reconstruction of stenotic laryngeal segments as well as organ transplants, reinnervation and remobilization of the paralyzed larynx. Vollrath's paper, delivered in the 1999 discussion report of the German Society for ENT Medicine, Head and Neck Surgery, provides an overview of all relevant causes of laryngotracheal stenoses in children [8]. The focus of the following text is a summary of the current therapeutic options for young and adult patients with the emphasis on reconstructive methods.
2. Stabilization
In respiration, the larynx is subjected to alternating pressure. The negative inspiratory pressure may result in soft tissue being sucked into the laryngeal lumen. Glottis and subglottis are protected from inspiratory collapse by the thyroid and cricoid cartilages. However, inspiratory instability attains to clinical significance in the area of the supraglottis. Neonates and small children up to up to 12 months of age are affected most frequently [9], [10], [11], [12], [13], [14], [15]. Jackson first used the current term laryngomalacia [16] for this clinical picture, which used to be designated in terms of its primary symptom, i.e. "congenital stridor".
Laryngomalacia may occur synchronously with a pharyngeal collapse in conjunction with obstructive sleep apnoea syndrome (OSA) [17], [18], [19]. Collapse of the supraglottis only in the area of the false vocal cords has also been observed in adults as a cause of sleep-related obstruction.
Patients in whom suction of the aryepiglottic cord or obstruction of the laryngeal opening by the epiglottis only occur during physical exertion are subsumed in Anglo-Saxon countries under the term exercise-induced laryngomalacia [20], [21], [22], [23]. This must be clearly differentiated from the clinical picture of vocal cord dysfunction (VCD), in which the airway obstruction is localized in the glottic area and a psychogenic cause is assumed [24], [25].
The term laryngomalacia suspects a softening in the sense of an immaturity of the laryngeal skeleton. If this assumption were true, premature infants would be expected to show a higher incidence of laryngomalacia than full-term infants. Belmont [26] refuted this notion. The typical endoscopic picture of this condition is also observed, albeit rarely, in older children and adults [27], [28], [29]. More recent findings suggest a multifactorial process. Current histological investigations of morphological correlates to "laryngomalacia" tend to point towards submucosal oedema and lymphangiectasis [30]. The literature frequently describes induction of the mucosal oedema by a laryngitis or gastrooesophageal reflux (GER) [31], [32], [33]. The discussion concerning the contribution of reflux to the causality of chronic laryngitis is currently en vogue but has not as yet reached any conclusions [34], [35], [36], [37]. A high level of coincidence and mutual negative influence are among the undisputed contentions [26], [38], [39], [40].
It may be that the mucosal changes are only a secondary consequence of the higher level of mechanical stress applied when the supraglottis collapses upon inspiration due to a reduced muscular tone. Analogous changes have been confirmed in the area of the uvula in cases of obstructive sleep apnoea [19]. The high coincidence of neuromuscular disorders and additional airway stenoses in affected children has been confirmed [13], [41], [42], [43], [44], [45], [46]. Some authors consider that the neurological disease itself may be the cause of the laryngomalacia [26], [47], [48], [49]. The example of an 11-year-old boy with the full endoscopic picture of a laryngomalacia after a pons infarction is cited in support of this idea [50].
The prognosis of laryngomalacia cases in infancy is generally favourable. Therapeutic intervention is required in 20% of cases with pronounced stridor, inhibited gas exchange or failure to thrive [51], [52]. In milder courses, a spontaneous improvement of the symptoms is observed by the end of the second year of life in most cases. In individual cases, support in the form of continuous positive airway pressure (CPAP) may be helpful [53].
The introduction of laser surgery in treatment of laryngomalacia has been a decisive factor in reducing the number of tracheotomies required in pronounced cases [11], [14], [54], whereby in some individual cases, especially in children showing neuropathologies, tracheotomy is the sole remaining therapeutic option.
2.1 Pneumatic dilatation (CPAP)
Analogously to the procedure for pharyngeal obstruction, "pneumatic stenting" of the airway lumen in the form of maintenance of continuous positive airway pressure (CPAP) during inspiration and expiration can prevent soft-tissue collapse in the supraglottic region. In milder courses of laryngomalacia requiring only occasional airway stabilization, and in cases of combined pharyngeal collapse, such respiratory support with single-phase or bi-phase positive airway pressure (BiPAP) does represent a therapeutic option [15], [55], [56]. The phase of weaning from the respirator in particular in previously respirated children can be positively influenced by the respiratory support of CPAP [57], [58].
The efficacy of the procedure should be visualized endoscopically, since a pre-existing valvular mechanism in some patients can be exacerbated by CPAP, also worsening the symptoms.
The positioning of infants with laryngomalacia also influences the degree of airway obstruction. Reclining of the neck and avoidance of dorsal positioning reduce the risk of obstruction [59], [60]. However, if there is an existing tendency towards reflux, the ventral position must be avoided due to the risk of reflux-induced glottic spasm. Jeffery [61] sees a possible cause of sudden infant death syndrome (SIDS) in glottic spasm.
2.2 Microsurgery
The introduction of surgical intervention (supraglottoplasty) in treatment of laryngomalacia by Lane facilitated treatment of severe forms and reduced the number of cases in which a tracheotomy is considered unavoidable [51]. Current review studies confirm a positive influence of surgical intervention on airway collapse [14], [54], [62], [63]. As Vollrath also mentions, this procedure can spare parents "torturing months of waiting for spontaneous normalization of breathing" [14].
Microsurgical supraglottoplasty, later termed epiglottoplasty by Cotton et al. [64], comprises essentially the excision of excessive mucosal tissue in the area of the arytenoid cartilage, the aryepiglottic cord and the lateral epiglottis. Also described are the incision of the aryepiglottic cords and epiglottopexy. The introduction of the laser enhanced the microsurgical character of the reconstructive treatment of laryngomalacia [65], [66]. The procedure does not involve a significant risk of occlusion due to swelling and can thus be performed without tracheotomy.
In children with an additional airway stenosis or pharyngeal collapse, however, therapeutic failure is likely [15], [55]. Such a complex collapse is also known as a discoordinate pharyngolaryngomalacia (DPLM) or pharyngeal wall inspiratory collapse (PWIC), underlining the necessity of thorough diagnostic endoscopy of the entire airway [42], [67], [68], [69], [70], [71], [72].
Holinger was the first to classify the polymorphic endoscopic findings in laryngomalacia [66]. Frequent occurrence of several different coincident findings defies classification under one of the five types. The indication for therapeutic intervention in laryngomalacia is derived from a clinical score worked out by Shah, which considers medical history data (stridor, reflux, aspiration, weight development and age of the child as well as neurological status) in addition to the laryngoscopic findings [44].
The algorithm acc. to Werner [54] appears to us best suited to clinical practice, since it matches the main endoscopic finding in each case to a concrete surgical procedure (see Table 1 (Tab. 1)).
Table 1. Laryngomalazia types and therapy (Werner 2002 [54]).
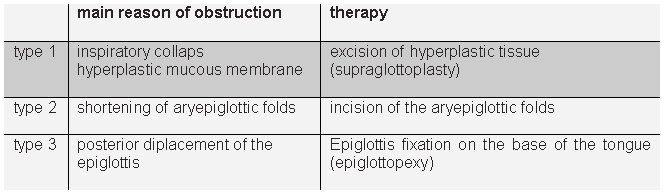
In practice, laser resection of hyperplastic mucosal segments is of greatest significance, since these tissues are the main cause of obstruction in 80% of cases [54]. Incision of shortened aryepiglottic cords and removal of enlarged cuneiform cartilage are also readily realized with laser surgery techniques [14].
Individual cases involving isolated suction of the epiglottis may necessitate its anterior fixation. Laser de-epithelialization of the median segments of the linqual epiglottis and corresponding retrolingual region as initiated by Werner, followed by wound margin suturing, facilitates a gentle epiglottopexy that is at first secured by the suture and later by the resulting synechia [11]. All three of these microlaser surgical procedures for laryngomalacia can be realized alone or in combination, depending on the specific findings.
3. Dilation
Congenital stenoses, tumors and deformities of the larynx are very rare. Since the extensive work published by Vollrath (1999) on childhood laryngotracheal stenoses [8], the literature has not revealed anything essentially new. This paper is focused on reconstructive methods applied in acquired laryngeal stenoses in children and adults.
On the one hand, the frequency of acquired glottic stenoses has dropped due to tube pressure applied in long-term translaryngeal respiration with improvement of the tube material and early laryngeal relief by tracheotomy. On the other hand, bilateral glottic motion impairment in connection with surgery of the neck and upper mediastinum remain a therapeutic challenge [73] worthy of separate consideration here.
3.1 Conventional surgery
Open laryngeal surgery methods are now of little relevance in cases of bilateral vocal cord motion impairment. In the international literature, Jackson (1922) is considered the father of glottis-dilating surgery [74]. It is less widely known that Pieniazek performed glottis surgery in bilateral vocal cord paralysis in Krakau beginning at the end of the 19th century [75]. The first endoscopic arytenoidectomy on record was performed by Thornell (1948) [76]. Laser surgery employing surgical microscopy is now the procedure of choice (see chapter 3.2).
However, conventional surgical methods are still used in treatment of acquired stenosis of the glottis and subglottis. As in laryngomalacia, the decisive indicative factor here is an accurate diagnosis. Evaluation of therapeutic success is only possible based on comparable assessments of the degree of stenosis over the course of treatment. The changes revealed by functional respiratory diagnostics in cases of low and medium-grade stenosis are not characteristic. The measured parameters only deviate significantly from normal in high-grade stenosis [77], [78], [79]. Spirometry, and in particular the flow-volume curve, is the method best suited for evaluation of respiratory function [80]. Other causes of respiratory insufficiency (secondary stenosis, obstructive bronchitis, gas exchange deficiency) overlap with the laryngeal stenosis. Isolated measurement of laryngeal airway resistance requires either an invasive procedure [81] or an existing tracheostoma [82].
A selective functional evaluation of laryngotracheal stenoses will be possible non invasive with methods of the finite element simulation. We are currently using a method of in situ measurement of airway resistance we developed independently of Wassermann [83], employing the working channel of the bronchoscope and a rhinomanometer.
The clinically established Cotton classification [84] is still the basis for endoscopic assessment of the stenosis grade, especially in cricoid stenoses in children.
In our view, however, the subjective estimation of the stenosis grade on which the severity classification is based presents problems. The estimation of stenosis grade from 50% to 71% is the basis for classification in grades I to IV. We know from our own experience that the degree of accuracy implied here is not attainable in practice. Error statistics for gastroenterological endoscopy-based ulcus and polyp size estimates are helpful here [85]: The statistics reveal deviations of up to 110 %.
Our ENDOSCAN method makes it possible to achieve objective and reproducible endoscopic measurement of a stenosis with classification according to Cotton grade and monitoring of therapeutic success [79], [86], [87], [88].
The main therapeutic options include bouginage, laryngofissure and cricotracheal resection (CTR). Chapter 4 addresses the reconstructive method. Publications by Vollrath and Schulz-Coulon [8], [89] contain an extensive and still current presentation of all conventional surgical methods used in treatment of childhood laryngeal stenoses. The principles applied to childhood cases are basically confirmed in adults as well.
In our experience, bouginage as the sole approach to stenosis treatment is also unsuitable in adults. Discussions of the dilation procedure in the literature are marked by controversy [90], [91], [92]. In general, rapid recurrence of the symptoms can be expected, as well as, in unfavourable cases, pronounced granulation due to mucosal tearing during bouginage. The combination with endoluminal laser incision results in a much larger dilation of the lumen, whereby granulation during wound healing remains a severe therapeutic problem nonetheless. Local application of corticosteroids [93], [94], growth factors [95], [96] and mitomycin C, which latter aims to reduce fibroblast proliferation by inhibiting DNA and protein synthesis and is a subject of intense current discussion in the literature [97], [98], [99], [100], [101], cannot prevent granulation in every case. Avoidance of circular mucosal defects should be a generally applied principle (star incisions).
The increase of the glottis gap achieved with dilatators usually requires stabilization by means of internal stenting. Stenting of the glottis is hardly an acceptable long-term method due to loss of voice and a tendency to aspirate. Subglottic stenting may be useful in individual cases. Problems include the risk of dislocation and the risk of secondary frictional damage to the glottis.
As a rule, definitive external surgical treatment is the only method that can promise permanent healing. The combination of bouginage and temporary stenting can, however, give the patient valuable time by delaying the necessity of tracheotomy, which can then in most cases be performed after 6 months when the submucosal inflammatory reaction has abated [102], [103]. Stent tolerance, the individual surgical risk, as well as patient compliance and willingness to undergo surgery, are among the factors that influence the decision to perform definitive interventional surgery.
Among open surgical methods used in treatment of acquired laryngeal stenoses there is a trend towards resection, even including removal of parts of the cricoid cartilage within the framework of cricotracheal resection [104]. This development is explained by the much shorter therapeutic periods and good results obtained with this approach. The risk of damage to the recurrent laryngeal nerve can be kept to a minimum, even in CTR, by knowledge of the anatomical course of this nerve in the area of the cricothyroid joint and use of intraoperative neural monitoring [105]. To prevent recidivation of the stenosis, the resection procedure must be carried out decisively so as to facilitate anastomosis of healthy mucosa. The calibre difference between the trachea and subglottic lumen can be balanced out in CTR by means of an angled incision in the segment with the smaller lumen [106].
Temporary stenting of the anastomosis is not usually required. In adults, the lumen to be anastomosed no longer has a round cross-section (as do the available stents). For this reason alone, use of a stent reduces the effective airway cross-section and presents a risk of secondary mucosal damage. Current animal experiments corroborate these experiential clinical data [107].
3.2 Laser surgery
Scar flaps and adhesions (synechias) in the glottic region are the domain of laser surgery [108], [109]. Problems are still caused by synechias in the area of the anterior commissure. The risk of recurrence is high, besides which the loci of these findings are not always readily accessible with the CO2 laser device.
To help prevent recurrence, direct contact with non-epithelialized wound surfaces must be avoided. An intervening plastic quill inserted through a laryngofissure has been the gold standard. Now, slitting of the stenosis with transoral introduction of a separating foil is reported as an outpatient procedure [109]. In such cases, we use a silicone foil, held in the anterior commissure by means of a transcutaneous fixation suture. The effectiveness of local mitomycin application in reducing excessive granulation [101] and mucosal patch transplantation to cover the epithelial defect [110], [111] cannot yet be assessed with any finality. Fixation of the small mucosal islands by means of laser welding is certainly an interesting innovation [112].
Anterior scar flaps and synechias that cannot be sharply focused in microlaryngoscopy [113], [114], [115] can be clearly imaged by means of endoscopy under local anaesthesia [116], [117] or general anaesthesia through a laryngeal mask [118], [119], [120], [121]. If the locus is accessible with a laser fibre pushed through the working channel of the endoscope, a KTP laser can reportedly be used as an alternative to the CO² laser to sever these structures [122]. We ourselves have achieved highly satisfactory results to date in 4 cases with flexible endoscopic laser ablation of scar flaps using the laryngeal mask and a diode laser.
Currently the most important field of application for laser techniques in glottis-dilating surgery is symptomatic treatment of (chronic) bilateral vocal cord motion impairment. The term, established by Kashima and Benjamin [123], [124], is important for its lack of the implied etiological interpretation, which would require further diagnostics, of the term normally used in the German literature: "bilateral paralysis of the recurrent laryngeal nerve." The parenthetically mentioned chronicity, as well as the functional reference, emphasize the necessity of treatment.
Since the publications of Ossoff and Eskew at the beginning of the eighties, use of the CO2 laser has been firmly established in surgery of bilateral vocal cord paralysis [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151]. Combined use of microscope and laser facilitates a microsurgical procedure that spares the healthy tissues of the larynx [152], [153], [154]. Compared to open surgery, postoperative laryngeal swelling and occlusion with respiratory relevance are rare, so that the procedure is normally possible without tracheotomy.
The introduction of laser surgery made possible both Thornell's arytenoidectomy and submucosal cordectomy, already familiar as an open surgical approach [128], [129], [132], [139], [143].
It is important to note that an arytenoidectomy always involves temporary aspiration [139] as well as in some cases a life-threatening risk of haemorrhage [155]. In our experience, patients at high risk for aspiration (chronic pulmonary diseases, immunosuppression, expectoration deficit) should not be considered for this procedure. Examination of axial CT section of the larynx at level of the glottis reveals that the arytenoid cartilage, ideally shown in some surgical textbooks as extending into the glottic lumen, in fact usually rests on the cricoid cartilage, whereby only the processus vocalis extends into the lumen of the cricoid cartilage. No wonder Eckel's morphometric examinations concluded that vocal cord resection increases the glottic cross-section more than removal of the arytenoid cartilage by a factor of 4 [5].
The current trend towards favouring vocal cord intervention procedures is understandable on the basis of these observations. The best-known method, posterior cordectomy, was developed by Dennis and Kashima [130]. The authors achieved an atraumatic glottis dilatation in the initial cases by means of a C-shaped resection in the posterior quarter of the vocal cord - originally without exposing the processus vocalis. However, their first publication of the method also mentioned the option of treating the opposite side as well after a given interval if improvement was insufficient. The same effect can be achieved by transverse cordotomy according to Kashima [123] or the separation of the vocal cord from the processus vocalis designated by Rontal and Rontal as muscular tenotomy [156]. Relaxation of the vocal cord results in a comparable excavation of the posterior vocal cord with a favourable approximation of the vocal cords in the anterior segment. This finding in particular is interpreted as an advantage of the method as regards the preservation of vocal quality. In practical terms, however, successful posterior dilation results in a phonatory air loss with typical aspiration and shortened tone duration [157].
In our experience, the posterior excavation situation as described is a brief episode. During the first two weeks following surgery, fibrin layers can form so as to restore, or even exacerbate, the respiratory situation as compared to preoperative status. If the effects are relevant in respiratory terms, these layers must be removed and patients require a close monitoring during this phase. In the further course of healing, shrinkage of the wound surface results in a favourable blunt pull on the vocal cord, which had initially deviated in the anterior direction, finally resulting in a scarified fixation of the vocal cord in a more lateral position. If the opening angle thus achieved is large enough, a satisfactory balance between respiratory and vocal functionality is usually achieved. The literature describes numerous variants of the Dennis-Kashima technique [136], [138], [140], [141], [158] involving enlargement of the incision or excision to enhance the effect of the operation. In the median arytenoidectomy described by Crumley (involvement of the processus vocalis in the posterior excision), the option of a bilateral procedure to counter insufficient efficacy is mentioned [136]. Such an assessment would suggest the insufficiency of the unilateral surgery in bilateral vocal cord paralysis.
Further variants that also fail to provide a satisfactory solution include complete arytenoidectomy and posterior cordectomy [159], as well as subtotal arytenoidectomy with incision of the posterior vocal cord and the vestibular ligament [147].
An approach that has received less attention in clinical practice to date is submucosal cordectomy using laser surgery techniques [128], [129], [132], [139], [143], [145]. This method, repeatedly recommended in Germany by Eckel, follows up on the good results obtained with classic open surgery according to Hoover [160] and resection of the vocal cord muscle performed endoscopically by Kleinsasser in combination with arytenoidectomy, preserving the covering mucosal layer [161], [162] and adding the advantages of modern microlaser surgery. In the morphometric studies mentioned above, Eckel demonstrated that a much greater degree of glottis dilatation can be achieved with submucosal resection of one vocal cord than with posterior cordotomy or arytenoidectomy. The clinical results obtained by Eckel and Reker confirm this theory. Tone duration is shortened compared to results obtained with posterior cordectomy [157]. This observation should, however, be considered evidence of a better glottis-dilating effect. Dysphagia and aspiration, as seen with arytenoidectomy, were not observed.
Presumably, it is the predominant opinion that the anterior portions of the glottis are the key loci for phonation that makes it difficult to accept a non-carcinomic indication for glottis-dilating surgery with involvement of parts of the anterior vocal cord. Phonation requires a tensing of the vocal cord, analogous to the string of a musical instrument. In our view, resection of a muscle insert by removing the arytenoid cartilage or in posterior cordectomy / tenotomy will render harmonic vibration of the vocal cord impossible. Studies by Jiang and Titze with animal models focusing on the canine hemilarynx [163] have confirmed that an intact connecting zone between the vocal cord and arytenoid cartilage is just as important as the status of the ligamentous structures. A review of voice quality results post oncologically indicated cordectomy [164], [165], [166] clearly shows that anterior resection, with formation of a cicatrized neoglottis, can also provide a basis for an acceptable level of residual vocal function. If the objective of surgery in bilateral vocal cord paralysis is a pronounced improvement in respiratory function, this may justify extending laser resection of the vocal cord in the anterior direction. In contrast to tumor surgery, the epithelial cover of the vocal cord can be spared for the most part in such cases, thus reducing the risk of synechias, whereby at the same time vocal quality will tend to suffer more from aspiration through the open glottis.
3.3 Suturing techniques
The suturing technique familiar from open surgery of lateralization of the vocal cord was initially also applied combination with endoscopic glottic dilation. Kirchner used the suturing technique in addition to electrosurgical ablation of the edge of the vocal cord [167] and Remsen used suturing to stretch the residual vocal fold laterally following subligamentous resection of the vocal cord tissue [129].
Lichtenberger took a different course entirely. He worked out the principle of reversible endo-extralaryngeal laryngomicrosurgical lateralization (REExL), whereby lateralization of the vocal cord is realized by means of one or more suture loops spanning the mucosa of the posterior segment of the vocal cord and the thyroid cartilage [168], [169], [170], [171], [172], [173], [174].
This technically complex suturing procedure above and below the vocal cord, and from endolaryngeal towards the periphery, is facilitated by a suture needle holder of his own devising [168]. In principle, the same suturing technique can also be realized using atraumatic suture material and a long, straight needle or a lumbar puncture needle as a trocar, as currently described by Mathur et al. [175].
The decisive advantage of this procedure is its reversibility, giving it a special place among the therapeutic options reviewed here. Whereas the methods described up to this point can only be applied following a sufficient recovery period to allow for functional recovery of the recurrent laryngeal nerve or early assessment of vocal cord motion impairment as permanent, a reversible laterofixation can be performed without delay.
Both of the preconditions for irreversible surgical procedures are fraught with practical difficulties: The length of the recovery period required for recovery of vocal cord function following paralysis of the recurrent laryngeal nerve varies widely from patient to patient. Whereas general practice foresees a chance of recovery within the first year, a current prospective study by Jamski et al. covering 100 patients over a period of 3 years revealed occurrence of functional reinnervations within a period of from 3 to 24 months after onset of the paralysis [176]. With the help of regular laryngeal EMG monitoring, an assessment leading to a prognosis that functional recovery is unlikely to occur is feasible after 9 months according to Schneider et al. [177]. While this may be true in the hands of this experienced working group, laryngeal EMG is not available at every facility. According to the working group in Erlangen, a one-time EMG examination arrives at a correct diagnosis in only 78% of cases. According to a current paper published by Munin et al., a clinically relevant prognosis that functional recovery will not occur is less certain than an expected functional recovery [178]. The publication by Munin confirms our own experience, i.e. that the fibrillations considered to be reliable neurological signs of an unfavourable prognosis are not always detectable in the vocalis muscle, and that spontaneous activity need not be classified as pathological in every case.
The method of transoral vocalis EMG in widespread use in Germany employing hooked-wire electrodes as leads [179] is based on a strictly localized assessment of muscular activity at the needle insertion point. A systematic analysis of the entire muscle is not possible. The results of transcutaneous needle lead EMG recording as favoured by Blitzer in New York are particularly reliable in cases in which no EMG potentials are detected [180], [181], [182]. The examiner can then be certain that the lack of potentials is not due to uncertainties deriving from the lead locus.
As is clear from the above presentation, the neural recovery time varies widely and prognosis remains uncertain at best. Medical histories contain only a very few cases in which the nerve has been severed on purpose, e.g. due to malignant disease.
Before we can make a final decision to perform an irreversible surgical procedure, the patient must be informed in detail concerning the actual circumstances and therapeutic alternatives and is called upon to contribute to the decision.
Within this decision-making context, temporary laterofixation of the vocal cord using the suturing technique according to Lichtenberger is a particularly interesting option. Especially in cases in which hope of rapid functional recovery or patient worries concerning the outcome of glottis-dilating surgery play a major role, and if the patient also rejects the tracheotomy as an interim solution circumventing the larynx, this is the recommended approach.
Lichtenberger himself mentions the possibility of the suture(s) being pulled right through the vocal cord [174]. In his view, this can only happen after prolonged endotracheal intubation has caused mucosal damage in the area of the laterofixation. In our experience, there is a general tendency for the sutures to behave in this manner. In most cases, the mucosal incision closes quickly over the suture, although this does not mean the function of the laterofixation is immediately neutralized. If a recovery of function parallels the reduction of the laterofixation effect, the suture can simply be removed. Otherwise, all of the options of glottis-dilating surgery remain open, besides which the patient can also still opt for a tracheotomy.
We do not offer this approach to patients with severe generalized diseases, obesity or pronounced secondary oedemas.
Assuming functional recovery is a concrete possibility, this procedure can be recommended to patients who have managed to get along for some time with bilateral vocal cord motion impairment and are very worried about the voice problems that can be expected to result from partial glottis resection surgery.
We do not see laterofixation as a permanent solution.
4. Reconstruction
Laryngectomy may be the most reliable airway reconstruction method in cases of malignant disease of the larynx, but it is doubtless also the worst method in terms of quality of life. Due to modern developments in extensive transoral laser surgery resections, it is now much less frequently used [183], [184], [185]. Radiochemotherapy with the objective of organ preservation is now the more likely response to a hypopharyngeal carcinoma with a poor prognosis [186], [187], [188]. While it is true that even large residual defects after laser surgery of the tumor heal satisfactorily without plastic reconstruction, key functional regions such as the cricoid cartilage still require reconstruction [189].
Laryngotracheoplasty (LTP) according to Evans and Todd [190], [191], [192] is the oldest reconstructive procedure for treatment of cricoid cartilage stenoses in children. The zip-like opening of the laryngotracheal transition with involvement of the cricoid cartilage and internal stenting with a self-expanding silastic roll - Swiss roll - made it possible to adapt the method so as to minimize interference with the continued growth of the airway in childhood. Nonetheless, the stenotically cicatrized cartilage structures often made the incisions next to impossible and the dilation effect did not prove attainable in every case.
Cotton is credited with the further development of Rethi's laryngofissure technique [193] to include the interposition of costal cartilage into the ventral, and later dorsal, cricoid cartilage incisions [194]. This laryngotracheal reconstruction (LTR) method became the most commonly applied procedure. Additional lateral incisions were recommended in severe cases in which the cricoid cartilage lumen was nearly obliterated [195], [196]. The drawback to all LTR methods, particularly those involving dorsal rib cartilage implantation, remains the long-term stenting required for fixation of the cartilage and stabilization of the dilating effect in the presence of the tracheostoma.
A clear therapeutic advantage using the more frequently encountered ventral interposition was achieved with single-stage laryngotracheal reconstruction (SSLTR) according to Prescott. It was possible to close the airway in the same session by reconstructing the orificial region at the same time.
Under the impression of the good therapeutic results achieved with the resection procedures described in chapter 3.1, these reconstructing methods, and cricotracheal resection (CTR) in particular, have lost some of their pre-eminence in recent years. In a current publication, however, the Montgomery working group has once again lauded this technique for patients at high risk for a CTR. A new aspect in the group's approach is the use of autologous thyroid cartilage for interposition and a fixation technique for these dorsal cartilage implants that employs special pins. The decannulation rate, in 21 patients, was 95% [197].
Even extensive laryngeal or hypopharyngeal carcinomas adjoining the hemilarynx can now be resected using the methods of laser surgery. If the cartilaginous skeleton is affected, and in particular posterior cricoid cartilage segments, a vertical hemilaryngectomy may become necessary. Especially if laryngeal stenosis of relevant proportions develops, a concept for surgical reconstruction will have to be found.
A listing of current trends among the many methods discussed follows. Cansiz et al. report on reconstruction of the hemilarynx using a regional bone-muscle flap taken from straight neck musculature and the hyoid bone [198]. Of 17 patients undergoing this procedure, 2 required reoperation and one a laryngectomy. This method does not take into account the epithelial covering layer in the region of the laryngeal defect.
Delaere describes a number of new experimental and practical surgical approaches to this problem in his book, published in 2004: "Laryngotracheal Reconstruction. From Lab to Clinic" [189].
Promising approaches include in particular composite graft autotransplantation of microvascularly autonomized tracheal segments with preserved mucosal lining in reconstruction of the defective side of the larynx [199], [200], [201], [202], [203], [204], [205], [206].
Delaere describes autonomization of the cranial tracheal segment, realized by exposing the segment, then enveloping it with a radialis flap that has been microvascularly anastomosed within the framework of hemilaryngectomy in tumor cases. Following temporary coverage of the resection defect and bypassing of laryngeal function by means of tracheotomy and percutaneous endoscopic gastrostomy (PEG), the homolateral tracheal wall with its vascularized covering is moved cranially through the radialis flap into position in the laryngeal defect in a second step (after 3-4 months). This reconstruction technique, required in rare cases only, is more of a look into the future. It does become clear, however, that complex transplants comprising a supporting element, mucosal covering and surrounding vascularized tissue are what is needed for airway reconstruction.
An intact mucosal covering prevents formation of scar tissue and restenosis [207], [208], [209]. Cartilage that is autologous, either proliferated using the methods of tissue engineering or newly formed, gives the airway reconstruction the necessary form and stability [210], [211], [212], [213], [214]. Further advances can be expected in this area in the years to come.
5. Transplantation
In a number of important organs, transplantation remains the method of last resort if no further reconstruction options are open. Kidney transplantation has become a routine procedure [215]. Nearly all transplantation centres now also perform lung and liver transplantations [216].
Why isn't this the case with laryngeal transplantation?
Development of a suitable larynx transplantation technique has been the object of an intensive search since the 1960s [217], [218], [219], [220]. Whereas the results of many larynx transplantations in rabbits, dogs and later other animal models were encouraging, successful transplantation of a human laryngeal skeleton was at first the sole preserve of the Belgian group working with Kluyskens in 1969 [221]. Transplantation of an entire human organ, including the necessary microanastomoses and postoperative immunosuppression, remained a hopeful vision until the work of the Cleveland Group under Marshall Strome, who not only performed the first successfully human laryngeal transplantation, but above all paved the way for this work in extensive studies on the suitability of different immunosuppressant substances for larynx transplantations [222], [223], [224], [225], [226], [227], [228], [229], [230], [231].
In 1998, in a milestone operation, Strome transplanted an entire larynx with cranial trachea, large sections of the hypopharynx and the thyroid gland into a 40-year-old patient who, following a motorcycle accident and multiple operations, was left with laryngeal damage that could not be otherwise reconstructed [227], [228]. Bilateral microvascular anastomosis was accompanied by an attempt at reinnervation by means of neurorrhaphy of the superior laryngeal nerve bilaterally and the recurrent laryngeal nerve on the right side (The left stump of the recurrent laryngeal nerve had been irrecoverably lost in previous surgical attempts).
In 2001, the working group published a follow-up on the first 40 months after the transplantation. An incipient immunological rejection reaction was brought under control by changing the immunosuppressant medication. The patient first reported sensations in the area of the larynx three months after the transplantation and eventually developed the ability to feed himself transorally. In the assessment of the treating physicians, vocal function improvement continued for 16 months after the transplantation. EMG revealed signs of reinnervation of the cricothyreoid muscle and the vocalis muscle. It did not prove feasible to achieve opening movements of the vocal cords sufficient for respiration by the end of the 40-month observation period. According to current reports, this objective has not been achieved to date, so the patient still relies on the tracheostoma. Currently, glottis resecting surgery, as performed in patients with bilateral vocal cord motion impairment from other causes, is being considered to facilitate decannulation.
In addition to Strome, Birchall et al. in the UK have also done extensive groundwork on establishment of laryngeal transplantation as a clinical standard in recent years. In numerous experimental studies, this working group has developed animal models to test methods of revascularization, reinnervation and the efficacy of immunosuppressants [232], [233], [234], [235], [236].
To sum up, we consider two problems in particular to have been the key hindrances to more rapid establishment of human laryngeal transplantation.
For one thing, most cases of total organ loss are due to malignant tumors. The immunosuppression necessary for every transplantation procedure must be critically assessed due to its negative influence on tumor defences. [237], [238], [239], [240], [241].
The second major difficulty is that the transplant is lacking in natural motor and sensory activity. If the technically demanding laryngeal transplantation leads to a marked improvement of the quality-of-life for the patient, further efforts will have to be made in the direction of aspiration-free swallowing, respiration capacities that suffice to support physical exertion and satisfactory vocalization. A thorough and functional reinnervation of the transplant is necessary to achieve these ends.
The ethical implications of transplantation of other people's voice must also be considered [242], [243].
It will surely take some time before laryngeal transplantations become routine clinical procedures. The side-effect profiles of modern immunosuppressants have been greatly improved so that, for example, liver transplantations in patients with hepatocellular carcinoma have now been performed under immunosuppression [244].
Post-laryngectomy patients should be offered the perspective of a laryngeal transplantation to follow the healing period. It appears unlikely, however, that patients who have received radiotherapy will be candidates for the procedure due to problems to be expected with revascularization and reinnervation.
6. Reanimation of the Larynx
With glottis-dilating surgery, as described in chapter 3, the best that can be achieved is a compromise in terms of the balance between vocal and respiratory laryngeal function. We know from clinical experience that patients with a minimum of residual mobility in one vocal cord with bilateral motion impairment rehabilitate more readily than patients with bilaterally fixated vocal cords. This reflects the special significance of preservation or recovery of a dynamic vocal cord position. For this reason, a number of groups are working on further development of methods of neuromuscular reinnervation of the larynx. As was made clear in the last chapter, reinnervation also plays a key role in the development of functionality in a transplanted larynx. There is also a group of scientists, currently still small, who are working on specific electrostimulation of the paralyzed larynx analogous to the way a pacemaker functions. Understood correctly, this procedure would not compete with reinnervation, but would rather complement it.
6.1 Reinnervation
Different laryngeal reinnervation techniques have different objectives [245], among them the recovery of sensory function, glottic closure and glottic opening as well as increasing vocal cord tension.
Here we would like to take a closer look at current developments in the field of abductor reinnervation, which are among the reconstructive procedures used to treat compromised laryngeal respiratory function.
A basic objective of all reinnervation techniques is recovery of an effector muscle connection, in this case the glottis opener, i.e. the posterior cricoarytenoid muscle (PCA), with a nerve that stimulates the muscle synchronously with inspiration.
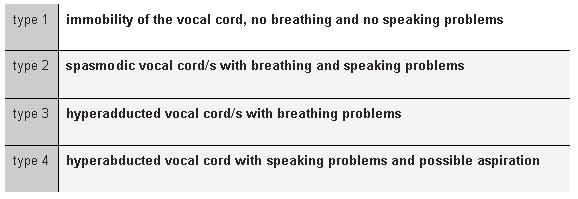
As in any case of neural severance, neurorrhaphy is the first reconstruction method up for discussion. Use of this method for the recurrent laryngeal nerve (RLN) was described as early as 1909 by Horsley [246]. In contrast to other nerves, the extralaryngeal RLN does not show the typical fascicular organization featuring distinct opener and closer bundles [247], [248]. This nerve is most frequently damaged along its extralaryngeal course in the thyroid gland region. The rehabilitation results of end-to-end anastomoses of the RLN for damage in this segment are therefore not reliably predictable. The reason for this is synkinetic reinnervation. Even though the post-reinnervation EMG reveals potentials, the glottic opening is stimulated synchronously by agonists and antagonists and vocal cord mobility remains impaired [249]. Since synkinetic reinnervation is not the rule, Chou et al. still recommend in a current publication, assuming probable RLN severance in a thyroid operation, revision of the surgical region and a primary neurorrhaphy procedure [250]. The revision may also help reveal and eliminate haematomas or ligations in the area around the dysfunctional RLN. In a case of bilateral traumatic severance of the RLN in our experience, we observed at least a residual opening of the larynx post neurorrhaphy, which movement sufficed for closure of the tracheotomy. However, a small proportion of patients who readily contemplate legal action can be expected to file subsequent damages suits against the physician performing the neural revision if function is not restored following iatrogenic neural damage. A highly detailed and critical discussion with the patient is recommended, including clear references to the uncertainty of therapeutic success. As with neurorrhaphy, synkinesias also occur in significantly greater numbers of cases than was previously assumed in cases of spontaneous reinnervation of the RLN following iatrogenic trauma or idiopathic paresis. In a multicentric retrospective analysis, colleagues in Innsbruck found EMG evidence of synkinesia in 67% of patients with unilateral paresis [251]. Zealear even mentions a figure as high as 85% [252]. The rarity of clinical observation of a pronounced atrophy of the muscles of the vocal cord in RLN paresis (for example in cases of damage to the vagus trunk) may be an indication that reinnervation is the rule. Judging from the extent of functional impairment resulting from pathological reinnervation, Crumley [253] differentiates four types of synkinesia (see Table 2 (Tab. 2)).
Table 2. Granding of synkinesis (Crumley 2000 [253]).
While this system reflects the wide spectrum of therapeutic situations encountered in practice, it fails to differentiate between unilateral and bilateral paresis.
In recent years, considerable progress has been made in suppressing collateral sprouting and growth into the false axon around the neurorrhaphy region in the facial nerve. Sprouting was reduced by means of local application of antibodies that block neural growth factors [254]. Wrapping the nerve anastomosis with olfactory epithelium releases mediators that enhance axonal pathfinding [255], [256].
Kanemaru et al. succeeded in a current animal experiment in realizing an RLN anastomosis at a distance of 10 mm from an implant corpus (absorbable polyglycolic acid tube) [257], [258]. Such a procedure is of useful relevance if otherwise an end-to-end anastomosis can only be realized under tension. Spanning greater distances becomes particularly interesting when the proximal neural stump is no longer available and a donor nerve is to be used to replace the RLN.
Many different nerves are used as donor material. Whereas the ansa cervicalis and the hypoglossal nerve are at the focus of a number of publications on reanimation of glottic closure [259], [260], [261], [262], [263], the phrenic nerve is best suited to achieve an improvement in respiratory function [264], [265], [266], [267]. However, the resulting unilateral phrenoparalysis, itself a hindrance to respiratory function, restricts its use. The great neural distance of intercostal nerves makes them unsuitable. The terminal branches of the ansa cervicalis to the omohyoid muscle and sternothyreoid muscle and the superior laryngeal nerve also show some inspiratory activity and are therefore potential candidates as well.
Something all donor nerves have in common is that an end-to-end anastomosis to the RLN with synchronous stimulation of the adductors does not open the glottis. Possible courses of action include either selective severing of the adductor branch of the RLN [268] with reinsertion of it into the PCA [265], or a motor block of the adductors using botulinum toxin.
Tucker is credited with using a nerve-muscle pedicle (NMP) as a alternative to neurorrhaphy for selective reinnervation of the PCA [269]. In this technique, developed with laryngeal transplantation in mind, the nerve, in this case the ansa cervicalis, is implanted directly into the venter of the muscle. Sprouting nerve fibres later reach the motor endplates of the target muscle, in this case the PCA. Although Tucker recorded successes in 74% of his patients, other surgeons were unable to duplicate his results. It may be that the surgical trauma results in cicatrizations with negative secondary effects on the functional gains. The muscle-nerve-muscle neurotization from an innervated to a denervated muscle described in 2001 by Hogikyan et al. represents a current update on this issue [270], [271]. We see potential uses for this technique in bilateral reanimation of the larynx, e.g. in transplantations in which a donor nerve is required for only one side and neurotization on the other side can be realized in the form of such an MNM bridge. Be that as it may, the validity of the method requires further testing.
A current development in reinnervation of the larynx is the application of growth factors to regenerate the denervated muscle using genetic therapy techniques [272], [273], [274], [275], [276]. We ourselves are planning applications of human stem cells in support of selective reorganization of the muscle tissue following reinnervation.
The Vanderbilt group working under David Zealear recently demonstrated that electrostimulation of the denervated muscle during the reinnervation process results in selective reinnervation [252], [277], [278], i.e. a reduction of synkinetic reinnervation.
If further studies confirm that the reinnervation process can be influenced by targeted electrostimulation and perhaps also enhanced by means of genetic therapy techniques, we will in future no longer have to wait for spontaneous "healing" with its inherent tendency towards synkinesia.
6.2 Electrostimulation (pacemaker)
Functional electrostimulation (FES) revolutionized medical practice with introduction of the cardiac pacemaker by Zoll [279]. The irreversible failure of neural control in important muscle systems, especially in the aftermath of strokes and transverse cord lesions with paraplegia, led to highly intense research on FES in the 70s and 80s.
Zealear and Dedo were the first to develop the concept of electrostimulation of the paralyzed larynx in 1977 [280]. The idea of inspiration-synchronized electrostimulation and the term "pacemaker" for laryngeal FES are attributed to Obert. Bergmann (Charité) developed a chest wall sensor to synchronize triggering of the PCA stimulation with respiration and performed extensive long-term stimulation experiments with dogs [281], [282], [283], [284], [285], [286], [287], [288], [289], [290]. Otto based development of an inspiration-synchronized trigger for PCA stimulation on diaphragm EMG signals [291], [292]. In search of further trigger mechanisms, Kim worked out the idea of making use of the changes in pharyngeal temperature that accompany respiration [293]. Zealear considered using intrathoracic pressure to synchronize electrostimulation [294].
The systematic analysis of the stimulus-muscle response characteristic of the PCA muscle in a dog model by Sanders proved an important milestone [295].
Zealear and the Vienna group working under Zrunek refined the parametric definitions as the basis for chronic stimulation [296], [297], [298], [299], [300].
Bergmann had tested his implant in long-term animal experiments and had obtained the necessary permits to begin testing it in humans when changes in healthcare regulations, in 1989, prevented him from continuing.
The first publication on temporary electrostimulation of the PCA muscle in humans is credited to Zealear et al. (1996) [301]. They applied stimulation during a thyroplasty procedure for unilateral paresis. Once the necessary advances in equipment engineering were in place to allow for clinical testing of the first fully implantable laryngeal pacemaker, a total of 7 patients received pacemaker implants from 1995 to 1997 within the framework of a transnational study [302]. All of the patients included in the study unterwent previously tracheotomy.
One (female) patient suffered an early infection that later led to removal of the implant. A stimulated opening movement was confirmed in 5 of the remaining 6 patients. Closure of the tracheostoma was possible in 4 of the patients, whereby one (female) patient rejected decannulation. Vocal quality did not suffer in any of the patients.
Although a number of problems remain to be solved, this pioneering study demonstrated that pacemaker applications in treatment of bilateral vocal cord motion impairment are feasible and effective in principle.
Some of the problems were of a technical nature, such as the electrode corrosion described in three of the patients. In view of the fact that the implant device used to date (Itrel II, Medtronic, USA) was developed for treatment of chronic pain, its electrode design and parametric flexibility are not optimally adapted to the requirements of laryngeal applications. It is not yet possible to synchronize triggering of the stimulation signals with the patient's respiration. The opening stimulation is generated at a fixed rate of 10 stimuli per minute, to which rate the patient must adapt his or her breathing. Therefore, interruptions of phonation, and theoretically aspiration as well, may occur when glottis opening is stimulated during swallowing. In Zealear's opinion, the strength of voluntary patient glottic closure is stronger than the stimulated opening, enabling the patient to "override" it [302].
In our view, the lack of respiration-synchronized triggering is an essential drawback to this system. In a research project sponsored by the BMBF, we are currently attempting to remedy this situation by using a newly developed implant. The objective is to avoid frustrated respiratory effort against a closed glottis when respiratory rate and rhythm change. Benefit in terms of quality of life must be measured in terms of the degree to which normal physical exertion becomes supportable. It must be possible to raise respiratory frequency in response to sporting or other physical effort, e.g. on the job. Abdominal pressure must not be interrupted by an involuntary opening of the glottis. (see Figure 2 (Fig. 2))
Figure 2. Concept on laryngeal pacing (pacemaker).
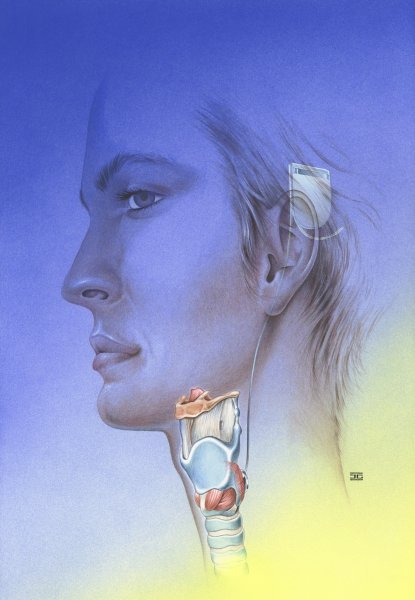
(Grafik: Jens Geiling, institut of anatomics, FSU Jena, 2003)
We are also concerned that the electrode design be better adapted to anatomical dimensions. The Itrel implant is only capable of single-channel stimulation. One of Zealear's (female) patients experienced a slight electrode dislocation during the long-term course following implantation. With development of array electrodes and multiple-channel stimulation, we want to make sure the optimum stimulation points in the PCA muscle, the segments designated as electrophysiological "hot spots" [300] with the strongest muscle response to the stimulus, are reached. Another objective is the possibility of later replacement of the stimulating array electrodes in case of minor dislocations.
Furthermore, bilateral stimulation should be made possible to provide for better adaptation to physical exertion and ensure emergency reserves in case of unilateral stimulation failure.
A pacemaker device suitable for routine clinical use will become available in the foreseeable future. Functional electrostimulation (FES) in treatment of bilateral vocal cord motion impairment has decisive advantages over irreversible static enlargement of the airway cross-section by means of glottis-dilating surgery, since it does not diminish vocal and swallowing functionality and dynamically increases the airway cross-section.
Pacemaker support is required to ensure the functional success of a laryngeal transplantation until functional reinnervation has been established.
Recent informations in the field of reinnervation make it clear that FES does not represent a competing procedure, but rather a useful supplementation, to reinnervation surgery, since the extent of synkinetic reinnervation can be reduced by this method.
Practice heretofore has involved waiting for possible reinnervation, during which period the muscle tissue is reorganized, the joint capsules shrink and uncontrolled (synkinetic) reinnervation has a chance to get started. Early application of the pacemaker (instead of tracheotomy) should be a main focus of the minimally invasive electrode application procedure.
References
- 1.Goethe JW. West-östlicher Divan. 1819. [Google Scholar]
- 2.Olofsson J. Laryngotracheal anatomy and physiology. Acta Otorhinolaryngol Belg. 1995;49:303–311. [PubMed] [Google Scholar]
- 3.Sasaki CT, Isaacson G. Functional anatomy of the larynx. Otolaryngol Clin North Am. 1988;21:595–612. [PubMed] [Google Scholar]
- 4.Proctor DF. The upper airways. II. The larynx and trachea. Am Rev Respir Dis. 1977;115:315–342. doi: 10.1164/arrd.1977.115.2.315. [DOI] [PubMed] [Google Scholar]
- 5.Eckel HE, Sittel C. Morphometric studies at the level of the glottis as a principle in larynx enlarging microlaryngoscopic surgical procedures in bilateral recurrent nerve paralysis. Laryngol Rhinol Otol. 1994;73:417–422. doi: 10.1055/s-2007-997164. [DOI] [PubMed] [Google Scholar]
- 6.Eckel HE, Sittel C. Morphometry of the larynx in horizontal sections. Am J Otolaryngol. 1995;16:40–48. doi: 10.1016/0196-0709(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 7.Shah MD, Shah SM. The applied physiology of cough. Indian J Pediatr. 2001;68 Suppl 2:3–10. [PubMed] [Google Scholar]
- 8.Vollrath M. Kehlkopf- und Trachealchirurgie bei Kindern. In: Hildmann H, Koch U, editors. Verhandlungsbericht 1999. Berlin, Heidelberg: Springer; 1999. pp. 145–227. [Google Scholar]
- 9.Holinger LD. Etiology of stridor in the neonate, infant and child. Ann Otol Rhinol Laryngol. 1980;89:397–400. doi: 10.1177/000348948008900502. [DOI] [PubMed] [Google Scholar]
- 10.Altman KW, Wetmore RF, Marsh RR. Congenital airway abnormalities in patients requiring hospitalization. Arch Otolaryngol Head Neck Surg. 1999;125:525–528. doi: 10.1001/archotol.125.5.525. [DOI] [PubMed] [Google Scholar]
- 11.Werner JA, Lippert BM, Ankermann T. Transoral treatment of severe laryngomalacia. Review and presentation of a modified surgical technique. Laryngol Rhinol Otol. 2000;79:416–422. doi: 10.1055/s-2000-4628. [DOI] [PubMed] [Google Scholar]
- 12.Masters IB, Chang AB, Patterson L, Wainwright C, Buntain H, Dean BW, Francis PW. Series of laryngomalacia, tracheomalacia, and bronchomalacia disorders and their associations with other conditions in children. Pediatr Pulmonol. 2002;34:189–195. doi: 10.1002/ppul.10156. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez I, Navarro H, Mendez M, Holmgren N, Caussade S. Clinical characteristics of children with tracheobronchial anomalies. Pediatr Pulmonol. 2003;35:288–291. doi: 10.1002/ppul.10256. [DOI] [PubMed] [Google Scholar]
- 14.Vollrath M. Laryngomalacia. Definition, diagnosis and therapy. HNO. 2004;52:336–343. doi: 10.1007/s00106-004-1055-7. [DOI] [PubMed] [Google Scholar]
- 15.Shatz A, Goldberg S, Picard E, Kerem E. Pharyngeal wall collapse and multiple synchronous airway lesions. Ann Otol Rhinol Laryngol. 2004;113:483–487. doi: 10.1177/000348940411300613. [DOI] [PubMed] [Google Scholar]
- 16.Jackson C, Jackson C. Diseases and injuries of the larynx. New York: MacMillan; 1942. pp. 63–69. [Google Scholar]
- 17.Catalfumo FJ, Golz A, Westerman ST, Gilbert LM, Joachims HZ, Goldenberg D. The epiglottis and obstructive sleep apnoea syndrome. J Laryngol Otol. 1998;112:940–943. doi: 10.1017/s0022215100142136. [DOI] [PubMed] [Google Scholar]
- 18.Golz A, Goldenberg D, Westerman ST, Catalfumo FJ, Netzer A, Westerman LM, Joachims HZ. Laser partial epiglottidectomy as a treatment for obstructive sleep apnea and laryngomalacia. Ann Otol Rhinol Laryngol. 2000;109:1140–1145. doi: 10.1177/000348940010901211. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen FP, Steven P, Tsokos M, Jungmann K, Muller A, Verse T, Pirsig W. Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am J Respir Crit Care Med. 2002;166:501–509. doi: 10.1164/rccm.2109099. [DOI] [PubMed] [Google Scholar]
- 20.Smith RJ, Bauman NM, Bent JP, Kramer M, Smits WL, Ahrens RC. Exercise-induced laryngomalacia. Ann Otol Rhinol Laryngol. 1995;104:537–541. doi: 10.1177/000348949510400707. [DOI] [PubMed] [Google Scholar]
- 21.Bent JP, 3rd, Miller DA, Kim JW, Bauman NM, Wilson JS, Smith RJ. Pediatric exercise-induced laryngomalacia. Ann Otol Rhinol Laryngol. 1996;105:169–175. doi: 10.1177/000348949610500301. [DOI] [PubMed] [Google Scholar]
- 22.Naito A, Niimi S. The larynx during exercise. Laryngoscope. 2000;110:1147–1150. doi: 10.1097/00005537-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Mandell DL, Arjmand EM. Laryngomalacia induced by exercise in a pediatric patient. Int J Pediatr Otorhinolaryngol. 2003;67:999–1003. doi: 10.1016/s0165-5876(03)00178-2. [DOI] [PubMed] [Google Scholar]
- 24.Kenn K, Schmitz M. Vocal cord dysfunction, an important differential diagnosis of severe and implausible bronchial asthma. Pneumologie. 1997;51:14–18. [PubMed] [Google Scholar]
- 25.Kothe C, Schade G, Fleischer S, Hess M. Vocal cord dysfunction. An important differential diagnosis to bronchial asthma. HNO. 2004;52:261–264. doi: 10.1007/s00106-003-1023-7. [DOI] [PubMed] [Google Scholar]
- 26.Belmont JR, Grundfast K. Congenital laryngeal stridor (laryngomalacia): etiologic factors and associated disorders. Ann Otol Rhinol Laryngol. 1984;93:430–437. doi: 10.1177/000348948409300502. [DOI] [PubMed] [Google Scholar]
- 27.Gessler EM, Simko EJ, Greinwald JH., Jr Adult laryngomalacia: an uncommon clinical entity. Am J Otolaryngol. 2002;23:386–389. doi: 10.1053/ajot.2002.126322. [DOI] [PubMed] [Google Scholar]
- 28.Siou GS, Jeannon JP, Stafford FW. Acquired idiopathic laryngomalacia treated by laser aryepiglottoplasty. J Laryngol Otol. 2002;116:733–735. doi: 10.1258/002221502760238073. [DOI] [PubMed] [Google Scholar]
- 29.Li HY, Fang TJ, Lin JL, Lee ZL, Lee LA. Laryngomalacia causing sleep apnea in an osteogenesis imperfecta patient. Am J Otolaryngol. 2002;23:378–381. doi: 10.1053/ajot.2002.128037. [DOI] [PubMed] [Google Scholar]
- 30.Chandra RK, Gerber ME, Holinger LD. Histological insight into the pathogenesis of severe laryngomalacia. Int J Pediatr Otorhinolaryngol. 2001;61:31–38. doi: 10.1016/s0165-5876(01)00541-9. [DOI] [PubMed] [Google Scholar]
- 31.McSwiney PF, Cavanagh NP, Languth P. Outcome in congenital stridor (laryngomalacia) Arch Dis Child. 1977;52:215–218. doi: 10.1136/adc.52.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride JT. Stridor in childhood. J Fam Pract. 1984;19:782–790. [PubMed] [Google Scholar]
- 33.Iyer VK, Pearman K, Raafat F. Laryngeal mucosal histology in laryngomalacia: the evidence for gastro-oesophageal reflux laryngitis. Int J Pediatr Otorhinolaryngol. 1999;49:225–230. doi: 10.1016/s0165-5876(99)00205-0. [DOI] [PubMed] [Google Scholar]
- 34.Bouchard S, Lallier M, Yazbeck S, Bensoussan A. The otolaryngologic manifestations of gastroesophageal reflux: when is a pH study indicated? J Pediatr Surg. 1999;34:1053–1056. doi: 10.1016/s0022-3468(99)90562-6. [DOI] [PubMed] [Google Scholar]
- 35.Bobin S, Attal P. Laryngotracheal manifestations of gastroesophageal reflux in children. Pediatr Pulmonol Suppl. 1999;18:73–75. [PubMed] [Google Scholar]
- 36.Yellon RF, Goldberg H. Update on gastroesophageal reflux disease in pediatric airway disorders. Am J Med. 2001;111 Suppl 8A:78S–84S. doi: 10.1016/s0002-9343(01)00861-0. [DOI] [PubMed] [Google Scholar]
- 37.Gilger MA. Pediatric otolaryngologic manifestations of gastroesophageal reflux disease. Curr Gastroenterol Rep. 2003;5:247–252. doi: 10.1007/s11894-003-0027-5. [DOI] [PubMed] [Google Scholar]
- 38.Jani P, Koltai P, Ochi JW, Bailey CM. Surgical treatment of laryngomalacia. J Laryngol Otol. 1991;105:1040–1045. doi: 10.1017/s0022215100118158. [DOI] [PubMed] [Google Scholar]
- 39.McClurg FL, Evans DA. Laser laryngoplasty for laryngomalacia. Laryngoscope. 1994;104:247–252. doi: 10.1288/00005537-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Roger G, Denoyelle F, Triglia JM, Garabedian EN. Severe laryngomalacia: surgical indications and results in 115 patients. Laryngoscope. 1995;105:1111–1117. doi: 10.1288/00005537-199510000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Wiggs WJ, Jr, DiNardo LJ. Acquired laryngomalacia: resolution after neurologic recovery. Otolaryngol Head Neck Surg. 1995;112:773–776. doi: 10.1016/S0194-59989570193-1. [DOI] [PubMed] [Google Scholar]
- 42.Mancuso RF, Choi SS, Zalzal GH, Grundfast KM. Laryngomalacia. The search for the second lesion. Arch Otolaryngol Head Neck Surg. 1996;122:302–306. doi: 10.1001/archotol.1996.01890150076014. [DOI] [PubMed] [Google Scholar]
- 43.Altman KW, Wetmore RF, Marsh RR. Congenital airway abnormalities requiring tracheotomy: a profile of 56 patients and their diagnoses over a 9 year period. Int J Pediatr Otorhinolaryngol. 1997;41:199–206. doi: 10.1016/s0165-5876(97)00089-x. [DOI] [PubMed] [Google Scholar]
- 44.Shah UK, Wetmore RF. Laryngomalacia: a proposed classification form. Int J Pediatr Otorhinolaryngol. 1998;46:21–26. doi: 10.1016/s0165-5876(98)00111-6. [DOI] [PubMed] [Google Scholar]
- 45.Senders CW, Navarrete EG. Laser supraglottoplasty for laryngomalacia: are specific anatomical defects more influential than associated anomalies on outcome? Int J Pediatr Otorhinolaryngol. 2001;57:235–244. doi: 10.1016/s0165-5876(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 46.Denoyelle F, Mondain M, Gresillon N, Roger G, Chaudre F, Garabedian EN. Failures and complications of supraglottoplasty in children. Arch Otolaryngol Head Neck Surg. 2003;129:1077–1080. doi: 10.1001/archotol.129.10.1077. [DOI] [PubMed] [Google Scholar]
- 47.Peron DL, Graffino DB, Zenker DO. The redundant aryepiglottic fold: report of a new cause of stridor. Laryngoscope. 1988;98:659–663. doi: 10.1288/00005537-198806000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Amin MR, Isaacson G. State-dependent laryngomalacia. Ann Otol Rhinol Laryngol. 1997;106:887–890. doi: 10.1177/000348949710601101. [DOI] [PubMed] [Google Scholar]
- 49.Worley G, Witsell DL, Hulka GF. Laryngeal dystonia causing inspiratory stridor in children with cerebral palsy. Laryngoscope. 2003;113:2192–2195. doi: 10.1097/00005537-200312000-00028. [DOI] [PubMed] [Google Scholar]
- 50.Archer SM. Acquired flaccid larynx. A case report supporting the neurologic theory of laryngomalacia. Arch Otolaryngol Head Neck Surg. 1992;118:654–657. doi: 10.1001/archotol.1992.01880060104021. [DOI] [PubMed] [Google Scholar]
- 51.Lane RW, Weider DJ, Steinem C, Marin-Padilla M. Laryngomalacia. A review and case report of surgical treatment with resolution of pectus excavatum. Arch Otolaryngol. 1984;110:546–551. doi: 10.1001/archotol.1984.00800340058017. [DOI] [PubMed] [Google Scholar]
- 52.Chen JC, Holinger LD. Congenital laryngeal lesions: pathology study using serial macrosections and review of the literature. Pediatr Pathol. 1994;14:301–325. doi: 10.3109/15513819409024262. [DOI] [PubMed] [Google Scholar]
- 53.Zwacka G, Scholle S. Experiences with therapy of pediatric sleep apnea syndrome and obstructive nasopharyngeal respiratory pattern with nasal BIPAP and CPAP therapy. Pneumologie. 1995;49 Suppl 1:152–154. [PubMed] [Google Scholar]
- 54.Werner JA, Lippert BM, Dunne AA, Ankermann T, Folz BJ, Seyberth H. Epiglottopexy for the treatment of severe laryngomalacia. Eur Arch Otorhinolaryngol. 2002;259:459–464. doi: 10.1007/s00405-002-0477-7. [DOI] [PubMed] [Google Scholar]
- 55.Froehlich P, Seid AB, Denoyelle F, Pransky SM, Kearns DB, Garabedian EN, Morgon A. Discoordinate pharyngolaryngomalacia. Int J Pediatr Otorhinolaryngol. 1997;39:9–18. doi: 10.1016/S0165-5876(96)01454-1. [DOI] [PubMed] [Google Scholar]
- 56.Fauroux B, Pigeot J, Polkey MI, Roger G, Boule M, Clement A, Lofaso F. Chronic stridor caused by laryngomalacia in children: work of breathing and effects of noninvasive ventilatory assistance. Am J Respir Crit Care Med. 2001;164:1874–1878. doi: 10.1164/ajrccm.164.10.2012141. [DOI] [PubMed] [Google Scholar]
- 57.Hoshi K, Ejima Y, Hasegawa R, Saitoh K, Satoh S, Matsukawa S. Differences in respiratory parameters during continuous positive airway pressure and pressure support ventilation in infants and children. Tohoku J Exp Med. 2001;194:45–54. doi: 10.1620/tjem.194.45. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez JA, Von Dessauer B, Duffau G. Non-invasive continuous positive airways pressure for post-extubation laryngitis in pediatric patients. Arch Bronconeumol. 2002;38:463–467. doi: 10.1016/s0300-2896(02)75266-6. [DOI] [PubMed] [Google Scholar]
- 59.Reed WR, Roberts JL, Thach BT. Factors influencing regional patency and configuration of the human infant upper airway. J Appl Physiol. 1985;58:635–644. doi: 10.1152/jappl.1985.58.2.635. [DOI] [PubMed] [Google Scholar]
- 60.Aubert G. Alternative therapeutic approaches in sleep apnea syndrome. Sleep. 1992;15:S69–S72. doi: 10.1093/sleep/15.suppl_6.s69. [DOI] [PubMed] [Google Scholar]
- 61.Jeffery HE, Megevand A, Page H. Why the prone position is a risk factor for sudden infant death syndrome. Pediatrics. 1999;104:263–269. doi: 10.1542/peds.104.2.263. [DOI] [PubMed] [Google Scholar]
- 62.Reddy DK, Matt BH. Unilateral vs. bilateral supraglottoplasty for severe laryngomalacia in children. Arch Otolaryngol Head Neck Surg. 2001;127:694–699. doi: 10.1001/archotol.127.6.694. [DOI] [PubMed] [Google Scholar]
- 63.Toynton SC, Saunders MW, Bailey CM. Aryepiglottoplasty for laryngomalacia: 100 consecutive cases. J Laryngol Otol. 2001;115:35–38. doi: 10.1258/0022215011906966. [DOI] [PubMed] [Google Scholar]
- 64.Zalzal GH, Anon JB, Cotton RT. Epiglottoplasty for the treatment of laryngomalacia. Ann Otol Rhinol Laryngol. 1987;96:72–76. doi: 10.1177/000348948709600118. [DOI] [PubMed] [Google Scholar]
- 65.Seid AB, Park SM, Kearns MJ, Gugenheim S. Laser division of the aryepiglottic folds for severe laryngomalacia. Int J Pediatr Otorhinolaryngol. 1985;10:153–158. doi: 10.1016/s0165-5876(85)80027-6. [DOI] [PubMed] [Google Scholar]
- 66.Holinger LD, Konior RJ. Surgical management of severe laryngomalacia. Laryngoscope. 1989;99:136–142. doi: 10.1288/00005537-198902000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Holinger LD. Diagnostic endoscopy of the pediatric airway. Laryngoscope. 1989;99:346–348. doi: 10.1288/00005537-198903000-00023. [DOI] [PubMed] [Google Scholar]
- 68.Altman KW, Wetmore RF, Mahboubi S. Comparison of endoscopy and radiographic fluoroscopy in the evaluation of pediatric congenital airway abnormalities. Int J Pediatr Otorhinolaryngol. 1998;44:43–46. doi: 10.1016/s0165-5876(98)00042-1. [DOI] [PubMed] [Google Scholar]
- 69.Landau LI. Investigation and treatment of chronic stridor in infancy. Monaldi Arch Chest Dis. 1999;54:18–21. [PubMed] [Google Scholar]
- 70.Bhat N, De R, Zeiton H. Paediatric airway endoscopy. Rev Laryngol Otol Rhinol (Bord) 2000;121:31–35. [PubMed] [Google Scholar]
- 71.De Beer D, Chambers N. Double trouble: prolapsing epiglottis and unexpected dual pathology in an infant. Paediatr Anaesth. 2003;13:448–452. doi: 10.1046/j.1460-9592.2003.00980.x. [DOI] [PubMed] [Google Scholar]
- 72.O'Sullivan BP, Finger L, Zwerdling RG. Use of nasopharyngoscopy in the evaluation of children with noisy breathing. Chest. 2004;125:1265–1269. doi: 10.1378/chest.125.4.1265. [DOI] [PubMed] [Google Scholar]
- 73.Steurer M, Passler C, Denk DM, Schneider B, Niederle B, Bigenzahn W. Advantages of recurrent laryngeal nerve identification in thyroidectomy and parathyroidectomy and the importance of preoperative and postoperative laryngoscopic examination in more than 1000 nerves at risk. Laryngoscope. 2002;112:124–133. doi: 10.1097/00005537-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 74.Jackson C. Ventriculocordectomy. A new operation for the cure of goitrous glottic stenosis. Arch Surg. 1922;4:257–274. [Google Scholar]
- 75.Pieniazek P. Verengungen der Luftwege. Leipzig und Wien: Franz Deuticke; 1901. p. 505. [Google Scholar]
- 76.Thornell WC. Intralaryngeal approach for arytenoidectomy in bilateral abductor vocal cord paralysis. Arch Otolaryngol. 1948;47:505–508. doi: 10.1001/archotol.1948.00690030527016. [DOI] [PubMed] [Google Scholar]
- 77.Demedts M, Melissant C, Buyse B, Verschakelen J, Feenstra L. Correlation between functional, radiological and anatomical abnormalities in upper airway obstruction (UAO) due to tracheal stenosis. Acta Otorhinolaryngol Belg. 1995;49:331–339. [PubMed] [Google Scholar]
- 78.Vössing M, Wassermann K, Eckel HE, Ebeling O. Peak flow measurement in patients with laryngeal and tracheal stenoses. A simple and valuable spirometric method. HNO. 1995;43:70–75. [PubMed] [Google Scholar]
- 79.Müller A. Modern diagnostics of tracheal stenosis. Laryngol Rhinol Otol. 2004;83:381–386. doi: 10.1055/s-2004-814585. [DOI] [PubMed] [Google Scholar]
- 80.Schuchardt P. Klinisch-experimentelle Studie zur Pathophysiologie von Stenosen des Larynx und der Trachea, in HNO-Klinik / Phoniatrie. Berlin: Humboldt-Universität; 1984. p. 197. [Google Scholar]
- 81.McHenry MA, Kuna ST, Minton JT, Vanoye CR. Comparison of direct and indirect calculations of laryngeal airway resistance in connected speech. J Voice. 1996;10:236–244. doi: 10.1016/s0892-1997(96)80004-x. [DOI] [PubMed] [Google Scholar]
- 82.Berdel D, Koch U. Spontaneous flow measurements of laryngotracheal resistance in 30 tracheostoma patients. Arch Otorhinolaryngol. 1984;240:133–137. doi: 10.1007/BF00453470. [DOI] [PubMed] [Google Scholar]
- 83.Wassermann K, Koch A, Warschkow A, Mathen F, Muller-Ehmsen J, Eckel HE. Measuring in situ central airway resistance in patients with laryngotracheal stenosis. Laryngoscope. 1999;109:1516–1520. doi: 10.1097/00005537-199909000-00029. [DOI] [PubMed] [Google Scholar]
- 84.Myer CM, 3rd, O'Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994;103:319–323. doi: 10.1177/000348949410300410. [DOI] [PubMed] [Google Scholar]
- 85.Margulies C, Krevsky B, Catalano MF. How accurate are endoscopic estimates of size? [see comments] Gastrointest Endosc. 1994;40:174–177. doi: 10.1016/s0016-5107(94)70162-8. [DOI] [PubMed] [Google Scholar]
- 86.Müller A, Schubert M. Endoluminales 3D-Scanning der oberen Luftwege. Laryngol Rhinol Otol. 1998;77:636–637. [Google Scholar]
- 87.Müller A, Schubert M, Beleites E. A Noncontact Three-Dimensional laser Measuring Device for Tracheoscopy. Ann Otol Rhinol Laryngol. 2002:111. doi: 10.1177/000348940211100911. [DOI] [PubMed] [Google Scholar]
- 88.Müller A. ENDOSCAN : Entwicklung eines Verfahrens zur endoskopischen Vermessung von Trachealstenosen, in HNO-Klinik. Jena: Friedrich-Schiller-Universität; 2003. p. 145. [Google Scholar]
- 89.Schultz-Coulon HJ. The management of postintubation stenoses in children. HNO. 2004;52:363–377. doi: 10.1007/s00106-004-1060-x. [DOI] [PubMed] [Google Scholar]
- 90.Narcy P, Contencin P, Fligny I, Francois M. Surgical treatment for laryngotracheal stenosis in the pediatric patient. Arch Otolaryngol Head Neck Surg. 1990;116:1047–1050. doi: 10.1001/archotol.1990.01870090063009. [DOI] [PubMed] [Google Scholar]
- 91.Benjamin B, Jacobson I, Eckstein R. Idiopathic subglottic stenosis: diagnosis and endoscopic laser treatment. Ann Otol Rhinol Laryngol. 1997;106:770–774. doi: 10.1177/000348949710600911. [DOI] [PubMed] [Google Scholar]
- 92.Giudice M, Piazza C, Foccoli P, Toninelli C, Cavaliere S, Peretti G. Idiopathic subglottic stenosis: management by endoscopic and open-neck surgery in a series of 30 patients. Eur Arch Otorhinolaryngol. 2003;260:235–238. doi: 10.1007/s00405-002-0554-y. [DOI] [PubMed] [Google Scholar]
- 93.Tcacencu I. Mifepristone (RU-486) impairs post-surgical wound healing of the larynx. Med Sci Monit. 2002;8:BR397–BR400. [PubMed] [Google Scholar]
- 94.Othersen HB., Jr Subglottic tracheal stenosis. Semin Thorac Cardiovasc Surg. 1994;6:200–205. [PubMed] [Google Scholar]
- 95.Walner DL, Cotton RT, Willging JP, Bove KE, Toriumi DM. Model for evaluating the effect of growth factors on the larynx. Otolaryngol Head Neck Surg. 1999;120:78–83. doi: 10.1016/S0194-5998(99)70373-6. [DOI] [PubMed] [Google Scholar]
- 96.Loewen MS, Walner DL, Caldarelli DD. Improved airway healing using transforming growth factor beta-3 in a rabbit model. Wound Repair Regen. 2001;9:44–49. doi: 10.1046/j.1524-475x.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 97.Correa AJ, Reinisch L, Sanders DL, Huang S, Deriso W, Duncavage JA, Garrett CG. Inhibition of subglottic stenosis with mitomycin-C in the canine model. Ann Otol Rhinol Laryngol. 1999;108:1053–1060. doi: 10.1177/000348949910801106. [DOI] [PubMed] [Google Scholar]
- 98.Rahbar R, Valdez TA, Shapshay SM. Preliminary results of intraoperative mitomycin-C in the treatment and prevention of glottic and subglottic stenosis. J Voice. 2000;14:282–286. doi: 10.1016/s0892-1997(00)80037-5. [DOI] [PubMed] [Google Scholar]
- 99.Garrett CG, Soto J, Riddick J, Billante CR, Reinisch L. Effect of mitomycin-C on vocal fold healing in a canine model. Ann Otol Rhinol Laryngol. 2001;110:25–30. doi: 10.1177/000348940111000105. [DOI] [PubMed] [Google Scholar]
- 100.Hartnick CJ, Hartley BE, Lacy PD, Liu J, Bean JA, Willging JP, Myer CM, 3rd, Cotton RT. Topical mitomycin application after laryngotracheal reconstruction: a randomized, double-blind, placebo-controlled trial. Arch Otolaryngol Head Neck Surg. 2001;127:1260–1264. doi: 10.1001/archotol.127.10.1260. [DOI] [PubMed] [Google Scholar]
- 101.Spector JE, Werkhaven JA, Spector NC, Huang S, Sanders D, Reinisch L. Prevention of anterior glottic restenosis in a canine model with topical mitomycin-C. Ann Otol Rhinol Laryngol. 2001;110:1007–1010. doi: 10.1177/000348940111001103. [DOI] [PubMed] [Google Scholar]
- 102.Baugnee PE, Marquette CH, Ramon P, Darras J, Wurtz A. Endoscopic treatment of post-intubation tracheal stenosis. Apropos of 58 cases. Rev Mal Respir. 1995;12:585–592. [PubMed] [Google Scholar]
- 103.Brichet A, Verkindre C, Ramon P, Marquette CH. Post-intubation tracheal stenosis. Rev Mal Respir. 1999;16:685–692. [PubMed] [Google Scholar]
- 104.van den Boogert J, Hans Hoeve LJ, Struijs A, Hagenouw RR, Bogers AJ. Single-stage surgical repair of benign laryngotracheal stenosis in adults. Head Neck. 2004;26:111–117. doi: 10.1002/hed.10364. [DOI] [PubMed] [Google Scholar]
- 105.Cavanaugh K, Park AH. Recurrent laryngeal nerve monitoring during cricotracheal resection. Ann Otol Rhinol Laryngol. 2000;109:654–657. doi: 10.1177/000348940010900708. [DOI] [PubMed] [Google Scholar]
- 106.Pearson FG, Gullane P. Subglottic resection with primary tracheal anastomosis: including synchronous laryngotracheal reconstructions. Semin Thorac Cardiovasc Surg. 1996;8:381–391. [PubMed] [Google Scholar]
- 107.Jewett BS, Cook RD, Johnson KL, Logan TC, Shockley WW. Effect of stenting after laryngotracheal reconstruction in a subglottic stenosis model. Otolaryngol Head Neck Surg. 2000;122:488–494. doi: 10.1067/mhn.2000.102112. [DOI] [PubMed] [Google Scholar]
- 108.Gallivan GJ. Bilateral vocal fold posterior glottic/subglottic stenotic web resected with contact tip Nd-YAG laser. J Voice. 2002;16:415–421. doi: 10.1016/s0892-1997(02)00113-3. [DOI] [PubMed] [Google Scholar]
- 109.Casiano RR, Lundy DS. Outpatient transoral laser vaporization of anterior glottic webs and keel placement: risks of airway compromise. J Voice. 1998;12:536–539. doi: 10.1016/s0892-1997(98)80062-3. [DOI] [PubMed] [Google Scholar]
- 110.Wang Z, Pankratov MM, Gleich LL, Rebeiz EE, Shapshay SM. New technique for laryngotracheal mucosa transplantation. 'Stamp' welding using indocyanine green dye and albumin interaction with diode laser. Arch Otolaryngol Head Neck Surg. 1995;121:773–777. doi: 10.1001/archotol.1995.01890070059013. [DOI] [PubMed] [Google Scholar]
- 111.Hsiung MW, Wang HW. Endoscopic buccal mucosal grafting to the anterior glottic web: a case report. Eur Arch Otorhinolaryngol. 2002;259:287–289. doi: 10.1007/s00405-002-0476-8. [DOI] [PubMed] [Google Scholar]
- 112.Wang Z, Pankratov MM, Rebeiz EE, Perrault DF, Jr, Shapshay SM. Endoscopic diode laser welding of mucosal grafts on the larynx: a new technique. Laryngoscope. 1995;105:49–52. doi: 10.1288/00005537-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 113.Klussmann JP, Knoedgen R, Wittekindt C, Damm M, Eckel HE. Complications of suspension laryngoscopy. Ann Otol Rhinol Laryngol. 2002;111:972–976. doi: 10.1177/000348940211101104. [DOI] [PubMed] [Google Scholar]
- 114.Müller A, Verges L, Schleier P, Wohlfarth M, Gottschall R. The incidence of microlaryngoscopy associated complications. HNO. 2002;50:1057–1061. doi: 10.1007/s00106-002-0640-x. [DOI] [PubMed] [Google Scholar]
- 115.Müller A, Verges L, Gottschall R. Prognostic value of a screening test for difficult microlaryngoscopy. HNO. 2002;50:727–732. doi: 10.1007/s00106-001-0585-5. [DOI] [PubMed] [Google Scholar]
- 116.Wallner F, Knoch H. The potential uses and limitations of the flexible laryngoscopy under local anesthesia in clinical practice. HNO. 1999;47:702–705. doi: 10.1007/s001060050448. [DOI] [PubMed] [Google Scholar]
- 117.Schade G, Hess M. Endolaryngeal biopsies with local anesthesia. HNO. 2002;50:940–942. doi: 10.1007/s00106-002-0706-9. [DOI] [PubMed] [Google Scholar]
- 118.Okada S, Yamauchi H, Sato S. Use of laryngeal mask in bronchoscopy or bronchoscopic treatment. Kyobu Geka. 1998;51:996–1000. [PubMed] [Google Scholar]
- 119.Jameson JJ, Moses RD, Vellayappan U, Lathi KG. Use of the laryngeal mask airway for laser treatment of the subglottis. Otolaryngol Head Neck Surg. 2000;123:101–102. doi: 10.1067/mhn.2000.107457. [DOI] [PubMed] [Google Scholar]
- 120.Yavascaoglu B, Tokat O, Basagan EM, Kaya FN, Erisen L, Kutlay O. The use of the laryngeal mask airway in children with subglottic stenosis. J Int Med Res. 2001;29:541–545. doi: 10.1177/147323000102900612. [DOI] [PubMed] [Google Scholar]
- 121.Gottschall R. The Laryngeal Mask – its Importance in Anaesthesia. Anasthesiol Intensivmed Notfallmed Schmerzther. 2004;39:497–501. doi: 10.1055/s-2004-825844. [DOI] [PubMed] [Google Scholar]
- 122.Kanagalingam J, Hurley R, Grant HR, Patel A. A new technique for the management of inaccessible anterior glottic lesions. J Laryngol Otol. 2003;117:302–306. doi: 10.1258/00222150360600922. [DOI] [PubMed] [Google Scholar]
- 123.Kashima HK. Bilateral vocal fold motion impairment: pathophysiology and management by transverse cordotomy. Ann Otol Rhinol Laryngol. 1991;100:717–721. doi: 10.1177/000348949110000905. [DOI] [PubMed] [Google Scholar]
- 124.Benjamin B. Vocal cord paralysis, synkinesis and vocal fold motion impairment. ANZ J Surg. 2003;73:784–786. doi: 10.1046/j.1445-2197.2003.02799.x. [DOI] [PubMed] [Google Scholar]
- 125.Eskew JR, Bailey BJ. Laser arytenoidectomy for bilateral vocal cord paralysis. Otolaryngol Head Neck Surg. 1983;91:294–298. doi: 10.1177/019459988309100317. [DOI] [PubMed] [Google Scholar]
- 126.Ossoff RH, Karlan MS, Sisson GA. Endoscopic laser arytenoidectomy. Lasers Surg Med. 1983;2:293–299. doi: 10.1002/lsm.1900020402. [DOI] [PubMed] [Google Scholar]
- 127.Lim RY. Laser arytenoidectomy. Arch Otolaryngol. 1985;111:262–263. doi: 10.1001/archotol.1985.00800060086013. [DOI] [PubMed] [Google Scholar]
- 128.Prasad U. CO2 surgical laser in the management of bilateral vocal cord paralysis. J Laryngol Otol. 1985;99:891–894. doi: 10.1017/s0022215100097863. [DOI] [PubMed] [Google Scholar]
- 129.Remsen K, Lawson W, Patel N, Biller HF. Laser lateralization for bilateral vocal cord abductor paralysis. Otolaryngol Head Neck Surg. 1985;93:645–649. doi: 10.1177/019459988509300514. [DOI] [PubMed] [Google Scholar]
- 130.Dennis DP, Kashima H. Carbon dioxide laser posterior cordectomy for treatment of bilateral vocal cord paralysis. Ann Otol Rhinol Laryngol. 1989;98:930–934. doi: 10.1177/000348948909801203. [DOI] [PubMed] [Google Scholar]
- 131.Ossoff RH, Duncavage JA, Shapshay SM, Krespi YP, Sisson GA, Sr Endoscopic laser arytenoidectomy revisited. Ann Otol Rhinol Laryngol. 1990;99:764–771. doi: 10.1177/000348949009901002. [DOI] [PubMed] [Google Scholar]
- 132.Eckel HE. Laser surgical microlaryngoscopic glottis dilatation in the treatment of recurrent bilateral nerve paralysis. Surgical technique and results. Laryngol Rhinol Otol. 1991;70:17–20. doi: 10.1055/s-2007-997976. [DOI] [PubMed] [Google Scholar]
- 133.Holm AF, Wouters B, Van Overbeek JJM. CO2 Laser cordectomy for bilateral vocal cord paralysis. Las Med Sci. 1989;4:93–96. [Google Scholar]
- 134.Rontal M, Rontal E. Endoscopic laryngeal surgery for bilateral midline vocal cord obstruction. Ann Otol Rhinol Laryngol. 1990;99:605–610. doi: 10.1177/000348949009900803. [DOI] [PubMed] [Google Scholar]
- 135.Bower CM, Choi SS, Cotton RT. Arytenoidectomy in children. Ann Otol Rhinol Laryngol. 1994;103:271–278. doi: 10.1177/000348949410300403. [DOI] [PubMed] [Google Scholar]
- 136.Bower CM, Choi SS, Cotton RT. Arytenoidectomy in children. Ann Otol Rhinol Laryngol. 1994;103:271–278. doi: 10.1177/000348949410300403. [DOI] [PubMed] [Google Scholar]
- 137.Merite Drancy A, Laccourreye O, Brasnu D, Laccourreye H. Partial posterior cordectomy with laser CO2 in bilateral recurrent paralysis. Ann Otolaryngol Chir Cervicofac. 1992;109:235–239. [PubMed] [Google Scholar]
- 138.Linder A, Lindholm CE. Vocal fold lateralization using carbon dioxide laser and fibrin glue. J Laryngol Otol. 1992;106:226–230. doi: 10.1017/s0022215100119127. [DOI] [PubMed] [Google Scholar]
- 139.Eckel HE, Thumfart M, Wassermann K, Vossing M, Thumfart WF. Cordectomy versus arytenoidectomy in the management of bilateral vocal cord paralysis. Ann Otol Rhinol Laryngol. 1994;103:852–857. doi: 10.1177/000348949410301105. [DOI] [PubMed] [Google Scholar]
- 140.Herberhold C, Huck P. Posterior cordotomy by CO2 laser surgery for bilateral vocal cord paralysis: Kashima's technique and modified technique. Adv Otorhinolaryngol. 1995;49:174–175. doi: 10.1159/000424366. [DOI] [PubMed] [Google Scholar]
- 141.Nawka T. Endoscopic management of bilateral recurrent nerve paralysis with the CO2 laser. Adv Otorhinolaryngol. 1995;49:170–173. [PubMed] [Google Scholar]
- 142.Bigenzahn W, Hoefler H. Minimally invasive laser surgery for the treatment of bilateral vocal cord paralysis. Laryngoscope. 1996;106:791–793. doi: 10.1097/00005537-199606000-00024. [DOI] [PubMed] [Google Scholar]
- 143.Eckel HE, Vossing M. Endolaryngeal surgical procedures in glottis expansion in bilateral recurrent nerve paralysis. Laryngol Rhinol Otol. 1996;75:215–222. doi: 10.1055/s-2007-997565. [DOI] [PubMed] [Google Scholar]
- 144.Remacle M, Lawson G, Mayne A, Jamart J. Subtotal carbon dioxide laser arytenoidectomy by endoscopic approach for treatment of bilateral cord immobility in adduction. Ann Otol Rhinol Laryngol. 1996;105:438–445. doi: 10.1177/000348949610500604. [DOI] [PubMed] [Google Scholar]
- 145.Reker U, Rudert H. Modified posterior Dennis and Kashima cordectomy in treatment of bilateral recurrent nerve paralysis. Laryngol Rhinol Otol. 1998;77:213–218. doi: 10.1055/s-2007-996963. [DOI] [PubMed] [Google Scholar]
- 146.Laccourreye O, Paz Escovar MI, Gerhardt J, Hans S, Biacabe B, Brasnu D. CO2 laser endoscopic posterior partial transverse cordotomy for bilateral paralysis of the vocal fold. Laryngoscope. 1999;109:415–418. doi: 10.1097/00005537-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 147.Maurizi M, Paludetti G, Galli J, Cosenza A, Di Girolamo S, Ottaviani F. CO2 laser subtotal arytenoidectomy and posterior true and false cordotomy in the treatment of post-thyroidectomy bilateral laryngeal fixation in adduction. Eur Arch Otorhinolaryngol. 1999;256:291–295. doi: 10.1007/s004050050248. [DOI] [PubMed] [Google Scholar]
- 148.Pia F, Pisani P, Aluffi P. CO(2) laser posterior ventriculocordectomy for the treatment of bilateral vocal cord paralysis. Eur Arch Otorhinolaryngol. 1999;256:403–406. doi: 10.1007/s004050050175. [DOI] [PubMed] [Google Scholar]
- 149.Misiolek M, Namyslowski G, Warmuzinski K, Karpe J, Rauer R, Misiolek H. The influence of laser arytenoidectomy on ventilation parameters in patients with bilateral vocal cord paralysis. Eur Arch Otorhinolaryngol. 2003;260:381–385. doi: 10.1007/s00405-003-0603-1. [DOI] [PubMed] [Google Scholar]
- 150.Motta S, Moscillo L, Imperiali M, Motta G. CO2 laser treatment of bilateral vocal cord paralysis in adduction. ORL J Otorhinolaryngol Relat Spec. 2003;65:359–365. doi: 10.1159/000076055. [DOI] [PubMed] [Google Scholar]
- 151.Bizakis JG, Papadakis CE, Karatzanis AD, Skoulakis CE, Kyrmizakis DE, Hajiioannou JK, Helidonis ES. The combined endoscopic CO(2) laser posterior cordectomy and total arytenoidectomy for treatment of bilateral vocal cord paralysis. Clin Otolaryngol. 2004;29:51–54. doi: 10.1111/j.1365-2273.2004.00779.x. [DOI] [PubMed] [Google Scholar]
- 152.Albrecht R. Value of colposcopic method of examination in leucoplakia and carcinoma of the mouth and the larynx. Arch Ohren Nasen Kehlkopfheilkd. 1954;165:459–463. [PubMed] [Google Scholar]
- 153.Kleinsasser O. Endolaryngeal microsurgery. J Otolaryngol Soc Aust. 1968;2:3–7. [PubMed] [Google Scholar]
- 154.Kleinsasser O. Microlaryngoscopy and endolaryngeal microsurgery. Philadelphia: Saunders; 1968. [Google Scholar]
- 155.Reidenbach MM. Anatomical bases of glottic widening surgery related to arytenoidectomy. Clin Anat. 1999;12:94–102. doi: 10.1002/(SICI)1098-2353(1999)12:2<94::AID-CA3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 156.Rontal M, Rontal E. Use of laryngeal muscular tenotomy for bilateral midline vocal cord fixation. Ann Otol Rhinol Laryngol. 1994;103:583–589. doi: 10.1177/000348949410300801. [DOI] [PubMed] [Google Scholar]
- 157.Eckel HE, Sittel C. Bilateral recurrent laryngeal nerve paralysis. HNO. 2001;49:166–179. doi: 10.1007/s001060050729. [DOI] [PubMed] [Google Scholar]
- 158.Saetti R, Silvestrini M, Galiotto M, Derosas F, Narne S. Contact laser surgery in treatment of vocal fold paralysis. Acta Otorhinolaryngol Ital. 2003;23:33–37. [PubMed] [Google Scholar]
- 159.el Chazly M, Rifai M, el Ezz AA. Arytenoidectomy and posterior cordectomy for bilateral abductor paralysis. J Laryngol Otol. 1991;105:454–455. doi: 10.1017/s0022215100116287. [DOI] [PubMed] [Google Scholar]
- 160.Hoover WB. Bilateral abductor paralysis: Operative treatment of submucous resection of the vocal cords. Arch Otolaryngol. 1932;15:339–355. [Google Scholar]
- 161.Kleinsasser O. Endolaryngeal arytenoidectomy and submucous hemichordectomy for the widening of the glottis in bilateral abductor paralysis. Monatsschr Ohrenheilkd Laryngorhinol. 1968;102:443–446. [PubMed] [Google Scholar]
- 162.Kleinsasser O, Nolte E. Report on the indication, technique and functional results of endolaryngeal arytenoidectomy and submucous partial chordectomy in bilateral paralysis of vocal cord. Laryngol Rhinol Otol (Stuttg) 1981;60:397–401. [PubMed] [Google Scholar]
- 163.Jiang JJ, Titze IR. A methodological study of hemilaryngeal phonation. Laryngoscope. 1993;103:872–882. doi: 10.1288/00005537-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 164.Xu W, Han D, Hou L, Zhang L, Yu Z, Huang Z, Ye J, Wang J. Study on voice quality after different layers injured in vocal fold with CO2 laser microsurgery. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16:267–269. [PubMed] [Google Scholar]
- 165.Peretti G, Piazza C, Balzanelli C, Cantarella G, Nicolai P. Vocal outcome after endoscopic cordectomies for Tis and T1 glottic carcinomas. Ann Otol Rhinol Laryngol. 2003;112:174–179. doi: 10.1177/000348940311200212. [DOI] [PubMed] [Google Scholar]
- 166.Lopez Llames A, Nunez Batalla F, Llorente Pendas JL, Puente Verez M, Aldama Barahona P, Suarez Nieto C. Laser cordectomy: oncologic outcome and functional results. Acta Otorrinolaringol Esp. 2004;55:34–40. doi: 10.1016/s0001-6519(04)78480-0. [DOI] [PubMed] [Google Scholar]
- 167.Kirchner FR. Endoscopic lateralization of the vocal cord in abductor paralysis of the larynx. Laryngoscope. 1979;89:1779–1783. doi: 10.1288/00005537-197911000-00010. [DOI] [PubMed] [Google Scholar]
- 168.Lichtenberger G. Endo-extralaryngeal needle carrier instrument. Laryngoscope. 1983;93:1348–1350. doi: 10.1002/lary.1983.93.10.1348. [DOI] [PubMed] [Google Scholar]
- 169.Lichtenberger G, Toohill RJ. The endo-extralaryngeal needle carrier. Otolaryngol Head Neck Surg. 1991;105:755–756. doi: 10.1177/019459989110500522. [DOI] [PubMed] [Google Scholar]
- 170.Lichtenberger G, Toohill RJ. Technique of endo-extralaryngeal suture lateralization for bilateral abductor vocal cord paralysis. Laryngoscope. 1997;107:1281–1283. doi: 10.1097/00005537-199709000-00023. [DOI] [PubMed] [Google Scholar]
- 171.Lichtenberger G. Reversible immediate and definitive lateralization of paralyzed vocal cords. Eur Arch Otorhinolaryngol. 1999;256:407–411. doi: 10.1007/s004050050176. [DOI] [PubMed] [Google Scholar]
- 172.Rovo L, Jori J, Brzozka M, Czigner J. Airway complication after thyroid surgery: minimally invasive management of bilateral recurrent nerve injury. Laryngoscope. 2000;110:140–144. doi: 10.1097/00005537-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 173.Lichtenberger G. Reversible lateralization of the paralyzed vocal cord without tracheostomy. Ann Otol Rhinol Laryngol. 2002;111:21–26. doi: 10.1177/000348940211100104. [DOI] [PubMed] [Google Scholar]
- 174.Lichtenberger G. Prevention and management of bilateral vocal cord paralysis by and after thyroid surgery. Otolaryngol Pol. 2004;58:165–171. [PubMed] [Google Scholar]
- 175.Mathur NN, Kumar S, Bothra R. Simple method of vocal cord lateralization in bilateral abductor cord paralysis in paediatric patients. Int J Pediatr Otorhinolaryngol. 2004;68:15–20. doi: 10.1016/j.ijporl.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 176.Jamski J, Jamska A, Graca M, Barczynski M, Wlodyka J. Recurrent laryngeal nerve injury following thyroid surgery. Przegl Lek. 2004;61:13–16. [PubMed] [Google Scholar]
- 177.Schneider W, Wolf SR, Krause JW. Electromyography study and follow-up of bilateral recurrent laryngeal nerve paralysis after thyroid gland operations. HNO. 1997;45:551–555. doi: 10.1007/s001060050130. [DOI] [PubMed] [Google Scholar]
- 178.Munin MC, Rosen CA, Zullo T. Utility of laryngeal electromyography in predicting recovery after vocal fold paralysis. Arch Phys Med Rehabil. 2003;84:1150–1153. doi: 10.1016/s0003-9993(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 179.Thumfart WF. Electromyography of the larynx and related technics. Acta Otorhinolaryngol Belg. 1986;40:358–376. [PubMed] [Google Scholar]
- 180.Blitzer A, Jahn AF, Keidar A. Semon's law revisited: an electromyographic analysis of laryngeal synkinesis. Ann Otol Rhinol Laryngol. 1996;105:764–769. doi: 10.1177/000348949610501002. [DOI] [PubMed] [Google Scholar]
- 181.Hillel AD, Benninger M, Blitzer A, Crumley R, Flint P, Kashima HK, Sanders I, Schaefer S. Evaluation and management of bilateral vocal cord immobility. Otolaryngol Head Neck Surg. 1999;121:760–765. doi: 10.1053/hn.1999.v121.a98733. [DOI] [PubMed] [Google Scholar]
- 182.Sulica L, Blitzer A. Electromyography and the immobile vocal fold. Otolaryngol Clin North Am. 2004;37:59–74. doi: 10.1016/S0030-6665(03)00168-3. [DOI] [PubMed] [Google Scholar]
- 183.Steiner W. Results of curative laser microsurgery of laryngeal carcinomas. Am J Otolaryngol. 1993;14:116–121. doi: 10.1016/0196-0709(93)90050-h. [DOI] [PubMed] [Google Scholar]
- 184.Moreau PR. Treatment of laryngeal carcinomas by laser endoscopic microsurgery. Laryngoscope. 2000;110:1000–1006. doi: 10.1097/00005537-200006000-00022. [DOI] [PubMed] [Google Scholar]
- 185.Steiner W, Vogt P, Ambrosch P, Kron M. Transoral carbon dioxide laser microsurgery for recurrent glottic carcinoma after radiotherapy. Head Neck. 2004;26:477–484. doi: 10.1002/hed.20009. [DOI] [PubMed] [Google Scholar]
- 186.Dietz A, Nollert J, Eckel H, Volling P, Schroder M, Staar S, Conradt C, Helmke B, Dollner R, Muller RP, Wannenmacher M, Weidauer H, Rudat V. Organ preservation in advanced laryngeal and hypopharyngeal carcinoma by primary radiochemotherapy. Results of a multicenter phase II study. HNO. 2002;50:146–154. doi: 10.1007/s001060100541. [DOI] [PubMed] [Google Scholar]
- 187.Marmiroli L, Ausili-Cefaro G, Nardone L, Fiorentino G, Genovesi D, Salvi G. Combined radiochemotherapy for organ preservation in head and neck cancer: review of literature and personal experience. Rays. 1997;22:425–440. [PubMed] [Google Scholar]
- 188.Sannazzari GL, Gabriele P, Redda MG, Verna R. Combined modality therapy for laryngeal and hypopharyngeal cancer. Rays. 1997;22:360–371. [PubMed] [Google Scholar]
- 189.Delaere PR. Laryngotracheal reconstruction. From lab to clinic. Berlin, Heidelberg, New York: Springer; 2004. p. 304. [Google Scholar]
- 190.Evans JN, Todd GB. Laryngo-tracheoplasty. J Laryngol Otol. 1974;88:589–597. doi: 10.1017/s0022215100079147. [DOI] [PubMed] [Google Scholar]
- 191.MacRae D, Barrie P. "Swiss roll" laryngotracheoplasty in young children. J Otolaryngol. 1986;15:116–118. [PubMed] [Google Scholar]
- 192.Schultz-Coulon HJ, Laubert A. [Laryngotracheoplasty in early childhood] HNO. 1988;36:1–12. [PubMed] [Google Scholar]
- 193.Rethi A. Chirurgie der Verengungen der oberen Luftwege. Stuttgart: Georg Thieme; 1959. pp. 55–61. [Google Scholar]
- 194.Cotton R. Management of subglottic stenosis in infancy and childhood. Review of a consecutive series of cases managed by surgical reconstruction. Ann Otol Rhinol Laryngol. 1978;87:649–657. doi: 10.1177/000348947808700509. [DOI] [PubMed] [Google Scholar]
- 195.Drake AF, Contencin P, Narcy F, Cotton RT. Lateral cricoid cuts as an adjunctive measure to enlarge the stenotic subglottic airway: an anatomic study. Int J Pediatr Otorhinolaryngol. 1989;18:129–137. doi: 10.1016/0165-5876(89)90065-7. [DOI] [PubMed] [Google Scholar]
- 196.Cotton RT, Mortelliti AJ, Myer CM., 3rd Four-quadrant cricoid cartilage division in laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 1992;118:1023–1027. doi: 10.1001/archotol.1992.01880100013005. [DOI] [PubMed] [Google Scholar]
- 197.Varvares MA, Montgomery WW. Repair of chronic subglottic stenosis with autogenous thyroid cartilage. Ann Otol Rhinol Laryngol. 2004;113:212–217. doi: 10.1177/000348940411300308. [DOI] [PubMed] [Google Scholar]
- 198.Cansiz H, Yener HM, Sekercioglu N, Gunes M. Laryngotracheal reconstruction with a muscle-pedicle hyoid bone flap: a series of 23 patients. Ear Nose Throat J. 2004;83:424–427. [PubMed] [Google Scholar]
- 199.Delaere PR, Liu Z, Pauwels P, Feenstra L. Experimental revascularization of airway segments. Laryngoscope. 1994;104:736–740. doi: 10.1288/00005537-199406000-00014. [DOI] [PubMed] [Google Scholar]
- 200.Delaere PR, Liu Z, Feenstra L. Tracheal autograft revascularization and transplantation. Arch Otolaryngol Head Neck Surg. 1994;120:1130–1136. doi: 10.1001/archotol.1994.01880340070012. [DOI] [PubMed] [Google Scholar]
- 201.Delaere PR, Poorten VV, Goeleven A, Feron M, Hermans R. Tracheal autotransplantation: a reliable reconstructive technique for extended hemilaryngectomy defects. Laryngoscope. 1998;108:929–934. doi: 10.1097/00005537-199806000-00025. [DOI] [PubMed] [Google Scholar]
- 202.Delaere PR, Vander Poorten V, Hermans R. Autotransplantation of the trachea: experimental evaluation of a reconstructive technique for extended hemilaryngectomy defects. Ann Otol Rhinol Laryngol. 1999;108:143–146. doi: 10.1177/000348949910800207. [DOI] [PubMed] [Google Scholar]
- 203.Delaere P. From concept to clinical reality: implementing tracheal autotransplantation in conservation laryngectomy. Acta Otorhinolaryngol Belg. 1999;53:181–188. [PubMed] [Google Scholar]
- 204.Hardillo JA, Vander Poorten V, Delaere PR. Transplantation of tracheal autografts: is a two-stage procedure necessary? Acta Otorhinolaryngol Belg. 2000;54:13–21. [PubMed] [Google Scholar]
- 205.Delaere PR, Hermans R. Tracheal autotransplantation as a new and reliable technique for the functional treatment of advanced laryngeal cancer. Laryngoscope. 2003;113:1244–1251. doi: 10.1097/00005537-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 206.Delaere PR, Vertriest R, Hermans R. Functional treatment of a large laryngeal chondrosarcoma by tracheal autotransplantation. Ann Otol Rhinol Laryngol. 2003;112:678–682. doi: 10.1177/000348940311200805. [DOI] [PubMed] [Google Scholar]
- 207.Lin P, Yang B. [Reconstruction with pyriform sinus mucosa in partial laryngectomy] Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16:665–666. [PubMed] [Google Scholar]
- 208.Glatz F, Neumeister M, Suchy H, Lyons S, Damikas D, Mowlavi A. A tissue-engineering technique for vascularized laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 2003;129:201–206. doi: 10.1001/archotol.129.2.201. [DOI] [PubMed] [Google Scholar]
- 209.Rees LE, Ayoub O, Haverson K, Birchall MA, Bailey M. Differential major histocompatibility complex class II locus expression on human laryngeal epithelium. Clin Exp Immunol. 2003;134:497–502. doi: 10.1111/j.1365-2249.2003.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wambach BA, Cheung H, Josephson GD. Cartilage tissue engineering using thyroid chondrocytes on a type I collagen matrix. Laryngoscope. 2000;110:2008–2011. doi: 10.1097/00005537-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 211.Huber JE, Spievack A, Simmons-Byrd A, Ringel RL, Badylak S. Extracellular matrix as a scaffold for laryngeal reconstruction. Ann Otol Rhinol Laryngol. 2003;112:428–433. doi: 10.1177/000348940311200508. [DOI] [PubMed] [Google Scholar]
- 212.Bucheler M, Haisch A. Tissue engineering in otorhinolaryngology. DNA Cell Biol. 2003;22:549–564. doi: 10.1089/104454903322405446. [DOI] [PubMed] [Google Scholar]
- 213.Omori K, Nakamura T, Kanemaru S, Kojima H, Magrufov A, Hiratsuka Y, Shimizu Y. Cricoid regeneration using in situ tissue engineering in canine larynx for the treatment of subglottic stenosis. Ann Otol Rhinol Laryngol. 2004;113:623–627. doi: 10.1177/000348940411300805. [DOI] [PubMed] [Google Scholar]
- 214.Kamil SH, Eavey RD, Vacanti MP, Vacanti CA, Hartnick CJ. Tissue-engineered cartilage as a graft source for laryngotracheal reconstruction: a pig model. Arch Otolaryngol Head Neck Surg. 2004;130:1048–1051. doi: 10.1001/archotol.130.9.1048. [DOI] [PubMed] [Google Scholar]
- 215.Ferguson RM, Henry ML, Elkhammas EA, Davies EA, Bumgardner GL, Pelletier RP, Rajab A. Twenty years of renal transplantation at Ohio State University: the results of five eras of immunosuppression. Am J Surg. 2003;186:306–311. doi: 10.1016/s0002-9610(03)00219-8. [DOI] [PubMed] [Google Scholar]
- 216.Hettiaratchy S, Butler PE. Extending the boundaries of transplantation. BMJ. 2003;326:1226–1227. doi: 10.1136/bmj.326.7401.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Takenouchi S, Ogura JH, Kawasaki M, Yagi M. Autogenous transplantation of the canine larynx. Laryngoscope. 1967;77:1644–1667. doi: 10.1288/00005537-196709000-00004. [DOI] [PubMed] [Google Scholar]
- 218.Mounier-Kuhn P, Haguenauer JP, Inuyama Y, Descotes J. [Feasibility of autologous graft of the larynx in dogs] Ann Otolaryngol Chir Cervicofac. 1968;85:637–654. [PubMed] [Google Scholar]
- 219.Mounier-Kuhn P, Haguenauer JP. [Autotransplantation of the larynx in the dog] Arch Ital Otol Rinol Laringol Patol Cervicofacc. 1970;81:341–352. [PubMed] [Google Scholar]
- 220.Silver CE, Rosen RG. Function of transplanted and denervated larynges. II. Arytenoidectomy with sensory reinnervation. Arch Otolaryngol. 1974;99:100–101. doi: 10.1001/archotol.1974.00780030106005. [DOI] [PubMed] [Google Scholar]
- 221.Kluyskens P, Ringoir S. Follow-up of a human larynx transplantation. Laryngoscope. 1970;80:1244–1250. doi: 10.1288/00005537-197008000-00006. [DOI] [PubMed] [Google Scholar]
- 222.Strome M, Strome S. Laryngeal transplantation. The future. Otolaryngol Clin North Am. 1991;24:1385–1389. [PubMed] [Google Scholar]
- 223.Strome S, Strome M. Laryngeal transplantation: ethical considerations. Am J Otolaryngol. 1992;13:75–77. doi: 10.1016/0196-0709(92)90003-c. [DOI] [PubMed] [Google Scholar]
- 224.Strome M, Strome S, Darrell J, Wu J, Brodsky G. The effects of cyclosporin A on transplanted rat allografts. Laryngoscope. 1993;103:394–398. doi: 10.1002/lary.5541030406. [DOI] [PubMed] [Google Scholar]
- 225.Strome M, Strome S. Laryngeal transplantation: a program for investigating new parameters. J Voice. 1994;8:92–94. doi: 10.1016/s0892-1997(05)80325-x. [DOI] [PubMed] [Google Scholar]
- 226.Strome M, Wu J, Strome S, Brodsky G. A comparison of preservation techniques in a vascularized rat laryngeal transplant model. Laryngoscope. 1994;104:666–668. doi: 10.1288/00005537-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 227.Strome M. Human laryngeal transplantation: considerations and implications. Microsurgery. 2000;20:372–374. doi: 10.1002/1098-2752(2000)20:8<372::aid-micr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 228.Strome M, Stein J, Esclamado R, Hicks D, Lorenz RR, Braun W, Yetman R, Eliachar I, Mayes J. Laryngeal transplantation and 40-month follow-up. N Engl J Med. 2001;344:1676–1679. doi: 10.1056/NEJM200105313442204. [DOI] [PubMed] [Google Scholar]
- 229.Lorenz RR, Dan O, Fritz MA, Nelson M, Strome M. Rat laryngeal transplant model: technical advancements and a redefined rejection grading system. Ann Otol Rhinol Laryngol. 2002;111:1120–1127. doi: 10.1177/000348940211101211. [DOI] [PubMed] [Google Scholar]
- 230.Nelson M, Fritz M, Dan O, Worley S, Strome M. Tacrolimus and mycophenolate mofetil provide effective immunosuppression in rat laryngeal transplantation. Laryngoscope. 2003;113:1308–1313. doi: 10.1097/00005537-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 231.Akst LM, Siemionow M, Dan O, Izycki D, Strome M. Induction of tolerance in a rat model of laryngeal transplantation. Transplantation. 2003;76:1763–1770. doi: 10.1097/01.TP.0000100398.39169.5B. [DOI] [PubMed] [Google Scholar]
- 232.Birchall MA. Laryngeal transplantation. Br J Surg. 1997;84:739–740. doi: 10.1002/bjs.1800840603. [DOI] [PubMed] [Google Scholar]
- 233.Birchall M. Human laryngeal allograft: shift of emphasis in transplantation. Lancet. 1998;351:539–540. doi: 10.1016/S0140-6736(05)78550-0. [DOI] [PubMed] [Google Scholar]
- 234.Stavroulaki P, Birchall M. Comparative study of the laryngeal innervation in humans and animals employed in laryngeal transplantation research. J Laryngol Otol. 2001;115:257–266. doi: 10.1258/0022215011907226. [DOI] [PubMed] [Google Scholar]
- 235.Birchall MA, Bailey M, Barker EV, Rothkotter HJ, Otto K, Macchiarini P. Model for experimental revascularized laryngeal allotransplantation. Br J Surg. 2002;89:1470–1475. doi: 10.1046/j.1365-2168.2002.02234.x. [DOI] [PubMed] [Google Scholar]
- 236.Birchall M, Idowu B, Murison P, Jones A, Burt R, Ayling S, Stokes C, Pope L, Terenghi G. Laryngeal abductor muscle reinnervation in a pig model. Acta Otolaryngol. 2004;124:839–846. doi: 10.1080/00016480410022507. [DOI] [PubMed] [Google Scholar]
- 237.Tucker HM. Laryngeal transplantation: current status 1974. Laryngoscope. 1975;85:787–796. doi: 10.1288/00005537-197505000-00003. [DOI] [PubMed] [Google Scholar]
- 238.Namyslowski G, Religa Z, Steszewska U, Misiollek M, Czecior E. [A case of laryngeal carcinoma as a result of immunosuppressive therapy with cyclosporin A following heart transplantation] Otolaryngol Pol. 1994;48:72–74. [PubMed] [Google Scholar]
- 239.Berenguer M, Prieto M, Bustamante M, Carrasco D, Lopez-Andujar R, Mir J, Berenguer J. [Incidence of de novo neoplasms after liver transplantation] Med Clin (Barc) 1998;111:481–484. [PubMed] [Google Scholar]
- 240.Garlicki M, Wierzbicki K, Przybylowski P, Drop D, Biernat M, Rudzinski P, Olszewska B, Dziatkowiak A. The incidence of malignancy in heart transplant recipients. Ann Transplant. 1998;3:41–47. [PubMed] [Google Scholar]
- 241.Pham SM, Kormos RL, Landreneau RJ, Kawai A, Gonzalez-Cancel I, Hardesty RL, Hattler BG, Griffith BP. Solid tumors after heart transplantation: lethality of lung cancer. Ann Thorac Surg. 1995;60:1623–1626. doi: 10.1016/0003-4975(95)00120-4. [DOI] [PubMed] [Google Scholar]
- 242.Berke GS, Ye M, Block RM, Sloan S, Sercarz J. Orthotopic laryngeal transplantation: is it time? Laryngoscope. 1993;103:857–864. doi: 10.1288/00005537-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 243.Genden EM, Urken ML. Laryngeal and tracheal transplantation: ethical limitations. Mt Sinai J Med. 2003;70:163–165. [PubMed] [Google Scholar]
- 244.Schwartz M. Liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2004;10:S81–S85. doi: 10.1002/lt.20048. [DOI] [PubMed] [Google Scholar]
- 245.Paniello RC. Laryngeal reinnervation. Otolaryngologic Clinics of North America. 2004;37:161–181. doi: 10.1016/S0030-6665(03)00164-6. [DOI] [PubMed] [Google Scholar]
- 246.Horsley J. Suture of the recurrent laryngeal nerve with report of a case. Trans South Surg Gynecol Assoc. 1909;22:161. [Google Scholar]
- 247.Gacek RR, Malmgren LT, Lyon MJ. Localization of adductor and abductor motor nerve fibers to the larynx. Ann Otol Rhinol Laryngol. 1977;86:771–776. [PubMed] [Google Scholar]
- 248.Malmgren LT, Gacek RR. Acetylcholinesterase staining of fiber components in feline and human recurrent laryngeal nerve. Topography of laryngeal motor fiber regions. Acta Otolaryngol. 1981;91:337–352. doi: 10.3109/00016488109138515. [DOI] [PubMed] [Google Scholar]
- 249.Siribodhi C, Sundmaker W, Atkins J, Bonner F. Electromyographic studies of laryngeal paralysis and regeneration of laryngeal motor nerves in dogs. Laryngoscope. 1963;73:148–164. [Google Scholar]
- 250.Chou FF, Su CY, Jeng SF, Hsu KL, Lu KY. Neurorrhaphy of the recurrent laryngeal nerve. J Am Coll Surg. 2003;197:52–57. doi: 10.1016/S1072-7515(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 251.Pototschnig C. Retrospektive multizentrische Analyse des Kehlkopf-EMG auf Synkinesie bei Patienten mit Rekurrensparese. Innsbruck: 2003. [Google Scholar]
- 252.Zealear DL, Billante CR. Neurophysiology of vocal fold paralysis. Otolaryngol Clin North Am. 2004;37:1–23. doi: 10.1016/S0030-6665(03)00165-8. [DOI] [PubMed] [Google Scholar]
- 253.Crumley RL. Laryngeal synkinesis revisited. Ann Otol Rhinol Laryngol. 2000;109:365–371. doi: 10.1177/000348940010900405. [DOI] [PubMed] [Google Scholar]
- 254.Streppel M, Azzolin N, Dohm S, Guntinas-Lichius O, Haas C, Grothe C, Wevers A, Neiss WF, Angelov DN. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. Eur J Neurosci. 2002;15:1327–1342. doi: 10.1046/j.1460-9568.2002.01971.x. [DOI] [PubMed] [Google Scholar]
- 255.Guntinas-Lichius O, Wewetzer K, Tomov TL, Azzolin N, Kazemi S, Streppel M, Neiss WF, Angelov DN. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002;22:7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Guntinas-Lichius O, Angelov DN, Tomov TL, Dramiga J, Neiss WF, Wewetzer K. Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons. Exp Neurol. 2001;172:70–80. doi: 10.1006/exnr.2001.7774. [DOI] [PubMed] [Google Scholar]
- 257.Nakamura T, Inada Y, Fukuda S, Yoshitani M, Nakada A, Itoi S, Kanemaru S, Endo K, Shimizu Y. Experimental study on the regeneration of peripheral nerve gaps through a polyglycolic acid-collagen (PGA-collagen) tube. Brain Res. 2004;1027:18–29. doi: 10.1016/j.brainres.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 258.Kanemaru S, Nakamura T, Omori K, Kojima H, Magrufov A, Hiratsuka Y, Ito J, Shimizu Y. Recurrent laryngeal nerve regeneration by tissue engineering. Ann Otol Rhinol Laryngol. 2003;112:492–498. doi: 10.1177/000348940311200602. [DOI] [PubMed] [Google Scholar]
- 259.Crumley RL. Update: ansa cervicalis to recurrent laryngeal nerve anastomosis for unilateral laryngeal paralysis. Laryngoscope. 1991;101:384–387. doi: 10.1002/lary.1991.101.4.384. [DOI] [PubMed] [Google Scholar]
- 260.Paniello RC, Lee P, Dahm JD. Hypoglossal nerve transfer for laryngeal reinnervation: a preliminary study. Ann Otol Rhinol Laryngol. 1999;108:239–244. doi: 10.1177/000348949910800304. [DOI] [PubMed] [Google Scholar]
- 261.Paniello RC. Laryngeal reinnervation with the hypoglossal nerve: II. Clinical evaluation and early patient experience. Laryngoscope. 2000;110:739–748. doi: 10.1097/00005537-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 262.Paniello RC, West SE, Lee P. Laryngeal reinnervation with the hypoglossal nerve. I. Physiology, histochemistry, electromyography, and retrograde labeling in a canine model. Ann Otol Rhinol Laryngol. 2001;110:532–542. doi: 10.1177/000348940111000607. [DOI] [PubMed] [Google Scholar]
- 263.Maronian N, Waugh P, Robinson L, Hillel A. Electromyographic findings in recurrent laryngeal nerve reinnervation. Ann Otol Rhinol Laryngol. 2003;112:314–323. doi: 10.1177/000348940311200405. [DOI] [PubMed] [Google Scholar]
- 264.Doyle PJ, Chepeha DB, Westerberg BD, Schwarz DW. Phrenic nerve reinnervation of the cat's larynx: a new technique with proven success. Ann Otol Rhinol Laryngol. 1993;102:837–842. doi: 10.1177/000348949310201103. [DOI] [PubMed] [Google Scholar]
- 265.Mahieu HF, van Lith-Bijl JT, Groenhout C, Tonnaer JA, de Wilde P. Selective laryngeal abductor reinnervation in cats using a phrenic nerve transfer and ORG 2766. Arch Otolaryngol Head Neck Surg. 1993;119:772–776. doi: 10.1001/archotol.1993.01880190068014. [DOI] [PubMed] [Google Scholar]
- 266.van Lith-Bijl JT, Stolk RJ, Tonnaer JA, Groenhout C, Konings PN, Mahieu HF. Selective laryngeal reinnervation with separate phrenic and ansa cervicalis nerve transfers. Arch Otolaryngol Head Neck Surg. 1997;123:406–411. doi: 10.1001/archotol.1997.01900040042007. [DOI] [PubMed] [Google Scholar]
- 267.van Lith-Bijl JT, Stolk RJ, Tonnaer JA, Groenhout C, Konings PN, Mahieu HF. Laryngeal abductor reinnervation with a phrenic nerve transfer after a 9-month delay. Arch Otolaryngol Head Neck Surg. 1998;124:393–398. doi: 10.1001/archotol.124.4.393. [DOI] [PubMed] [Google Scholar]
- 268.Rice DH. Laryngeal reinnervation. Laryngoscope. 1982;92:1049–1059. [PubMed] [Google Scholar]
- 269.Tucker HM. Human laryngeal reinnervation. Laryngoscope. 1976;86:769–779. doi: 10.1288/00005537-197606000-00004. [DOI] [PubMed] [Google Scholar]
- 270.El-Kashlan HK, Carroll WR, Hogikyan ND, Chepeha DB, Kileny PR, Esclamado RM. Selective cricothyroid muscle reinnervation by muscle-nerve-muscle neurotization. Arch Otolaryngol Head Neck Surg. 2001;127:1211–1215. doi: 10.1001/archotol.127.10.1211. [DOI] [PubMed] [Google Scholar]
- 271.Hogikyan ND, Johns MM, Kileny PR, Urbanchek M, Carroll WR, Kuzon WM, Jr Motion-specific laryngeal reinnervation using muscle-nerve-muscle neurotization. Ann Otol Rhinol Laryngol. 2001;110:801–810. doi: 10.1177/000348940111000901. [DOI] [PubMed] [Google Scholar]
- 272.Shiotani A, O'Malley BW, Jr, Coleman ME, Alila HW, Flint PW. Reinnervation of motor endplates and increased muscle fiber size after human insulin-like growth factor I gene transfer into the paralyzed larynx. Hum Gene Ther. 1998;9:2039–2047. doi: 10.1089/hum.1998.9.14-2039. [DOI] [PubMed] [Google Scholar]
- 273.Flint PW, Shiotani A, O'Malley BW., Jr IGF-1 gene transfer into denervated rat laryngeal muscle. Arch Otolaryngol Head Neck Surg. 1999;125:274–279. doi: 10.1001/archotol.125.3.274. [DOI] [PubMed] [Google Scholar]
- 274.Shiotani A, O'Malley BW, Jr, Coleman ME, Flint PW. Human insulinlike growth factor 1 gene transfer into paralyzed rat larynx: single vs multiple injection. Arch Otolaryngol Head Neck Surg. 1999;125:555–560. doi: 10.1001/archotol.125.5.555. [DOI] [PubMed] [Google Scholar]
- 275.Flint PW, Nakagawa H, Shiotani A, Coleman ME, O'Malley BW., Jr Effects of insulin-like growth factor-1 gene transfer on myosin heavy chains in denervated rat laryngeal muscle. Laryngoscope. 2004;114:368–371. doi: 10.1097/00005537-200402000-00035. [DOI] [PubMed] [Google Scholar]
- 276.Nakagawa H, Shiotani A, O'Malley BW, Jr, Coleman ME, Flint PW. Timing of human insulin-like growth factor-1 gene transfer in reinnervating laryngeal muscle. Laryngoscope. 2004;114:726–732. doi: 10.1097/00005537-200404000-00024. [DOI] [PubMed] [Google Scholar]
- 277.Zealear DL, Billante CL, Chongkolwatana C, Herzon GD. The effects of chronic electrical stimulation on laryngeal muscle reinnervation. ORL J Otorhinolaryngol Relat Spec. 2000;62:87–95. doi: 10.1159/000027723. [DOI] [PubMed] [Google Scholar]
- 278.Zealear DL, Rodriguez RJ, Kenny T, Billante MJ, Cho Y, Billante CR, Garren KC. Electrical stimulation of a denervated muscle promotes selective reinnervation by native over foreign motoneurons. J Neurophysiol. 2002;87:2195–2199. doi: 10.1152/jn.00451.2001. [DOI] [PubMed] [Google Scholar]
- 279.Zoll PM, Frank HA, Linenthal AJ. Four-Year Experience with an Implanted Cardiac Pacemaker. Ann Surg. 1964;160:351–365. doi: 10.1097/00000658-196409000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zealear DL, Dedo HH. Control of paralyzed axial muscles by electrical stimulation. Trans Sect Otolaryngol Am Acad Opthamol Otolaryngol. 1977;84:310. [PubMed] [Google Scholar]
- 281.Bergmann K, Warzel H, Eckhardt HU, Gerhardt HJ. [Respiration rhythm-controlled electric stimulation of paralyzed laryngeal muscles – animal experiment findings] Z Exp Chir Transplant Kunstliche Organe. 1983;16:344–351. [PubMed] [Google Scholar]
- 282.Bergmann K, Warzel H, Eckhardt HU, Gerhardt HJ. Respiratory rhythmically regulated electrical stimulation of paralyzed laryngeal muscles. Laryngoscope. 1984;94:1376–1380. doi: 10.1288/00005537-198410000-00022. [DOI] [PubMed] [Google Scholar]
- 283.Bergmann K, Warzel H, Eckhardt HU, Hopstock U, Peters W, Gerhardt HJ. Die atemrhythmusgesteuerte Elektrostimulation der gelähmten Kehlkopfmuskulatur – tierexperimentelle Grundlagen. Wissenschaftliche Zeitschrift der Humboldt-Universität zu Berlin. 1984;XXXIII:263–265. [Google Scholar]
- 284.Bergmann K. Elektromyographische Diagnostik bei Bewegungsstörungen der Kehlkopfmuskulatur. Wissenschaftliche Zeitschrift der Humboldt-Universität zu Berlin. 1984;XXXIII:175–177. [Google Scholar]
- 285.Bergmann K. Tierexperimentelle Untersuchungen zur atemrhythmusgesteuerten Elektrostimulation der gelähmten Kehlkopfmuskulatur. 1984. [Google Scholar]
- 286.Bergmann K. [Indication and Limits of the Significance of EMG Recording from Larynx Muscles] HNO-Praxis. 1986;11:83–88. [Google Scholar]
- 287.Bergmann K. [Functional Disorders of the Superior Laryngeal Nerve in Patients Suffering from Laryngeal Nerve Palsies] HNO-Praxis. 1986;11:153–159. [Google Scholar]
- 288.Bergmann K, Warzel H, Eckhardt HU, Hopstock U, Gerhardt HJ. [Chronic Implantation of a System for Electrical Stimulation of Paralyzed Laryngeal Muscles in Dogs] HNO-Praxis. 1986;11:231–239. [Google Scholar]
- 289.Bergmann K, Herrmann V. [Histochemical Characteristics of the Normal and Paretic Dog Laryngeal Muscle] HNO-Praxis. 1987;12:259–266. [Google Scholar]
- 290.Bergmann K, Warzel H, Eckhardt HU, Hopstock U, Hermann V, Gerhardt HJ. Long-term implantation of a system of electrical stimulation of paralyzed laryngeal muscles in dogs. Laryngoscope. 1988;98:455–459. doi: 10.1288/00005537-198804000-00020. [DOI] [PubMed] [Google Scholar]
- 291.Otto RA, Davis W, Betten JR, Downen P, Otto PM. Electrophysiologic pacing of vocal cord abductors in bilateral recurrent laryngeal nerve paralysis. Am J Surg. 1985;150:447–451. doi: 10.1016/0002-9610(85)90151-5. [DOI] [PubMed] [Google Scholar]
- 292.Otto RA, Templer J, Davis W, Homeyer D, Stroble M. Coordinated electrical pacing of vocal cord abductors in recurrent laryngeal nerve paralysis. Otolaryngol Head Neck Surg. 1985;93:634–638. doi: 10.1177/019459988509300512. [DOI] [PubMed] [Google Scholar]
- 293.Kim KM, Choi HS, Kim GR, Hong WP, Chun YM, Park YJ. Laryngeal pacemaker using a temperature sensor in the canine. Laryngoscope. 1987;97:1207–1210. doi: 10.1288/00005537-198710000-00016. [DOI] [PubMed] [Google Scholar]
- 294.Zealear DL, Herzon GD. Progress toward the development of a chronically implantable laryngeal pacemaker. In: Fujimura O, editor. Vocal physiology: voice production, mechanisms and functions. New York: Raven Press; 1988. [Google Scholar]
- 295.Sanders I. Electrical stimulation of laryngeal muscle. Otolaryngol Clin North Am. 1991;24:1253–1274. [PubMed] [Google Scholar]
- 296.Zrunek M, Carraro U, Catani C, Scabolcs M, Gruber H, Streinzer W, Mayr W, Thoma H. [Functional electrostimulation of the denervated posticus muscle in an animal experiment: histo- and biochemical results] Laryngol Rhinol Otol (Stuttg) 1986;65:621–627. [PubMed] [Google Scholar]
- 297.Zrunek M, Mayr W, Streinzer W, Thoma H, Losert U, Schneider B, Unger E. Laryngeal pacemaker: activity of the posterior cricoarytenoid muscle (PCM) and the diaphragm during respiration in sheep. Acta Otolaryngol. 1989;108:311–316. doi: 10.3109/00016488909125533. [DOI] [PubMed] [Google Scholar]
- 298.Zrunek M, Mayr W, Bigenzahn W, Unger E, Thoma H. [Respiration-synchronous bilateral direct electric posticus stimulation in animal experiments] Biomed Tech (Berl) 1990;35 Suppl 2:145–147. doi: 10.1515/bmte.1990.35.s2.145. [DOI] [PubMed] [Google Scholar]
- 299.Zrunek M, Bigenzahn W, Mayr W, Unger E, Feldner-Busztin H. A laryngeal pacemaker for inspiration-controlled, direct electrical stimulation of the denervated posterior cricoarytenoid muscle in sheep. Eur Arch Otorhinolaryngol. 1991;248:445–448. doi: 10.1007/BF00627631. [DOI] [PubMed] [Google Scholar]
- 300.Zealear DL, Hamdan AL, Rainey CL. Effects of denervation on posterior cricoarytenoid muscle physiology and histochemistry. Ann Otol Rhinol Laryngol. 1994;103:780–788. doi: 10.1177/000348949410301007. [DOI] [PubMed] [Google Scholar]
- 301.Zealear DL, Rainey CL, Herzon GD, Netterville JL, Ossoff RH. Electrical pacing of the paralyzed human larynx. Ann Otol Rhinol Laryngol. 1996;105:689–693. doi: 10.1177/000348949610500904. [DOI] [PubMed] [Google Scholar]
- 302.Zealear DL, Billante CR, Courey MS, Netterville JL, Paniello RC, Sanders I, Herzon GD, Goding GS, Mann W, Ejnell H, Habets AM, Testerman R, Van de Heyning P. Reanimation of the paralyzed human larynx with an implantable electrical stimulation device. Laryngoscope. 2003;113:1149–1156. doi: 10.1097/00005537-200307000-00010. [DOI] [PubMed] [Google Scholar]


