Abstract
Aim:
Arterial extracellular matrix (ECM) remodeling by matrix metalloproteinases (MMPs) is one of the major hallmarks of atherosclerosis. Mimecan, also known as osteoglycin has been implicated in the integrity of the ECM. This study assessed the validity of an enzyme-linked immunosorbent assay (ELISA) developed to measure a specific MMP12-derived fragment of mimecan, MMCN-151, in apolipoprotein-E knockout (ApoE-KO) mice.
Methods and results:
A mouse monoclonal antibody raised against MMCN-151 was used to develop a competitive ELISA. The assay was validated using samples from 20 ApoE-KO and 20 wild type [C57 BL/6] male mice fed a normal or high-fat diet (HFD) for up to 20 weeks. The technical reliability of the assay was established with intra-assay variability <2% and inter-assay variability <10%. The lowest limit of quantification of MMCN-151 was 0.5 ng/ml. ApoE-KO mice fed a HFD for 20 weeks had four-fold increased circulating levels of MMCN-151 compared to baseline, whereas MMCN-151 levels in control mice on HFD increased two-fold compared with baseline. After 10 weeks of a HFD, a significant difference in MMCN-151 levels was observed between ApoE-KO and control mice (P = 0.005) and became more significant at 20 weeks (P = 0.002).
Conclusions:
The newly developed assay is a reliable detector of MMCN-151 levels which ultimately may be useful indicators of arterial remodeling in patients affected by atherosclerotic disease.
Keywords: MMCN-151, mimecan, biomarker, atherosclerosis, proteoglycans, extracellular matrix, matrix metalloproteinases
Introduction
Even though research into the treatment of cardiovascular diseases (CVD) has advanced in recent years and many diverse therapeutic approaches have been developed, CVD still remains the leading cause of death in most parts of the world.1 Atherosclerosis is one of the major underlying causes of cardiovascular-related deaths. In humans, atherosclerotic plaques consist of a fibrous cap mainly composed of smooth muscle cells (SMCs) and excessive extracellular matrix (ECM).2 Fibrillar collagens such as type I and type III are the most predominant proteins of the ECM while the rest of the ECM is composed of proteoglycans such as mimecan, biglycan, decorin, perlecan and versican.3 Type III collagen is thought to be associated with extensibility of the wall of the artery, whereas type I collagen may be responsible for arterial stiffness.4,5 The ECM is critically important for maintaining the physicochemical structure of the artery, providing tensile strength as well as viscoelastic properties.6 The ECM also contributes to cellular and organ functions. Proteins making up the ECM in the fibrous cap of an atherosclerotic plaque may be degraded at least in part by matrix metallo proteases (MMPs) when these are present in abnormally high quantities. MMPs are produced by macrophage foam cells and other inflammatory cells. As a result of MMP activity, the fibrous cap may be eroded, releasing the highly thrombogenic lipid core of the plaque to the circulation, resulting in thrombus formation.7–10 MMP-12 in particular has a broad range of substrates, especially collagen type I, III, elastin and proteoglycans. Recently, a proteoglycan, mimecan, also known as osteoglycin, has been implicated in regulation of collagen fibrillogenesis, artheriogenesis and in regulation of left ventricular mass.11–13 Mimecan is one of the small leucin-rich proteoglycans (SLRP). Other members of this family are biglycan, decorin, fibromodulin and lumican.
The atherosclerotic plaque is continuously remodelled, releasing in the process a range of degradation products of ECM proteins, the so-called neoepitopes.14 These neoepitopes may be specific to the tissue of origin and therefore may prove useful as novel molecular biochemical markers. The neoepitope-based biochemical markers measurable in urine and serum are receiving increased attention for their diagnostic and prognostic potential.15
Previously, assays determining type III collagen synthesis by measurements of the N-terminal peptide (PIIIP) demonstrated significant increases in serum PIIIP in atherosclerotic patients compared with controls.16 While collagen synthesis may be important in plaque progression, an important factor leading to plaque instability is degradation of collagens in the atherosclerotic lesion, especially in the areas of plaque shoulders by proteases such as MMPs produced locally by macrophages and other cells.17–20 We previously developed biochemical markers of type I and type II collagen degradation by proteases. These markers are now being used in preclinical and clinical settings to evaluate the efficacy of intervention in osteoporosis and osteoarthritis.21–23
The purposes of the current study were to: (1) investigate specific ECM protein fragments released from atherosclerotic plaques; (2) perform in vitro protein digestion by MMPs and identify the released fragments by mass-spectrophotometry (3) develop an enzyme-linked immunosorbent assay (ELISA) based on a specific monoclonal antibody against a specific peptide of mimecan generated by the action of MMP-12 (4) validate the assay in the gold standard mouse model of atherosclerosis, the apolipoprotein E knock-out (ApoE KO) mouse.
Material and Methods
In vitro cleavage
Recombinant mouse mimecan (cat. No. 2949-MC-050, R&D Systems, Denmark) was cleaved with activated MMP-12 (cat.no.ab54058, Abcam, UK). To facilitate MMP-12 cleavage of mimecan, 1 mg/mL mimecan was filtered to remove proteins below 10,000 kDa (Microcon Ultracel YM-10, cat. no. 42407, Millipore, Billerica, MA, USA) after which 100 μg mimecan was mixed with 1 μg of enzyme (MMP-12) in MMP buffer (100 mM Tris-HCl, 100 mM NaCl, 10 mM CaCl2, 2 mM Zn acetate, pH 8.0). As a control, 100 μg of mimecan was mixed with MMP buffer only. The solutions containing mimecan were incubated for 24 hrs at 37 °C. All cleavage activity was terminated using EDTA. Finally the cleavage was verified visually using the SilverXpress® Silver Staining Kit (cat. no. LC6100, Invitrogen, Carlsbad, Ca, USA) according to the manufacturer’s instructions.
Peptide identification
Peptide fragments in the in vitro cleaved samples were identified using matrix-assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) and liquid chromatography (LC) coupled with electro spray ionization (ESI) tandem mass spectrometry (LC-MS/MS). MALDI-TOF samples were purified using C18 zip-tips (cat.no.ZTC18SO24, Millipore, Billerica, MA, USA) according to the manufacturer’s specifications and 0.1 μg of material was eluted onto a MTP 384 ground steel target plate (Bruker-Daltonics, Bremen, Germany). MALDI tandem mass spectra were recorded on a Bruker ultraflex MALDI-TOF/TOF mass spectrometer (Bruker-Daltonics, Bremen, Germany) in positive ion reflector mode. Mass spectra were externally calibrated in the m/z range of 800–4000 using peptides generated by tryptic digestion of bovine β-lactoglobulin. The m/z software “Flex-analysis” (Bruker-Daltonics, Bremen, Germany) was used to analyze spectra. LC-MS samples were ultra-filtrated to remove proteins above 10 kDa, the pH was adjusted to 2.0 using formic acid, and a 4 μL sample was analyzed by LC-MS/MS. LC was performed on a nanoACQUITY UPLC BEH C18 column (Waters, Milford, MA, USA) using a formic acid/acetonitril gradient. MS and MS/MS were performed on a Synapt High Definition Mass Spectrometry quadruple time of flight MS (QUAD-TOF; Waters, Milford, MA, USA), with an acquisition range of 350–1600 m/z in MS and 50–2000 m/z, in MS/MS. The software “ProteinLynx Global SERVER (PLGS)” (Waters, Milford, MA, USA) was used to analyze spectra and generate peak lists. To identify peptides, MS and MS/MS data were searched against the mimecan (FASTA) protein database using the Mascot 2.2 (Matrix Science, Boston, MA, USA) software with either the MALDI-TOF/TOF or ESI-QUAD-TOF settings.
Selection of peptide for immunizations
The first six amino acids of each free end of the peptide sequences identified by MS were regarded as neo-epitopes generated by the protease in question. All obtained protease-generated sequences were analyzed for homology and distance to other cleavage sites and then blasted for homology using the NPS@: network protein sequence analysis.
Reagents and peptides
All reagents were standard high-quality chemicals from companies such as Merck and Sigma Aldrich. The following synthetic peptides used for monoclonal antibody production and validation were purchased from the Chinese Peptide Company, Beijing, China: (a) immunogenic peptide: Ovalbumine-GGC-EDIEDGTFSK (OVA), (b) screening peptide EDIEDGTFSK, (c) de-selection peptide EDIEDGTF-SKL which had been elongated with one amino acid in the C-terminus. Peptide conjugation reagents were obtained from Pierce, Thermofisher, (Denmark).
Buffers
Buffer used for dissolving the coating peptide was composed of the following: 40 mM NaHPO4, 12HO, 7 mM KH PO4, 137 mM NaCl, 2,7 mM KCl, 25 mM EDTA, 0,1% Tween 20, 1% BSA, 10% sorbitol, pH 7.
Buffer containing the following chemicals was used for incubation of the serum/plasma: 100 mM TRIZMA, 0,05% Tween 20, 0,1% BSA, 0,36% Bronidox L5, pH 7,4. For washing steps, we used a buffer composed of: 25 mM TRIZMA, 50 mM NaCl, 0,036% Bronidox L5, 0,1% Tween 20, and reaction stopping buffer composed of 0,1% H SO4.
ELISA-plates used for the assay development were streptavidin-coated from Roche Diagnostics cat.: 11940279. All ELISA plates were analyzed with the ELISA reader from Molecular Devices, Spectra-Max M, (CA, USA).
Development of the ELISA
We followed the previously described methods for monoclonal antibody development.24 Briefly, 4- 6-week-old Balb/C mice were immunized subcutaneously with 200 μl emulsified antigen and 50 μg MMCN-151 (EDIEDGTFSK). Consecutive immunizations were performed at 2-week intervals until stable sera titer levels were reached in Freund’s incomplete adjuvant. The mice were bled from the 2nd immunization on. At each bleeding, the serum titer was detected and the mouse with the highest antiserum titer was selected for fusion. The selected mouse was rested for 1 month followed by intravenous boosting with 50 μg MMCN-151 in 100 μl 0.9% sodium chloride solution 3 days before isolation of the spleen for cell fusion.
Fusion
The fusion procedure previously described25 was followed with SP2/0 as myeloma cells. The fusion cells were cloned in 35-mm cell culture dishes using the semi-solid medium method and the dishes were incubated in a CO2-incubator. Next, clones were plated into 16 96-well microtiter plates and left for three days, followed by screening of culture supernatants.
Antibody screening
Supernatants were screened in a competitive ELISA setting. Peptide EDIEDGTFSK was used as the selection peptide and the EDIEDGTFSKL as the elongated peptide. Cell lines specific to the selection peptide and without cross-reactivity to the elongated peptide were selected and the antibodies were purified.
MMCN-151 ELISA methodology
In preliminary experiments, we optimized the reagents, their concentrations and the incubation periods by performing several checkerboard analyses. The MMCN-151 ELISA was developed as follows: A 96-well ELISA plate pre-coated with streptavidin was further coated with 1.25 ng/ml of the synthetic peptide Biotin-EDIEDGTFSK which had been dissolved in PBS-TBE buffer at 20 °C for 30 min by constant shaking at 300 rpm. After the plate was washed five times in washing buffer, 20 μl of sample was added, followed by 100 μl of peroxidase conjugated anti-human mAb-MMCN-151 solution (30 ng/mL). The plate was incubated for 1 h at 20 °C in 100 mM Tris-BT buffer during which time it was shaken at 300 rpm.
The plate was again washed five times followed by the addition of 100 μl tetramethylbenzinidine (TMB) (Kem-En-Tec cat.438OH). The plate was incubated for 15 min in darkness and shaken at 300 rpm. To cease the reaction,100 μl of stopping solution (95%–97% H2SO4, Merck Cat. No.: 1.00731) was added and the plate was analyzed in the ELISA reader at 450 nm with 650 nm as the reference.
Standards
A standard curve was obtained by serial dilution of biotinylated-MMCN-151. Standard concentrations were 0, 0.3125, 6.25, 12.5, 25, 50, 100, 200 ng/mL.
Samples for testing native reactivity of the antibodies
For assay development and validation, serum from healthy adult volunteers aged 23–50 years and of either gender was used. The positive control used in the assays was the material obtained from in vitro cleavage of mimecan with purified human MMP-12. We also tested serum samples from different species including mouse, rat, rabbit, monkey and pig to determine the level of interspecies cross-reactivity.
Technical evaluation of the ELISA
All ELISAs were developed according to the Nordic Bioscience standard operating procedures which require:
Intra-assay precision and accuracy: 10 independent runs on 10 different plates were performed on the test samples of human serum.
Inter-assay precision and accuracy: This was determined by 10 independent assay runs of each validation sample on 10 different plates.
Lower Limit of Detection (LLD): In an analytical run, 40 determinations were made of the lowest standard (the zero standards). This was repeated three times. On each plate, the LLD was estimated using the following formula: LLD = mean – 3 × SD. The average LLD concentration was calculated using the 4-parametric-fit equation.
Recovery: Four different samples covering more than 50% of the standard curve, from all dilutions, were back-calculated from diluted to undiluted samples in order to estimate the recovery percentage.
Animal samples
20 ApoE KO and 20 wild type C57 BL/6 male mice aged 10 weeks from Taconic, Europe A/S (Lille Skensved, Denmark) were fed either a standard maintenance diet of Altromin (product ID: 1320, Brogaarden, Denmark) or ad libitum, a high fat diet containing 60% fat (product ID: D12492, Brogaarden, Denmark). Ethical guidelines for experimental investigations in animals were followed and the protocol was approved by the local Experimentation Ethics Committee “Dyreforsogstilsynet”, in accordance with European Commission guidelines. The study also conformed to the “Guide for care and use of laboratory animals” published by the US National Institutes of Health. When mice were aged 10 (baseline), 20 and 30 weeks, plasma samples were collected by blood sampling using retro orbital puncture or, in the case of sacrifice at weeks 20 or 30, by syringe aspiration of around 1 mL from the jugular vein. Blood heparinized with lithium heparin was centrifuged and the plasma frozen at −20 °C until assayed. Plasma cholesterol was measured by enzymatic assays using an analyzer from Molecular Devices, SpectraMax M, (CA, USA).
Densitometry
Densitometry measurements were performed using UN-SCAN-IT Version 6.1 from Silk Scientific, according to the manufacturer’s guidelines.
Histology image analysis
Sections of mouse aorta were stained with Alcian blue, a standard staining which binds to glycosaminoglycans on proteoglycabs. Stained mouse sections were analysed using Visiopharm software Version 3.2.8.0 (Hørsholm, Denmark). The Visiopharm application quantified specific tissue areas containing positive staining. Images were acquired using the Pixelink PL-A623C microscope digital camera.
Statistical analysis
For assay validation, optical density (OD) was matched against analyte concentrations by applying a four-parameter logistic regression to the calibration curve. Average, standard deviations, percentage coefficient of variation (%CV), and differences from theoretical values were calculated for all standards and samples. Quantitative data were analyzed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Significant differences between means were determined using the Student’s two-tailed unpaired t-test, not assuming Gaussian distribution. Correlations between serum MMCN-151 values and the rest of the variables studied were analyzed with Pearson’s two-tailed test. Data were expressed as mean ± standard error of the mean and differences were considered significant at a P level of 0.05 or lower.
Results
ELISA technical specifications
The antibody, raised against MMCN-151 antigen, which had the best native reactivity, affinity and stability generated after the fusion of spleen and myeloma cells was NB206-15B6-F8. Consequently, this clone was chosen for antibody purification and the subsequent development of the ELISA.
Standard curve and recovery
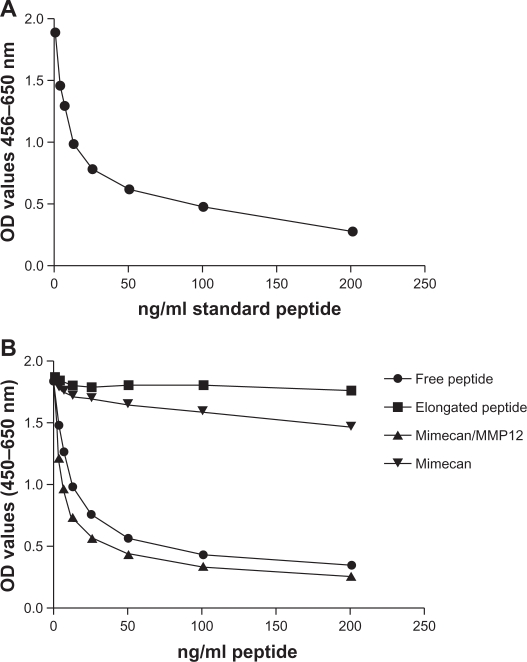
A typical standard curve is presented in Figure 1A, showing chosen standards and the 4-parametric fit equation for determination of sample concentration.
Figure 1.
(A) Example of a 4-parametric fitted standard curve in a competitive ELISA setting with peptide concentrations of 0, 3.125, 6.25, 12.5, 25, 50, 100 and 200 ng/ml. (B) Competitive ELISA test of MMCN-151 antibody reaction towards free peptide, elongated peptide, mimecan cleaved with MMP12 and intact mimecan. The x-axis is representative of the concentrations of free and elongated peptide. Concentrations of samples containing mimecan and mimecan cleaved with MMP12 are 43 ug/ml as the highest concentration followed by 2-fold dilutions of samples.
Determination of the linearity or recovery by dilution in different samples resulted in the following: the average recoveries back-calculated from samples diluted 1:1, 1:2, 1:4, 1:8 and 1:16 to undiluted sample were close to 100% and within the recommended ±10% (data not shown).
To ensure that the antibody was specific to the neo-epitope of mimecan and was not cross-reacting with either elongated peptide or whole mimecan, we compared the assay reactivity of MMP-12 cleaved mimecan with uncleaved mimecan and elongated peptide. As illustrated in Figure 1B, the MMCN-151 antibody was specific to the synthetic-free peptide, resulting in the inhibition of the signal from approximately 2.0 optical density (OD) (450–650) to 0.5. Similar results were seen for mimecan cleaved in vitro by MMP12, where the signal inhibition followed the synthetic peptide curve. Moreover, no inhibition of the signal was detected in the sample containing the synthetic peptide elongated with one amino-acid. Very weak competition with intact mimecan was observed at the highest concentration.
The lowest limit of detection (LLD), defined as the lowest analyte concentration that could be quantitatively determined with suitable accuracy, was 0.5 ng/ml.
Precision—intra-assay variability and inter-assay variability
The average intra-assay variability, calculated as the average % coefficient of variation (%CV) in readings of 10 replicates of four human serum samples which had an average MMCN-151 concentration of 5 ng/ml, was 1.3%. The 10 replicates of one rat serum sample had an average MMCN-151 concentration of 13 ng/ml with an average intra-assay variability of 1.1%.
Inter-assay mean %CVs were determined from the same sets of samples as the intra-assay and standards but on 10 different plates on 10 different days. The average inter-assay variability, between the highest and lowest levels detected, was 10.2%.
Plasma MMCN-151 values reflect early atherosclerosis in ApoE KO mice
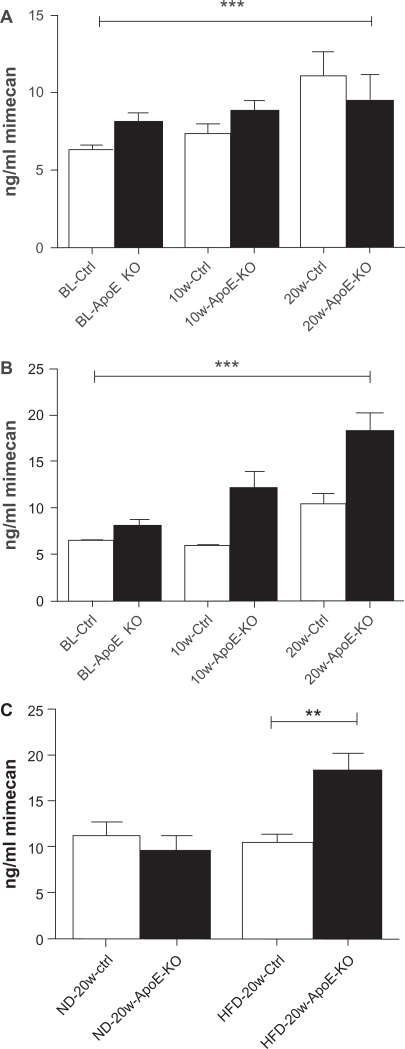
Plasma MMCN-151 levels, measured in ApoE KO mice and in the C57 bl6 controls at baseline and after 10 or 20 weeks of feeding on either normal diet (ND) or HFD, are shown in Figure 2A and B, respectively. The effect of the two diets on mimecan levels in ApoE KO mice compared with control mice at 20 weeks is presented in Figure 2C.
Figure 2.
Plasma levels of MMCN-151 in control (ctrl) and ApoE-KO mice, at baseline (BL), after 10 weeks and after 20 weeks of feeding either standard chow (A) or high fat diet (B), and (C) effect of diet on circulating levels of MMCN-151 neoepitope after 20 weeks of feeding.
Notes: *P < 0.05; **P < 0.01; ***P < 0.001.
The one-way ANOVA was significant (P < 0.0001) at 10 and 20 weeks in both the standard diet group and in HFD group. Results of MMCN-151 levels at 10 and 20 weeks are shown in Table 1 below. At 10 weeks of feeding, MMCN-151 values were already significantly increased (P > 0.001) in ApoE KO animals fed HFD compared to their controls and this difference was even more prominent at 20 weeks. At 20 weeks, there was no significant difference in control mice fed standard or HFD.
Table 1.
Levels of circulating MMCN-151 peptide after 10 and 20 weeks.
| A. | |||
| Standard diet | Controls | ApoE-KO | P (ApoE KO vs. controls) |
| 10 weeks | 5.7 ng/ml | 8.8 ng/ml | P < 0.05 |
| 20 weeks | 10.4 ng/ml | 9.1 ng/ml | ns |
| B. | |||
| High fat diet | Controls | ApoE-KO | P (ApoE KO vs. controls) |
| Standard diet | 5.3 ng/ml | 12.1 ng/ml | P < 0.001 |
| High fat diet | 10.2 ng/ml | 18.2 ng/ml | P < 0.001 |
In the HFD groups, the levels of circulating MMCN-151 were significantly higher (P = 0.002) in ApoE-KO mice than in HFD-fed controls after 20 weeks. No changes in MMCN-151 levels were seen in animals consuming a ND over 20 weeks (Fig. 2C).
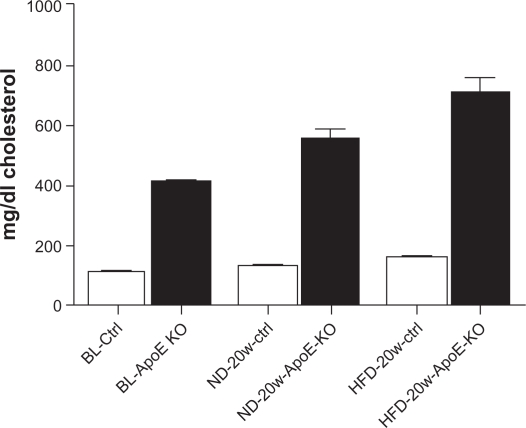
Plasma cholesterol levels in control and ApoE KO mice
Cholesterol levels in ApoE KO and control mice at baseline and after 20 weeks of feeding on either a normal or HFD are illustrated in Figure 3. Cholesterol levels increased significantly between baseline and the 20-week timepoint in control mice fed a HFD (P < 0.001), but not in mice fed a ND. In ApoE KO animals, cholesterol increased significantly between baseline and after 20 weeks of a ND (P = 0.001) and aHFD (P = 0.001). Furthermore, cholesterol levels were significantly higher (P < 0.001) in ApoE KO than control mice at baseline, and in ApoE KO mice fed either a ND or HFD. (P < 0.001).
Figure 3.
Cholesterol levels measured in mice plasma at baseline (BL) and after 20 weeks of feeding normal diet (ND) or high fat diet (HFD) in either control mice (ctrl) or in apolipoportein-E knock-out (ApoE-KO) mice.
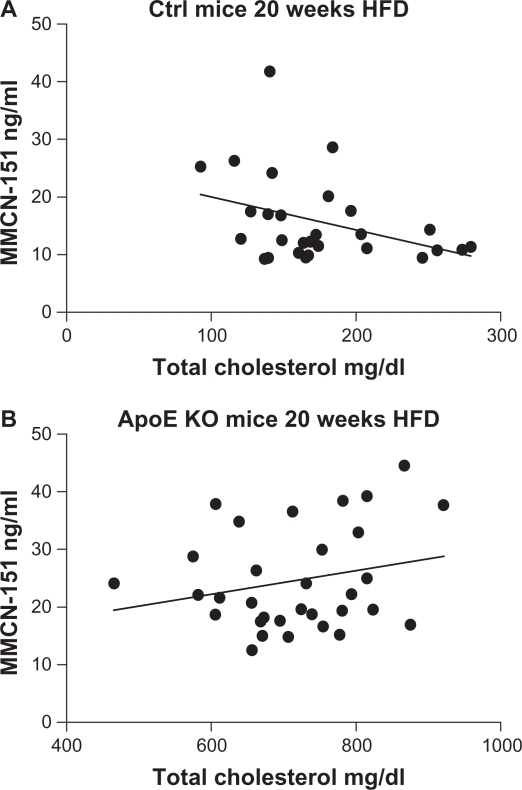
The linear relationship between plasma MMCN-151 values and total cholesterol after 20 weeks of a HFD in control mice (r = 0.48; P = 0.276), and ApoE-KO animals (r = 0.04; P = 0.931) is shown in Figure 4.
Figure 4.
Linear correlation between (A) plasma cholesterol of control mice fed HFD and their plasma levels of MMCN-151 and (B) plasma cholesterol of ApoE KO mice fed HFD and the levels of MMCN-151.
Total proteoglycan content correlates with plasma MMCN-151 levels
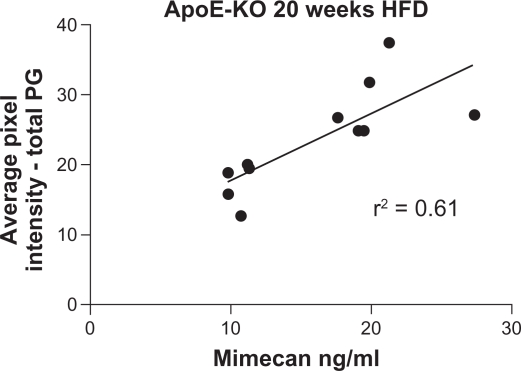
Visiopharm evaluation of the percentage of pixel intensity in a certain area, showed the total proteoglycan content in the abdominal aorta of ApoE KO mice after 20 weeks of a HFD was significantly correlated with plasma MMCN-151 levels (Fig. 5).
Figure 5.
Linear correlation between plasma levels of MMCN-151 and average pixel intensity (%) corresponding to the total proteoglycan area of each mouse aorta. The average pixel intensity is reflected as the intensity of Alcian blue, which is a standard proteoglycan staining. Alcian blue binds to glycosaminoglycan chains on proteoglycans.
Discussion
Biochemical markers consisting of protein degradation fragments from tissues undergoing extensive remodeling may indicate disease pathology and may be useful for diagnostic and prognostic purposes. Furthermore, these markers may detect changes resulting from intervention strategies and serve as surrogate markers of efficacy.26
The current study is, to our knowledge, the first to present the development of an assay detecting a specific fragment of mimecan (also known asosteoglycin) generated by MMP-12. Our main findings were: (1) The monoclonal antibody selected for assay development was highly specific towards MMCN-151, a mimecan neo-epitope generated by MMP-12. When tested in a competitive ELISA setting the antibody did not indicate cross-reactivity with the synthetically generated form of the epitope elongated by one amino-acid, nor to uncleaved mimecan from the in vitro test; (2) The MMCN-151 assay had strong native reactivity towards human serum and plasma and due to sequence homology also to rat and mouse sera and plasma; (3) A technically robust assay was developed with acceptable inter-, and intra-assay variation, dilution recovery and a low limit of detection; (4) In the in vivo part of the study, MMCN-151 plasma levels were significantly higher in mice fed a HFD for 20 weeks than those fed a normal diet. Furthermore, plasma levels of MMCN-151 in ApoE KO mice were significantly elevated at baseline, after 10 and 20 weeks of feeding compared with levels in control mice; (5) There was no significant correlation between MMCN-151 and plasma cholesterol levels after 20 weeks of feeding.
Selection of clones and ELISA development
Characterization of the selected monoclonal antibody revealed strong reactivity towards human and rodent sera and plasma. Additionally, the antibody demonstrated specificity towards the MMCN-151 peptide, whether generated synthetically or in vitro by cleavage of mimecan by MMP-12, indicating that the antibody recognizes this particular peptide of mimecan in native samples. This was confirmed by the experiments showing no cross-reactivity towards the elongated synthetic peptide or to the intact mime-can protein.
Plasma MMCN-151 in atherosclerosis in ApoE KO mice
In the ApoE KO model of atherosclerosis, plasma MMCN-151 levels increased significantly between baseline and 10 weeks, and between 10 weeks and 20 weeks, of feeding on a HFD. The same pattern was observed in control mice, but in this group of animals there was only a significant increase between baseline and 20 weeks of feeding on a HFD. In general, levels of MMCN-151 were higher at all time-points in ApoE KO mice than in control animals. This finding is supported by the literature, which shows ApoE mice develop atherosclerosis over time even when fed only a normal diet.
The study indicates that the MMCN-151 neo-epitope measured by this novel assay may be useful for the evaluation of atherosclerosis. These data are in agreement with the literature showing that mimecan is implicated in the formation of atherosclerotic plaques.27 MMP12 is just one of a range of MMPs identified in atherosclerotic plaques where they are most often located in smooth muscle cells, macrophages and macrophage foam cells bordering the fibrous cap and within plaque shoulders.8,28–30 A gelatinase-family member, MMP-9, has been shown to have an effect on the size and composition of atherosclerotic plaques.30 MMP-12, which is also known as elastase, is of special interest due to its selective secretion by macrophages.10,30,31 MMP-12 has also been shown to accelerate the initiation of atherosclerosis and progression of fatty streaks.32
We previously found that both MMP-9 and -12 generate the MMCN-151 fragment in cleavages of human atherosclerotic plaque material (unpublished data). The in vitro-cleaved purified mimecan and the lack of fragments we identified using the current ELISA might be due to the fact that 72 hours of cleavage resulted in further destruction of the neo-epitope by MMP-9. Further in vitro, time-dependent experiments are needed to investigate whether other proteases, including MMP-9, are able to generate the MMCN-151 fragment of mimecan.
Remarkably, we did not observe a correlation between levels of the MMCN-151 marker and plasma cholesterol in control or ApoE KO mice. These data suggest that the specific degradation of mimecan in atherosclerotic tissue might reflect very early pathological changes in the arteries, before plasma cholesterol levels begin to increase. Moreover, it is interesting that we found no difference in MMCN-151 plasma levels between ApoE KO and control mice fed a normal diet. However, we saw that the increase in MMCN-151 is not mediated by a high cholesterol diet, since only ApoE KO mice had elevated plasma biomarker levels. Moreover, it is interesting that we found no difference in MMCN-151 plasma levels in ApoE KO and control mice which received normal diet as we have seen previously with our collagen III, CO3-610 urine biomarker where significant biomarker increase was demonstrated in ApoE KO mice fed standard rodent chow.33 However, we have seen that the increase in MMCN-151 in ApoE KO mice seems to be mediated by a high cholesterol diet, whereas no significant increase in the circulating levels of MMCN-151 were observed in control mice when comparing the two diet regimens.
In conclusion, we developed a technically robust competitive assay using a specific monoclonal antibody for the detection in human, rat and mouse sera and plasma of MMCN-151, a fragment of mimecan derived from MMP-12 degradation. We demonstrated that MMCN-151 was significantly elevated in ApoE KO mice fed a HFD for 20 weeks in comparison with control mice.
More studies, in particular human longitudinal studies, are needed to further evaluate the potential of this marker in cardiovascular diseases associated with underlying atherosclerosis. Lastly, this marker may provide additional insights into mimecan and its function in both healthy and diseased states.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.American Heart Association . Heart Disease and Stroke Statistics—2007 Update. Dallas, Texas: American Heart Association; 2007. http://wwwamericanheart.org/downloadable/heart/1166712318459HS_StatsInsideTextpdf. [Google Scholar]
- 2.Kragel AH, Reddy SG, Wittes JT, Roberts WC. Morphometric analysis of the composition of atherosclerotic plaques in the four major epicardial coronary arteries in acute myocardial infarction and in sudden coronary death. Circulation. 1989 Dec;80(6):1747–56. doi: 10.1161/01.cir.80.6.1747. [DOI] [PubMed] [Google Scholar]
- 3.Wagner WD. Proteoglycan structure and function as related to atherosclerosis. Ann NY Acad Sci. 1985;454:52–68. doi: 10.1111/j.1749-6632.1985.tb11844.x. [DOI] [PubMed] [Google Scholar]
- 4.McCullagh KG, Duance VC, Bishop KA. The distribution of collagen types I, III and V (AB) in normal and atherosclerotic human aorta. J Pathol. 1980 Jan;130(1):45–55. doi: 10.1002/path.1711300107. [DOI] [PubMed] [Google Scholar]
- 5.Shekhonin BV, Domogatsky SP, Muzykantov VR, Idelson GL, Rukosuev VS. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res. 1985 Sep;5(4):355–68. doi: 10.1016/s0174-173x(85)80024-8. [DOI] [PubMed] [Google Scholar]
- 6.Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 2003;10(5):267–74. doi: 10.5551/jat.10.267. [DOI] [PubMed] [Google Scholar]
- 7.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994 Aug;90(2):775–8. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 8.Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res. 1999 Feb;41(2):369–75. doi: 10.1016/s0008-6363(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 9.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007 Mar;5(2):265–82. doi: 10.1586/14779072.5.2.265. [DOI] [PubMed] [Google Scholar]
- 11.Kampmann A, Fernandez B, Deindl E, et al. The proteoglycan osteoglycin/mimecan is correlated with arteriogenesis. Mol Cell Biochem. 2009 Feb;322(1–2):15–23. doi: 10.1007/s11010-008-9935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tasheva ES, Corpuz LM, Funderburgh JL, Conrad GW. Differential splicing and alternative polyadenylation generate multiple mimecan mRNA transcripts. J Biol Chem. 1997 Dec 19;272(51):32551–6. doi: 10.1074/jbc.272.51.32551. [DOI] [PubMed] [Google Scholar]
- 13.Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997 Oct 31;27244:28089–95. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- 14.Karsdal MA, Henriksen K, Leeming DJ, et al. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009 May;14(3):181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 15.Veidal SS, Bay-Jensen AC, Tougas G, Karsdal MA, Vainer B. Serum markers of liver fibrosis: combining the BIPED classification and the neo-epitope approach in the development of new biomarkers. Dis Markers. 2010;28(1):15–28. doi: 10.3233/DMA-2010-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Host NB, Jensen LT, Bendixen PM, Jensen SE, Koldkjaer OG, Simonsen EE. The aminoterminal propeptide of type III procollagen provides new information on prognosis after acute myocardial infarction. Am J Cardiol. 1995 Nov 1;76(12):869–73. doi: 10.1016/s0002-9149(99)80251-3. [DOI] [PubMed] [Google Scholar]
- 17.Uemura S, Matsushita H, Li W, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. 2001 Jun 22;88(12):1291–8. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 18.Death AK, Fisher EJ, McGrath KC, Yue DK. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis. 2003 Jun;168(2):263–9. doi: 10.1016/s0021-9150(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 19.Bellosta S, Baetta R, Canavesi M, et al. Raloxifene inhibits matrix metalloproteinases expression and activity in macrophages and smooth muscle cells. Pharmacol Res. 2007 Aug;56(2):160–7. doi: 10.1016/j.phrs.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers. 2002;66(1):19–32. doi: 10.1002/bip.10201. [DOI] [PubMed] [Google Scholar]
- 21.Sondergaard BC, Wulf H, Henriksen K, et al. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthritis Cartilage. 2006 Aug;14(8):759–68. doi: 10.1016/j.joca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Karsdal MA, Byrjalsen I, Leeming DJ, Christiansen C. Tibolone inhibits bone resorption without secondary positive effects on cartilage degradation. BMC Musculoskelet Disord. 2008;9:153. doi: 10.1186/1471-2474-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenquist C, Fledelius C, Christgau S, et al. Serum CrossLaps One Step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem. 1998 Nov;44(11):2281–9. [PubMed] [Google Scholar]
- 24.Barascuk N, Veidal SS, Larsen L, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010 Apr 7; doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Gefter ML, Margulies DH, Scharff MD. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–6. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- 26.Karsdal MA, Henriksen K, Leeming DJ, et al. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009 May;14(3):181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 27.Shanahan CM, Cary NR, Osbourn JK, Weissberg PL. Identification of osteoglycin as a component of the vascular matrix. Differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1997 Nov;17(11):2437–47. doi: 10.1161/01.atv.17.11.2437. [DOI] [PubMed] [Google Scholar]
- 28.Halpert I, Sires UI, Roby JD, et al. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9748–53. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mecham RP, Broekelmann TJ, Fliszar CJ, Shapiro SD, Welgus HG, Senior RM. Elastin degradation by matrix metalloproteinases. Cleavage site specificity and mechanisms of elastolysis. J Biol Chem. 1997 Jul 18;272(29):18071–6. doi: 10.1074/jbc.272.29.18071. [DOI] [PubMed] [Google Scholar]
- 30.Luttun A, Lutgens E, Manderveld A, et al. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004 Mar 23;109(11):1408–14. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Wang KY, Tanimoto A, et al. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol. 2008 May;172(5):1419–29. doi: 10.2353/ajpath.2008.070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada S, Wang KY, Tanimoto A, et al. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol. 2008 May;172(5):1419–29. doi: 10.2353/ajpath.2008.070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barascuk N, Vassiliadis E, Larsen L, et al. Development and validation of an enzyme-linked immunosorbent assay for the quantification of a specific MMP-9 mediated degradation fragment of type III collagen—A novel biomarker of atherosclerotic plaque remodeling. Clin Biochem. 2011 Jul;44(10–11):900–6. doi: 10.1016/j.clinbiochem.2011.04.004. [DOI] [PubMed] [Google Scholar]