Abstract
The incidence of olfactory disorders is appoximately 1-2% and they can seriously impact on the quality of life. Quantitative disorders (hyposmia, anosmia) are distinguished from qualitative disorders (parosmia, phantosmia). Olfactory disorders are classified according to the etiology and therapy is planned according to the underlying pathophysiology. In ENT patients olfactory disorders caused by sinonasal diseases are the most common ones, followed by postviral disorders. Therapy consists of topical and systemic steroids, whereas systemic application seems to be of greater value. It is very difficult to predict the improvement of olfactory function using surgery, moreover, the long term - success in surgery is questionable.
Isolated taste disorders are rare and in most often caused by underlying diseases or side effects of medications. A meticulous history is necessary and helps to choose effective treatment. In selected cases zinc might be useful.
Keywords: olfactory disorders, taste disorders, etiology, therapy
1. Introduction - Olfaction
Smelling is the sensation experienced when the olfactory epithelium of the nose is stimulated by volatile substances. Although human sensory perception has become increasingly focused on visual and auditive impulses, the sense of smell remains of fundamental importance. A functioning olfactory system prevents us from consuming spoilt food, alerts us to the smell of burning, and contributes substantially to the quality of life by enabling flavor perception with food and drink as well as the appreciation of scents, such as perfume or a fresh sea breeze.
1.1 Anatomy and Physiology
The human olfactory epithelium consists of about 6 million neurons, spread over an area of about 2 cm2, located in the uppermost region of the nasal cavity (in the area of the upper septum, lamina cribrosa, and superior turbinate) where they are well protected. The dimensions of the olfactory epithelium vary considerably between individuals [1], [2]. In biopsies, olfactory epithelium is frequently determined in dorsoposterior sections of the septum, with abundancies in the area of the superior turbinate [3], [4] and middle turbinate [5] showing greater variability.
The olfactory epithelium consists of various cell types [6]. Bipolar olfactory receptor neurons, embedded in a formation of supporting cells, have immotile cilia at their apical surface that constitute the site of sensory transmission [7]. Normal life span of olfactory neurons ranges from 30 to 90 days [8], but olfactory performance decreases with age, possibly because of accelerated apoptosis [9], and olfactory epithelium is increasingly replaced by respiratory epithelium [10], [11]. Olfactory receptor neurons are continuously regenerated from basal cells that are sometimes referred to as "multipotent stem cells" [12]. Like the cells ensheathing axon bundles, the so-called "olfactory ensheathing cells", basal cells are regarded as a promising target for transplantations in spinal nerve injuries [13], [14].
The axons of bipolar receptor cells combine into about 40 bundles called fila olfactoria that project into the olfactory bulb via the lamina cribrosa. After synaptic transmission within specific glomeruli, the information is forwarded to the olfactory cortex. Central activity after odor stimulation can be visualized by positron emission tomography (PET) [15] or functional magnetic resonance imaging (fMRI) [16].
1.2 Olfactory Performance and Quality of Life
Both quantitative olfactory disorders, such as hyposmia and anosmia, or qualitative disorders, such as phantosmia and parosmia, can seriously impact on the quality of life, often leading to weight loss and even depression [17], [18], [19], [20]. Moreover, olfactory loss is associated with a markedly increased risk of exposure to hazardous events in every-day living, such as intake of spoilt food, burning of meat etc. leading to a fire, or gas leakage [21]. For many affected individuals, the main problem lies in the consumption of bland and tasteless food. Patients with post-traumatic anosmia have meanwhile been shown to also have an elevated gustatory threshold [22].
2. Olfactory Function Tests
2.1 Self-Assessment of Olfactory Function
Despite the importance of olfaction in every-day living, most people can only inadequately assess their own olfactory performance. This is not only true for patients with neurodegenerative diseases [10], [23], but equally applies to subjects with normal olfactory function [24]. Somewhat better correlation between self-assessment and objectively measured olfactory performance is seen in subjects consulting a physician because of an olfactory disorder (personal data). For these reasons, subjective reporting of olfactory performance, e.g., a 'significant improvement', based solely on self-assessment, warrants careful interpretation.
2.2 Objective Assessment of Olfactory Function
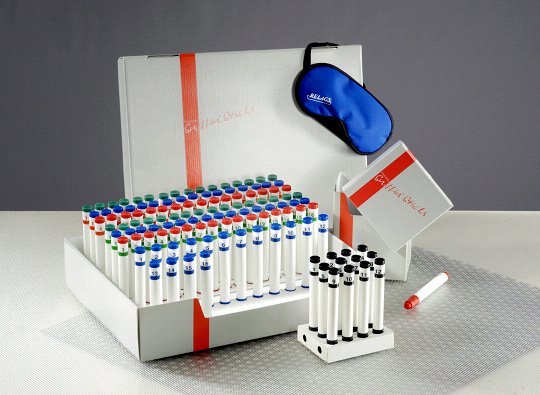
Nowadays, a large number of validated test procedures are available. Here, these are described only briefly. Most Anglo-Saxon studies employ either the University of Pennsylvania Smell Identification Test (UPSIT) developed by Doty et al. [25], an abridged version termed 'Cross-Cultural Smell Identification Test' (CC-SIT) [26], or the CCCRC test [27]. European centers tend to use the "Sniffin' Sticks" test battery that assesses odor discrimination, odor identification, and olfactory threshold (Figure 1 (Fig. 1)) [28], [29], [30]. In addition, the Aachen rhinotest [31] and smell diskettes are available screening tests [32], [33]. In Japan, T&T olfactometry [34] or the intravenous Alinamin® test are used [35]. Specialized centers measure evoked potentials after chemosensory stimulation to objectively test olfactory performance [36], [37].
Figure 1. Sniffin´Sticks® test battery.
3. Classification of Olfactory Disorders
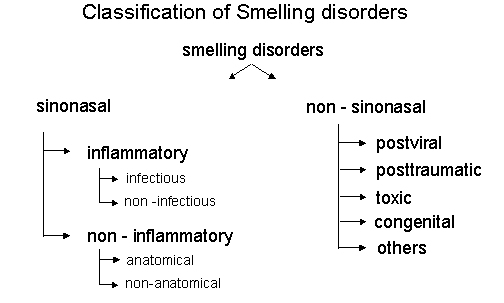
To effectively treat an olfactory or gustatory disorder and to re-establish olfactory function, it is essential to classify its etiology as accurately as possible. The system to classify olfactory disorders generally used today is in close agreement with the guidelines of the "Arbeitsgemeinschaft Olfaktologie/Gustologie der Deutschen Gesellschaft für HNO Heilkunde, Kopf- und Halschirurgie" [38], [39], see Figure 2 (Fig. 2).
Figure 2. Classification of smelling disorders.
3.1 Epidemiology
In the USA, the incidence of olfactory disorders is estimated to be 1.4% [40], but more recent studies suggest that the actual figure is probably higher [41], [42]. Based on a survey among German, Austrian, and Swiss ear, nose, and throat clinics, olfactory disorders in 72% of all treated patients have a sinonasal origin [43]. Mott & Leopold [44] and Nordin et al. [45] reported similar figures, with the stated proportions of postviral and post-traumatic disturbances depending on the specialization of the respective clinic [17], [20], [46].
4. Treatments
4.1 Olfactory Disorders of Sinonasal Etiology
These olfactory disorders comprise impairments caused by illnesses of the nose or the paranasal sinuses. The olfactory disorder occurs as a result or an accompaniment of the upper respiratory tract infection. Olfactory dysfunction may be conductive in nature or may be caused by damage to the olfactory mucosa; the latter may persist even if the conductive disorder has been corrected. Because the nomenclature used in studies is inconsistent, olfactory disorders of sinonasal etiology are discussed as a group to facility overview. Chronic rhinitis or rhinosinusitis often lead to deterioration of the olfactory threshold [47], [48], while neither the visibility of the olfactory cleft [49], [50] nor nasal breathing [47], [51] correlate with olfactory function as such.
4.1.2 Conservative Therapies
4.1.2.1 Topical Steroids
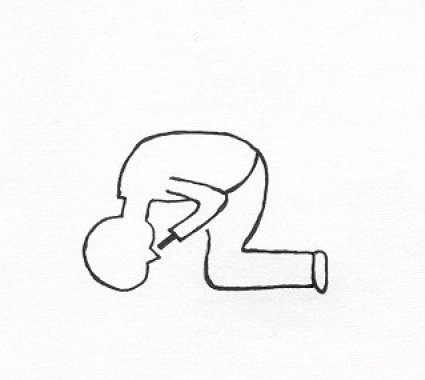
Two open studies documented a significant improvement of olfactory performance after betamethasone drops [52] or flunisolide drops [53] administered by the so-called "head down forward technique" (see Figure 3 (Fig. 3)). However, Heilmann et al. did not find any significant improvement after administration of topical steroids [54].
Figure 3. Application of nasal spray in the so called: "head down forward position".
In two prospective, double-blind studies, both Tos et al. [55] and Lund et al. [56] reported a significant improvement of the subjective olfactory sensitivity in response to budesonide, but objective olfactory performance was not determined. In contrast to this finding, budesonide spray failed to achieve any significant improvement of olfaction assessed with three substances in a study reported by Lildholdt et al.[57]. Similarly, elNaggar et al. found no real difference in the UPSIT between one side of the nose treated postoperatively with beconase and the untreated control side [58]. Treatment of allergic rhinitis with mometasone spray in 2 double-blind studies using different olfactory tests yielded contradictory results. While Meltzer et al. reported a significant improvement of odor identification at an unchanged odor threshold in the active group [59], Stuck et al. found an improved odor threshold in the absence of improved identification or discrimination [60]. No correlation between olfactory performance and improved nasal breathing was established. Similarly, Hedén Blomqvist et al. [61] documented no further improvement in patients initially responding to a 10-day combination therapy (oral and topical steroids) receiving follow-up treatment with topical steroids in a double-blind study.
4.1.2.2 Summary and Assessment of Topical Steroids
No significant improvement was demonstrated in any of the prospective, double-blind studies in which olfactory performance was determined by means of standardized tests before and after study start, with the exception of an improved odor threshold or identification seen after short-term use of mometasone spray in allergic rhinitis. Application of nasal sprays or drops in the 'head forward' position may possibly be advantageous, as shown by Benninger et al. [62].
4.1.2.3 Oral Steroids
Oral steroids were used successfully already in the fifties in patients with nasal obstruction, polyps, and olfactory disorders [63]. Below, the term 'steroid-dependent anosmia' is used for cases who rapidly experience clear improvement of olfaction after oral steroids but who frequently require a maintenance dose of steroids after surgery to maintain olfactory function [64], [65]. Because olfactory performance often fluctuates as seen in medical histories, the use of oral steroids may serve as a diagnostic tool [66]. In a retrospective study in 55 patients, Heilmann et al. reported a significant improvement of olfactory performance in response to oral steroids, while topical steroids had no measurable effect [54]. In a study by Ikeda et al., oral steroids improved olfactory function in 12 patients with ethmoid sinus disease as determined radiologically [67]. Pathological findings confined to the olfactory cleft have been termed "olfactory cleft disease" by Biacabe et al., who reported improved odor threshold in 50% of cases treated with oral steroids [68].
4.1.2.4 Summary and Assessment of Oral Steroids
Oral steroids are often effective in cases that do not respond to topical steroids. Their specific mechanism of action on olfactory performance remains unclear, but effects via glucocorticoid receptors in the olfactory mucosa [69], [70] or via regulation of adenosine triphosphate (ATP) activity in the olfactory mucosa [71] are being discussed. So far, no placebo-controlled, double-blind studies have been performed.
4.1.2.5 Leukotrienes
Leukotrienes, produced by mast cells and eosinophils, play an important pathophysiologic role in the early phase of allergies [72]. In open studies, leukotriene synthesis inhibitors (Zileuton) and leukotriene antagonists (Montelukast, Zafirlukast) have been shown to alleviate the complaints in patients with polyps [73], but data on olfactory performance are either limited to single observations [73] or rare [74].
4.1.2.6 Antibiotics
In animal models, macrolides reduce the symptoms of chronic inflammation (i.e., enhanced mucociliary transport, reduced secretion of goblet cells, accelerated apoptosis of neutrophils, and diminished gene expression of interleukin-6 [IL-6] and IL-8). In a review, Cervin [75] discusses several clinical studies conducted in Japan in which chronic sinusitis improved in response to prolonged therapy (in some cases for months) with low-dose macrolides. The success rate ranged from 60% to 80%, and neither previous steroid therapy nor surgery had been successful in any of the cases.
No definitive data on the effects of leukotrienes or macrolides on olfactory performance are available.
4.1.3 Surgical Interventions
Usually, surgical therapy of sinonasal olfactory disorders primarily serves to improve drainage (to facilitate healing of the inflammation) or nasal breathing. Thus, surgery aims to directly or indirectly improve olfactory performance by accelerating healing of the inflammation and restoring conduction [76]. Consequently, it is difficult to define predictive factors for the success of olfactory surgery [77], which is reflected in the variable success rates. The successful combination of surgery with subsequent oral steroid therapy [64] encouraged Jafek and Hill [78] to recommend pretreatment with oral steroids. However, even the use of steroid therapy after surgery often only achieves hyposmia [79], [80]. Delank and Stoll achieved normosmia in only 25% of hyposmic patients and in 5% of patients with initial anosmia [81]. These figures are in agreement with data reported by Downey et al. and Kimmelmann et al., who achieved improvements in olfactory function after surgery in only 50% and 66% of cases, respectively [82], [83]. The result was not substantially better if an antibiotic (Co-amoxiclav) was added on to oral steroids in the follow-up treatment [84]. Moreover, all three authors reported postsurgical deterioration of olfactory threshold in up to 34% of cases [83]. This high percentage may be due to the early testing after surgery; ideally, testing of olfactory performance should be done only after approx. 3 months [85]. Significantly improved olfaction was demonstrated both in the "Sniffin' Sticks" screening test and the subjective assessments in 70 patients [86]. If olfactory performance was based on subjective assessments only, a significant improvement was demonstrated in 178 patients receiving topical steroids for one year after surgery [87].
Long-term maintenance of olfactory function after surgery remains critical. Jankowski et al. attempted to achieve this by radical intervention, i.e., 'nazalisation' [88]. The authors failed to document any difference in olfactory performance one year after surgery between patients pretreated with oral steroids for one week and those not receiving any steroid pretreatment. Both patient groups had received an intramuscular depot formulation of steroid (Triamconolon, 80 mg) immediately after surgery [89]. These data are in contrast to the findings reported by Hedén Blomqvist et al. [90]. Patients with symmetrical intranasal conditions received oral cortisone and a 1month therapy with topical steroids before undergoing unilateral endonasal surgery with subsequent topical steroid therapy for 12 months. One year after surgery, there was no significant difference between the nasal sides with respect to olfactory function, while obstruction and secretion were improved on the corrected side.
4.1.3.1 Septoplasty and Septorhinoplasty
Literature data on this topic are scarce. Twenty years ago, Stevens and Stevens documented olfactory threshold improvements [olfactometric method: Elsberg threshold test [91], regarded as inadequate nowadays] after various nasal surgeries (septoplasty, septorhinoplasty, turbinate resection) in 100 patients [92]. While Ophir et al. reported an improved olfactory threshold in 24 patients undergoing inferior turbinectomy [93], Damm et al. primarily found improved odor identification and discrimination after septoplasty and turbinectomy in 30 patients [94].
4.1.3.2 Olfactory Cleft Surgery
In certain cases with pathological findings confined to the olfactory cleft, lateralization of the middle turbinate may be of benefit (see Figure 4 a/b (Fig. 4)), although conclusive data are still missing.
Figure 4.
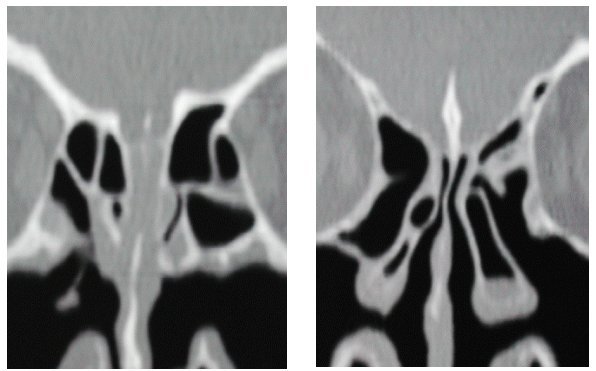
a) Preoperative findings in a cat scan demonstrating the closed olfactory cleft
b) Postoperative findings demonstrating the opening of the olfactory cleft after lateralisation of the middle turbinate
4.1.3.3 Summary and Assessment of Surgical Interventions
Factors that predict surgical success with respect to olfactory performance have yet to be identified. Similarly, no conclusive data on the correlation between olfactory performance and nasal airflow are available [95]. Comparison of the available studies is hampered by the variable severity of disorders present before surgery, the missing psychometric tests in some cases, and the highly varied conservative therapies applied in addition to surgery. In particular, the data by Hedén Blomqvist et al. [90] indicate that the decision to use surgery in chronic rhinosinusitis to improve olfactory performance should be made with caution for the time being, especially in the absence of additional randomized, controlled studies. Septoplasty to improve olfactory performance remains controversial.
4.1.4 Histology and Experimental Drugs
In chronic sinusitis and steroid-dependent anosmia, initial histologic appearance of the olfactory epithelium may be quite normal [64], [96], while irreversible olfactory receptor damage, epithelial metaplasia, and fibrosis are commonly seen subsequently [97], [98]. Progressing olfactory impairment leads to a loss of olfactory epithelium and olfactory receptor neurons with subsequent destruction of the epithelial structure [99].
Unilateral experimental infection with staphylococci in an animal model revealed that the inflammatory reaction (including epithelial thinning, loss of supporting cells, cilia, and dendrites) as well as apoptotic cell death occur not only on the infected nasal side but are virtually mirrored with a slight delay on the other nasal side [100]. Although the regulatory mechanisms activating apoptosis on the side initially not infected are not understood, it is known that a number of enzymes are responsible for apoptosis, with caspase-3, usually present in cells as inactive procaspase-3, thought to play a key role [101]. Kern et al. [102] measured modest caspase activity in healthy olfactory receptor neurons, whereas elevated caspase activity in olfactory receptor neurons and fila olfactoria as well as epithelial inflammation were evident in sinusitis even if olfaction was clinically normal. If olfactory dysfunction was also present, the inflammatory reaction was more marked and caspase-3 activity responsible for cell death continued to increase [102]. Caspase inhibitors tested successfully in animal models (i.e., for the treatment of ischemia or post-traumatic brain damage) may be the drugs of the future [103].
4.2 Olfactory Disorders of Non-Sinonasal Etiology
4.2.1 Postviral Olfactory Disorders
Every acute infection of the upper respiratory tract may potentially lead to an olfactory disorder, but the exact pathogenesis remains unclear [104]. Elderly subjects and women are affected more often [17], [46], and parosmia as well as dysosmia are common [105], [106]. Akerlund et al. [107] observed an elevated olfactory threshold correlating with nasal congestion in 9 volunteers experimentally infected with coronavirus with subsequent development of a common cold. In 36 volunteers suffering from a common cold, reduced amplitudes of the initial components in olfactory potentials were seen in addition to elevated thresholds, even after use of the nasal decongestant oxymetazoline [108]. This finding indicates that olfactory impairment may, in part, be independent of nasal congestion, thus explaining the failure of oxymetazoline to improve olfaction [109].
4.2.1.1 Histological Findings in Postviral Olfactory Disorders
Biopsies usually reveal a patchwork pattern, i.e., olfactory and respiratory epithelia alternate, and the number of olfactory receptor neurons is reduced. It remains controversial whether the extent of damage observed correlates with olfactory dysfunction [110], [111]. Dendrites of the olfactory receptor neurons often have cytoplasmic inclusions whose function is not yet understood [98].
4.2.1.2 Outlook
In mice, experimental infection with the neurotropic influenza A virus induced apoptosis of infected olfactory neurons, but viral penetration into the central nervous system (CNS) via the olfactory bulb did not occur and the animals survived (inoculation of the virus in the CNS is fatal in 100% of cases) [112]. Consequently, virus-induced apoptosis of olfactory cells via caspase-3 activation may be viewed as a mechanism to protect the organism from viral penetration into the CNS [113].
4.2.1.3 Treatment of Postviral Olfactory Disorders
Treatment with alpha-lipoid acid seems promising. In an open, prospective study, patients [n=23, 19 hyposmic patients, 4 functionally anosmic patients, based on "Sniffin' Sticks" test results [29], [30], received alpha-lipoid acid (600 mg/day) for 4.5 months on average [114]. Six patients experienced mild improvement and 8 patients clear improvement of olfactory performance. However, the authors stated that confirmation of these findings warrants a double-blind study because spontaneous recovery and regeneration are common in postviral olfactory disorders and may occur up to 2 years after viral exposure [105].
4.2.1.4 Treatment of Parosmia / Phantosmia
Apart from various drugs, e.g., antiepileptics, antidepressants, and local anesthetics [115], surgical removal of the olfactory epithelium, reported for the first time in 1991 by Leopold et al. [116], may be of use. Long-term follow-up (for 5 years after surgery) revealed that 7 of 8 patients were completely free of complaints, with only 2 patients showing reduced olfactory performance [117]. Bulbectomy may also be of use in specific cases [118].
4.2.2 Post-Traumatic Olfactory Disorders
Post-traumatic olfactory dysfunction correlates with the severity of trauma [119], but even minor traumas may lead to impaired olfaction. Possible mechanisms are middle-face fractures or traumas directly or indirectly damaging the olfactory region, shearing lesions of the fila olfactoria, or intracranial injuries. Severing of the axons from olfactory receptor neurons eventually leads to retrograde cell death [120]. Nevertheless, spontaneous improvement is possible, as confirmed by Doty et al. in 24 of 66 patients who were re-analyzed after 1 to 13 years [121] as well as Duncan and Seiden in 7 of 20 patients after 2 to 3 years [105]. Using MRI, post-traumatic changes were characterized as encephalomalacias [122] and reduced volumes of the olfactory bulb [121], [123]. Spontaneous recovery is possible because damaged neurons are able to regenerate [124]. Basal cells differentiate into olfactory receptor neurons whose axons extend to the olfactory bulb via the lamina cribrosa to form synapses. The bulb supplies the olfactory receptor neurons with trophic factors essential for their survival [125]. If no contact is established and synapses fail to develop, olfactory receptor neurons will die within a few days [126]. If, however, connection with the bulb is successful, neuronal function is restored [127], [128]. Vitamin A has been shown to markedly accelerate neuronal regeneration in mice [129].
4.2.2.1 Histology of Olfactory Epithelium in Post-Traumatic Olfactory Disorders
Olfactory receptor neurons are shriveled and have only few cilia, suggesting a reduced number of connections with the olfactory bulb [130]. Typically, there are many axons in the vicinity of the basal membrane that appear not to have made any connection with the bulb [131]. The ultrastructural changes correlate with the type and extent of damage [132].
4.2.2.2 Treatment of Post-Traumatic Olfactory Disorders
In a prospective study in 95 patients suffering from post-traumatic olfactory disorders, Aiba et al. reported significant improvement of self-assessed olfactory performance in 2 of 4 patients receiving zinc sulfate (300 mg/day) for > 1 month [133]. In an open, prospective study in anosmic patients receiving either oral caroverine (120 mg/day) or zinc sulfate (400 mg/day) for 4 weeks, Quint et al. [134] reported a significantly improved olfactory threshold in patients receiving caroverine compared with those receiving zinc sulfate. The authors discussed a possible intrabulbous repair mechanism and a neuroprotective effect of the glutamate receptor inhibitor, caroverine, similar to the one present in the inner ear [135]. However, the authors recommended a double-blind study to confirm this notion. In an open, prospective study conducted in Japan, the use of local dexamethasone (4 mg/0.5 mL, 8 times, injected into the mucosa of the upper septum in 2-week intervals), was discussed [136]. All 27 patients treated also received vitamin B12 (750 µg/day to 1500 µg/day) and ATP (300 mg/day). In 6 patients, the recognition threshold was improved, while 4 patients experienced an improved identification threshold. However, the authors did not exclude spontaneous recovery and suggested additional studies.
4.2.3 Congenital Olfactory Disorders
Overall, congenital olfactory disorders are very rare. They tend to show between the age of 5 and 10 years. The best known genetic olfactory disorder is Kallmann's syndrome (hypogonadotropic hypogonadism with anosmia) [137], but isolated anosmia is also known [138]. Neuroradiologic examination revealed marked hypoplasia or the total absence of the olfactory bulb or tract (68-84%) [139], in addition to clearly reduced sulcus depth [140].
4.2.3.1 Histological Findings in Congenital Olfactory Disorders
While Jafek et al. did not find any olfactory epithelium in 36 biopsies of 7 patients with congenital anosmia [141], Leopold et al. described a pathological epithelium with immature olfactory receptor neurons and missing cilia in 2 of 5 patients [142]. In contrast, Rawson et al. succeeded in demonstrating mature olfactory receptor neurons [143].
4.2.3.2 Treatment of Congenital Olfactory Disorders
At present, no treatment options are known.
4.2.4 Toxic Olfactory Disorders
Many substances, such as metals (e.g., lead or cadmium), organic substances (e.g., solvents or formaldehyde), inorganic substances (e.g., chlorine, CO, ammonium chloride), and other substances (e.g., cement dust) may be olfactotoxic [144], [145], [146]. Although the olfactory epithelium is located away from the main airway, 10% to 15% of the inhaled air reaches the olfactory epithelium even without sniffing, thus causing exposure to potentially toxic substances [147]. Chronic exposure is more likely to induce lasting damage than acute exposure [148].
4.2.4.1 Treatment of Toxic Olfactory Disorders
No treatments with proven success exist, and larger studies documenting a clear relationship between exposure and olfactory loss are missing. Nevertheless, careful assessment of the work-place history is essential for all olfactory disorders.
4.2.5 Olfactory Disorders of Other Etiologies
Multiple internal disorders (e.g., hypothyreosis, renal failure, diabetes mellitus), neurologic diseases (e.g., Alzheimer's disease, Parkinson's disease), and psychiatric diseases (e.g., schizophrenia, depression) are accompanied by olfactory disorders [104]. Although literature information is controversial in part, dialysis in chronic renal failure, for example, does not appear to improve olfactory performance [149], nor does antiparkinson medication improve olfaction in Parkinson's disease. In these situations, the primary steps must be to identify and treat the underlying disease. Drugs themselves may induce olfactory disorders but these tend to be transient and reversible in most cases [150], [151].
For a long time, laryngectomized patients were also thought to be anosmic [152]. However, more recent studies showed that the olfactory epithelium usually remains intact even years after laryngectomy [153], [154], and that patients can draw air to the olfactory cleft and regain olfaction by adopting a special yawning technique [155], [156].
4.3 Experimental Drugs and Other Treatments
4.3.1 Hormonal Therapy
Studies by Deems et al. showing that only a small percentage of postmenopausal women with olfactory disorders receive hormonal therapy triggered speculation about a protective effect of hormones [17]. The smaller bulb volume in men suffering from post-traumatic olfactory disorders, relative to that in women [121], as well as olfactory performance in schizophrenic women shown to differ in relation to their menopausal status was interpreted along the same lines [157]. In animal experiments, ovarectomized mice exposed to olfactotoxic substances recovered more quickly if they received hormonal replacement than did those not receiving any hormones [158]. However, a longitudinal study in 62 women failed to confirm these findings; the only finding was a correlation between loss of olfaction and age progression [159].
4.3.2 Dopamine
Although it is established that the neurotransmitter dopamine is present in the olfactory bulb, existence of D2 receptors in the olfactory epithelium has only recently been described [160]. Dopamine triggers neuronal differentiation and maturation in the epithelium in vitro, while on stimulation of the lamina propria, dopamine induces the liberation of substances that block neuronal differentiation [160]. Dopamine led to a significantly reduced rate of apoptosis in olfactory biopsies of schizophrenic patients but clearly accelerated apoptosis in the olfactory epithelium of control subjects [161]. In Parkinson's patients, Huisman et al. determined a doubling of dopaminergic cells in the olfactory bulb and interpreted this as a possible cause of the olfactory disorders seen in Parkinson's disease [162].
4.3.3 Acupuncture
Data confirming the benefit of acupuncture are missing. In an open study, auricular acupuncture improved olfactory threshold in 23 healthy volunteers [163]. In addition, a case report documented restored olfaction after acupuncture in a female patient who had been suffering from anosmia for 2 years [164].
4.3.4 Theophylline
Levy et al. [165] used theophylline (250 to 500 mg, given for 4 to 6 months) in 4 hyposmic patients (allergic rhinitis, n=3; post-traumatic hyposmia, n=1) and reported normal post-treatment olfaction in 2 patients, improved olfaction in 1 patient, and unchanged olfaction in the remaining patient. Functional MRI in 3 patients indicated enhanced central activation after treatment. No additional data are available.
4.3.5 Growth Factors (Transforming Growth Factor)
Olfactory receptor neurons are continuously replaced during the course of life, with the rate highest at younger age [9]. Because the rate of neurogenesis can be manipulated by external factors (e.g., doubling after ablation of one olfactory bulb [166], decrease after unilateral nostril occlusion [167]), acceleration by using growth hormones appears feasible. Intraperitoneal administration of transforming growth factor-alpha (TGA-alpha) resulted in enhanced cell proliferation not only in fetal but also in adult mice [168]. So far, no studies in humans have been performed.
4.3.6 Vitamin A
Vitamin A has been reported to normalize olfactory performance in malabsorption conditions or A-β-lipoproteinemia [169]. Moreover, Garrett-Laster et al. [170] reported a significant improvement of olfactory threshold for pyridine and taste threshold for bitter and salty substances in 37 patients with vitamin A deficiency due to alcoholic liver cirrhosis undergoing a 4-week therapy with oral vitamin A (10 mg/day). No additional data are available.
4.3.7 Zinc
Zinc sulfate (100 mg) did not have any significant effects in 106 patients participating in a double-blind study reported by Henkin et al. [171] (see also Quint et al. [134]). High doses of local intranasal zinc may even be olfactotoxic [172].
5. Introduction - Taste Disturbances
After biting into an apple, we identify the piece in the mouth as 'apple', based on its consistency, temperature, spiciness, retronasal olfactory sensation, and the slightly acidic taste. The combination of all perceptions is known as 'flavor', although the only genuine taste qualities defined are sweetness, acidity, saltiness, and bitterness.
5.1 Anatomy and Physiology
The site for taste perception has been identified as the taste buds located in the area of the tongue, soft palate, oropharyngeal mucosa, and also the epiglottis. The taste buds, approx. 4600 on average, consist of 20 to 50 cells arranged like slices of an orange exposing the taste pore in the center. The microvilli of neuroepithelial sensory cells extend into the taste pore [173]. The life span of taste buds ranges from about 10 to 20 days [174]. The majority of taste buds are located on taste papillae classified as vallate papillae, filiform papillae, and fungiform papillae [175], [176]. Basically, each type of papilla is sensitive to several, if not all, taste modalities [177]. Innervation takes place via the chorda tympani nerve, glossopharyngeal nerve, and vagus nerve. Excitatory transmission for acidic and salty tastes occurs directly via ions, while sweet and bitter tastes trigger secondary-messenger systems via membrane-specific receptors [178], [179].
6. Assessment of Gustatory Function
Gustatory function is assessed in terms of total or regional taste perception [180], [181]. Taste threshold is typically assessed with the 3-drop method using saccharose, citric acid, table salt, and quinidine hydrochloride solution [182], [183], or by using the so-called 'taste strips' [184]. Differences between the sides are assessed by electrogustometry that determines the electrical perception threshold [185]. Specialized ear, nose, and throat centers employ contact endoscopy to visualize the morphologic changes.
6.1 Classification and Epidemiology of Taste Disorders
Taste disorders are classified as quantitative and qualitative taste disorders. Quantitative disorders include hypogeusia and ageusia, while qualitative disorders are parageusia and phantogeusia. The classification as epithelial, neuronal, or central taste disturbance, depending on its cause, is in close agreement with the guidelines of the "Arbeitsgemeinschaft Olfaktologie/Gustologie der Deutschen Gesellschaft für HNO Heilkunde, Kopf- und Halschirurgie" [186]. Taste disturbances are less common than olfactory disorders, and qualitative changes are clearly more frequent than quantitative alterations.
7. Treatments
Because taste disorders are often coupled with a concomitant illness that can be diagnosed and treated, initial identification of the illness by means of detailed analysis of the medical history and clinical examination is of primary importance. The possible therapies of concomitant illnesses are not discussed here; for review see Bromley and Doty [187]. Below, some disorders and their possible treatments are discussed only as examples.
7.1 Taste Disorders after Radio-Chemotherapy
Radiotherapy leads to transient hypogeusia (especially for bitter and salty tastes) or even ageusia, which is most pronounced approx. 2 months after irradiation [188]. The taste disturbance can persist for 1 to 2 years after radiotherapy [189]. In a randomized clinical study, Ripamonti et al. [190] demonstrated faster recovery of taste function in patients receiving zinc sulfate (3 x 45 mg/day, given during radiotherapy and for 1 month afterwards) than in those receiving placebo. Similarly, zinc infusions had a positive effect on the electrogustometric threshold in chemotherapeutically treated patients [191]. Simultaneous application of amifostine during radiochemotherapy reduces xerostomia and dysgeusia [192].
7.2 Postoperative Taste Disorders
Taste disorders after tonsillectomy caused by pressure on the lingual branch of the glossopharyngeal nerve are rare (0.31%) and commonly disappear spontaneously [193]. Just et al. [194] studied 118 patients with a strained or severed chorda tympani nerve after various surgeries. The subjective complaints in these patients were highly variable and did not necessarily correlate with measured taste perception. Saito et al. [195] also demonstrated better long-term recovery of clinical (subjective) taste perception than of objective taste function measured by electrogustometry. After 2 years, only 2.7% of all patients (n=113) reported subjective taste impairment. This low rate was thought to be caused by the loss of central inhibition [196]. In patients with lingual nerve injuries excision of neurinomas and end-to-end suture resulted in improved postoperative taste perception in 40% of cases [197].
7.3 Drug-Induced Taste Disorders
Drug-induced dysgeusia, but also hypogeusia or ageusia, are common and especially associated with the ACE inhibitor captopril (20% dysgeusia) or the diuretic azetazolamide (100% dysgeusia) [150], [151]. In elderly patients, taste disturbances are often not the consequence of old age but may be associated with concomitant diseases or side effects of drugs taken [198]. Age-dependent impairment of taste varies considerably, and the debate of its clinical relevance is controversial [199].
7.4 Therapeutic Use of Zinc in Taste Disorders
Numerous studies document the favorable effect of zinc on taste perception. In an observational study, Stoll and Oepen reported improved taste perception in 5 psychiatric patients, but the authors failed to indicate their test method [200]. In an open study (n=119; idiopathic taste disturbance, n=45; drug-induced taste disturbance, n=38; zinc deficiency, n=36), taste improvement by 50% was achieved after 4 weeks and by 80% after 8 weeks of treatment with zinc sulfate (100 mg, three times daily) [201]. In a double-blind, placebo-controlled study (n=73; idiopathic taste disturbance, n=48; lowered zinc levels, n=25), treatment with zinc picolinate (30 mg, three times daily) for 3 months did not improve subjective taste assessment or taste performance in the entire mouth, although the group receiving zinc picolinate performed significantly better than the placebo group in the filter paper test [202]. However, both the double-blind study by Henkin et al. [171] and the double-blind study in 65 patients by Yoshida et al. [203] failed to confirm this difference. Nevertheless, if the patients with drug-induced taste disturbances were excluded and only the patients with idiopathic taste disturbances and zinc deficiency were analyzed, the result was significant [203]. A double-blind study in hemolized patients (n=22) with low zinc levels demonstrated a significant improvement in response to zinc (50 mg/day) given for 12 weeks [204]. Similarly, preliminary findings from a double-blind study with zinc gluconate by Heckmann et al. seemed promising in idiopathic dysgeusia [205], [206].
7.5 Experimental Drugs
Dysgeusia is the most frequent form of taste disturbance and is often idiopathic. A link between dysgeusia and depression has been established. In two thirds of cases, dysgeusia resolves spontaneously after about 10 months [207]. In an open observational study in 44 patients with burning mouth syndrome, treatment with alpha-lipoic acid (3 x 200 mg/day) for 2 months significantly improved taste function as measured by symptoms scores [208]. In cases of severe dysgeusia topical anesthetics, either as solution, spray or gel (lidocaine 2%, or lidocaine spray 10% or lidocaine gel) may be helpful [209].
7.6 Importance of Saliva in Taste Function
The entire oral surface, particularly the surface of the taste receptors, is covered with saliva whose quantitative excretion is controlled by the autonomic nervous system [210]. Saliva is produced by pairs of the major salivary glands (i.e., parotid, submandibular, and sublingual salivary glands) as well as the minor salivary glands (known as the Ebner glands) that secrete saliva into the crypts of the circumvallate and foliate papillae on the posterior of the tongue. Saliva secreted by the Ebner glands contains the Ebner gland protein [211] that was postulated to bind bitter, hydrophobic substances thus facilitating their recognition by the taste papillae. Meanwhile, it is established that this notion was incorrect; the protein is now thought to play a role in the management of inflammatory processes [212]. A further salivary protein, i.e., gustin [213], has meanwhile been identified as carbonic anhydrase VI [214]. In an open study in 14 patients with postviral taste and smell disorders and low levels of carbonic anhydrase VI, 4-month treatment with zinc sulfate (100 mg/day) clearly increased salivary levels of carbonic anhydrase VI and improved taste and olfactory functions in 10 patients [215].
7.6.1 Sjögren's Syndrome and Taste Disorders
This autoimmune disease is associated with decreased salivary secretion, which explains the taste disturbances often reported by Sjögren's patients. Henkin et al. demonstrated elevated taste thresholds in patients with Sjögren´s syndrome [216], but Weiffenbach et al. observed normal thresholds [217]. Reduced salivary volume can, but does not necessarily have to, lead to taste impairment [218]. Salivary secretion can be stimulated using parasympathomimetics, typically pilocarpine (5-10 mg/ 3-4x/d) [219], [220]. Interferon - α either systemically or as low dose lozenge (150 IU, 3x/d) has been shown to reduce xerostomia and increase salivary output [221],[222]. Moreover, artificial salivas, oral rinses and gels have been proposed to treat xerostomia, even though their effect is only transient and so far no effect on taste function has been shown. Specific dietary measures (e.g., consumption of raw vegetables or salty cookies, e.g., bretzels) may help to enhance salivary production. A similar method was already practiced by the redskin Indians who sucked pebbles to increase salivary output if no drinking water was available.
Acknowledgement
I wish to thank Prof. Dr. Rudolf Probst for his continuous support and challenging questions. Much constructive criticism and valuable recommendations by Prof. Dr. Thomas Hummel and Prof. Dr. Markus Wolfensberger have gone into this manuscript, for which I am most grateful to them. My thanks also go to PD. Dr. Daniel Simmen for his permission to use Figure 4 (Fig. 4).
References
- 1.von Brunn A. Beiträge zur mikroskopischen Anatomie der menschlichen Nasenhöhle. Arch Mikr Anat. 1892;39:632–651. [Google Scholar]
- 2.Read EA. A contribution to the knowledge of the olfactory apparatus in dog, cat and man. Am J Anat. 1908;8:17–47. [Google Scholar]
- 3.Féron F, Perry C, McGrath J, Mackay-Sim A. New techniques for biopsy and culture of human olfactory epithelia neurons. Arch Otolaryngol Head Neck Surg. 1998;124:861–866. doi: 10.1001/archotol.124.8.861. [DOI] [PubMed] [Google Scholar]
- 4.Say P, Leopold DA, Cochran G, Smith L, Greiner T. Resection of the inferior superior turbinate: Does it affect olfactory ability or contain olfactory neuronal tissue? Am J Rhinol. 2004;18:157–160. [PubMed] [Google Scholar]
- 5.Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G. Anterior distribution of human olfactory epithelium. Laryngoscope. 2000;110:417–421. doi: 10.1097/00005537-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis and rhinosinusitis. Laryngoscope. 2001;111:409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 8.Costanzo RM, Graziadei PPC. Development and plasticity of the olfactory system. In: Finger TE, Silver WL, editors. Neurobiology of smell and taste. New York: John Wiley & Sons; 1987. pp. 233–250. [Google Scholar]
- 9.Conley DB, Robinson AM, Shinners MJ, Kern RC. Age-related olfactory dysfunction: cellular and molecular characterization in the rat. Am J Rhinol. 2003;17:169–175. [PubMed] [Google Scholar]
- 10.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 11.Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy: the influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118:731–738. doi: 10.1001/archotol.1992.01880070061012. [DOI] [PubMed] [Google Scholar]
- 12.Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- 13.Perry C, Mackay-Sim A, Féron F, McGrath J. Olfactory neural cells: an untapped diagnostic and therapeutic resource. Laryngoscope. 2002;112:603–607. doi: 10.1097/00005537-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Benito FF, Ramón-Cueto A. Olfactory ensheathing glia transplantation: a therapy to promote repair in the mammalian central nervous system. Anat Rec. 2003;271B:77–85. doi: 10.1002/ar.b.10015. [DOI] [PubMed] [Google Scholar]
- 15.Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- 16.Yousem DM, Williams SCR, Howard RO, et al. Functional MR imaging during Odor stimulation: preliminary data. Radiology. 1997;204:833–838. doi: 10.1148/radiology.204.3.9280268. [DOI] [PubMed] [Google Scholar]
- 17.Deems DA, Doty RL, Settle G, et al. Smell and taste disorders, a study of 750 patients from the university of Pennsylvania smell and taste center. Arch Otolaryngol Head Neck Surg. 1991;117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 18.Miwa T, Furukawa M, Tsukatani T, Costanzo RM, DiNardo LJ, Reiter ER. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg. 2001;127:497–503. doi: 10.1001/archotol.127.5.497. [DOI] [PubMed] [Google Scholar]
- 19.Hedén Blomqvist E, Brämerson A, Stjärne P, Nordin S. Consequences of olfactory loss and adopted coping strategies. Rhinology. [PubMed] [Google Scholar]
- 20.Temmel AFP, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128:635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 21.Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130:317–319. doi: 10.1001/archotol.130.3.317. [DOI] [PubMed] [Google Scholar]
- 22.Hummel T, Nesztler C, Kallert S, Kobal G, Bende M, Nordin S. Gustatory sensitivity in patients with anosmia. Chem Senses. 2001;26:1118. [Google Scholar]
- 23.Nordin S, Monsch AU, Murphy C. Unawareness of smell loss in normal aging and Alzheimer´s disease: discrepancy between self-reported and diagnosed smell sensitivity. J Gerontol B Psychol Sci Soc Sci. 1995;50B:187–192. doi: 10.1093/geronb/50b.4.p187. [DOI] [PubMed] [Google Scholar]
- 24.Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. Ratings of overall olfactory function. Chem Senses. 2003;28:691–694. doi: 10.1093/chemse/bjg061. [DOI] [PubMed] [Google Scholar]
- 25.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function UPSIT. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 26.Doty RL, Marcus A, Lee WW. Development of the 12-Item cross-cultural smell identification test CC-SIT. Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Cain WS, Gent JF, Goodspeed RB, Leonard G. Evaluation of olfactory dysfunction in the conneticut chemosensory clinical research center. Laryngoscope. 1988;98:83–88. doi: 10.1288/00005537-198801000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf SR. "Sniffin´Sticks": Screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- 29.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. "Sniffin´Sticks": olfactory performance assessed by the combined testing of odor identification, odor discrimination, and olfactory thresholds. Chemical Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 30.Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257:205–211. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
- 31.Mösges R, Bartsch M, Hetzenecker A, et al. Eine pragmatische Geruchsprüfung. HNO. 1990;38:459–461. [PubMed] [Google Scholar]
- 32.Simmen D, Briner HR, Hess K. Screening of olfaction with smell diskettes. Laryngo-Rhino-Otol. 1999;78:125–130. doi: 10.1055/s-2007-996844. [DOI] [PubMed] [Google Scholar]
- 33.Briner HR, Simmen D. Smell diskettes as screening test of olfaction. Rhinology. 1999;37:145–148. [PubMed] [Google Scholar]
- 34.Zusho H, Asaka H, Okamoto M. Diagnosis of olfactory disturbance. Auris Nasus Larynx. 1981;8:19–26. doi: 10.1016/s0385-8146(81)80011-9. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa M, Kamide M, Miwa T, Umeda R. Significance of intraveneous olfaction test using thiamine propyldisuldie Alinamin in olfactometry. Auris Nasus Larynx. 1988;15:25–31. doi: 10.1016/s0385-8146(88)80006-3. [DOI] [PubMed] [Google Scholar]
- 36.Kobal G, Plattig KH. Methodische Anmerkungen zur Gewinnung olfaktorischer EEG-Antworten des wachen Menschen objektive Olfaktometrie. Z EEG-EMG. 1978;9:135–145. [PubMed] [Google Scholar]
- 37.Welge-Luessen A, Wolfensberger M, Kobal G, Hummel T. Grundlagen, Methoden und Indikationen der objektiven Olfaktometrie. Laryngo-Rhino-Otol. 2002;81:661–667. doi: 10.1055/s-2002-34449. [DOI] [PubMed] [Google Scholar]
- 38.Universität Düsseldorf. Riechstörungen – Leitlinie zur Epidemiologie, Pathophysiologie, Klassifikation, Diagnose und Therapie. Düsseldorf: Universität; 2004. Available from: http://www.uni-duesseldorf.de/AWMF/II/hno_II50.htm. [Google Scholar]
- 39.Förster G, Damm M, Gudziol H, et al. Riechstörungen: Epidemiologie, pathophysiologische Klassifikation, Diagnose und Therapie. HNO. 2004;52:679–684. doi: 10.1007/s00106-004-1117-x. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann HJ, Ishii EK, MacTurk RH. Age-related changes in the prevalence of smell/taste problems among the United States adult population:results of the 1994 disability supplement to the National Health Interview Survey NHIS. Ann N Y Acad Sciences. 1998;855:716–722. doi: 10.1111/j.1749-6632.1998.tb10650.x. [DOI] [PubMed] [Google Scholar]
- 41.Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: The Skövde population-based study. Laryngoscope. 2004;114:733–737. doi: 10.1097/00005537-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 42.Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004 doi: 10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Damm M, Temmel A, Welge-Luessen A, et al. Epidemiologie und Therapie von Riechstörungen in Deutschland, Österrreich und der Schweiz. HNO. 2004;52:112–120. doi: 10.1007/s00106-003-0877-z. [DOI] [PubMed] [Google Scholar]
- 44.Mott AE, Leopold DA. Disorders in taste and smell. Med Clin N Am. 1991;75:1321–1353. doi: 10.1016/s0025-7125(16)30391-1. [DOI] [PubMed] [Google Scholar]
- 45.Nordin S, Murphy C, Davidson TM, Quinonez C, Jalowayski AA, Ellison DW. Prevalence and assessment of qualitative olfactory dysfunction in different age groups. Laryngoscope. 1996;106:739–742. doi: 10.1097/00005537-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Quint C, Temmel AF, Schickinger B, Pabinger S, Ramberger P, Hummel T. Patterns of non-conductive olfactory disorders in eastern Austria: a study of 120 patients from the Department of Otorhinolaryngology at the University of Vienna. Wien Klin Wochenschr. 2001;113:52–57. [PubMed] [Google Scholar]
- 47.Cowart BJ, Flynn-Rodden K, McGeady SJ, Lowry LD. Hyposmia in allergic rhinitis. J Allergy Clin Immunol. 1993;91:747–751. doi: 10.1016/0091-6749(93)90194-k. [DOI] [PubMed] [Google Scholar]
- 48.Simola M, Malmberg H. Sense of smell in allergic and nonallergic rhinitis. Allergy. 1998;53:190–194. doi: 10.1111/j.1398-9995.1998.tb03869.x. [DOI] [PubMed] [Google Scholar]
- 49.Apter AJ, Mott AE, Frank ME, Clive JM. Allergic rhinitis and olfactory loss. Ann Allergy Asthma Immunol. 1995;75:311–316. [PubMed] [Google Scholar]
- 50.Apter AJ, Gent JF, Frank ME. Fluctuating olfactory sensitivity and distorted odor perception in allergic rhinitis. Arch Otolaryngol Head Neck Surg. 1999;125:1005–1010. doi: 10.1001/archotol.125.9.1005. [DOI] [PubMed] [Google Scholar]
- 51.Klimek L. Das Riechvermögen bei allergischer Rhinitis. Pneumologie. 1998;52:196–202. [PubMed] [Google Scholar]
- 52.Golding-Wood DG, Holmstrom M, Darby Y, Scadding GK, Lund VJ. The treatment of hyposmia with intranasal steroids. J Laryngol Otol. 1996;110:132–135. doi: 10.1017/s0022215100132967. [DOI] [PubMed] [Google Scholar]
- 53.Mott AE, Cain WS, Lafreniere D, Leonard G, Gent JF, Frank ME. Topical corticosteroid treatment of anosmia associated with nasal and sinus disease. Arch Otolaryngol Head Neck Surg. 1997;123:367–372. doi: 10.1001/archotol.1997.01900040009001. [DOI] [PubMed] [Google Scholar]
- 54.Heilmann S, Huttenbrink KB, Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am J Rhinol. 2004;18:29–33. [PubMed] [Google Scholar]
- 55.Tos M, Svendstrup F, Arndal H, et al. Efficacy of an aqueous and a powder formulation of nasal budenoside compared in patients with nasal polyps. Am J Rhinol. 1998;12:183–189. doi: 10.2500/105065898781390217. [DOI] [PubMed] [Google Scholar]
- 56.Lund VJ, Black JH, Szabó LZ, Schrewelius C, Akerlund A. Efficacy and tolerability of bedenoside aqueous nasal spray in chronic rhinosinusitis patients. Rhinology. 2004;42:57–62. [PubMed] [Google Scholar]
- 57.Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polys: a double-blind, placebo-controlled study of bedenoside. Clin Otolaryngol. 1995;20:26–30. doi: 10.1111/j.1365-2273.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 58.elNaggar M, Kale S, Aldren C, Martin F. Effect of beconase spray on olfactory function in post-nasal polypectomy patients: a prospective controlled trial. J Laryngol Otol. 1995;109:941–944. doi: 10.1017/s002221510013172x. [DOI] [PubMed] [Google Scholar]
- 59.Meltzer EO, Jalowayski AA, Orgel A, Harris AG. Subjective and objective assessments in patients with seasonal allergic rhinitis: Effects of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol. 1998;102:39–49. doi: 10.1016/s0091-6749(98)70053-3. [DOI] [PubMed] [Google Scholar]
- 60.Stuck BA, Blum A, Hagner E, Hummel T, Klimek L, Hörmann K. Mometasone furoate nasal spray improves olfactory performance in seasonal allergic rhinitis. Allergy. 2003;58:1195–1216. doi: 10.1034/j.1398-9995.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 61.Hedén Blomqvist E, Lundblad L, Bergstedt H, Stjärne P. Placebo-controlled, randomized,double-blind study evaluating the efficacy of fluticasone propionate nasal spray for the treatment of patients with hyposmia/anosmia. Acta Otolaryngol. 2003;123:862–868. doi: 10.1080/00016480310002140. [DOI] [PubMed] [Google Scholar]
- 62.Benninger MS, Hadley JA, Osguthorpe JD, et al. Techniques of intranasal steroid use. Otolaryngol Head Neck Surg. 2004;130:5–24. doi: 10.1016/S0194-5998(03)02085-0. [DOI] [PubMed] [Google Scholar]
- 63.Hotchkiss WI. Influence of prednisone on nasal polyposis with anosmia. Ann Allergy. 1956;24:278–283. doi: 10.1001/archotol.1956.03830180028006. [DOI] [PubMed] [Google Scholar]
- 64.Jafek BW, Moran DT, Eller PM. Steroid-dependent anosmia. Arch Otolaryngol. 1987;113:547–549. doi: 10.1001/archotol.1987.01860050093023. [DOI] [PubMed] [Google Scholar]
- 65.Stevens MH. Steroid-dependent Anosmia. Laryngoscope. 2001;111:200–203. doi: 10.1097/00005537-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Seiden AM, Duncan HJ. The diagnosis of a conductive olfactory loss. Laryngoscope. 2001;111:9–14. doi: 10.1097/00005537-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Ikeda K, Sakurada T, Suzaki Y, Takasaka T. Efficacy of systemic corticosteroid treatment for anosmia with nasal and paranasal sinus disease. Rhinology. 1995;33:162–165. [PubMed] [Google Scholar]
- 68.Biacabe B, Faulcon P, Amanou L, Bonfils P. Olfactory cleft disease: An analysis of 13 cases. Otolaryngol Head Neck Surg. 2004;130:202–208. doi: 10.1016/j.otohns.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Robinson AM, Kern RC, Foster JD, Fong KJ, Pitovski DZ. Expression of glucocorticoid receptor mRNA and protein in the olfactory mucosa; physiologic and pathophysiologic implications. Laryngoscope. 1998;108:1238–1242. doi: 10.1097/00005537-199808000-00026. [DOI] [PubMed] [Google Scholar]
- 70.Robinson AM, Kern RC, Foster JD, Krozowski ZS, Pitovski DZ. Mineralocorticoid receptors in the mammalian olfactory tissue. Ann Otol Rhinol Laryngol. 1999;108:974–981. doi: 10.1177/000348949910801009. [DOI] [PubMed] [Google Scholar]
- 71.Fong KJ, Kern RC, Foster JD, Zhao JC, Pitovski DZ. Olfactory secretion and sodium, potassium-adenosine triphosphatase: regulation by corticosteroids. Laryngoscope. 1999;109:383–388. doi: 10.1097/00005537-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Bisgaard H. Pathophysiology of the cysteinyl leukotriens and effects of leukotriene receptor antagonist in asthma. Allergy. 2001;56:7–11. doi: 10.1034/j.1398-9995.56.s66.2.x. [DOI] [PubMed] [Google Scholar]
- 73.Scadding GK. Comparison of medical and surgical tratment of nasal polyposis. Curr Allergy Astma Rep. 2002;2:494–499. doi: 10.1007/s11882-002-0090-2. [DOI] [PubMed] [Google Scholar]
- 74.Parnes SM, Chuma AV. Acute effects of antileukotriens on sinonasal polyposis and sinusitis. Ear Nose Throat J. 2000;79:18–25. [PubMed] [Google Scholar]
- 75.Cervin A. The anti-inflammatory effect of erythromycin and its derivates, with special reference to nasal polyposis and chronic sinusitis. Acta Otolaryngol. 2001;121:83–92. doi: 10.1080/000164801300006326. [DOI] [PubMed] [Google Scholar]
- 76.Wolfensberger M, Hummel T. Anti-inflammatory and surgical therapy of olfactory disorders related to sino-nasal disease. Chem Senses. 2002;27:617–622. doi: 10.1093/chemse/27.7.617. [DOI] [PubMed] [Google Scholar]
- 77.Damm M, Vent J, Schmidt M. Intranasal volume and olfactory function. Chem Senses. 2002;27:831–839. doi: 10.1093/chemse/27.9.831. [DOI] [PubMed] [Google Scholar]
- 78.Jafek BW, Hill DP. Surgical Management of chemosensory disorders. Ear Nose Throat J. 1989;68:398–404. [PubMed] [Google Scholar]
- 79.Eichel BS. Improvement of olfaction following pansinus surgery. Ear Nose Throat J. 1994;73:248–253. [PubMed] [Google Scholar]
- 80.Delank KW, Stoll W. Die Riechfunktion vor und nach endonasaler Operation der chronisch polypösen Sinusitis. HNO. 1994;42:619–623. [PubMed] [Google Scholar]
- 81.Delank KW, Stoll W. Olfactory function after functional endoscopic sinus surgery for chronic sinusitis. Rhinology. 1998;36:15–19. [PubMed] [Google Scholar]
- 82.Downey LL, Jacobs JB, Lebowitz RA. Anosmia and chronic sinus disease. Otolaryngol Head Neck Surg. 1996;115:24–28. doi: 10.1016/S0194-5998(96)70131-6. [DOI] [PubMed] [Google Scholar]
- 83.Kimmelmann CP. The risk to olfaction from nasal surgery. Laryngoscope. 1994;104:981–988. doi: 10.1288/00005537-199408000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Rowe-Jones JM, Mackay IS. A prospective study of olfaction following endoscopic sinus surgery with adjuvant medical treatment. Clin Otolaryngol. 1997;22:377–381. doi: 10.1046/j.1365-2273.1997.00004.x. [DOI] [PubMed] [Google Scholar]
- 85.Klimek L, Moll B, Amedee RG, Mann WJ. Olfactory function after microscopic endonasal surgery in patients with nasal polyps. Am J Rhinol. 1997;11:251–255. doi: 10.2500/105065897781446621. [DOI] [PubMed] [Google Scholar]
- 86.Abdel-Hak B, Gunkel A, Kanonier G, Schrott-Fischer A, Ulmer H, Thumfart W. Ciliary beat frequency, olfaction and endoscopic sinus surgery. ORL. 1998;60:202–205. doi: 10.1159/000027594. [DOI] [PubMed] [Google Scholar]
- 87.Perry BP, Kountakis SE. Subjective improvement of olfactory function after endoscopic sinus surgery for chronic rhinosinusitis. Am J Otolaryngol. 2003;24:366–369. doi: 10.1016/s0196-0709(03)00067-x. [DOI] [PubMed] [Google Scholar]
- 88.Jankowski R, Pigret D, Decroocq F. Comparison of functional results after ethmoidectomy and nasalization for diffuse and severe nasal polyposis. Acta Otolaryngol Stockh. 1997;117:601–608. doi: 10.3109/00016489709113445. [DOI] [PubMed] [Google Scholar]
- 89.Jankowski R, Bodino C. Olfaction in patients with nasal polyposis:effects of systemic steroids and radical ethmoidectomy with middle turbinate resection nasalisation. Rhinology. 2003;41:220–230. [PubMed] [Google Scholar]
- 90.Hedén Blomqvist E, Lundblad L, Änggard A, Haraldsson P-O, Stjärne P. A randomized controlled study evaluating medical treatment versus surgical treatment in addition to medical treatment of nasal polyposis. J Allergy Clin Immunol. 2001;107:224–228. doi: 10.1067/mai.2001.112124. [DOI] [PubMed] [Google Scholar]
- 91.Elsberg CA, Levy I. The sense of smell: I. A new and simple method of quantitative olfactometry. Bull Neurol Inst NY. 1935;4:4–19. [Google Scholar]
- 92.Stevens CN, Stevens MH. Quantitative effects of nasal surgery on olfaction. Am J Otolaryngol. 1985;6:264–267. doi: 10.1016/s0196-0709(85)80053-3. [DOI] [PubMed] [Google Scholar]
- 93.Ophir D, Gross-Isseroff R, Lancet D, Marshak G. Changes in olfactory acuity induced by total inferior turbinectomy. Arch Otolaryngol. 1986;112:195–197. doi: 10.1001/archotol.1986.03780020075017. [DOI] [PubMed] [Google Scholar]
- 94.Damm M, Eckel HE, Jungehülsing M, Hummel T. Olfactory changes at threshold and suprathreshold levels following septoplasty with partial inferior turbinectomy. Ann Otol Rhinol Laryngol. 2003;112:91–97. doi: 10.1177/000348940311200117. [DOI] [PubMed] [Google Scholar]
- 95.Damm M, Eckel HE, Streppel M, Jungehülsing M, Stennert E. Abhängigkeit des uni-und bilateralen Riechvermögens von der nasalen Strömung bei Patienten mit chronischer Rhinosinusitis. HNO. 2000;48:436–443. doi: 10.1007/s001060050594. [DOI] [PubMed] [Google Scholar]
- 96.Jafek BW, Murrow B, Johnson EW. Olfaction and endoscopic sinus surgery. Ear Nose Throat J. 1994;73:548–552. [PubMed] [Google Scholar]
- 97.Seiden AM. Olfactory loss secondary to nasal and sinus pathology. In: Seiden AM, editor. Taste and Smell Disorders. New York: Thieme; 1997. pp. 52–71. [Google Scholar]
- 98.Jafek BW, Murrow B, Michaels R, Restrepo D, Linschoten M. Biopsies of human olfactory epithelium. Chem Senses. 2002;27:623–628. doi: 10.1093/chemse/27.7.623. [DOI] [PubMed] [Google Scholar]
- 99.Lee SH, Lim HH, Lee HM, Park HJ, Choi JO. Olfactory mucosal findings in patients with persistent anosmia after endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2000;109:720–725. doi: 10.1177/000348940010900804. [DOI] [PubMed] [Google Scholar]
- 100.Ge Y, Tsukatani T, Nishimura T, Furukawa M, Miwa T. Cell death of olfactory receptor neurons in a rat with nasosinusitis infected artificially with staphylococcus. Chem Senses. 2002;27:521–527. doi: 10.1093/chemse/27.6.521. [DOI] [PubMed] [Google Scholar]
- 101.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 102.Kern RC, Conley DB, Haines GK, Robinson AM. Pathology of the olfactory mucosa: implications for the treatment of olfactory dysfunction. Laryngoscope. 2004;114:279–285. doi: 10.1097/00005537-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 103.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 104.Murphy C, Doty RL, Duncan HJ. Clinical disorders of olfaction. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York, Basel: Marcel Dekker; 2003. pp. 461–478. [Google Scholar]
- 105.Duncan HJ, Seiden AM. Long term-follow up of olfactory loss secondary to head trauma and upper respiratory tract infection. Arch Otolaryngol Head Neck Surg. 1995;123:367–372. doi: 10.1001/archotol.1995.01890100087015. [DOI] [PubMed] [Google Scholar]
- 106.Hummel T. Therapie von Riechstörungen. Laryngo-Rhino-Otol. 2003;82:552–554. doi: 10.1055/s-2003-41238. [DOI] [PubMed] [Google Scholar]
- 107.Akerlund A, Bende M, Murphy C. Olfactory threshold and nasal mucosal changes in experimentally induced common cold. Acta Otolaryngol. 1995;115:88–92. doi: 10.3109/00016489509133353. [DOI] [PubMed] [Google Scholar]
- 108.Hummel T, Rothbauer C, Barz S, Grosser K, Pauli E, Kobal G. Olfactory function in acute rhinitis. Ann N Y Acad Sci. 1998;855:616–624. doi: 10.1111/j.1749-6632.1998.tb10632.x. [DOI] [PubMed] [Google Scholar]
- 109.Hummel T, Rothbauer C, Pauli E, Kobal G. Effects of the nasal decongestant oxymetazoline on human olfactory and intranasal trigeminal function in acute rhinitis. Eur J Clin Pharmacol. 1998;547:521–528. doi: 10.1007/s002280050507. [DOI] [PubMed] [Google Scholar]
- 110.Jafek BW, Hartman D, Eller PM, Johnson EW, Strahan RC, Moran DT. Postviral olfactory dysfunction. Am J Rhinol. 1990;4:91–100. [Google Scholar]
- 111.Yamagishi M, Fujiwara M, Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology. 1994;32:113–118. [PubMed] [Google Scholar]
- 112.Mori I, Goshima F, Imai Y, et al. Olfactory receptor neurons prevent dissemination of neurovirulent influenza A virus into the brain by undergoing virus-induced apoptosis. J Gen Virol. 2002;83:2109–2116. doi: 10.1099/0022-1317-83-9-2109. [DOI] [PubMed] [Google Scholar]
- 113.Mori I, Nishiyama Y, Yokochi T, Kimura Y. Virus-induced neuronal apoptosis as pathological and protective responses of the host. Rev Med Virol. 2004;14:209–216. doi: 10.1002/rmv.426. [DOI] [PubMed] [Google Scholar]
- 114.Hummel T, Heilmann S, Hüttenbrink K-B. Lipoid acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope. 2002;112:2076–2080. doi: 10.1097/00005537-200211000-00031. [DOI] [PubMed] [Google Scholar]
- 115.Leopold DA. Distortion of olfactory perception: Diagnosis and treatment. Chem Senses. 2002;27:611–615. doi: 10.1093/chemse/27.7.611. [DOI] [PubMed] [Google Scholar]
- 116.Leopold DA, Schwob JE, Youngentob SL, Hornung DE, Wright H, Mozell MM. Successfull treatment of phantosmia with preservataion of olfaction. Arch Otolaryngol Head Neck Surg. 1991;117:1402–1406. doi: 10.1001/archotol.1991.01870240094016. [DOI] [PubMed] [Google Scholar]
- 117.Leopold DA, Loehrl TA, Schwob JE. Long-term follow-up of surgically treated phantosmia. Arch Otolaryngol Head Neck Surg. 2002;128:642–647. doi: 10.1001/archotol.128.6.642. [DOI] [PubMed] [Google Scholar]
- 118.Kern RC, Quinn B, Rosseau G, Farbman AI. Post-traumatic olfactory dysfunction. Laryngoscope. 2000;110:2106–2109. doi: 10.1097/00005537-200012000-00025. [DOI] [PubMed] [Google Scholar]
- 119.Heywood PG, Zasler ND, Costanzo RM. Proceedings of the 14th Annual Conference on Rehabilitation of the Brain Injured 1990; Williamsburg, Virginia. 1990. Olfactory screening test for assessment of smell loss following traumatic brain injury. [Google Scholar]
- 120.Farbman AI. Cell Biology of Olfaction. New York: Cambridge Press; 1992. [Google Scholar]
- 121.Doty RL, Yousem DM, Pham LT, Kreshak AA, Geckle RJ, Lee WW. Olfactory dysfunction in patients with head trauma. Arch Neurol. 1997;54:1131–1140. doi: 10.1001/archneur.1997.00550210061014. [DOI] [PubMed] [Google Scholar]
- 122.Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL. Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol. 1996;17:1171–1179. [PMC free article] [PubMed] [Google Scholar]
- 123.Yousem DM, Geckle RJ, Bilker WB, Kroger H, Doty RL. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol. 1999;6:264–272. doi: 10.1016/s1076-6332(99)80449-8. [DOI] [PubMed] [Google Scholar]
- 124.Costanzo RM, DiNardo LJ, Reiter ER. Head Injury and Olfaction. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York, Basel: Marcel Dekker; 2003. pp. 629–638. [Google Scholar]
- 125.Schwob JE, Szumowski KE, Stasky AA. Olfactory sensory neurons are trophically dependent on the olfactory bulb for their prolonged survival. J Neurosci. 1992;12:3896–3919. doi: 10.1523/JNEUROSCI.12-10-03896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carr VM, Farbman AI. The dynamics of cell death in the olfactory epithelium. Exp Neurol. 1993;124:308–314. doi: 10.1006/exnr.1993.1201. [DOI] [PubMed] [Google Scholar]
- 127.Costanzo RM. Neural regeneration and functional reconnection following olfactory nerve transsection in hamster. Brain Res. 1985;361:258–266. doi: 10.1016/0006-8993(85)91297-1. [DOI] [PubMed] [Google Scholar]
- 128.Yee KK, Costanzo RM. Restoration of olfactory mediated behavior after olfactory bulb deafferentation. Physiol Behav. 1995;58:959–968. doi: 10.1016/0031-9384(95)00159-g. [DOI] [PubMed] [Google Scholar]
- 129.Yee KK, Rawson NE. Retinoid acid enhances the rate of olfactory recovery after olfactory nerve transsection. Develop Brain Res. 2000;124:129–132. doi: 10.1016/s0165-3806(00)00108-5. [DOI] [PubMed] [Google Scholar]
- 130.Moran DT, Jafek BW, Rowley JC, Eller PM. Electron microscopy of olfactory epithelia in two patients with anosmia. Arch Otolaryngol. 1985;111:122–126. doi: 10.1001/archotol.1985.00800040086013. [DOI] [PubMed] [Google Scholar]
- 131.Jafek BW, Eller PM, Esses BA, Moran DT. Post-traumatic anosmia. Arch Neurol. 1989;46:300–304. doi: 10.1001/archneur.1989.00520390066018. [DOI] [PubMed] [Google Scholar]
- 132.Jafek BW, Johnson EW, Eller PM, Murrow B. Olfactory mucosal biopsy and related histology. In: Seiden AM, editor. Taste and Smell disorders. New York: Thieme; 1997. pp. 107–127. [Google Scholar]
- 133.Aiba T, Sugiura M, Mori J, et al. Effect of zinc sulfate on sensorineural olfactory disorder. Acta Otolaryngol Stockh. 1998;Suppl 538:202–204. doi: 10.1080/00016489850182936. [DOI] [PubMed] [Google Scholar]
- 134.Quint C, Temmel AFP, Hummel T, Ehrenberger K. The quinoxaline derivative caroverine in the treatment of sensorineural smell disorders: a proof-of-concept study. Acta Otolaryngol. 2002;122:877–881. [PubMed] [Google Scholar]
- 135.Ehrenberger K, Felix D. Caroverine depresses the activity of cochlear glutamate receptors in ginua pigs: in vivo model for drug-induced neuroprotection. Neuropharmacology. 1992;31:1259–1263. doi: 10.1016/0028-3908(92)90054-s. [DOI] [PubMed] [Google Scholar]
- 136.Fujii M, Fukazawa K, Takayasu S, Sakagami M. Olfactory dysfunction in patients with head trauma. Auris Nasus Larynx. 2002;29:35–40. doi: 10.1016/s0385-8146(01)00118-3. [DOI] [PubMed] [Google Scholar]
- 137.Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidisms. Am J Mental Defic. 1944;48:203–221. [Google Scholar]
- 138.Assouline S, Shevell MI, Zatorre RJ, Jones-Gotman M, Schloss M, Oudjhane K. Children who can´t smell the coffee: isolated congenital anosmia. J Child Neurol. 1998;13:168–172. doi: 10.1177/088307389801300404. [DOI] [PubMed] [Google Scholar]
- 139.Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL. MR Evaluation of patients with congenital hyposmia or anosmia. A J R. 1995;166:439–443. doi: 10.2214/ajr.166.2.8553963. [DOI] [PubMed] [Google Scholar]
- 140.Abolmaali ND, Hietschold V, Vogl TJ, Huttenbrink KB, Hummel T. MR evaluation in patients with isolated anosmia since birth or early childhood. Am J Neuroradiol. 2002;23:157–164. [PMC free article] [PubMed] [Google Scholar]
- 141.Jafek BW, Gordon AS, Moran DT, Eller PM. Congenital Anosmia. Ear Nose Throat J. 1990;69:331–337. [PubMed] [Google Scholar]
- 142.Leopold DA, Hornung DE, Schwob JE. Congenital lack of olfactory ability. Ann Otol Rhinol Laryngol. 1992;101:229–236. doi: 10.1177/000348949210100306. [DOI] [PubMed] [Google Scholar]
- 143.Rawson NE, Brand JG, Cowart BJ, et al. Functionally mature olfactory neurons from two anosmic patients with Kallmann syndrome. Brain Res. 1995;29:58–64. doi: 10.1016/0006-8993(95)00283-v. [DOI] [PubMed] [Google Scholar]
- 144.Amoore JE. Effects of chemical exposure on olfaction in humans. In: Barrow CS, editor. Toxicology of the Nasal Passages. Washington, DC: Hemisphere Publishing; 1986. pp. 155–190. [Google Scholar]
- 145.Doty RL, Hastings L. Neurotoxic exposure and olfactory impairment. Clinic Occupat Environ Med Neurotoxicology. 2001;1:547–575. [Google Scholar]
- 146.Hastings L, Miller MM. Influence on enviromental toxicants on olfactory function. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York, Basel: Marcel Dekker; 2003. pp. 575–591. [Google Scholar]
- 147.deVries H, Stuiver M. The absolute sensitivity of the human sense of smell. In: Rosenblith WA, editor. Sensory Communication. New York: Wiley and Sons; 1961. pp. 159–167. [Google Scholar]
- 148.Klimek L, Muttray A, Moll B, Konietzko J, Mann W. Riechstörungen durch inhalative Schadstoffexposition. Laryngo-Rhino-Otol. 1999;78:620–626. doi: 10.1055/s-1999-8762. [DOI] [PubMed] [Google Scholar]
- 149.Frasnelli J, Temmel AFP, Quint C, Oberbauer R, Hummel T. Olfactory function in chronic renal failure. Am J Rhinol. 2002;16:275–279. [PubMed] [Google Scholar]
- 150.Henkin RI. Drug-induced taste and smell disorders.Incidence,mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994;11:318–377. doi: 10.2165/00002018-199411050-00004. [DOI] [PubMed] [Google Scholar]
- 151.Ackerman BH, Kasbekar N. Disturbances of taste and smell induced by drugs. Pharmacotherapy. 1997;17:482–496. [PubMed] [Google Scholar]
- 152.Henkin RI, Larson AL. On mechanisms of hyposmia following laryngectomy in man. Laryngoscope. 1972;82:836–843. doi: 10.1288/00005537-197205000-00010. [DOI] [PubMed] [Google Scholar]
- 153.Welge-Lussen A, Kobal G, Wolfensberger M. Assessing olfactory function in laryngectomees using the sniffin´sticks test battery and chemosensory evoked potentials. Laryngoscope. 2000;110:303–307. doi: 10.1097/00005537-200002010-00022. [DOI] [PubMed] [Google Scholar]
- 154.Fujii M, Fukazawa K, Hatta C, Yasuno H, Sakagami M. Olfactory acuity after total laryngectomy. Chem Senses. 2002;27:117–121. doi: 10.1093/chemse/27.2.117. [DOI] [PubMed] [Google Scholar]
- 155.Gudziol H, Beleites E. Riechvermögen nach Laryngektomie. Wiss Z Humboldt. 1991;40:43–44. [Google Scholar]
- 156.Hilgers FJM, van Dam FSAM, Keyzers S, Koster MN, van As CJ, Muller MJ. Rehabilitation of olfaction after laryngectomy by means of a nasal airflow-inducing maneuver. Arch Otolaryngol Head Neck Surg. 2000;126:726–732. doi: 10.1001/archotol.126.6.726. [DOI] [PubMed] [Google Scholar]
- 157.Kopala LC, Good K, Honer WG. Olfactory identification ability in pre- and postmenopausal women with schizophrenia. Biol Psychiatry. 1995;38:57–63. doi: 10.1016/0006-3223(94)00224-Q. [DOI] [PubMed] [Google Scholar]
- 158.Dhong H-J, Chung S-K, Doty RL. Estrogen protects against 3-methylindole-induced olfactory loss. Brain Res. 1999;824:312–315. doi: 10.1016/s0006-8993(99)01241-x. [DOI] [PubMed] [Google Scholar]
- 159.Hughes LF, McAsey ME, Donathan CL, Smith T, Coney P, Struble RG. Effects of hormone replacement therapy on olfactory sensitivity:cross-sectional and longitudinal studies. Climacteric. 2002;5:140–150. [PubMed] [Google Scholar]
- 160.Féron F, Vincent A, Mackay-Sim A. Dopamine promotes differentiation of olfactory neuron in vitro. Brain Res. 1999;845:252–259. doi: 10.1016/s0006-8993(99)01959-9. [DOI] [PubMed] [Google Scholar]
- 161.Féron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion,proliferation and death in neural cultures from adults with schizophrenia. Schizophrenia Research. 1999;40:211–218. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- 162.Huisman E, Uylings HBM, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson´s Disease. Movement Disorders. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 163.Tanaka O, Mukaino Y. The effect of auricular acupuncture on olfactory acuity. Am J Chin Med. 1999;27:19–24. doi: 10.1142/S0192415X99000045. [DOI] [PubMed] [Google Scholar]
- 164.Michael W. Anosmia treated with acupuncture. Acupuncture in Medicine. 2003;21:153–154. doi: 10.1136/aim.21.4.153. [DOI] [PubMed] [Google Scholar]
- 165.Levy L, Henkin RI, Lin CS, Hutter A, Schellinger D. Increased brain activation in response to odors in patients with hyposmia after theophylline treatment demonstrated by fMRI. J Comp Ass Tomography. 1998;22:760–770. doi: 10.1097/00004728-199809000-00019. [DOI] [PubMed] [Google Scholar]
- 166.Carr VM, Farbman AI. Ablation of the olfactory bulb up-regulates the rate of neurogenesis and induces precocious cell death in olfactory epithelium. Exp Neurol. 1992;115:55–59. doi: 10.1016/0014-4886(92)90221-b. [DOI] [PubMed] [Google Scholar]
- 167.Farbman AI, Brunjes PC, Rentfro L, Michas J, Ritz S. The effect of unilateral naris occlusion on cell dynamics in the developing rat olfactory epithelium. J Neurosci. 1988;8:3290–3295. doi: 10.1523/JNEUROSCI.08-09-03290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farbman AI, Ezeh PI. TGF-alpha and olfactory marker protein enhance mitosis in rat olfactory epithelium in vivo. NeuroReport. 2000;11:3655–3658. doi: 10.1097/00001756-200011090-00051. [DOI] [PubMed] [Google Scholar]
- 169.Henkin RI, Laster L. Relationship between vitamin A metabolism and decreased olfactory detection sensitivity in patients with abetalipoproteinemia and other types of malabsorption. J Clin Invest. 1967;46:1069. [Google Scholar]
- 170.Garrett-Laster M, Russell RM, Jacques PF. Impairment of taste and olfaction in patients with cirrhosis: the role of vitamin A. Hum Nutr Clin Nutr. 1984;38C:203–214. [PubMed] [Google Scholar]
- 171.Henkin RI, Schecter PJ, Friedewald WT, Demets DL, Raff M. A double blind study of the effects of zinc sulfate on taste and smell dysfunction. Am J Med Sci. 1976;272:285–299. doi: 10.1097/00000441-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 172.Jafek BW, Linschoten M, Murrow B. Anosmia after intranasal zinc gluconate use. Am J Rhinol. 2004;18:137–141. [PubMed] [Google Scholar]
- 173.Miller IJJ, Bartoshuk LM. Taste perception, taste bud distribution, and spatial relationships. In: Getchell ML, Bartoshuk LM, Doty RL, Snow JBJ, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 205–233. [Google Scholar]
- 174.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miller IJJ, Reedy FEJ. Variations in human taste bud density and taste intensity perception. Physiol Behav. 1990;47:1213–1219. doi: 10.1016/0031-9384(90)90374-d. [DOI] [PubMed] [Google Scholar]
- 176.Cullen MM, Leopold DA. Disorders of smell and taste. Med Clin N Am. 2004;83:57–74. doi: 10.1016/s0025-7125(05)70087-0. [DOI] [PubMed] [Google Scholar]
- 177.Smith DV. Basic anatomy and physiology of taste. In: Seiden AM, editor. Taste and Smell Disorders. New York: Thieme; 1997. pp. 128–145. [Google Scholar]
- 178.Kinnamon SC. Taste transduction: a diversity of mechanisms. Trends Neurosci. 1988;11:491–496. doi: 10.1016/0166-2236(88)90010-0. [DOI] [PubMed] [Google Scholar]
- 179.Kinnamon SC, Margolskee RF. Mechanisms of taste transduction. Curr Opin Neurobiol. 1996;6:506–513. doi: 10.1016/s0959-4388(96)80057-2. [DOI] [PubMed] [Google Scholar]
- 180.Welge-Lüssen A, Gudziol H. Schmeckstörungen – Ursachen, Diagnostik und Therapie. Therapeutische Umschau. 2004;5:302–307. doi: 10.1024/0040-5930.61.5.302. [DOI] [PubMed] [Google Scholar]
- 181.Ahne G, Erras A, Hummel T, Kobal G. Assessment of gustatory function by means of tasting tablets. Laryngoscope. 2000;1108:1396–1401. doi: 10.1097/00005537-200008000-00033. [DOI] [PubMed] [Google Scholar]
- 182.Henkin RI, Gill JRJ, Bartter FC. Studies on taste thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids and serum sodium concentration. J Clin Invest. 1963;42:727–735. doi: 10.1172/JCI104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hummel T, Knecht M. Smell and Taste disorders. In: Calhoun KH, editor. Expert Guide to Otolyryngology. Philadelphia, PA: American College of Physicians; 2001. pp. 650–664. [Google Scholar]
- 184.Mueller C, Kallert S, Renner B, et al. Quantitative assessment of gustatory function in a clinical context using impregnated "taste-strips". Rhinology. 2003;41:2–6. [PubMed] [Google Scholar]
- 185.Haberland EJ, Fikentscher R, Roseburg B. Ein neues Elektrogustometer. Mschr Ohrenheilk. 1974;108:254–258. [Google Scholar]
- 186.Universität Düsseldorf. Schmeckstörungen – Leitlinie zur Epidemiologie, Pathophysiologie, Klassifikation, Diagnose und Therapie. Düsseldorf: Universität; 2004. Available from: http://www.uni-duesseldorf.de/AWMF/II/hno_II46.htm. [Google Scholar]
- 187.Bromley SM, Doty RL. Clinical Disorders affecting taste:evaluation and management. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York: Marcel Dekker; 2003. pp. 935–957. [Google Scholar]
- 188.Conger AD. Loss and recovery of taste acuity in patients irridiated to the oral cavity. Radiat Res. 1973;53:338–347. [PubMed] [Google Scholar]
- 189.Maes A, Huygh I, Weltens C, et al. De Gustibus: time scale of loss and recovery of tastes caused by radiotherapy. Radiother Oncol. 2002;63:195–201. doi: 10.1016/s0167-8140(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 190.Ripamonti C, Zecca E, Brunelli C, et al. A randomized,controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer. 1998;82:1938–1945. doi: 10.1002/(sici)1097-0142(19980515)82:10<1938::aid-cncr18>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 191.Yamagata T, Nakamura H, Yamagata Y, et al. The pilot trial of the prevention of the increase in electrical taste thresholds by zinc containing fluid infusion during chemotherapy to treat primary lung cancer. J Exp Clin Cancer Res. 2003;22:557–563. [PubMed] [Google Scholar]
- 192.Büntzel J, Schuth J, Küttner K, Glatzel M. Radiochemotherapy with amifostine cytoprotection for head and neck cancer. Support Care Cancer. 1998;6:155–160. doi: 10.1007/s005200050150. [DOI] [PubMed] [Google Scholar]
- 193.Tomita H, Ohtuka K. Taste disturbance after tonsillectomy. Acta Otolaryngol. 2002;S 546:164–172. doi: 10.1080/00016480260046571. [DOI] [PubMed] [Google Scholar]
- 194.Just T, Homoth J, Graumüller S, Pau HW. Schmeckstörung und Erholung der Schmeckfunktion nach Mittelohroperation. Laryngo-Rhino-Otol. 2002;82:494–500. doi: 10.1055/s-2003-40899. [DOI] [PubMed] [Google Scholar]
- 195.Saito T, Manabe Y, Shibamori Y, et al. Long-Term follow-up results of electrogustometry and subjective taste disorder after middle ear surgery. Laryngoscope. 2001;111:2064–2070. doi: 10.1097/00005537-200111000-00037. [DOI] [PubMed] [Google Scholar]
- 196.Lehman CD, Bartoshuk LM, Catalanotto FC, Kveton JF, Lowlicht RA. Effect of anesthesia of the chorda tympani nerve on taste perception in humans. Physiol Behav. 1995;57:943–951. doi: 10.1016/0031-9384(95)91121-r. [DOI] [PubMed] [Google Scholar]
- 197.Robinson PP, Loescher AR, Smith KG. A prospective, quantitative study on the clinical outcome of lingual nerve repair. Br J Oral Maxillofac Surg. 2002;38:255–263. doi: 10.1054/bjom.2000.0463. [DOI] [PubMed] [Google Scholar]
- 198.Schiffman SS. Taste and smell losses in normal aging and disease. JAMA. 1997;278:1357–1362. [PubMed] [Google Scholar]
- 199.Mattes RD. The chemical senses and nutrition in aging: challenging old assumptions. J Am Diet Assoc. 2002;102:192–196. doi: 10.1016/s0002-8223(02)90047-7. [DOI] [PubMed] [Google Scholar]
- 200.Stoll AL, Oepen G. Zinc Salts for the treatment of olfactory and gustatory symptoms in psychiatric patients: a case series. J Clin Psychiatry. 1994;55:309–311. [PubMed] [Google Scholar]
- 201.Kitagoh H, Tomita H, Ikui A, Ikeda M. Course of recovery from taste receptor disturbance. Acta Otolaryngol. 2002;S 546:83–93. doi: 10.1080/00016480260046454. [DOI] [PubMed] [Google Scholar]
- 202.Sakai F, Yoshida S, Endo S, Tomita H. Double-blind,placebo-controlled trial of zinc picolinate for taste disorders. Acta Otolaryngol. 2002;S 545:129–133. doi: 10.1080/00016480260046517. [DOI] [PubMed] [Google Scholar]
- 203.Yoshida S, Endop S, Tomita H. A double-blind study of therapeutic efficacy of zinc gluconate on taste disorders. Auris Nasus Larynx. 1991;18:153–161. doi: 10.1016/s0385-8146(12)80219-7. [DOI] [PubMed] [Google Scholar]
- 204.Mahajan SK, Prasad AS, Lambujon J, Abbasi AA, Briggs WA, McDonald FD. Improvement of uremic hypogeusia by zinc: a double-blind study. Am J Clin Nutr. 1980;33:1517–1521. doi: 10.1093/ajcn/33.7.1517. [DOI] [PubMed] [Google Scholar]
- 205.Heckmann SM, Habiger S, Wichmann MG, Hummel T. Zinc gluconat in the treatment of dysgeusia: a double blinded controlled study. Chem Senses. 2003;28:E24. [Google Scholar]
- 206.Heckmann JG, Heckmann SM, Lang CJ, Hummel T. Neurological aspects of taste disorders. Arch Neurol. 2003;60:667–671. doi: 10.1001/archneur.60.5.667. [DOI] [PubMed] [Google Scholar]
- 207.Deems DA, Yen DM, Kreshak AA, Doty RL. Spontaneous resolution of dysgeusia. Arch Otolaryngol Head Neck Surg. 1996;122:961–963. doi: 10.1001/archotol.1996.01890210037009. [DOI] [PubMed] [Google Scholar]
- 208.Femiano F, Scully C. Burning mouth syndrome BMS: double blind controlled study of alpha-lipoic acid thioctic acid therapy. J Oral Pathol Med. 2002;31:267–269. doi: 10.1034/j.1600-0714.2002.310503.x. [DOI] [PubMed] [Google Scholar]
- 209.Bull TR. Taste and the chorda tympani. J Laryngol Otol. 1965;79:479–493. doi: 10.1017/s0022215100063969. [DOI] [PubMed] [Google Scholar]
- 210.Bradley RM, Beidler LM. Saliva: Its role in taste function. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York, Basel: Marcel Dekker; 2003. pp. 639–650. [Google Scholar]
- 211.Bläker M, Kock K, Ahlers C, Buck F, Schmale H. Molecular cloning of human von Ebner's gland protein, a member of the lipocalin superfamily highly expressed in lingual salivary glands. Biochem Biophys Acta Gene Struct Expression. 1993;1172:131–137. doi: 10.1016/0167-4781(93)90279-m. [DOI] [PubMed] [Google Scholar]
- 212.Van´t Hof W, Blankenvoorde MFJ, Veerman ECI, Nieuw Amerongen AV. The salivary lipocalin von Ebner´s gland protein is a cysteine proteinase inhibitor. J Biol Chem. 1997;272:1837–1841. doi: 10.1074/jbc.272.3.1837. [DOI] [PubMed] [Google Scholar]
- 213.Henkin RI, Lippoldt R, Bilstad J, Edelhoch H. A zinc protein isolated from human parotid saliva. Proc Natl Acad Sci USA. 1975;72:488–492. doi: 10.1073/pnas.72.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thatcher BJ, Doherty AE, Orvisky E, Martin BM, Henkin RI. Gustin from human parotid saliva is carbonic anhydrase VI. Biochem Biophys Res Commun. 1998;250:635–641. doi: 10.1006/bbrc.1998.9356. [DOI] [PubMed] [Google Scholar]
- 215.Henkin RI, Martin BM, Agarwal RP. Efficacy of exogeneous oral zinc in treatment of patients with carbonic anhydrase VI deficiency. Am J Med Sci. 1999;318:392–404. doi: 10.1097/00000441-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 216.Henkin RI, Talal N, Larson AL, Mattern CFT. Abnormalities of taste and smell in Sjögren´s syndrome. Ann Intern Med. 1972;76:375–383. doi: 10.7326/0003-4819-76-3-375. [DOI] [PubMed] [Google Scholar]
- 217.Weiffenbach JM, Fox PC, Baum BJ. Taste and salivary function. Proc Natl Acad Sci USA. 1986;83:6103–6106. doi: 10.1073/pnas.83.16.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Catalanotto FA, Sweeney EA. Oral Conditions affecting chemosensory function. In: Getchell ML, Doty RL, Bartoshuk LM, Snow JBJ, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 643–651. [Google Scholar]
- 219.Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:28–46. doi: 10.1016/j.tripleo.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 220.Fox PC. Salivary enhancement therapies. Caries Res. 2004;38:241–246. doi: 10.1159/000077761. [DOI] [PubMed] [Google Scholar]
- 221.Ferraccioli GF, Salaffi F, De Vita S, et al. Interferon alpha-2 IFN alpha 2 increases lacrimal and salivary function in Sjögren's syndrome patients:preliminary results of an open pilot trial versus OH-chloroquine. Clin Exp Rheumatol. 1996;14:367–371. [PubMed] [Google Scholar]
- 222.Ship JA, Fox PC, Michalek JE, Cummins MJ, Richards AB. Treatment of primary Sjögren's syndrome with low-dose natural human interferon-alpha administered by the oral mucosal route: a phase II clinical trial. J Interferon Cytokine Res. 1999;19:943–951. doi: 10.1089/107999099313497. [DOI] [PubMed] [Google Scholar]


