Abstract
A 59-year-old man presented with a skin eruption and bilateral swelling of the legs. Soon after the initial presentation, he developed acute respiratory distress syndrome (ARDS) with miliary lung nodules. Culture of samples from the skin ulcers, sputum, and bronchoalveolar lavage fluid all revealed Mycobacterium szulgai infection. The patient was successfully treated with antituberculosis drugs. M. szulgai infection is very rarely reported worldwide, and disseminated infection usually occurs in immunocompromised patients. However, the present patient was a non-immunocompromised case, although he was a hepatitis B virus carrier. While the progression to ARDS from M. tuberculosis infection is well known, this is the first case of M. szulgai infection progressing to ARDS.
Keywords: cutaneous infection, Mycobacterium szulgai, acute respiratory distress syndrome
Introduction
The incidence of non-tuberculosis mycobacterial (NTM) infection has increased recently, and this condition currently accounts for 10%–15% of all mycobacterial infection cases.1,2 While the Mycobacterium avium complex (MAC) and M. kansasii are responsible for more than 90% of cases of mycobacterial infection, Mycobacterium species have diversified and now include species such as M. szulgai.3 M. szulgai is an uncommon mycobacterial pathogen in humans.4 It causes pulmonary disease resembling the common type of M. tuberculosis infection, as well as extrapulmonary infection. Disseminated infection of M. szulgai is rare and usually occurs in immunocompromised patients.5
Here, we report an unusual case of disseminated mycobacterial infection progressing to acute respiratory distress syndrome (ARDS) in a non-immunocompromised hepatitis B virus (HBV) carrier.
Case Report
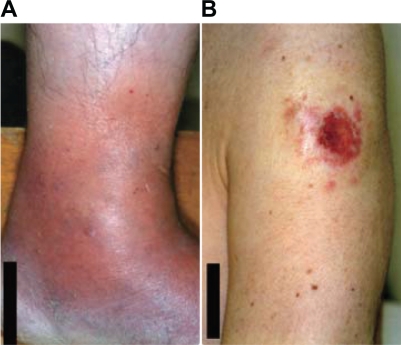
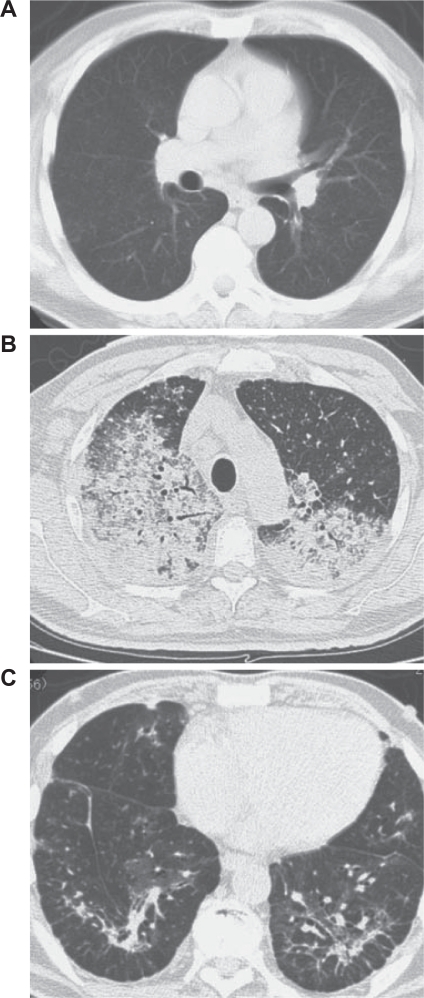
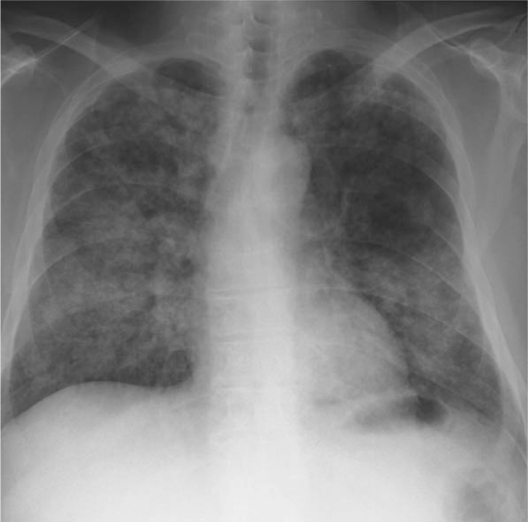
A 59-year-old man was admitted to our hospital because he was experiencing severe respiratory distress. He had been diagnosed with intestinal catarrh when he was 1 year old, nephritic syndrome when he was 13 years old, and pleuritis when he was 57 years old. He was an HBV carrier, but not immunocompromised. He did not have a history of tuberculosis. He lived in a rural district composed mainly of paddy fields and worked as an engineer in an office. He was an ex-smoker, having quit smoking at the age of 30 years. Six months before hospital admission, he presented with edema of the left leg and low-grade fever. At the time of presentation, he tested positive for anti-Chlamydia pneumoniae immunoglobulin antibodies and was treated with clarithromycin. He experienced symptom relief with this treatment within a few weeks. However, 4 months before hospital admission, soon after the treatment was discontinued, he developed low-grade fever, a skin eruption resembling folliculitis in the left upper arm, and adenopathy of multiple lymph nodes. Two weeks before hospital admission, he presented with bilateral swelling of the fingers and feet (Fig. 1A). Further, the skin eruption had progressed to ulceration (Fig. 1B). Isolates from the ulcer exudate were cultured and revealed cutaneous infection with mycobacterial species. He was therefore referred to our department. Chest X-ray (CXR) findings were normal, but computed tomography (CT) revealed the presence of tiny miliary nodules in both lung fields (Fig. 2A). Five days before admission, the patient developed a fever (temperature >38 °C). He was urged to admit himself to our hospital because of severe respiratory distress. Physical examination revealed tachypnea and 80% oxygen saturation in room air. Prominent inspiratory crackles were heard over both lower lung fields. CXR showed diffuse bilateral infiltration (Fig. 3), and CT revealed the presence of multiple nodules and infiltrates in both lung fields (Fig. 2B). Blood gas analysis in room air revealed a partial pressure of oxygen in arterial blood (PaO2) of 46 mmHg and a pH of 7.46. The other blood parameters were as follows: hemoglobin level, 11.3 g/dL, was within normal limits; leukocyte count, 14.4 × 103 cells/L, was high; and C-reactive protein level, 20.8 mg/L, was high. The brain natriuretic peptide level was 30.0 pg/mL, which was within the normal limits.
Figure 1.
(A) Swelling of the feet and (B) skin ulcer observed 2 weeks before hospital admission.
Figure 2.
(A) Thoracic computed tomography (CT) obtained 2 weeks before hospital admission, showing tiny nodules with no abnormal shadows. (B) CT scan obtained upon admission, showing shadows and nodules on both lung fields. (C) CT scan obtained shortly before discharge from the hospital, showing almost complete disappearance of the shadows.
Figure 3.
Posteroanterior chest radiograph obtained upon hospital admission, showing diffuse bilateral infiltration.
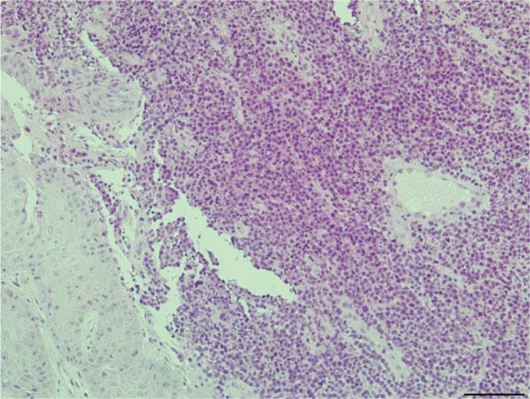
We initiated treatment with a broad-spectrum panel of antimicrobial agents, namely, meropenem and ciproxacin, along with methylprednisolone pulse therapy. However, over the next 24 hours, the patient developed severe respiratory failure (PaO2/fraction of inspired oxygen [PF ratio] <200) and required intubation and mechanical ventilation with positive end-expiratory pressure. Intubation was followed by bronchoscopy with bronchoalveolar lavage (BAL). Positive results were obtained in Ziehl-Neelsen staining of a sputum smear and BAL fluid, but negative results were obtained for Gram staining. We therefore strongly suspected mycobacterial infection. Although M. tuberculosis and the MAC were not detected in polymerase chain reaction analysis of the patient’s samples, we continued to suspect a miliary mycobacterial infection. Therefore, we initiated treatment with a 4-drug regimen comprising rifampicin 450 mg/day, isoniazid 300 mg/day, pyrazinamide 1500 mg/day, and streptomycin 1 g/day. Meropenem and ciproxacin were discontinued 17 days after hospital admission because the patient’s condition improved. Skin biopsy which was done at 1 weak after admission revealed diffuse infiltration of mixed inflammatory cells in the dermis, which was consistent with the diagnosis of cutaneous NTM infection (Fig. 4).6 CXR and CT revealed a gradual improvement in the patient’s condition, and the patient was weaned off mechanical ventilation 23 days after admission. About 1 month after admission, we identified M. szulgai in cultures of the sputum, skin ulcer exudate, and BAL fluid using the colorimetric microdilution plate hybridization method.7 About 2 months after admission, the bilateral infiltrates had almost completely disappeared from the lungs (Fig. 2C), and the patient was discharged.
Figure 4.
Biopsy of the skin ulcer (hematoxylin and eosin, 200×), revealing infiltration of mixed inflammatory cells, small vessel proliferation in the dermis, and epidermal proliferation. Dermal granulomas were not observed.
Discussion
M. szulgai infection is very rare, and this bacterium accounts for <0.5% of all isolates obtained from human patients with NTM infection.8,9 In Japan, only 27 cases of M. szulgai infection have been reported between 1989 and 2008, most of which involved pre-existing disease.10 In a study in Turkey, mycobacterial growth was positive in 10041 of 45981 samples from 19553 patients with a prediagnosis of pulmonary tuberculosis between November 2004 and January 2009; M. szulgai was isolated from only 3 patients among this study population.11
Cultures yielding M. szulgai usually have a pathologic significance, because this bacterium is rarely recovered from the environment.8,9 Consequently, in appropriate clinical circumstances, 1 positive culture may suffice for diagnosing M. szulgai infection. In the present case, cultures of the skin ulcer exudate, sputum, and BAL fluid all revealed M. szulgai infection only, implicating this bacterium as the sole cause of the miliary infection.
The clinical features of this case, including the acute onset, bilateral shadows, low PF ratio (<200), and no clinical evidence of elevated left atrial pressure, were compatible with the diagnostic criteria for ARDS.12 NTM infection-induced ARDS is very rare.13 In contrast, miliary tuberculosis is a well-known cause of ARDS.14,15 This case is the first reported case of M. szulgai infection progressing to ARDS. Miliary tuberculosis accounts for 2.3% of all cases of tuberculosis infection in Japan.14 Additionally, in a series study in South Korea, only about 20% of miliary tuberculosis patients developed ARDS.16 Thus, miliary infection-induced ARDS probably accounts for less than 1% of all tuberculosis cases. On the other hand, the incidence of miliary infection among all cases of M. szulgai infection cannot be easily estimated, because infection with M. szulgai is very rare.
In conclusion, M. szulgai can cause severe disease by hematogenous spread. M. szulgai infection-induced ARDS is extremely rare, but can be successfully treated with antituberculosis drugs.
Footnotes
Author Contributions
Hiromitsu Ohta analyzed and interpreted the patient data and wrote this article.
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material. Written consent was obtained from the patient for publication of this case.
References
- 1.Diagnosis and treatment of disease caused by nontuberculous mycobacteria This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997;156:S1–25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 2.Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest. 2008;133:243–51. doi: 10.1378/chest.07-0358. [DOI] [PubMed] [Google Scholar]
- 3.Fujita J, Hibiya K, Haranaga S, Higa F, Tateyama M. Overview of respiratory infection caused by nontuberculous mycobacteria. Kekkaku. 2007;82:721–7. [PubMed] [Google Scholar]
- 4.Schaefer WB, Wolinsky E, Jenkins PA, Marks J. Mycobacterium szulgai-a new pathogen. Serologic identification and report of five new cases. Am Rev Respir Dis. 1973;108:1320–6. doi: 10.1164/arrd.1973.108.6.1320. [DOI] [PubMed] [Google Scholar]
- 5.Meyer JJ, Gelman SS. Multifocal osteomyelitis due to Mycobacterium szulgai in a patient with chronic lymphocytic leukemia. J Infect. 2008;56:151–4. doi: 10.1016/j.jinf.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Song H, Lee H, Choi G, Shin J. Cutaneous nontuberculous mycobacterial infection: a clinicopathological study of 7 cases. Am J Dermatopathol. 2009;31:227–31. doi: 10.1097/DAD.0b013e318196187a. [DOI] [PubMed] [Google Scholar]
- 7.Kusunoki S, Ezaki T, Tamesada M, et al. Application of colorimetric microdilution plate hybridization for rapid genetic identification of 22 Mycobacterium species. J Clin Microbiol. 1991;29:1596–603. doi: 10.1128/jcm.29.8.1596-1603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortoli E, Besozzi G, Lacchini C, Penati V, Simonetti MT, Emler S. Pulmonary infection due to Mycobacterium szulgai, case report and review of the literature. Eur Respir J. 1998;11:975–7. doi: 10.1183/09031936.98.11040975. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Alarcos JM, De Miguel-Diez J, Bonilla I, Sicilia JJ, Alvarez-Sala JL. Pulmonary infection due to Mycobacterium szulgai. Respiration. 2003;70:533–6. doi: 10.1159/000074214. [DOI] [PubMed] [Google Scholar]
- 10.Sekine A, Hagiwara E, Ogura T, et al. Four cases of pulmonary infection due to Mycobacterium szulgai with a review of previous case reports in Japan. Nihon Kokyuki Gakkai Zasshi. 2008;46:880–8. [PubMed] [Google Scholar]
- 11.Bicmen C, Coskun M, Gunduz AT, Senol G, Cirak AK, Tibet G. Nontuberculous mycobacteria isolated from pulmonary specimens between 2004 and 2009: causative agent or not? New Microbiol. 2010;33:399–403. [PubMed] [Google Scholar]
- 12.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 13.Baylor P, Larson R. Mycobacterium avium septicemia with ARDS in a patient with diabetes mellitus and no other known immune-compromising condition. J Intensive Care Med. 2009;24:140–3. doi: 10.1177/0885066608330103. [DOI] [PubMed] [Google Scholar]
- 14.Nojima D, Ozaki S, Fujii Y, et al. A case of miliary tuberculosis presenting with acute respiratory distress syndrome. Nihon Kokyuki Gakkai Zasshi. 2009;47:195–9. [PubMed] [Google Scholar]
- 15.Dyer RA, Chappell WA, Potgieter PD. Adult respiratory distress syndrome associated with miliary tuberculosis. Crit Care Med. 1985;13:12–5. doi: 10.1097/00003246-198501000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Park YB, Kim YS, et al. Miliary tuberculosis and acute respiratory distress syndrome. Int J Tuberc Lung Dis. 2003;7:359–64. [PubMed] [Google Scholar]