Abstract
When it comes to restoring impaired neural function by means of surgical reconstruction, sensory nerves have always been in the role of the neglected child when compared with motor nerves. Especially in the head and neck area, with its either sensory, motor or mixed cranial nerves, an impaired sensory function can cause severe medical conditions. When performing surgery in the head and neck area, sustaining neural function must not only be highest priority for motor but also for sensory nerves. In cases with obvious neural damage to sensory nerves, an immediate neural repair, if necessary with neural interposition grafts, is desirable.
Also in cases with traumatic trigeminal damage, an immediate neural repair ought to be considered, especially since reconstructive measures at a later time mostly require for interposition grafts.
In terms of the trigeminal neuralgia, commonly thought to arise from neurovascular brainstem compression, a pharmaceutical treatment is considered as the state of the art in terms of conservative therapy. A neurovascular decompression of the trigeminal root can be an alternative in some cases when surgical treatment is sought after. Besides the above mentioned therapeutic options, alternative treatments are available.
Keywords: trigeminal nerve, trigeminal neuralgia, neural reconstruction, neurovascular decompression, neural interposition graft
1. Introduction
The sensory innervation of the head is mainly covered by the trigeminal nerve, also known as cranial nerve 5 (CN-V). Besides the skin of the head (exception: occipital area and maxillary joint area), the nerve feeds the mucosa of the orbital, nasal and oral cavities. As the key player in sensory function of the head he is, various damages, both disease and trauma related, to his main branch and to his divisions are possible.
Nevertheless past research efforts have neglected aspects of trigeminal nerve rehabilitation. Neural rehabilitation has so far focused on motor nerve function, and the facial nerve function in particular. Since impaired aesthesia of the face and mucous membranes is associated with a severe discomfort and often relevant consequences, it seems more than appropriate to discuss options for regaining and restoring impaired trigeminal function. However, May and colleagues [1] already stated back in 1980 that therapeutic options are limited.
2. Anatomic remarks
Before dealing with reconstructive procedures involving sensory innervations, it has to be stated that detailed anatomic knowledge is essential. However, particular discussion of this topic will exceed the purpose of this review. In depth information on the anatomy of cranial nerves can be found in manuscripts by Samandari [2], Wilson-Pauwels et al. [3], Leblanc [4], [5] and Lang [6].
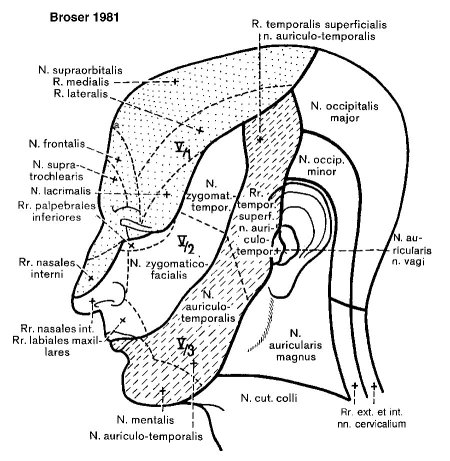
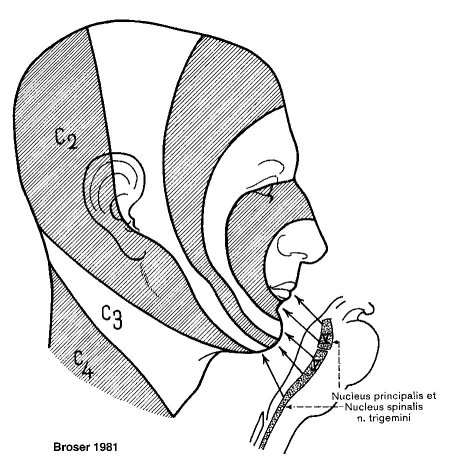
Peripheral sensoric areas are organized in dermatomes (Figure 1 (Fig. 1)) in respect to their corresponding innervation. Other "somato-optic" visualizations are depicted according to the "onionskin-like" construction of the spinal nucleus, separated by the Laehr-Soelder lines (Figure 2 (Fig. 2)). Most cranially terminate perioral fibers, followed by fibers from the anterior and medial facial skin in the medial section of the nucleus and fibers from the lateral facial skin terminating in the most caudal parts of the nucleus.
Figure 1. Sensoric innervation of the face for illustration of peripheral innervation pattern of the trigeminal nerve.
Figure 2. Central innervation pattern of the trigeminal nerve (lines of Laehr-Soelder).
Considering this distribution, it is possible to determine peripheral and central lesions by analyzing the involved facial area. It is also important that the spinal trigeminal nucleus mainly contains proteopathic fibers - therefore when dealing with impaired trigeminal function it is necessary to examine not only tactile responses but also pain or temperature recognition.
Perioral sensory deficit due to reclination of the head may be a characteristic symptom of vertebrobasilar insufficiency and is caused by impaired perfusion in the region of the cranial portion of the spinal trigeminal nucleus.
3. Causes for trigeminal dysesthesia
Before considering possible reconstructive measures, we need to cover possible causes of nerve damage and impaired function.
Assessing nerve function has to start with a bilateral sensoric examination (touch, temperature, pain and proprioception), followed by a mastication test, completing a thorough trigeminal topodiagnostic evaluation. An examination of the corneal reflex is mandatory for recognition and prevention of ceratitis neuroparalytica. Loescher et al. [7] reviewed in depth diagnostic steps for a structured trigeminal dysesthesia assessment.
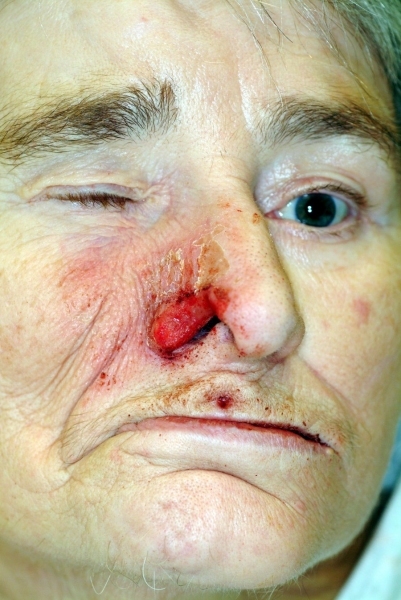
When examining a patient with trigeminal dysesthesia, special attention has to be devoted to habitual bites on lips, cheek mucous membranes and tongue as well as to neurotropic ulcerations (Figure 3 (Fig. 3)). Neurotropic ulcerations are observed variably weeks or even years after trigeminal nerve damage [8]. Typical causes for these ulcerations are brain stem infarcts (i.e. Wallenberg syndrome), cerebello-pontine angle tumors and iatrogenic nerve damages (i.e. trigeminal neuralgia surgery). Neural damage, in the chain of events leading to neurotropic ulcerations, is followed by repeated manipulation. Manipulation in combination with altered autonomous innervation starts a vicious circle of impaired wound healing and ends in neurotropic ulcerations.
Figure 3. Neurotrophic ulceration at the right nasal alar of a 69 years old woman with vascular dementia, paraparesis, facial paralysis on left side and trigeminal neuropathia on right side. The lesion was decided for biopsy to exclude basal cell carcinoma. By observation of the patient habitual manipulations at the nasal site using the left hand were detected. After application of a splint to the left arm the lesion healed almost completely.
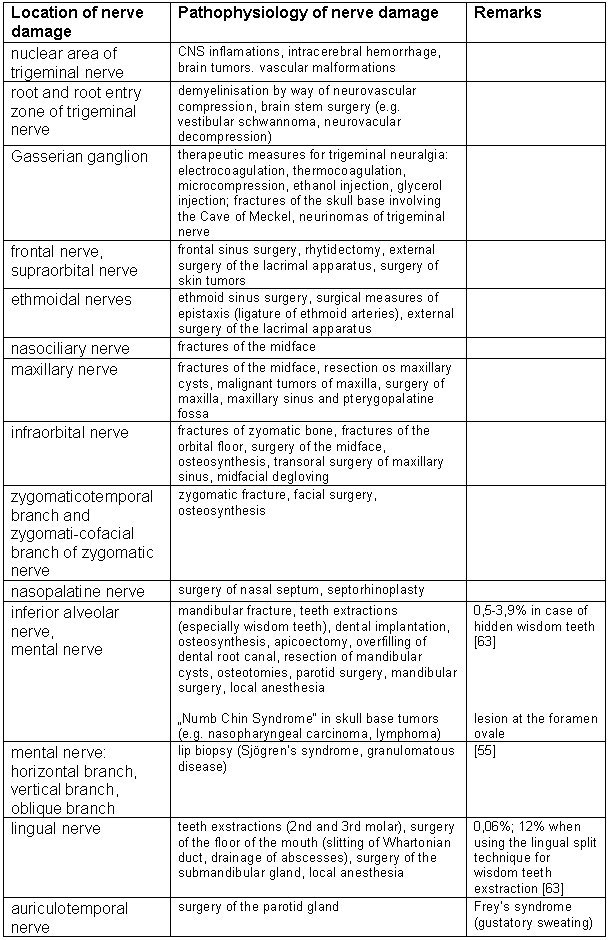
Iatrogenic damage to the trigeminal root on a greater scale is seldomly reported, since the nerve enters the brain stem not with a single fiber, but rather in a fan-shaped manner. Therefore traumatic as well as iatrogenic lesions to the nerve involve only single roots or at the most a couple of strands. A complete neural transaction is only feasible at the trigeminal ganglion. Table 1 (Tab. 1) gives an overview over possible causes for trigeminal damage.
Table 1. Lesions of the trigeminal nerve.
Gregg [9] reported that 8.4% (0%-19%) of patients undergoing dental implantology suffer from persistent trigeminal irritations. He hypothesized that neural compression by the implant results in a neuroma formation, which in turn causes chronic neuropathic pain sensations. Kraut and Chahal [10] pointed out a higher risk of damage for the lingual nerve and lesser risks for the inferior alveolar nerve in dental implantology. The inferior alveolar nerve is mostly clearly visible in orthopantomograms and therefore at a lower risk of damage.
A couple of syndromes are characterized by impaired trigeminal nerve function - such as: Gradenigo syndrome, Garcin symptomatology, Raeder symptomatology, Tolosa-Hunt symptomatology, Wallenberg syndrome, superior orbital fissure syndrome.
Postherpetic neuralgia mostly affects the elderly over 60 years of age and is characterized by a burning sensation with corresponding hypoesthesia of the affected trigeminal root (anesthesia dolorosa). Allodynia, pain arising from stimuli which are normally not painful, is also often reported in this context. Common shingle rashes or blisters are not mandatory. The ophthalmic nerve has a 4-fold higher chance to be affected by post herpetic neuralgia than all other branches. Therefore attention should be drawn on ceratitis neuroparalytica.
The "typical" trigeminal neuralgia has been associated with neurovascular compression, mostly caused by the superior cerebellar artery located at the trigeminal entry zone of the sensory root (Oberheimer-Redlich zone). Permanent pressure upon the nerve causes a focal demyelinization of Ab fibers right at the transition from peripheral to central myelin. This causes a shortcut-like ephaptic signal transmission from Ab-fibers onto Ad- and C-fibers [11], [12], resulting in excruciating pain sensations.
Other central mechanisms like epileptic foci [11], [13] located within trigeminal nuclei have been discussed.
Knowledge on the pathology dispenses older distinctions between "typical = idiopathic" and atypical trigeminal neuralgia. However we have to differentiate between disease with spontaneous lightning-like pain shots followed by variable pain-free intervals and trigeminal neuropathy. Trigeminal neuropathy is characterized as a permanent trigeminal impairment with or without pain sensations as in trigeminal neuralgia. Some authors see trigeminal neuralgia as predecessor form of trigeminal neuropathy. Symptomatic trigeminal neuralgia as well as neuropathy has been reported in association with encephalomyelitis disseminata, Wallenberg syndrome, pontine cysts, aneurysms, gliomas of the pons, acoustic neuromas und syringobulbia [13], [14]. Trigeminal neuralgia affects both genders but predominantly females (m : f = 2 : 3) with increasing incidence in the elderly (overall incidence: 4/100000; age > 70 years: 25/100000). For whatever reason, the right side is statistically twice as often affected as the left side. Painful attacks can be spontaneous, but they may also be provoked stereotypically by chewing or changes in temperature and even mild stimulation around the face, including brushing teeth, shaving, speaking, swallowing or putting on makeup. The trigeminal roots V3 and V2 are more often affected than V1. Table 2 (Tab. 2) gives an overview on possible causes for trigeminal neuropathy and affections [14], [15].
Table 2. Uncommon causes of trigeminal impairment.

Little is known so far on trigeminal denervations of the nasal mucous membranes. Due to its overlapping sensoric and secretory innervations in this area, iatrogenic or traumatic damage mostly stays undetected. Only when performing nasal septum surgery, damage to the nasopalatine nerve often results in hypesthesia of the frontal teeth and hard palate [16]. Damage to the nasopalatine nerve may also occur after dental interventions on the incisor- and canine tooth [17].
Isolated neuralgia of the nasociliary nerve is called Charlin-Sluder-neuralgy and characterized by neuralgia-like pain sensation of the nasociliary nerve dermatome. This shall not be mistaken with the Sluder-neuralgy, a syndrome of the pterygopalatine ganglion with impaired parasympathic function.
Chronic and sometimes neuralgia-like pain is also reported after surgery of the nose, paranasal sinuses and nasal traumas [18], mostly associated by traumatic nerve damage or excessive scaring.
4. Pathology and physiology of neural damage and regeneration
Peripheral neural damage can be classified by Seddon's criteria as neurapraxia, axonotmesis and neurotmesis [19].
Neurapraxia is the physiological interruption of an anatomically intact nerve. In this condition there is minimal damage. The axons are intact but conduction is lost because of segmental demyelinization. This is a transient lesion and recovery is spontaneous after a few days or weeks.
Axonotmesis is the separation of axons of a peripheral nerve. In this condition there is severe damage of the axon and the distal portions of the axons degenerate. The sheaths of the nerve remain intact and recovery, though delayed, is likely. Axonotmesis is a typical surgery related damage caused by retractors or hooks.
Neurotmesis is a condition where there is complete division of the nerve and spontaneous recovery is unlikely. Persistent defects after healing already occur sometimes after axonotmesis and always after neurotmesis.
Sunderland differentiates 5 degrees of neural damage with grade A being neurapraxia, grade B axonotmesis and grade E neurotmesis [20], [21].
After complete neural division, a swelling of the neural stumps due to the continuing axonal transport is seen. The distal stump degenerates (Wallerian degeneration) with a sequence of axonal and myelin degeneration. Schwann cells proliferate and form the bands of Buengner [22]. Also the denervated cells degenerate, followed by macrophage infiltration. Pathologic changes that occur at the cell body of proximal to an axonal lesion are characterized by central chromatolysis. If no "regeneration-program" with consecutive reconstruction and axonal reconnection is performed, cell death is imminent [22]. A microglia scar is formed in case of retrograde degeneration. Cellular degradation, when started, may overlap and incorporate adjacent neural structures. The experimental application of NGF (Nerve Growth Factor) was able to show an inhibitory effect on the extent of cellular degradation [23]. NGF in this context was associated with a more directed axonal growth at the proximal neural stump and can be seen as a decisive factor in arranging neural regeneration.
If the neural sheath stays intact (neurapraxia, axonotmesis), a proper regeneration is likely. Macrophages stimulate Schwann cells, which in turn produce NGF and thereby promote axonal growth. Siemionow & Sari [21] give an overview in their review on state of the art research on peripheral neural repair mechanisms.
5. Conservative rehabilitation of trigeminal function
Studies on the animal model were able to show a trigeminal plasticity also in adults, ensuring rehabilitation of neural function after nerve damage without surgical intervention. This phenomenon is partly explained by a functional adoption of adjacent neural branches, that extent their innervation and cover areas of the damaged branch. Of course genuine nerve function after regeneration is also possible with the period of time as well as quality of reconstructed function depending on the extent of damage.
Research has focused on supporting these self-healing processes by physical as well as pharmaceutical means. The following techniques and substances were tested for their ability to support reconstruction and sustain nerve growth:
· Electro stimulation
· Neurotrophic vitamins
· Antioxidants
· Alpha lipoic acid
· Neurotrophins (i.e. NGF)
Among experimental measures for regaining sensoric function after nerve dissection is one setup from Rosén and Lundborg [24], who propose a training program aimed at remodeling cortical sensoric projection fields after nerve damage of the hand. It calls for an early use of a glove-like device that has integrated microphones at the fingertips and sends auditory signals to the ear. This auditory feedback is supposed to compensate for the lost incoming tactile information. Clinical studies are still ongoing, but if proven successful, this technique might be modified and applied for the rehabilitation in impaired trigeminal function. Other than this, there is no evidence supporting the benefit of electrical stimulation in neural reconstruction and rehabilitation.
The B-vitamins B1, B2, B6 and B12 - especially vitamin B6 - are traditionally named and used as neurotrophic substances and still given in exorbitant high dosage after nerve damage. Experimental as well as clinical data supporting this hypothesis are still pending. We therefore advise against the use of it, especially the intravenous application if no deficiency is evident. In contrast to the vitamin B, Iskandar et al. [25] was able to show in animal studies a benefit from the application of folic acid after spine and CNS damage. To date alpha lipoic acid is frequently used in the treatment of diabetic polyneuropathy. Application fields are currently extended, however no clinical data support the use of alpha lipoic acid in other fields and diseases so far.
Nerve growth factor (NGF) is currently not available for clinical use. It is one of the body's peptides and belongs to the family of neurotrophins. It functions as a chemokine on the growing axon of peripheral nerves. Positive effects have also been reported on its impact in the CNS. After initial damage to a peripheral nerve, NGF is produced locally by the effected nerve. It induces the increased expression of a NGF receptor trk-a (tyrosin receptor kinase A, [26]). Parallel the brain derived neurotrophic factor (BDNF) is increasingly expressed and binds to its receptor trk-b. The interplay of both factors and receptors communicate neural reconstruction and its related pain sensations [27]. It is to be expected, that in depth knowledge on the molecular mechanisms involved in neural repair and reconstruction will lead to the clinical application of factors and mediators for the treatment of damaged neural structures.
The group of Siemionow developed a technique to harvest axoplasmatic fluids from the injured neural stump and enrich the cytokines expressed. In order to test it for its neuroregenerative abilities, they injected it in neural anastomosis sites after surgical repair (see review of Siemionow & Sari [21]). According to their results, a positive effect was observed in terms of neural repair. Other substances that were brought in connection with neural repair were tested for their ability to positively affect neural regeneration. VEGF (vascular endothelial growth factor) and DHEA (dehydroepiandrosterone) were able to positively impact ongoing repair. DHEA is supposed to function as antioxidant in this context. Other antioxidants (i.e. ascorbic acids) are used on an empirical basis.
Trigeminal neuralgia is characterized by neural malfunction. However the associated pain sensations pose a far greater discomfort for the patient than the isolated loss of sensoric functionality.
Diagnostic procedures and therapeutics are an interdisciplinary approach by both ENT specialists as well as neurologists. Often ENT specialists are consulted regarding a causal clarification, such as paranasal sinus affections and septum pathologies. The ENT specialist therefore must be familiar with pathophysiology, diagnosis and therapy of trigeminal neuralgia. An initial conservative approach is often considered before a definite causal neurosurgical treatment option is sought.
Carbamazepine is to date the most effective conservative medication on the market for classic trigeminal neuralgia. It belongs to the family of anticonvulsive drugs and functions by membrane stabilization mechanisms. Success rates are reported with up to 60-70% response to initial treatment. Carbamazepine dosage should be increased over time with a typical starting dosage of 100 mg in an 8 hours interval. Equivalent to the treatment of seizure disorders, a plasma level dependent calibration of carbamazepine is recommended even though a more symptom oriented medication plan is mostly in order. Blood cell counts and analysis of AST and ALT are recommended. However, the effectiveness of carbamazepine over time decreases, and may at some point not be compensated by dosage increases without major side effects anymore.
Therapeutic alternatives, if carbamazepine is not tolerated, are: oxycarbazepine (900-1800mg/d), Gabapentin (600-3000mg/d), lamotrigine (Lamicatal), Phenytoine (second choice) and finally Baclofen as an add-on-medication [28].
American authors also reported on the use of Clonazepame (sedation side-effect) and Amitryptiline. Nonopioid analgetics, triptans, beta-blockers, calcium and serotonine antagonists are ineffective. Opioids are reserved for special indications.
Many drugs have so far been tried, only few have been effective; interestingly misoprostole, a prostaglandine E1-analogue which is so far only approved for protection of gastric ulcer in NSAID treatment, was by accident once successfully used in treatment of trigeminal neuralgia [29].
Charlin-Sluder-neuralgy has often been treated with topic sympathomimetics and local anesthetics. However the positive effect is only of short time and topic corticoids have been reported more successful. The use of capsaicine is discussed controversially, especially in the light of its possible retrograde axonal transport into the CNS. Alternatively botulinum toxin was successfully used and therefore can be considered as well as a turbinoplasty of the medial turbinate.
In case of post herpetic neuralgia, carbamazepine and tricyclic antidepressants can be tried since classic analgetics mostly don't work. Sympathicus blockage only works in very early stages and the use of a transcutaneous electro nerve stimulator (TENS) can be considered. Local application aspirin and capsaicine is discussed, however successful treatments are reported. Since there is no generally recognized treatment available so far, we have to point out the importance of a thorough antiviral treatment, since the incidence of post herpetic neuralgia after adequate antiviral therapy is considerably low. Post herpetic neuralgia is generally a self-limited disease. Symptoms tend to abate over time and few have pain at one year after initial presentation. Patients in an immunocompromised state are clearly at a higher risk for developing post herpetic neuralgia.
6. Surgical rehabilitation of trigeminal function
This paragraph is dedicated to surgical measures for reconstructing trigeminal function. Surgical procedures available for the treatment of trigeminal neuralgia and for recovering traumatic or iatrogenic trigeminal nerve damage are discussed.
6.1. Experimental procedures
In 1996 Holland [30] recapitulated electrophysiological and morphological findings after traumatic dissection and consecutive reconstruction of the inferior alveolar nerve and lingual nerve. He differentiated between neural contusion (axonotmesis) and dissection (neurotmesis). Results after neural contusion were in all cases better than after dissection, however functional deficits always occurred. Following neural dissection, he observed smaller receptor fields (density), higher excitation thresholds and a greater number of non-myelinated fibers.
Clinical observations after loss of sensory supply on the tongue (lingual nerve, chorda tympani) specify quantitative and qualitative variations in taste bulbs. Holland observed a subsequent epithelial re-differentiation after neural reconstruction, whereas mechanoreceptors prevail in terms of quantity and number. In a gerbil-animal model he observed a regeneration of taste bulbs after contusion of up to 60% and only 20% after neural dissection. These data confirm results from Tyndall [31] who showed in a rat-animal model, that regeneration of the infraorbital nerve was better for contusion than for neural dissection. These data emphasize and support clinical perceptions, that a neural regeneration after damage with intact neural sheath is better than after complete dissection, even though there will not be a complete recovery ("restitutio ad integrum") in any case.
In terms of the used neural reconstruction technique, a direct neural suture was functionally superior to a combination of suture and sifted polyethylene capillary tube.
Experiments on cats showed, that after dissection of the inferior alveolar nerve a spontaneous functional rehabilitation was observed after three to nine months without surgical or conservative measures. A recurrence of the jaw-opening reflex was even observed when capping both neural stumps after dissection. This was due to a spontaneous reinnervation of the dental pulp from contralateral neural fibers. Holland pointed out, that this phenomenon was never before seen in any other part of the body.
Zuniga [32] investigated the regeneration and reorganization of the trigeminal ganglion in adult rats after neural dissection of the mental nerve followed by surgical reconstruction. Best functional results were observed after immediate surgical reconstruction even though a complete recovery was not possible. A neural support from fibers of the contralateral side was not observed. All data support the superiority of an immediate neural reconstruction after dissection.
In contrast with above mentioned results, Zuniga und O'Connor [33] were not able to reproduce results with a superiority of the immediate compared to a delayed surgical reconstruction with relatively short (3mm) neural interpositions. In 1999 Zuniga [34] observed a demise of neural cells at the trigeminal ganglion after dissection of the mental nerve. After surgical reconstruction, the ganglion retrieved its original size and dimensions. He attributed this effect to either an increased axonal branching or glia-cell proliferation.
Smith and Robinson [35] did electrophysiological testing on cats after lingual nerve reconstruction with reanastomosis by epineural suture or entubulation. Reanastomosis by epineural suture was superior to entubulation in terms of postoperative regained functionality, even though a delayed conduction velocity and higher thresholds remained. In contrast reanastomosis by entubulation resulted in a higher specifity of the regenerated axons. Different materials were tested for entubulation, such as: polyethylene, collagen, autologe veins, polyester, PGA, silicone, Goretex, etc [35]. In order not to damage the cut surface by sutures, both neural stumps were fixed in the tube with sutures apart from the cut surface and reanastomosis site.
Both authors also investigated the outcome of sensoric re-innervation on the chorda tympani after reanastomosis [36]. Results indicated a superiority of the epineural suture compared to the entubulation technique. Observations indicated an increased number of mechano-sensoric than chemo-sensoric fibers after reanastomosis. After dissection of the lingual nerve, both authors found only a slight advantage of the spontaneous recovery compared to 12 weeks delayed reconstruction [37]. When comparing technical aspects of a reanastomosis surgery, Smith and Robinson tested the neural suture under tension against neural interpositions and frozen preserved muscle specimen [38]. Results clearly showed a superiority of the direct neural suture over all other techniques. However a tension free neural anastomosis was not included in this study.
In 1998 Curtis [39] described two techniques for an endosteal reanastomosis of the inferior alveolar nerve inside the mandibular canal in the rat animal model. He applied standard suture techniques as well as an ICG- and IR-diode laser. Both techniques were viewed as equivalent.
Recently, a biodegradable composite co-polymer guiding neurotube (NVR tube, Neural and Vascular Reconstruction Laboratories) was introduced by Rochkind [40] in 2004. It is designed for the treatment of complete peripheral nerve injuries where the nerve defect is significant. The tube consists of a biodegradable co-polymer containing viscous gel with regenerative factors, neuroprotective agents and Schwann cells and was used for reconnection of nerve defects up to 2 cm in length. Initial results are promising and more thorough preclinical tests are conducted at the moment.
Siemionow & Sari [21] publicized the use of neural tubes in order to provide the growing nerve with an ideal microenvironment and high concentrations of neurotrophines and to protect the regenerating nerve's stumps by an epineural sleeve neurorrhaphy. Both authors furthermore dismiss the unique use of fibrin glue and propagandize a 10-0 Nylon suture.
Belkas et al. [41] reviewed and compared to dates entubulation techniques and materials. They concluded that the technique of entubulation has great potential and is tightly connected with advancements in bioengineering.
Geuna et al. [42] successfully used a vein-muscle transplant as tube for bridging a 10 mm neural defect in rats.
As previously mentioned, a sensoric loss of the nasal mucous membranes does not seem to be associated with grave clinical relevance. Whicker and Kern [43] investigated the effect of a bilateral interruption of the sensoric nasal innervation in a dog model and found no pathologies associated with (pulmonal) compliance and resistance. However, the interaction between anesthesia, ventilation and physiology of the lung must be considered.
As discussed before, NGF might be of further relevance in nerve reconstruction.
A new approach was introduced by Cuevas [44], who used autologous bone marrow tissue and reported on its positive effect on neural healing in terms of regeneration-speed and functionality.
6.2. Clinical aspects
6.2.1. Trigeminal neuralgia
As discussed above, a vascular abnormality can be seen as cause for the classic trigeminal neuralgia [12]. Microvascular decompression, as described by Janetta is the first choice of a causal therapeutic. In this technique, the trigeminal nerve can be accessed by a suboccipital, retroauricular approach and abnormal vascular structures (A. cerebelli superior or pontine veins) can be removed and an "insulator" (muscle, teflon, Ivalon) can be inserted. It is a standard neurosurgical procedure and with an up to 90% success rate the most promising surgical technique in treatment of trigeminal neuralgia (for historical review see [45]). However a conservative treatment should precede a surgical intervention.
With the introduction of the vascular decompression, following techniques have become obsolete: removal of peripheral trigeminal branches, resection of the trigeminal ganglion, ethanol injection into the trigeminal ganglion (Haertel), subtemporal dura-dissection (trigeminal ganglion decompression - Taarnhoj), retroganglionic extradural dissection of sensoric roots (Spiller & Frazier) and intradural dissection of sensoric roots (Dandy- Rhizotomy).
The tractotomy by Sjöqvist, in which pain fibers within the medulla oblongata are dissected, has a fairly high surgical risk associated with it [46]. As an alternative to the Sjöqvist procedure, the nucleotractotomy is performed by a radiofrequency-lesion to the dorsal root entry zone (DREZ lesion) close to the sub-nucleus of the spinal nucleus. This technique has not yet been tested in a greater clinical trial.
Today the Dandy-Rhizotomy is only performed when no vascular abnormality can be found within Janetta's procedure and symptoms remain resistant to all other therapeutic options. The percutaneous thermorhizotomy procedure is only performed as alternative in older patients or when no other therapeutic option is feasible [12]. This procedure is aimed at selectively destroying noziceptive Aδ und C-fibers without damaging Aβ-fibers. Success rates are as high as 72% ([47], N=1200), whereas the effect lasts a couple of years only. Side effects are hypesthesia, anesthesia (>2%) and anesthesia dolorosa (0.2-4%). 17% of all patients report of hypersalivation. Temporary weakness of the mastication muscle is commonly observed [12]. Therapy related lethality in a clinical study with 22000 participants was 0.08% [48].
Another non-invasive treatment option is the percutaneous balloon-microcompression that was first described by Meglio, Cioni and Lobato. This treatment is predominantly performed in the Mediterranean countries. Pain recurrence is similar to radiofrequency treatment. Numbness in the face and paralysis of the mastication muscles are unfortunately high [12]. The use of boiling water for alteration of Gasserian ganglion is also reported.
Percutaneous injection of glycerol into the Gasserian ganglion and Meckle's cave (Hakanson) is another non-invasive treatment option. By injecting glycerol, a selective elimination of noziceptive fibers at the Gasserian ganglion is postulated. Eighty-five percent of patients achieve pain relief and persisting side effects are unusual [11]. Recurrence rates are relatively high and 50% of all patients recur after a couple of years [12]. Re-injection may be performed, but glycerol injections become less effective.
The value of stereotactic radio-surgery (Gamma knife, Cone beam) with a dosage of up to 70-80 Gy has so far not been evaluated properly. In a preliminary report treatment results with complete pain relief in 53% and good pain relief in 35% of all patients [48].
In case of anesthesia dolorosa after trigeminal neuralgia treatment, electro stimulation of the Gasserian ganglion or the central grey matter is possible as last option [12]. Case reports describe a successful ganglionic (superior cervical ganglion) treatment with opioids (GLOA-GCS). A trigemino-vascular-complex (Moskowitz et al.) is thought to positively effect noziceptive projections from the trigeminal nerve [12]. Pain influenced by blocking the sympathetic system is termed as sympathetic triggered pain syndrome.
The otorhinolaryngologist has to critically contemplate sense and nonsense of causally determined surgical procedures to the nose and paranasal sinuses in case of trigeminal neuralgia. We whish to commemorate the times, when patients with trigeminal neuralgia frequently had teeth extractions done without any clinical and statistical evidence supporting this connection [11]. It is imperative to differentiate between a classic trigeminal neuralgia, caused by a vascular compression, and a symptomatic trigeminal affection. Manipulations on the peripheral trigeminal branches can therefore not pose any form of causal treatment for a classic trigeminal neuralgia. However paranasal infections, scars, and nasal pathologies in general may constitute as triggers for eliciting the typical sharp, lancinating pain sensations.
The physician has to pay special attention to nasal septum pathologies, especially septum-spurs and turbinate-septum contacts. The authors had several patients with trigeminal septum-spur headaches who benefited from septum surgery and turbinotomy and are now pain free. The diagnosis of trigeminal neuralgia is made after assessing the patient's symptoms and therefore imaging studies, such as a computed tomography (CT) or magnetic resonance imaging (MRI) scan are mandatory in ruling out neoplasias [49]. Only if there are verifiable pathologies, a surgical intervention is in order. However, a success should never be guaranteed.
Facial neuralgias following nasal trauma and/or surgery should at first be treated conservatively with topical corticoids, carbamazepine and gabapentine. Surgical interventions in these cases tend to worsen symptoms.
Persistent dysesthesia associated with the infraorbital and supraorbital nerve are generally not related to a classic trigeminal neuralgia. Until proven otherwise, CT imaging ought to be mandatory for ruling out paranasal neoplasias.
6.2.2. Neuralgia following transmaxillary sinus surgery
The so-called "post Caldwell-Luc-Syndrome" [50], [51] following transmaxillary sinus surgery still poses a therapeutic challenge. However indications for this surgical technique in the treatment of inflammatory paranasal sinus disorders have long been replaced by endonasal sinus surgery. As in all neuropathic pains, an initial conservative approach with carbamazepine is in order.
If conservative measures fail, a decompression of the infraorbital nerve can be considered [50], however a clear identification of the nerve within the postoperative scar tissue is sometimes troublesome and an accidental dissection poses a serious surgical risk. Alternatively, a transconjunctival and infraorbital approach can be considered if an enoral approach is less promising. A volitional dissection of the infraorbital or maxillary nerve bears only additional side effects, such as hypesthesia and anesthesia dolorosa without proven benefit in terms of pain relief [52]. In this case nociception by unmyelinated C-fibers plays an important role.
Gregg [53] pointed out, that a successful pain relief after local anesthesia application is a good predictive indicator for success of microvascular nerve decompression.
6.2.3. Other cephalgias
Besides the already discussed trigeminal neuralgia, several forms of cephalgias exist. Among them are the already mentioned neural-, and -ganglionic syndromes, cluster headaches, ice-cream-headaches and many others. An in depth discussion of all of them would clearly exceed the limits of this review, and only a few therapeutic and surgical options are described in brief:
· Dissection of the major petrous nerve by a transtemporal approach
· Neurectomy of the pterygopalatine nerve (Vidian nerve) by a transantral approach
· Resection of the pterygopalatine ganglion
· Extraction (exhairesis) of the ethmoidal nerve by a transorbital approach
All mentioned techniques are used in special indications only. Surgical techniques are described and depicted in depth in Denecke & Ey surgical atlas [54].
6.2.4. Reconstruction of trigeminal branches
To date head and neck surgery has evolved towards a microsurgical field, where maintaining a functional and physiological state postoperatively is of highest priority. In this context also sensory nerves are worth to be preserved. An in depth knowledge on the human anatomy in combination with a meticulous surgical preparation guard best against accidental dissections of trigeminal branches. As an example we wish to commemorate lip biopsies for histological work up when suspecting Sjögren-syndrome. After this diagnostic procedure, dysesthesia of sensoric nerves is observed in 0.8-3.9% of all cases [55]. Functionally adverse effects are easily to be imagined. Alsaad investigated the sensoric innervation grids of the lip and established three patterns with reference to surgical incision lines.
Special attention has to be paid to the great auricular nerve when performing parotid gland or neck surgery. Besides its sensoric function, the nerve might be of value in cases when a neural interposition is needed.
Of course there are many situations when a nerve cannot be salvaged, especially when performing oncological surgery. Whenever a nerve runs through a tumor mass, it has to be sacrificed for the higher good, in this case a radical tumor removal. Reconstruction procedures can be considered after a complete tumor removal. One might be declined to reduce "unnecessary" anesthesia time by not reconstructing sensory nerves, but this perception has to be revised in knowledge of the benefits associated with sensory nerve reconstructions; especially for lesions of the ophthalmic nerve, where a ceratitis neuroparalytica poses a severe and troublesome complication.
Positive outcome after rehabilitation of peripheral trigeminal nerve lesions have been reported with the inferior alveolar nerve, the lingual and the infraorbital nerve [56]. Out of 46 patients undergoing reconstruction of the inferior alveolar and the lingual nerve, 55% were pleased with the postoperative results [57].
The same principles apply for reconstructive surgery on sensor as well as motor nerves. Techniques on end-to-end reanastomosis have been described manifold and shall not be repeated in this context. Hausamen [58], like many other authors, pointed out the necessity of an operating microscope when performing reconstructive neural surgery.
The following principles of neural sutures are herein after highlighted in brief:
1. A tension free anastomisis is mandatory for a rapid and directed reinnervation [21]. Otherwise formation of neuromas with associated dysesthesia might occur due to undirected axonal growth.
2. The earlier the reconstruction, the better the results.
3. Primary and tension free end-to-end anastomosis is superior to neural interpositions.
4. Microscopic epineural sutures are superior to entubulation techniques (to date).
6.2.4.1. Eligible interposition grafts
As highlighted above, the end-to-end anastomosis is the neural reconstruction technique of choice. When not applicable despite neural rerouting, interpositions can be considered. So far two main sources have been used for harvesting nerve structures for interposition grafts.
· Great auricular nerve
· Sural nerve
Meyer [59] and Wolford [60] did in depth analyses on pros and cons for both interpositions. The interposition ought to be 20-25% longer than the bridged defect and a correct positioning of the graft in terms of the axoplasmatic flow should be made [59]. However the latter point is discussed controversially. Number and patterns of the nerve fascicles (polyfascicular-grouped vs. polyfascicular-not grouped vs. oligofascicular) seem to be less important.
Wolford [60] suggested a "cable graft" technique, where the great auricular nerve is doubled in order to equalize possible gap size differences between graft and reconstructed nerve.
Eppley and Snyder [61] concluded after their microanatomic analysis, that of all possible graft sources, the great auricular nerve is most similar to the trigeminal nerve and therefore the ideal interposition material.
Other sources of interposition grafts are:
- Medial cutaneous nerve of forearm
- Lateral cutaneous nerve of forearm
- Long thoracic nerve (easily recovered when performing microvascular flaps).
All three nerves are suitable for reconstruction of the inferior alveolar nerve [59].
Great auricular nerve
The great auricular nerve is typically suited for transplants with up to 4cm in size. The nerve is generally 1.5mm in diameter and holds up to eight fascicles. After harvesting the great auricular nerve, a sensoric defect is permanent on the ear lobe and parotid gland region.
Sural nerve
The sural nerve is used when bridging greater distances of up to 20-30cm. The nerve can be dissected free through one long calf incision or by utilizing multiple small incisions (rope ladder technique [58]). A resulting sensoric defect on the calf is inevitable. The nerve consists of up to 11-12 fascicles and has a median diameter of 2.1mm.
6.2.4.2. Reconstructing the inferior alveolar nerve
Damage to the inferior alveolar nerve is mostly traumatic or iatrogenic and reconstructive techniques are mostly described in dentistry or maxillo-facial surgery literature.
Schmelzle and Schwenzer [62] pointed out, that one year after trauma 30% of patients with mandible fractures still suffer from a lack of sensitivity. With respect to reconstructive measures, the course of the inferior alveolar nerve within the mandibular canal can be seen as both blessing and curse [63]. A tension free reanastomosis is mostly impossible within the canal, but a genuine guiding influence of the canal was described for a spontaneous nerve recurrence. It is generally accepted to wait as long as 6-8 months for a spontaneous healing before considering surgical interventions [63].
When performing surgical reconstruction, Hausamen et al. [64] suggested a neural relocation away from the avascular bone into the submandibular soft tissue. This technique is based on the theory, that until neural vessels are rebuilt, the nerve is nourished by diffusion only. For the same reason the authors favor smaller diameter grafts.
Zuniga [56] stated that the transoral approach has to be favored over the transcutaneous approach when doing neural reconstruction surgery on the inferior alveolar nerve. This is mainly due to the fact that a more complete delineation of the nerve is assured by the transoral technique. When no obvious damage is found after surgical disclosure, a "silent" damage in terms of a drug-induced fibrosis (corticoids, local anesthetics) or dental filling materials has to be considered. Schmelzle and Schwenzer [62] found that some filling materials have a neurotoxic adverse reaction. Neurotoxic effects on the inferior alveolar nerve occur mostly when the filling canal is overloaded with filling. Damage sites can be carefully palpated and are characterized as ridifications. These ought to be cut out and the defect has to be bridged by either an interposition or adapted - if possible - by a tension free end-to-end anastomosis [56]. It is recommended to verify a normal histological aspect of both nerve endings by frozen section.
Gregg [9] suggested a neural decompression in order to prevent a centralization of neuropathic pain at the first signs of dysesthesia after newly set dental implants.
6.2.4.3. Reconstructing the lingual nerve
The lingual nerve is mostly damaged on its path through the floor of the mouth and past the mandibular teeth (transoral salivary stone removal, abscess drainage). According to Schmelzle and Schwenzer [62], the nerve has in 60% direct contact to the wisdom tooth's root canal. Damage scenarios include tooth extractions, osteotomies, drilling procedures and falsely placed sutures. In case of an apparent neural dissection, an instantaneous end-to-end reanastomosis should be carried out. Since most procedures bearing potential damage to the lingual nerve are carried out under local anesthesia, even severe damage to the nerve is almost exclusively noticed postoperatively.
When reconstructing the nerve in this area, one must be aware that fibers of the chorda tympani have already joined the lingual nerve. However a clear distinction between chorda and lingual fibers is almost never possible. On the animal model, experiments have shown that predominantly mechanosensitive fibers regenerate after dissection and subsequent reanastomosis. This fact has to be considered when performing lingual nerve reconstruction.
Potter et al. [65] recommend the neural reconstruction to be carried out under general anesthesia for reasons of quality. Loescher et al. [7] stated that a clear indication for surgery exists three to four months after nerve damage following tooth extraction without spontaneous recovery. Renton [66] criticized all prior manuscripts on lingual nerve reconstruction concerning their lack of diagnostic as well as therapeutic criteria and demanded a unique therapeutic regimen. Dodson and Kaban [67] have come to the same conclusion by applying principles of Evidenced Based Medicine.
6.2.4.4. Reconstructing the infraorbital nerve
Divergent statements were made on the occurrence of persisting infraorbital nerve dysesthesia and cheekbone fractures. All data, the author's included, indicate that an incidence of 76% as reported by Fogaca et al. [68] is definitely too high. A common weak point of all studies is their limited informational value on the mere incidence of nerve damage associated with cheekbone fractures. Most studies only evaluated patients in need for surgery and therefore suffered from a more severe trauma. Patients with a minor trauma are commonly not included in these studies.
Brand et al. [69] found in 418 patients a dysesthesia in 58% before and 39% after trauma surgery. The author's experience with isolated cheekbone fractures [70] on 65 patients showed that 40% exhibited infraorbital dysesthesia before and 0% after surgery.
Dysesthesia of the infraorbital nerve has to be viewed as an indication for surgery. Brand et al. [69] reported his best functional results when operating within 3 days after the initial trauma. An interim anti-phlogistic/anti-inflammatory medication ought to be administered. Anyway not much research has been done on the rehabilitation of infraorbital nerve damage following cheekbone and orbital floor fractures and no generally recognized therapeutic regimen exists. When performing surgery, a best possible reconstitution of the infraorbital nerve and its surrounding is as desirable as a stable osteosynthesis [71] and the avoidance of iatrogenic nerve damage when clinically not impaired before surgery.
When approaching the infraorbital canal, bone fragments, splinters as well as sharp edges have to be removed in order to avoid subsequent damage and ensure nerve decompression. When performing osteosynthesis with miniplates, drill holes have to be made in a safe distance away from the canal [72].
In cases when the fracture involves the canal or the foramen and a compression of the nerve has to be expected, a decompression step should be performed before fixing the fracture. After a rare comminuted fracture, a complete rerouting including a construction of a neo-foramen might be indicated [73].
When the nerve is torn off, a tension-free end-to-end reanastomosis can only be accomplished after rerouting.
Revision surgery is carried out following the same principles as mentioned above (see 6.2.2.). The infraorbital nerve has to be approached from as proximal as possible, since the nerve mostly branches off right at the foramen [60].
6.2.4.5. Reconstructing other trigeminal branches
A torn supraorbital nerve is reconstructed following the same principles as described for the infraorbital nerve
The buccal nerve is mostly damaged following wisdom tooth extractions. Incidences are not yet reported [7] and surgical reconstructions have not been attempted. A spontaneous regeneration can be expected to a certain degree.
The auriculotemporal syndrome (Frey syndrome, gustatory sweating) features facial flushing or sweating limited to the distribution of the auriculotemporal nerve and develops after trauma to the parotid gland or in association with their surgical removal. Based on its pathophysiology, it belongs to the group of autonomous neural disorders and is not discussed in this context.
6.2.4.6. Special cases
Sometimes when exploring a peripheral trigeminal branch in a patient with or without neuralgia, an amputation neuroma is found without visible corresponding distal nerve ending. The question remains unanswered as to whether or not a removal of the neuroma benefits the patient. A sole resection without further doing will result most likely in a recurrence. The following treatment strategies were outlined by Zuniga to prevent recurrence of an amputation neuroma:
1. Resection of the neuroma and interconnection of the neural stump with a surrounding muscle
2. Resection of the neuroma, construction of an epineural flap and forming of an occlusive suture („epineural envelope").
If only the distal stump of the inferior alveolar nerve is found, an end-to-end anastomosis with the proximal end of the great auricular nerve can be done [59].
Mucci und Dellon [74] described a case report, in which sensoric functions of the lower lip recurred in a patient with central trigeminal lesion after anastomosis with the supraclavicular nerve.
6.2.5. Alternative treatment options
Neurotrophic ulcerations can be stopped if the causal manipulation irritation ceases. Transcutaneous electro stimulation has a supportive effect on wound healing [8]. If necessary a regional skin flap with intact sensory innervation (ideally from the contralateral side) has to be considered.
7. Conclusion
In the year 2005 sensory nerves deserve the same attention as motor nerves. When performing surgery, sensory nerves ought to be protected if somehow possible. In cases where nerves have to be sacrificed and after traumatic or iatrogenic damage, an immediate or soonest possible reconstruction is desirable.
So far the best reconstruction technique seems to be a tension free end-to-end anastomisis. In cases where a rerouting is not possible, a sural or great auricular nerve interposition graft is mandatory. To date not enough data exists on entubulation techniques, but rapid progress is been made in this research area.
As for trigeminal neuralgia, many conservative and surgical treatment options exist. The treatment of trigeminal neuralgia is an interdisciplinary task and the otorhinolaryngologist has to communicate with neurologists and neurosurgeons.
References
- 1.May M. Management of Cranial Nerves I Through VII Following Skull Base Surgery. Otolaryngol Head Neck Surg. 1980;88:560–575. doi: 10.1177/019459988008800509. [DOI] [PubMed] [Google Scholar]
- 2.Samandari F. Funktionelle Anatomie der Hirnnerven und des vegetativen Nervensystems. Berlin: de Gruyter; 1984. [Google Scholar]
- 3.Wilson-Pauwels L, Akesson EJ, Stewart PA, Spacey SD. Cranial Nerves in Health and Disease. Hamilton: BC Decker; 2002. [Google Scholar]
- 4.Leblanc A. The Cranial Nerves: Anatomy, Imaging, Vascularisation. Berlin: Springer; 1995. [Google Scholar]
- 5.Leblanc A. Encephalo-Peripheral Nervous System. Vascularisation, Anatomy, Imaging. Berlin: Springer; 2001. [Google Scholar]
- 6.Lang J. Neuroanatomie der Nn. opticus, trigeminus, facialis, glossopharyngeus, vagus, accessorius und hypoglossus. Arch Otorhinolaryngol. 1981;231:1–69. doi: 10.1007/BF00465556. [DOI] [PubMed] [Google Scholar]
- 7.Loescher AR, Smith KG, Robison PP. Nerve Damage and Third Molar Removal. Dent Update. 2003;30:375–382. doi: 10.12968/denu.2003.30.7.375. [DOI] [PubMed] [Google Scholar]
- 8.Koch M, Constantinidis J, Hornung J, Winter M. Das neurotrophische Ulkus des N. trigeminus. HNO. 2004;52:447–450. doi: 10.1007/s00106-003-0845-7. [DOI] [PubMed] [Google Scholar]
- 9.Gregg JM. Neuropathic Complications of Mandibular Implant Surgery: Review and Case Presentations. Ann Roy Australia Coll Dent Surg. 2000;15:176–180. [PubMed] [Google Scholar]
- 10.Kraut RA, Chahal O. Management of patients with trigeminal nerve injuries after mandibular implant placement. JADA. 2002;133:1351–1354. doi: 10.14219/jada.archive.2002.0050. [DOI] [PubMed] [Google Scholar]
- 11.Brandt T. Kopf- und Gesichtsneuralgien: Trigeminusneuralgie und Glossopharyngeusneuralgie. In: Brandt T, Dichgans J, Diener HC, editors. Therapie und Verlauf neurologischer Erkrankungen. 3. Auflage. Stuttgart: Kohlhammer; 1998. pp. 50–56. [Google Scholar]
- 12.Görge HH. Operative Behandlungsmöglichkeiten der Trigeminusneuralgie. Schmerz. 2001;15:48–58. doi: 10.1007/s004820170048. [DOI] [PubMed] [Google Scholar]
- 13.Brandt T. Trigeminusneuralgie. In: Hopf HC, Deuschl G, Diener HC, Reichmann H, editors. Neurologie in Praxis und Klinik, Band I. 3. Auflage. Stuttgart: Thieme; 1999. pp. 266–269. [Google Scholar]
- 14.Berlit P. Erkrankungen der Hirnnerven und des Hirnstamms. In: Berlit P, editor. Klinische Neurologie. Berlin: Springer; 1999. pp. 352–407. [Google Scholar]
- 15.Glocker FX, Hopf HC. Läsionen des N. trigeminus. In: Hopf HC, Deuschl G, Diener HC, Reichmann H, editors. Neurologie in Praxis und Klinik, Band II. 3. Auflage. Stuttgart: Thieme; 1999. pp. 227–233. [Google Scholar]
- 16.Rettinger G, Engelbrecht-Schnur S. Palatine Gefühlsstörungen nach Septumkorrektur. Laryngorhinootologie. 1995;74:282–285. doi: 10.1055/s-2007-997740. [DOI] [PubMed] [Google Scholar]
- 17.Filippi A, Pohl Y, Tekin U. Sensory disorders after separation of the nasopalatine nerve during removal of palatal displaced canines: prospective investigation. Br J Oral Maxillofac Surg. 1999;37:134–136. doi: 10.1054/bjom.1997.0092. [DOI] [PubMed] [Google Scholar]
- 18.Golding-Wood DG, Brookes GB. Post-traumatic external nasal neuralgia – an often missed cause of facial pain? Postgrad Med. 1991;67:55–56. doi: 10.1136/pgmj.67.783.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colin W, Donoff RB. Restoring Sensation after Trigeminal Nerve Injury. JADA. 1992;123:80–85. doi: 10.14219/jada.archive.1992.0322. [DOI] [PubMed] [Google Scholar]
- 20.Sunderland S. Nerves and Nerve Injuries. 2nd edition. London: Churchill Livingstone; 1978. [Google Scholar]
- 21.Siemionow M, Sari A. A contemporary overview of peripheral nerve research from the Cleveland Clinic Microsurgery Laboratory. Neurol Res. 2004;26:218–225. doi: 10.1179/016164104225013860. [DOI] [PubMed] [Google Scholar]
- 22.Kreutzberg GW. Neurobiologische Aspekte der Nervenregeneration. Arch Otorhinolaryngol. 1981;231:71–88. doi: 10.1007/BF00465557. [DOI] [PubMed] [Google Scholar]
- 23.Greenstein B, Greenstein A. Color Atlas of Neuroscience. Neuroanatomy and Neurophysiology. Stuttgart: Thieme; 2000. [Google Scholar]
- 24.Rosén B, Lundborg G. Sensory Re-Education after Nerve Repair. Aspects of Timing. Handchir Mikrochir Plast Chir. 2004;36:8–12. doi: 10.1055/s-2004-815808. [DOI] [PubMed] [Google Scholar]
- 25.Iskandar BJ, Nelson A, Resnick D, Skene JHP, Gao P, Johnson C, Cook TD, Nariharan N. Folic Acid Supplementation Enhances Repair of the Adult Central Nervous System. Ann Neurol. 2004;56:221–227. doi: 10.1002/ana.20174. [DOI] [PubMed] [Google Scholar]
- 26.Sullins JS, Carnes DL, Kaldestad RN, Wheeler F. Time Course of the Increase in trk A Expression in Trigeminal Neurons After Tooth Injury. Journal of Endotontics. 2000;26:88–91. doi: 10.1097/00004770-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Behnia A, Zhang L, Makepeace C, Gold MS. Changes in Trk B-like Immunoreactivity in Rat Trigeminal Ganglion After Tooth Injury. Journal of Endodontics. 2003;29:135–140. doi: 10.1097/00004770-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Fox JM, Jörg J. Neurologische Pharmakotherapie. Stuttgart: Thieme; 2004. [Google Scholar]
- 29.Davies NM, Longstreth J, Jamali F. Misoprostol Therapeutics Revisited. Pharmacotherapy. 2001;21:60–73. doi: 10.1592/phco.21.1.60.34442. [DOI] [PubMed] [Google Scholar]
- 30.Holland GR. Experimental Trigeminal Nerve Injury. Crit Rev Oral Biol Med. 1996;7:237–258. doi: 10.1177/10454411960070030301. [DOI] [PubMed] [Google Scholar]
- 31.Tyndall DA, Gregg JM, Hanker JS. Evaluation of Peripheral Nerve Regeneration Following Crushing or Transection Injuries. J Oral Maxillofac Surg. 1984;42:314–318. doi: 10.1016/0278-2391(84)90111-3. [DOI] [PubMed] [Google Scholar]
- 32.Zuniga JR, Pate JD, Hegtvedt AK. Regenerative Organization of the Trigeminal Ganglion Following Mental Nerve Section and Repair in the Adult Rat. Journal of Comparative Neurology. 1990;295:548–558. doi: 10.1002/cne.902950404. [DOI] [PubMed] [Google Scholar]
- 33.Zuniga JR, O'Connor B. Primary and secondary microneuroanastomotic repair of the mental nerve in the rat. Int J Oral Maxillofac Surg. 1987;16:465–472. doi: 10.1016/s0901-5027(87)80086-3. [DOI] [PubMed] [Google Scholar]
- 34.Zuniga JR. Trigeminal Ganglion Cell Response to Mental Nerve Transection and Repair in the Rat. J Oral Maxillofac Surg. 1999;57:427–437. doi: 10.1016/s0278-2391(99)90284-7. [DOI] [PubMed] [Google Scholar]
- 35.Smith KG, Robinson PP. An experimental study of lingual nerve repair using epineurial sutures or entubulation. British Journal of Oral and Maxillofacial Surgery. 1995;33:211–219. doi: 10.1016/0266-4356(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 36.Smith KG, Robinson PP. The Reinnervation of the Tongue and Salivary Glands after two Methods of Lingual Nerve Repair in the Cat. Archs oral Biol. 1995;40:373–383. doi: 10.1016/0003-9969(94)00189-i. [DOI] [PubMed] [Google Scholar]
- 37.Smith KG, Robinson PP. An experimental study on the recovery of the lingual nerve after injury with or without repair. Int J Oral Maxillofac Surg. 1995;24:372–379. doi: 10.1016/s0901-5027(05)80496-5. [DOI] [PubMed] [Google Scholar]
- 38.Smith KG, Robinson PP. An Experimental Study of Three Methods of Lingual Nerve Defect Repair. J Oral Maxillofac Surg. 1995;53:1052–1062. doi: 10.1016/0278-2391(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 39.Curtis NJ, Trickett RI, Owen E, Lanzetta M. Intraosseous Repair of the Inferior Alveolar Nerve in Rats: An Experimental Model. J Reconstruct Microsurg. 1998;14:391–395. doi: 10.1055/s-2007-1000197. [DOI] [PubMed] [Google Scholar]
- 40.Rochkind S, Astachov L, El-Ani D, Hayon T, Graif M, Barsky L, Alon M, Odvak I, Nevo Z, Shahar A. Further development of reconstructive and cell tissue-engineering technology for treatment of complete peripheral nerve injury in rats. Neurological Research. 2004;26:161–166. doi: 10.1179/016164104225013905. [DOI] [PubMed] [Google Scholar]
- 41.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurological Research. 2004;26:151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 42.Geuna S, Tos P, Battiston B, Giacobini-Robecchi G. Bridging peripheral nerve defect with muscle-vein combined guides. Neurosurgical Research. 2004;26:139–144. doi: 10.1179/016164104225013752. [DOI] [PubMed] [Google Scholar]
- 43.Whicker JH, Kern EB. Effect of Denervation of Nasal Mucosa on Pulmonary Mechanics. Ann Otol. 1973;82:724–728. doi: 10.1177/000348947308200517. [DOI] [PubMed] [Google Scholar]
- 44.Cuevas P, Carceller F, Garcia-Gómez I, Yan M, Dujovny M. Bone marrow stromal cell implantation for peripheral nerve repair. Neurol Res. 2004;26:230–232. doi: 10.1179/016164104225013897. [DOI] [PubMed] [Google Scholar]
- 45.Sweet WW. Clinical Neurosurgery. Proceedings of the Congress of Neurological Surgeons. New York: Williams & Wilkins; 1984. The History of the Development of Treatment for Trigeminal Neuralgia; pp. 294–318. [Google Scholar]
- 46.Penzholz H, Kühner A, Sturm V. Die intrakraniellen Operationen bei Erkrankungen des N. trigeminus. Arch Otorhinolaryngol. 1981;231:401–449. [Google Scholar]
- 47.Taha JM, Tew JM. Radiofrequency rhizotomy for trigeminal and other cranial neuralgias. In: Gildenberg PI, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1996. pp. 1687–1696. [Google Scholar]
- 48.Greenberg MS. Handbook of Neurosurgery. 5th edition. New York: Thieme; 2001. pp. 364–385. [Google Scholar]
- 49.El Gammal T, Brooks BS. Trigeminal Neuralgia [letter] Radiology. 2000;231:284. doi: 10.1148/radiol.2311031388. [DOI] [PubMed] [Google Scholar]
- 50.Thumfart W, Steiner W, Jaumann MP. Diagnose und Indiaktion zur Revision der radikal voroperierten Kieferhöhle. HNO. 1978;26:289–295. [PubMed] [Google Scholar]
- 51.Draf W. Der Gesichtsschmerz nach Caldwell-Luc-Operation. Laryngol Rhinol Otol. 1980;59:308–311. [PubMed] [Google Scholar]
- 52.Braun TW, Sotereanos GC. Transantral maxillary neurectomy for intractable neuralgia. J Oral Surg. 1977;35:583–584. [PubMed] [Google Scholar]
- 53.Gregg JM. Studies of Traumatic Neuralgia in the Maxillofacial Region: Symptom Complexes and Response to Microsurgery. J Oral Maxillofac Surg. 1990;48:134–140. doi: 10.1016/s0278-2391(10)80200-9. [DOI] [PubMed] [Google Scholar]
- 54.Denecke HJ, Ey W. Die Operationen an der Nase und im Nasopharynx. Berlin: Springer; 1984. pp. 275–285. [Google Scholar]
- 55.Alsaad K, Lee TC, McCartan B. An Anatomical study of the cutaneous branches of the mental nerve. Int J Oroal Maxillofac Surg. 2003;32:325–333. doi: 10.1054/ijom.2002.0334. [DOI] [PubMed] [Google Scholar]
- 56.Zuniga JR. Surgical Management of Trigeminal Neuopathic Pain. Atlas of the Oral and Maxillofacial Surgery Clinics of North America. 2001;9:59–75. [PubMed] [Google Scholar]
- 57.Lam NP, Donoff BR, Kaban LB, Dodson TB. Patient satisfaction after trigeminal nerve repair. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:538–543. doi: 10.1067/moe.2003.163. [DOI] [PubMed] [Google Scholar]
- 58.Hausamen JE. Principles and Clinical Application of Microberve Surgery and Nerve Transplantation in the Maxillofacial Area. Ann Plast Surg. 1981;7:428–433. doi: 10.1097/00000637-198112000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Meyer RA. Nerve Harvesting Procedures. Atlas of the Oral and Maxillofacial Clinics of North America. 2001;9:77–91. [PubMed] [Google Scholar]
- 60.Wolford L. Autogenous Nerve Graft Repairs of the Trigeminal Nerve. Oral Maxillofac Surg Clin N Am. 1992;4:447–457. [Google Scholar]
- 61.Eppley BL, Snyders RV. Microanatomic Analysis of the Trigeminal Nerve and Potential Nerve Graft Donor Sites. J Oral Maxillofac Surg. 1991;49:612–618. doi: 10.1016/0278-2391(91)90343-k. [DOI] [PubMed] [Google Scholar]
- 62.Schmelzle R, Schwenzer N. Schädigungen des Nervus mandibularis. In: Schwenzer N, Grimm G, editors. Zahn-Mund-Kiefer-Heilkunde Band 2: Spezielle Chirurgie. 2. Auflage. Stuttgart: Thieme; 1990. pp. 245–252. [Google Scholar]
- 63.Ruggiero SL. Trigeminal Nerve Injury and Repair. NY State Dental J. 1996;62:36–40. [PubMed] [Google Scholar]
- 64.Hausamen JE, Samii M, Schmidseder R. Indication and Technique for the Reconstruction of Nerve Defects in Head and Neck. J max fac Surg. 1974;2:159–167. doi: 10.1016/s0301-0503(74)80036-6. [DOI] [PubMed] [Google Scholar]
- 65.Potter BE, Yarington CT, Walike W. Management of Intraoral Injuries. Am Fam Physician. 1978;18:96–102. [PubMed] [Google Scholar]
- 66.Renton T. Lingual Nerve Assessment and Repair Outcomes. Ann Roy Australas Coll Dent Surg. 2002;16:113–114. [PubMed] [Google Scholar]
- 67.Dodson TB, Kaban LB. Recommendations for Management of Trigeminal Nerve Defects Based on a Critical Appraisal of the Literature. J Oral Maxillofac Surg. 1997;55:1380–1386. doi: 10.1016/s0278-2391(97)90632-7. [DOI] [PubMed] [Google Scholar]
- 68.Fogaca WC, Fereira MC, Dellon AL. Infraorbital Nerve Injury Associated with Zygoma Fractures: Documentation with Neurosensory Testing. Plast Reconstr Surg. 2004;113:834–838. doi: 10.1097/01.prs.0000105335.41930.41. [DOI] [PubMed] [Google Scholar]
- 69.Brand G, Dreesen W, Wangerin K. Zeitwahl der operativen Versorgung der Jochbeinfrakturen. Fortschr Kiefer Gesichtschir. 1991;36:85–86. [PubMed] [Google Scholar]
- 70.Iro H. Funktionelle Ergebnisse nach operativer Versorgung von isolierten Orbitabodenfrakturen (Blow-out-Frakturen) HNO. 1989;37:292–294. [PubMed] [Google Scholar]
- 71.Vriens JP, Moos KF. Morbidity of the infraorbital nerve following orbitozygomatic complex fractures. J Craniomaxillofac Surg. 1995;23:363–368. doi: 10.1016/s1010-5182(05)80131-3. [DOI] [PubMed] [Google Scholar]
- 72.Westermark A, Jensen J, Sindet-Pedersen S. Zygomatic fractures and infraorbital nerve disturbances, Miniplate osteosynthesis vs. other treatment modalities. Oral Surg Oral Diagn. 1992;3:27–30. [PubMed] [Google Scholar]
- 73.Rath EM. Surgical treatment of maxillary nerve injuries. The infraorbital nerve. Atlas Oral Maxillofac Surg Clin North Am. 2001;9:31–41. [PubMed] [Google Scholar]
- 74.Mucci SJ, Dellon AL. Restoration of lower-lip sensation: Neurotization of the mental nerve with the supraclavicular nerve. J Reconstr Micorsurg. 1997;13:151–155. doi: 10.1055/s-2007-1000241. [DOI] [PubMed] [Google Scholar]