Abstract
A pivotal role in guiding mesenchymal stem cell (MSC) differentiation has recently been attributed to the primary cilium. This solitary, non-motile microtubule-based organelle emerging from the cell surface acts as a sensorial membrane structure reflecting developmental and adaptive processes associated with pathologies including human cystic kidney disease, skeletal malformations, obesity and cancer. Given that the intrinsic hypoxic adaptation of MSC remains poorly understood within ischemic tissues or hypoxic tumours, we questioned whether the hypoxia inducible factor-1α (HIF-1α) might be a downstream effector regulating cilium maintenance. We show that murine bone marrow-derived MSC cultured under hypoxic conditions (1.2% O2) lose their primary cilia in a time-dependent manner. Gene silencing of HIF-1α prevented cilia loss in hypoxic cultures, and generation of MSC expressing a constitutively active HIF-1α (MSC-HIF) was found to decrease primary cilium formation. A Wnt pathway-related gene expression array was also performed on MSC-HIF and indicated that the secreted Frizzled-related proteins (sFRP)-1, -3 and -4 were down-regulated, while sFRP-2 was up-regulated. Overexpression of recombinant sFRP-2 or gene silencing of sFRP-1, -3 and -4 in MSC led to primary cilium disruption. These results indicate a molecular signalling mechanism for the hypoxic disruption of the primary cilium in MSC involving an HIF-1α/sFRP axis. This mechanism contributes to our understanding of the adaptive processes possibly involved in the oncogenic transformation and tumour-supporting potential of MSC. Our current observations also open up the possibility for the primary cilia to serve as a biomarker in MSC adaptation to low oxygen tension within (patho)physiological microenvironments.
Keywords: cilia, hypoxia, mesenchymal stem cells, hypoxia-inducible factor (HIF)
Introduction
Most commonly isolated from the bone marrow, mesenchymal stem cells (MSC) represent a population of pluripotent adult stem cells that can differentiate into many mesenchymal phenotypes, and that can adapt to low oxygen environments such as those encountered within ischemic tissues or hypoxic tumours.1,2 This adaptive property has been exploited to study the therapeutic efficacy of genetically-modified MSC.3–6
Homing of MSC to tumours was recently reported in a mouse model where injected human MSC could be found preferentially migrating to implanted human melanoma tumours.6 In fact, recruitment of MSC by experimental vascularizing tumours also resulted in the incorporation of MSC within the tumor architecture6,7 which, combined with intrinsic immunomodulatory mechanisms, suggests that they must also respond to tumour-derived growth factor cues.8,9 Consequently, their potential contribution to tumour development implies that MSC must metabolically adapt to the low oxygen environment and nutrient deprivation that characterizes hypoxic tumours. Moreover, the sum of this evidence, in line with their increased ability to migrate under an atmosphere of low oxygen,10 suggests that MSC may be active participants in the development of hypoxic solid tumours.
In order to survive within the stressful hypoxic microenvironment, cells have developed a coordinated set of responses orchestrating their adaptation to hypoxia. In cancer cells, the resultant of such cellular responses to hypoxia is often associated with aggressive disease and resistance to therapy.11 A critical mediator of the hypoxic response is the transcription factor hypoxia inducible factor 1 (HIF-1) which upregulates expression of proteins that promote angiogenesis, anaerobic metabolism, and many other survival pathways.12 Regulation of HIF-1α, a component of the HIF-1 heterodimer, occurs at multiple levels including translation, degradation, and transcriptional activation, and serves as a testimony to the central role of HIF-1. More recently, the canonical Wnt pathway was shown to be activated in stem cells under low oxygen culture conditions via HIF-1α.13 How such HIF/Wnt signalling affects the MSC adaptive mechanisms remains poorly documented.
Stem cell differentiation and proliferation are among the important processes regulated through the Wnt pathway. Its activation in hematopoietic stem cells and in MSC enhances cell proliferation, maintains pluripotency and prevents induction of apoptosis.14–16 Moreover, expression of Dickkopf (DKK), a Wnt inhibitor, prevented osteogenic differentiation of cultured human MSC.17 Among the Wnt molecular players, secreted Wnt antagonists were also found to be important in stem cell homeostasis in an in vivo gastro-intestinal cancer model.18 Autocrine Wnt signaling also operates in MSC populations to regulate mesenchymal lineage specification.19,20 The molecular mechanisms that regulate self-renewal, lineage-specific differentiation and/or adaptation still remain to be linked to specific biomarker expression.
The primary cilium is a sensory membrane structure which transduces surrounding mechanical and chemical signals and which serves as a control center for many protein signalling complexes.21 Despite its purpose in development where a role in guiding lineage commitment was reported,22 the primary cilium mainly serves as a cell surface biomarker associated with a growing number of pathologies, including human cystic kidney disease, skeletal malformations, obesity and cancer.23 Recent evidence links the tumour suppressor pVHL, a protein involved in the nuclear translocation of HIF-1α, to cilium integrity maintenance in cystic kidney disease.24 We therefore examined the biomarker potential of the primary cilium, as well as the impact of Wnt signalling in MSC adaptation to hypoxic cues.
Experimental Procedures
Materials
Sodium dodecylsulfate (SDS) and bovine serum albumin (BSA) were purchased from Sigma (Oakville, ON). Cell culture media was obtained from Invitrogen (Burlington, ON). All other reagents were from Sigma-Aldrich Canada.
Cell culture and experimental hypoxic conditions
Bone marrow-derived MSC were isolated from the whole femur and tibia bone marrow of C57BL/6 female mice; cells were cultured and characterized as previously described.25 Analysis by flow cytometry, performed at passage 14, revealed that MSC expressed CD44 yet were negative for CD45, CD31, KDR/flk1 (VEGF-R2), flt-4 (VEGF-R3) and Tie2 (angiopoietin receptor) (data not shown). Hypoxic culture conditions were attained by incubation of 65%–80% confluent cells in an anaerobic box. The oxygen was maintained at 1%, as described by others,26–28 using a compact gas oxygen controller Proox model 110 (Reming Bioinstruments Co., Red-field, NY) with a residual gas mixture composed of 94% N2 and 5% CO2.
cDNA construct generation and transduction of the MSC-HIF-1α
The human full-length HIF-1α cDNA construct was generously provided by Dr Gregg L. Semenza (Johns Hopkins University, Baltimore, MD, USA), and was used as a template for generating an HIF-1α mutant which lacked its oxygen-dependent degradation domain (ODD401–603). The deletion mutant (HIF-1α ΔODD) was constructed by overlap extension using PCR. The deletion was confirmed by DNA sequencing, and the 1.95 kb HIF-1α ΔODD cDNA was subcloned into pcDNA3.1. For generation of retroviral particles, the HIF-1α ΔODD construct was digested out of the pcDNA3.1 vector using BamHI and HpaI restriction enzymes and subcloned into the multiple cloning site of the bicistronic retrovector pIRES-GFP. 293-GP2 viral packaging cells were transfected with either the HIF-1α ΔODD-pIRES-GFP or null-pIRES-GFP plasmids and the viral supernant was collected at 48 and 72 hours post-transfection. MSC were subjected to 8 rounds of viral transduction. Following viral transduction each GFP(+)MSC (AP2-MSC) and HIF-1α-ΔODD-GFP(+) MSC population were each subjected to high speed cell sorting using a BD FacsAria flow cytometer to obtain polyclonal pooled clones of retrovirally-transfected MSC that were 100% GFP(+) and similar in regards to GFP signal intensity.
Total RNA isolation, cDNA synthesis and real-time quantitative RT-PCR
Total RNA was extracted from cell monolayers using TriZol reagent (Invitrogen). For cDNA synthesis, 2 μg of total RNA were reverse-transcribed into cDNA using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). cDNA was stored at −80°C prior to PCR. Gene expression was quantified by real-time quantitative PCR using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). DNA amplification was carried out using an iCycler iQ5 (Bio-Rad, Hercules, CA) and product detection was performed by measuring binding of the fluorescent dye SYBR Green I to double-stranded DNA. The QuantiTect primer sets were provided by Qiagen (Valencia, CA): sFRP-1, -2, -3, -4 (Mm_Sfrp1_1_SG QT00167153, Mm_Sfrp2_1_SG QT00101759, Mm_Frzb_1_SG QT00169232, Mm_Sfrp4_1_SG QT00120491) and β-actin (Hs_Actb_2_SG QT01680476). The relative quantities of target gene mRNA against the internal control β-actin RNA were measured by following a ΔCT method employing an amplification plot (fluorescence signal vs. cycle number). The difference (ΔCT) between the mean values in the triplicate samples of target gene and those of β-actin mRNAs were calculated by iQ5 Optical System Software version 2.0 (Bio-Rad, Hercules, CA) and the relative quantified value (RQV) was expressed as 2ΔCT.
Mouse Wnt signaling pathway PCR array
The Mouse Wnt Signaling Pathway RT2 Profiler PCR Arrays (PAMM-043, SA Biosciences, Frederick, MD) were used according to the manufacturer’s protocol. Quantitative RT-PCR was performed and relative gene expressions were calculated using the 2−ΔΔCt method, in which Ct indicates the fractional cycle number where the fluorescent signal reaches detection threshold. The ‘delta–delta’ method uses the normalized ΔCt value of each sample, calculated using a total of five endogenous control genes (B2M, HPRT1, RPL13A, GAPDH, and ACTB). Fold change values are then presented as average fold change = 2 (averageΔΔCt) for genes in MSC-HIF relative to control MSC. Detectable PCR products were obtained and defined as requiring <35 cycles. Using real-time PCR, we reliably analyzed expression of a focused panel of genes related to Wnt-mediated signal transduction with these arrays. The resulting raw data were then analyzed using the PCR Array Data Analysis Template (http://www.sabiosciences.com/pcrar-raydataanalysis.php). This integrated web-based software package automatically performs all ΔΔCt based fold-change calculations from our uploaded raw threshold cycle data.
Immunofluorescent microscopy
Cells were seeded on 1.5-mm thick glass coverslips in 6-well culture plates. After hypoxic treatment, media were removed and cells fixed in 10% formalin phosphate buffer (Fisher Scientific, Ottawa, ON) for 20 min, permeabilized in 0.5% Triton X-100/PBS for 5 min, then blocked 1 h in 1% BSA/PBS. Immunostaining was performed for 1 h with a monoclonal anti-acetylated tubulin antibody (clone6-11B-1) 1:200 in 1% BSA/PBS (Sigma, St-Louis, MO), followed by 1:200 anti-mouse-RedX (Invitrogen). Nuclei were stained using 5 μg/mL DAPI and then glass coverslips were mounted on slides using Pro-Long Gold Antifade reagent (Invitrogen, ON) before fluorescence was examined by microscopy, using a Nikon Eclipse TE2000-U microscope coupled to a QImaging Retiga 1300 camera.
Transfection method and RNA interference
Cells were transiently transfected with 20 nM siRNA (Qiagen) against HIF-1α (Mm_Hif1a_2 Flexitube siRNA, SI00193018), sFRP-1, -2, -3, -4 (Mm_Sfrp1_1 SI01415855, Mm_Sfrp2_1 SI00182567, Mm_Frzb_1SI01005991,Mm_Sfrp4_1SI00209818) or scrambled sequences (AllStar Negative Control siRNA, 1027281) using Lipofectamine 2000 (Invitrogen, ON). Specific gene knockdown was evaluated by qRT-PCR as described above. Small interfering RNA and mismatch siRNA were synthesized by Qiagen and annealed to form duplexes.
Morphological analysis of apoptotic and necrotic cells
To visualize nuclear morphology and chromatin condensation by fluorescence microscopy, cells were stained with 0.06 mg/mL Hoechst (33258, blue fluorescence) for apoptotic cells or with 50 μg/mL propidium iodide (red fluorescence) for necrotic cells.25
Statistical data analysis
Data are representative of three or more independent experiments. Statistical significance was assessed using Student’s unpaired t-test. Probability values were considered significant when an asterisk identifies such significance in the figures. * = P < 0.05; ** = P < 0.01; and *** = P < 0.001.
Results
The primary cilium is expressed in MSC and hypoxic culture conditions diminish ciliogenesis
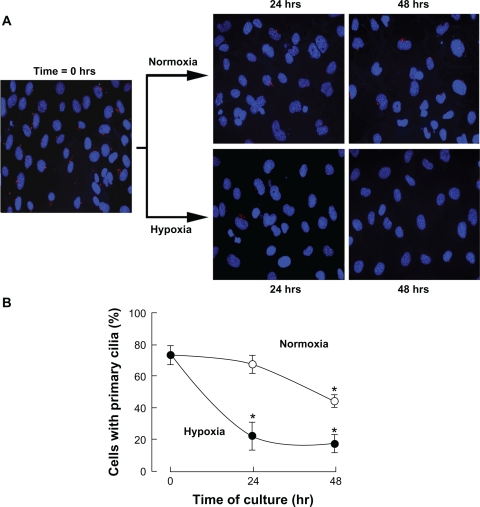
We first wished to monitor whether any primary cilia were expressed in MSC. MSC were seeded into petri dishes and cultured under normoxic conditions. Immunofluorescent staining was perfomed as described in the Methods section with the anti-acetylated tubulin antibody. We found that approximately 70% of the cells expressed a single plasma membrane protrusion attributable to the primary cilium (Fig. 1A). When MSC were cultured under hypoxic conditions for 24 and 48 hours, ciliogenesis was time-dependently and significantly decreased in hypoxic MSC cultures (Fig. 1B, closed circles) when compared to normoxic MSC cultures (Fig. 1B, open circles). Hoechst-33258 and propidium iodide staining only revealed basal levels of respective apoptotic and necrotic cells (<10%, data not shown) in accordance to previous studies,25 and suggests that the effects observed were not due to hypoxia-increased cell death. Significant decrease in ciliogenesis was also observed at 48 hours under normoxic culture conditions and may, although speculative, be attributable to low nutrient supply. Collectively, these observations still confirm that MSC express the primary cilium and support some hypoxia-mediated signalling events that would regulate such expression.
Figure 1.
Hypoxic culture conditions diminish ciliogenesis in MSC. Mesenchymal stem cells (MSC) were cultured under normoxic or hypoxic conditions as described in the Methods section for 24 and 48 hours. (A) Cells were fixed and cilia staining was performed. (B) Quantification of ciliogenesis in MSC cultured under normoxic (open circles) or hypoxic (black circles) conditions. Data are representative of three independent experiments. Three independent fields were quantified per experiment. Probability values of less than 0.05 were considered significant in hypoxic cultures, and an asterisk (*) identifies such significance relative to the normoxic culture condition.
Hypoxia inducible factor-1α expression is crucial in the hypoxic downregulation of ciliogenesis
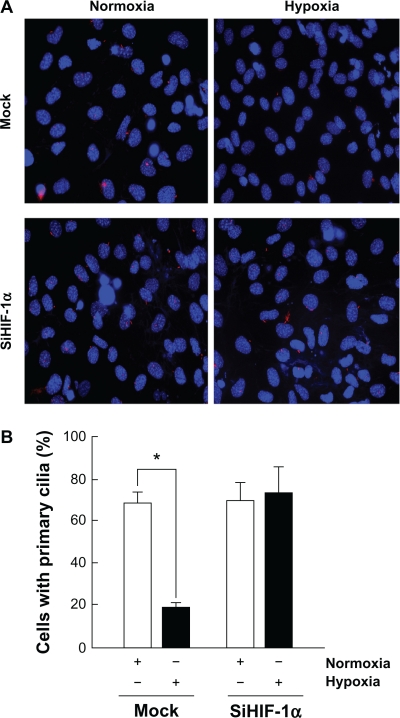
In order to assess the impact of the hypoxia inducible factor-1α (HIF-1α) in the hypoxia-mediated decrease in MSC ciliogenesis, MSC were transiently transfected with a scrambled siRNA sequence (Fig. 2A, upper panels; Mock) or with an siRNA designed to downregulate HIF-1α (Fig. 2A, lower panels; siHIF-1α) as described in the Methods section, then put in culture under normoxic (white bars) or hypoxic (black bars) conditions for 24 hours. Typical knockdown in HIF-1α gene expression was over 80% (not shown) and in accordance with our previous studies.29 Cilium staining was performed for each experimental condition and cilia were decreased, in agreement with the data in Figure 1, in hypoxic Mock-transfected cells (Fig. 2B, black bars). This decrease in cilium expression in hypoxic MSC was prevented from diminishing in siHIF-1α-transfected cells (Fig. 2B, black bars). These observations prompt for a crucial involvement of HIF-1α-mediated regulation of MSC ciliogenesis.
Figure 2.
Hypoxia inducible factor-1α expression is crucial in the hypoxic downregulation of ciliogenesis. MSC were transiently transfected with a scrambled siRNA sequence (Mock) or an siRNA designed to downregulate HIF-1α (siHIF-1α) as described in the Methods section. (A) Cells were then cultured either under normoxic or hypoxic conditions for 24 hours and cilia staining was performed. (B) Quantification of ciliogenesis in MSC cultured under normoxic (open bars) or hypoxic (black bars) conditions. Data are representative of three independent experiments. Probability values of less than 0.05 were considered significant, and an asterisk (*) identifies such significance relative to the respective control treatment.
HIF-1α stable expression dowregulates ciliogenesis
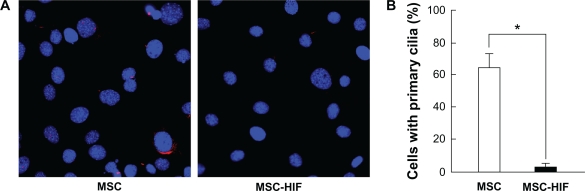
In order to rule out the sole effect of hypoxia and to firmly establish the direct HIF-1α’s impact on MSC ciliogenesis, cells were engineered to stably express a ΔODD HIF-1α mutant (MSC-HIF) as described in the Methods section. MSC and MSC-HIF were then cultured under normoxic conditions for 24 hours and cilia staining was performed (Fig. 3A). Cilium staining quantification confirmed the significant decrease in primary cilia expression in the MSC-HIF, therefore establishing HIF-1α as a major molecular actor in cilia downregulation.
Figure 3.
HIF-1α overexpression dowregulates ciliogenesis. MSC stably expressing a ΔODD HIF-1α mutant (MSC-HIF) were generated as described in the Methods section. (A) Cells were then cultured under normoxic conditions for 24 hours and cilia staining performed. (B) Quantification of ciliogenesis in MSC (open bars) or MSC-HIF (black bars) was performed. Data are representative of three independent cell cultures. Probability values of less than 0.05 were considered significant, and an asterisk (*) identifies such significance between MSC and MSC-HIF.
Gene array analysis reveals involvement of members from the secreted frizzled-related proteins in the hypoxic adaptation of MSC
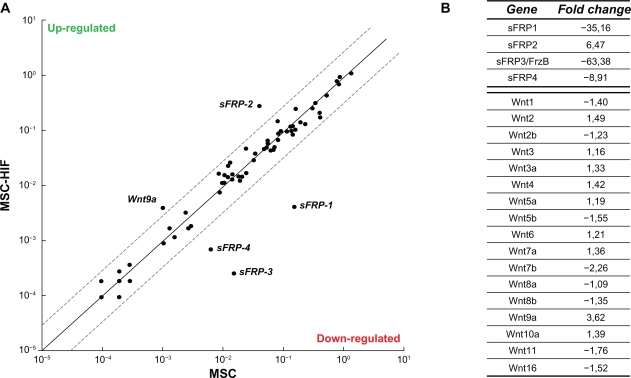
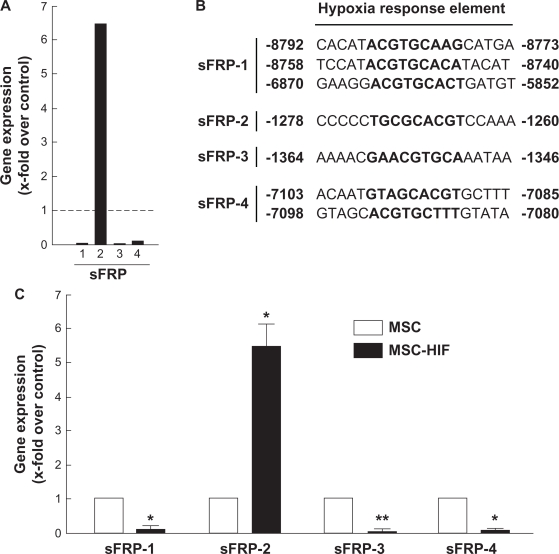
Given that the Wnt signalling pathway has been reported to regulate ciliogenesis,30,31 we performed a Wnt pathway-related gene expression array. Total RNA was isolated from MSC and from MSC-HIF, and cDNA was synthesized as described in the Methods section. A schematic representation of the genes which were up-regulated and downregulated (threshold in dotted lines) is depicted (Fig. 4A). A value between −3.00 and 3.00 was considered not significant. While most of the Wnt family gene members were unaffected (Wnt-1 to -16; Fig. 4B), Wnt-9a gene expression was the only one to be upregulated in MSC-HIF. Interestingly, gene expression of four members of the secreted Frizzled-related proteins (sFRP) were found modulated. While sFRP-2 was upregulated, the expression of sFRP-1, -3, and -4 was significantly downregulated (Fig. 4B). Collectively, these observations prompted us to explore whether sFRP transcriptional regulation may effectively occur within MSC.
Figure 4.
Gene array analysis reveals sFRP family involvement in hypoxic adaptation of MSC. Total RNA was isolated from MSC and from MSC-HIF and cDNA was synthesized as described in the Methods section. (A) Graphic representation of the array gene expression levels (thresholds in dotted lines). (B) Representative values of the sFRP1-4 and Wnt family gene expression. Values between −3.00 and 3.00 are not considered significant.
sFRP family members possess putative hypoxia responsive elements within their promoter region and are downregulated in hypoxic MSC
We next needed to validate the gene expression array data obtained previously. The data from Figure 4B were first used to generate a representative histogram of the sFRP-1, -3, and -4 gene down-regulation and of the sFRP-2 gene up-regulation (Fig. 5A). Specific involvement of HIF-1α in directing sFRP-1 to -4 gene regulation was next explored through sequence promoter analysis. 9,000 bp sequences upstream of the ATG coding sequence of the murine sFRP-1, -2, -3, and -4 gene promoter sequences was analyzed for HIF putative transcription factor binding sites with PROMO 3.0 (http://alggen.lsi.upc.es/) using version 8.3 of the TRANSFAC database. Several core consensus sequences of the hypoxia responsive elements (HRE) (A_G)CGT(G_C)C were found in the murine sFRP sequences analyzed (Fig. 5B). Quantitative qRT-PCR validation of the sFRP gene expression levels was finally performed in order to validate the gene array data and confirmed those gene expression data characterizing sFRP levels in MSC-HIF compared to MSC (Fig. 5C).
Figure 5.
sFRP family members possess putative Hypoxia Responsive Elements within their promoter regions. (A) Histogram of the sFRP-1, -3, and -4 genes downregulation and the sFRP-2 gene upregulation. (B) Sequences of the putative HRE found within the promoter regions of the four sFRP genes. NCBI sources : sFRP-1, NC_000074.5, chromosome 8; sFRP-2, NC_000069.5, chromosome 3; sFRP-3, NC_000068.6, chromosome 2; sFRP-4, NC_000079.5, chromosome 13. (C) qRT-PCR validation of sFRP gene expression levels.
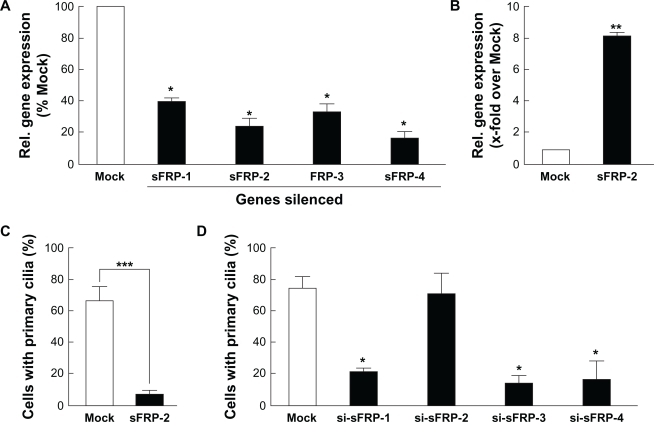
Overexpression of sFRP-2 or gene silencing of sFRP-1, -3, -4 family members dowregulates ciliogenesis
We next proceeded to validate the gene expression array significance of sFRP-2 gene upregulation and of sFRP-1, -3, and -4 gene dowregulation in MSC-HIF. We performed transient gene silencing of all sFRP members (Fig. 6A), and transiently transfected sFRP-2 cDNA (Fig. 6B) in MSC. We next went on to immunostain and assess the primary cilium expression in each of these conditions. We found that overexpression of recombinant sFRP-2 led to a significant decrease in transfected MSC (Fig. 6C). In parallel experiments, gene silencing of sFRP-1, -3, or -4 led to decreased ciliogenesis, while that of sFRP-2 did not change primary cilium expression (Fig. 6D).
Figure 6.
Overexpression of sFRP-2 or gene silencing of sFRP-1, -3, -4 family members dowregulates ciliogenesis. (A) Gene silencing was performed using specific siRNA for each of the sFRP-1, -2, -3, or -4 members as described in the Methods section. Total RNA was extracted and qRT-PCR performed in order to evaluate efficiency and validate gene expression. (B) Cells were transfected with Mock or sFRP-2 cDNA plasmid, and sFRP-2 gene expression validated by qRT-PCR. (C) Primary cilium staining was performed in Mock- (white bars) and in sFRP-2- (black bars) transfected cells. (D) Gene silencing was performed with a scrambled sequence (siScr; Mock), or with the respective sFRP-1, -2, -3, -4 siRNA sequences. Primary cilium staining was performed in Mock- (white bars) and in si-sFRP-transfected cells (black bars).
Discussion
Hypoxia is thought to be a tissue-specific condition that could promote oncogenic processes. In this study we have demonstrated that hypoxia significantly impacts ciliogenesis via a HIF-1α/Wnt signalling axis. While the Wnt family of proteins is known to influence the MSC phenotype through both canonical and non-canonical signalling pathways,20,32 our data support the importance of the Wnt pathway in the maintenance of cilium integrity. Accordingly, we also identified the crucial joint action of several sFRP members on MSC ciliogenesis. Our current observations also open up the possibility for the primary cilia to serve as a biomarker for MSC adaptation to low oxygen tension within (patho)physiological microenvironments.
The complex nature of the hypoxic tumor microenvironment may result in pro-oncogenic conditions for MSC. As such, mobilization and migration of MSC to the peripheral blood,33 and to tumors34,35 in response to hypoxic cues have recently been evidenced. These events are in part explained by the fact that hypoxic cancer cells secrete various cytokines including IL-6, VEGF, PDGF and FGF, that attract and promote MSC proliferation and differentiation into tumor-supporting cells.36,37 Recently, chemosensory response to PDGF-AA in fibroblasts was shown to require the primary cilium.38 In contrast, in the bone marrow, the hypoxic niche is important for maintaining stemness, cell cycle, cell survival and metabolism of MSC and HSC.39 The function of MSC in tumors is likely to parallel the role of MSC in wound healing.40,41 Accordingly, MSC derived from bone marrow are thought to endogenously support wound healing and hematopoiesis, but many of their native functions under hypoxic conditions remain poorly understood. Under such conditions the low oxygen tension is believed to protect the genomic integrity of stem cell populations by limiting the production of reactive oxygen species by mitochondrial respiration.42
Primary cilium identification as a biomarker may allow efficient monitoring of hypoxic MSC adaptation processes and help increase our understanding of the mechanisms involved in their oncogenic regulation. In combination with other MSC cell surface markers, hypoxic disruption of the primary cilium may also represent a usefull marker for clinical purposes. In support of this, the recent discovery of the importance of primary cilia in a variety of cell functions raises the possibility that this structure may indeed have a role in a variety of cancers. Recently, the formation of the primary cilium was disrupted in cells derived from astrocytoma/glioblastoma tumors.43 This observation is among the first evidence that altered primary cilium expression and function may be part of some malignant phenotypes.44,45 MSC were also shown to integrate, engraft and differentiate within hypoxic brain tumors,46 which further highlights the fact that the primary cilia could serve as a diagnostic tool and provide new insights into the mechanism of tumorigenesis.
Whether MSC are pro- or anti-tumorigenic is a subject of controversial reports that is in part explained by the complexity of their homing, engraftment, and differentiation mechanisms within the tumor microenvironement.47 The differentiation of MSC into lineage-specific cells is controlled by external factors in the environment, including cell–cell and cell–ECM adhesion and cytokine, chemokine, and growth factor availability.48,49 Several signalling pathways have recently been identified in MSC proliferation and differentiation control including canonical and non-canonical Wnt, RhoA/ROK, and Erk.50–52 Our current study highlights the increased levels of sFRP-2 in hypoxic MSC. Accordingly, while sFRP-1 and sFRP-2 are produced by the majority of longterm and ex vivo malignant glioma cell lines, only sFRP-2 was shown to strongly promote the growth of intracranial glioma xenografts in nude mice.53 We also report that sFRP-1, -3, and -4 downregulation correlates with the hypoxic MSC phenotype that leads to decreased ciliogenesis. Intracellular Wnt effectors, such as Fuzzy and Inturned, were found to disrupt the primary cilium structure in Drosophila melanogaster embryos.54 Interestingly, sFRP-1 is frequently silenced in many types of cancer leading to aberrant activation of Wnt signaling.55 Given they all possess HRE in their promoter region, it remains to be clarified how different sFRPs may have opposing effects on the same process and how they affect cell cycle progression and/or cell differentiation.56 Of note, primary cilia are coupled to the cell cycle and, in vertebrates, cilia believed to only present on non-proliferating cells, and resorbed during cell cycle progression to release the centrosome from the ciliary basal body, which makes it available for duplication and organization of the mitotic spindle.
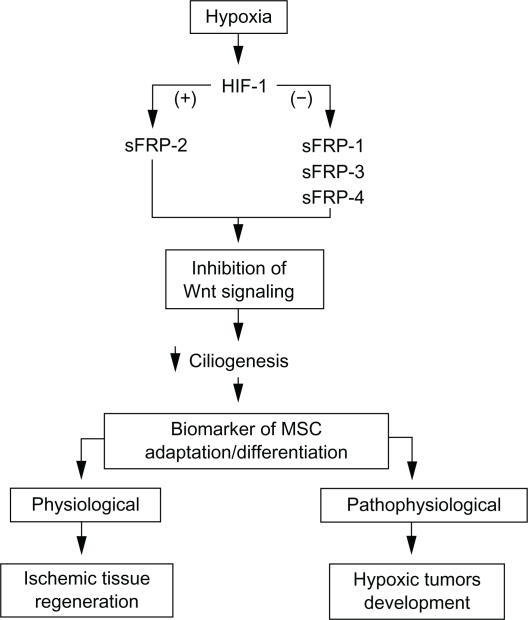
Our current observations may also impact on the potential of MSC to promote tissue repair in a diverse array of diseases, including ischemic heart disease, diabetes, and Parkinson’s disease.57 When engrafted at sites of tissue injury, MSC differentiate into connective tissue elements, support vasculogenesis, and secrete cytokines and growth factors that facilitate healing. Accordingly, MSC were confirmed as promising tools for cell therapy, as proven effective in US FDA-approved clinical trials for myocardial infarction, stroke, meniscus injury, limb ischemia, graft-versus-host disease and autoimmune disorders.58 The hypoxia/ischemia alteration of MSC within these pathological states still remains poorly understood. Clinical trials for MSC injection into the CNS to treat traumatic brain injury and stroke are also ongoing. One may envision that ischemic/hypoxic regulation of primary cilium expression may help monitor intravenous infusion of MSC to in vivo cerebral ischemia tissues.59 Neuroprotective, trophic support and therapeutic efficacy through MSC mobilization was also demonstrated in several ischemia/reperfusion models.60–62 In conclusion, our study enabled us to identify secreted Wnt-related proteins that contribute to hypoxic regulation of ciliogenesis in MSC. A schematic summary of the hypoxia-mediated regulation of ciliogenesis in MSC under (patho) physiological conditions is provided (Fig. 7). Altogether, we also provide new insight into stem cells biology and stem cell adaptation to hypoxia.
Figure 7.
Schematic summary of the hypoxia-mediated regulation of ciliogenesis in MSC. Hypoxic conditions trigger HIF-1α nuclear translocation which mediates transcriptional downregulation (−) of sFRP-1, -3, -4, and transcriptional upregulation (+) of sFRP-2. These molecular partners lead to inhibition of Wnt signaling and contribute to decreased ciliogenesis. Collectively, the primary cilium may be considered as a differentiation biomarker of cells that must adapt to low oxygen tension microenvironments. Such hypoxic conditions can be found within pathophysiological conditions such as those encountered in hypoxic tumour development, or in physiological regeneration of ischemic tissues.
Acknowledgments
BA holds a Canada Research Chair in Molecular Oncology from the Canadian Institutes of Health Research (CIHR). SPB is a Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) awardee. This study was funded by a grant from the NSERC to BA. Special thanks to Denis Flipo for assistance with the microscopy data collection.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 3.Seo SH, Kim KS, Park SH, Suh YS, Kim SJ, Jeun SS, et al. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011;18(5):488–95. doi: 10.1038/gt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romieu-Mourez R, François M, Abate A, Boivin MN, Birman E, Bailey D, et al. Mesenchymal stromal cells expressing ErbB-2/neu elicit protective antibreast tumor immunity in vivo, which is paradoxically suppressed by IFN-gamma and tumor necrosis factor-alpha priming. Cancer Res. 2010;70(20):7742–7. doi: 10.1158/0008-5472.CAN-10-0296. [DOI] [PubMed] [Google Scholar]
- 5.Kucic T, Copland IB, Cuerquis J, Coutu DL, Chalifour LE, Gagnon RF, et al. Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am J Physiol Renal Physiol. 2008;295(2):F488–96. doi: 10.1152/ajprenal.00044.2008. [DOI] [PubMed] [Google Scholar]
- 6.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–8. [PubMed] [Google Scholar]
- 7.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 8.Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91(6):1146–58. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- 9.Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83(3):241–7. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 10.Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21(3):337–47. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 11.Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3(3):e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh MY, Spivak-Kroizman TR, Powis G. HIF-1alpha and cancer therapy. Recent Results Cancer Res. 2010;180:15–34. doi: 10.1007/978-3-540-78281-0_3. [DOI] [PubMed] [Google Scholar]
- 13.Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, et al. O2 regulates stem cells through Wnt/ß-catenin signalling. Nat Cell Biol. 2010;12(10):1007–13. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–14. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 15.De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10(3–4):393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- 16.Derfoul A, Carlberg AL, Tuan RS, Hall DJ. Differential regulation of osteogenic marker gene expression by Wnt-3a in embryonic mesenchymal multipotential progenitor cells. Differentiation. 2004;72(5):209–23. doi: 10.1111/j.1432-0436.2004.07205003.x. [DOI] [PubMed] [Google Scholar]
- 17.Gregory CA, Gunn WG, Reyes E, Smolarz AJ, Munoz J, Spees JL, et al. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci. 2005;1049:97–106. doi: 10.1196/annals.1334.010. [DOI] [PubMed] [Google Scholar]
- 18.Byun T, Karimi M, Marsh JL, Milovanovic T, Lin F, Holcombe RF. Expression of secreted Wnt antagonists in gastrointestinal tissues: potential role in stem cell homeostasis. J Clin Pathol. 2005;58(5):515–9. doi: 10.1136/jcp.2004.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22(5):849–60. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 20.Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433(1–2):1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15(1):105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 22.Tummala P, Arnsdorf EJ, Jacobs CR. The Role of Primary Cilia in Mesenchymal Stem Cell Differentiation: A Pivotal Switch in Guiding Lineage Commitment. Cell Mol Bioeng. 2010;3(3):207–12. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev. 2009;19(3):220–9. doi: 10.1016/j.gde.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol. 2007;9(5):588–95. doi: 10.1038/ncb1579. [DOI] [PubMed] [Google Scholar]
- 25.Currie JC, Fortier S, Sina A, Galipeau J, Cao J, Annabi B. MT1-MMP down-regulates the glucose 6-phosphate transporter expression in marrow stromal cells: a molecular link between pro-MMP-2 activation, chemotaxis, and cell survival. J Biol Chem. 2007;282(11):8142–9. doi: 10.1074/jbc.M610894200. [DOI] [PubMed] [Google Scholar]
- 26.Raheja LF, Genetos DC, Wong A, Yellowley CE. Hypoxic regulation of mesenchymal stem cell migration: the role of RhoA and HIF-1alpha. Cell Biol Int. 2011. (in press). [DOI] [PubMed]
- 27.Tamama K, Kawasaki H, Kerpedjieva SS, Guan J, Ganju RK, Sen CK. Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem. 2011;112(3):804–17. doi: 10.1002/jcb.22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen JG, Frøbert O, Pilgaard L, Kastrup J, Simonsen U, Zachar V, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13(3):318–28. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 29.Proulx-Bonneau S, Guezguez A, Annabi B. A Concerted HIF-1a/MT1-MMP Signalling Axis Regulates the Expression of the 3BP2 Adaptor Protein in Hypoxic Mesenchymal Stromal Cells. PLoS One. 2011;6(6):e21511. doi: 10.1371/journal.pone.0021511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerdes JM, Katsanis N. Ciliary function and Wnt signal modulation. Curr Top Dev Biol. 2008;85:175–95. doi: 10.1016/S0070-2153(08)00807-7. [DOI] [PubMed] [Google Scholar]
- 31.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25(3):201–13. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karow M, Popp T, Egea V, Ries C, Jochum M, Neth P. Wnt signalling in mouse mesenchymal stem cells: impact on proliferation, invasion and MMP expression. J Cell Mol Med. 2009;13(8B):2506–20. doi: 10.1111/j.1582-4934.2008.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Yu Q, Lin J, Lai X, Cao W, Du K, et al. Hypoxia-Inducible factor-1a is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev. 2011. (in press). [DOI] [PubMed]
- 34.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15(2):57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Rattigan Y, Hsu JM, Mishra PJ, Glod J, Banerjee D. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res. 2010;316(20):3417–24. doi: 10.1016/j.yexcr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29(2):285–93. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25(2–3):279–92. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 40.Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ. Mesenchymal stem cells. Arch Med Res. 2003;34(6):565–71. doi: 10.1016/j.arcmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99(4):622–31. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman DS. HIF hits Wnt in the stem cell niche. Nat Cell Biol. 2010;12(10):926–7. doi: 10.1038/ncb1010-926. [DOI] [PubMed] [Google Scholar]
- 43.Moser JJ, Fritzler MJ, Rattner JB. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer. 2009;9:448. doi: 10.1186/1471-2407-9-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15(9):1062–5. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20(1):58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birnbaum T, Hildebrandt J, Nuebling G, Sostak P, Straube A. Glioblastoma-dependent differentiation and angiogenic potential of human mesenchymal stem cells in vitro. J Neurooncol. 2011. (in press). [DOI] [PubMed]
- 47.Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9:129. doi: 10.1186/1476-4598-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner W, Ho AD. Mesenchymal stem cell preparations—comparing apples and oranges. Stem Cell Rev. 2007;3(4):239–48. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 49.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 50.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21(10):1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 51.Meriane M, Duhamel S, Lejeune L, Galipeau J, Annabi B. Cooperation of matrix metalloproteinases with the RhoA/Rho kinase and mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase signaling pathways is required for the sphingosine-1-phosphate-induced mobilization of marrow-derived stromal cells. Stem Cells. 2006;24(11):2557–65. doi: 10.1634/stemcells.2006-0209. [DOI] [PubMed] [Google Scholar]
- 52.Liang X, So YH, Cui J, Ma K, Xu X, Zhao Y, et al. The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK/ERK pathway in rat cultured mesenchymal stem cells. J Radiat Res (Tokyo) 2011;52(3):380–6. doi: 10.1269/jrr.10121. [DOI] [PubMed] [Google Scholar]
- 53.Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, et al. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000;19(37):4210–20. doi: 10.1038/sj.onc.1203783. [DOI] [PubMed] [Google Scholar]
- 54.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38(3):303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 55.Jiang GX, Liu W, Cui YF, Zhong XY, Tai S, Wang ZD, et al. Reconstitution of secreted frizzled-related protein 1 suppresses tumor growth and lung metastasis in an orthotopic model of hepatocellular carcinoma. Dig Dis Sci. 2010;55(10):2838–43. doi: 10.1007/s10620-009-1099-3. [DOI] [PubMed] [Google Scholar]
- 56.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(6):737–46. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 57.Brooke G, Cook M, Blair C, Han R, Heazlewood C, Jones B, et al. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007;18(6):846–58. doi: 10.1016/j.semcdb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5(6):933–46. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perasso L, Cogo CE, Giunti D, Gandolfo C, Ruggeri P, Uccelli A, et al. Systemic administration of mesenchymal stem cells increases neuron survival after global cerebral ischemia in vivo (2VO) Neural Plast. 2010;2010:534925. doi: 10.1155/2010/534925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24(1):59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, et al. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 2011;6(4):e19195. doi: 10.1371/journal.pone.0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Zhang R, Li Y, He G, Zhang D, Zhang F. Simvastatin augments the efficacy of therapeutic angiogenesis induced by bone marrow-derived mesenchymal stem cells in a murine model of hindlimb ischemia. Mol Biol Rep. 2011. (in press). [DOI] [PubMed]