Abstract
Background:
Osteoporosis and related fragility fractures are one of the most common complications seen in patients with rheumatoid arthritis (RA) and dramatically affect quality of life.
Objective:
To evaluate changes in bone mineral density in patients with recent onset rheumatoid arthritis (<1 year) and its correlation if any with a modified DAS-28 score and simple erosion narrowing score (SENS).
Methods:
This study included 30 patients with recent-onset rheumatoid arthritis fulfilling the new American College of Rheumatology/European League Against Rheumatism diagnostic criteria for rheumatoid arthritis and 20 healthy volunteers as controls. All were subjected to a complete blood count, erythrocyte sedimentation rate, C-reactive protein, liver function tests, renal function tests, rheumatoid factor, and plain x-rays of the hands and feet. Dual-energy x-ray absorptiometry DEXA was used to measure bone mineral density (BMD) of the left proximal femur, lumbar spine (L1–L4), and lower distal radius at the time of recruitment.
Results:
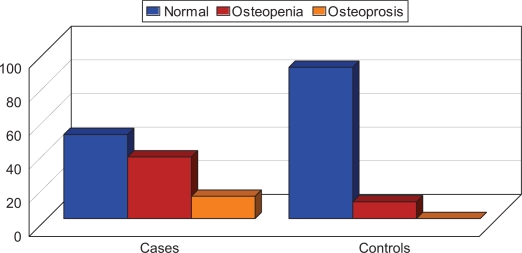
In the RA patients, 13.3% had osteoporosis, 50% had osteopenia, and 36.7% had normal BMD. The most common site of osteoporosis was the lumbar spine (four patients, 13.3%) followed by the femur (two patients, 6.6%), and forearm (only one patient, 3.3%). There was a significantly higher percentage of osteoporosis among RA males than females and the difference was statistically significant (P = 0.009). Osteoporosis was more common in patients treated with corticosteroids and disease modifying antirheumatic drugs (DMARDs) than in patients treated with only nonsteroidal anti-inflammatory drugs (P = 0.004). Higher disease activity (DAS-28) was found in RA patients with osteoporosis compared to RA patients with normal BMD or osteopenia, but the difference was not statistically significant. Osteoporotic RA patients were found to have a higher SENS score for radiological damage than nonosteoporotic ones.
Conclusion:
BMD changes do occur in patients with early RA, and are not necessarily correlated with disease activity (DAS-28). However, a significant negative correlation was found between BMD and the score of radiological damage (SENS). Dual energy x-ray absorptiometry is an important investigation to assess BMD in early RA patients.
Keywords: BMD, recent onset, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that causes chronic inflammation of the synovium with subsequent destruction and deformity of the joints. The etiology of RA remains unclear, but it is known to be associated with genetic and environmental factors.1 The prevalence of RA is approximately 0.8% (0.3%–2.1%).2
Osteoporosis is more common in patients with RA than in the general population. The prevalence of concurrent osteoporosis is 50%. Osteoporosis can cause pain and loss of height, and increases the risk of fractures after falling.3 The chronic synovial inflammation in RA can promote osteoclastogenesis, leading directly to both focal and generalized bone loss and increased risk of fractures. In addition, many indirect factors associated with inflammatory arthritis contribute to the risk of osteoporosis. These include immobility, weight loss, and use of medications known to promote bone loss, such as glucocorticoids.4
The International Society for Clinical Densitometry (ISCD) and National Osteoporosis Foundation (NOF) has recommended dual-energy x-ray absorptiometry (DEXA) testing for all adults RA patients, as well as women over 65 years old, those who sustain fractures after the age of 50 years or suffer a fragility fracture, those on chronic glucocorticoids therapy, and anyone at high risk of low bone mass, bone loss, or fracture.5 The aim of this study was to evaluate changes in bone mineral density (BMD) in patients with recent-onset RA (less than 12 months) and its correlation, if any, with RA disease activity and radiological joint damage scores.
Methods
Study population
This was a cross-sectional case control study that included 30 patients with recent onset RA (<12 months) and 20 healthy age and sex matched volunteers as controls. All patients fulfilled the American College of Rheumatology/European League Against Rheumatism EULAR/ACR criteria for RA.6 They were either inpatients in the Internal Medicine and Rheumatology Department or were attending the rheumatology outpatient clinic for regular follow-up at Ain Shams University Hospital. Informed consent was obtained from participants.
All participants underwent full history-taking and a thorough physical examination, including a detailed musculoskeletal assessment. Using the modified DAS-28 score to evaluate RA disease activity, a score ≤ 2.6 was considered to be disease remission, 2.6–3.2 as low disease activity, 3.3–4.9 as moderate disease activity, and >5.1 as severe disease activity.7 We determined the DAS cutoff value according to the median DAS score for our patients. Functional class assessment was done using a validated Arabic version of the health assessment questionnaire (HAQ).8
Laboratory investigations were performed, including a full blood count (CBC), erythrocyte sedimentation rate (ESR, mm/hour) in the first hour, C-reactive protein (CRP, mg/dL), and complete urine analysis, with full blood chemistry, including renal and liver function tests. Rheumatoid factor (RF IgM, U/L) was measured using the Biotec RA factor latex agglutination slide for qualitative determination of RF in serum.
All RA patients underwent plain X-ray films on the hands, feet and wrists joints, with calculation of radiological damage using SENS. The x-ray films were examined by an expert radiologist. The SENS cutoff value was determined according to the median SENS score for the RA patients.9
BMD was measured for all RA patients at the Radiodiagnosis Department using DEXA at the proximal femur, lumbar spine (L1–L4), and distal radius. A trained technician performed all scans using the same Lunar DEXA machine. The threshold for establishing a diagnosis of osteoporosis was based on the World Health Organization (WHO) definition, ie, BMD ≥2.5 standard deviations below the young adult mean (or T score ≤ −2.5). Osteopenia was defined as BMD ≤ −1.0 SD and > −2.5 SD from this mean, with low bone mass including all participants with osteopenia or osteoporosis.10
Statistical analysis
Analysis of data was done using the Statistical Program for Social Sciences version 12 (SPSS Inc, Chicago, IL). Values are expressed as mean ± standard deviation differences in values between the two groups and were analyzed using the unpaired t-test for numeric variables. The Chi-square test was used to analyze categorical variables. Correlations were established using the Spearman correlation test (r). The Mann–Whitney test was used for nonparametric data. The Kruskal-Wallis test (F) was used to compare more than two groups with regard to quantitative variables for nonparametric data. The difference was considered nonsignificant if P > 0.05, significant if P < 0.05, and highly significant if P < 0.001.
Results
Demographic data, DAS-28 score, HAQ score, SENS score, and ESR for the study subjects are summarized in Table 1. The 30 patients with RA (group 1) comprised 20 females and 10 males, aged 23–54 (35.7 ± 7.6) years with a mean disease duration of 8.6 ± 3.6 months. The 20 controls (group 2) comprised 17 (85%) females and three (15%) males aged 23–50 (mean 35.7 ± 7) years. Six (20%) patients in group 1 were smokers and two (10%) people in group 2 were smokers. Seventeen patients (56.6%) received non steroidal anti-inflammatory drugs (NSAIDs) alone and 13 (43.3%) received steroids with disease-modifying antirheumatic drugs (DMARDS), with 12 receiving methotrexate and one receiving leflunamide.
Table 1.
Demographic, clinical, and laboratory data in 30 patients with rheumatoid arthritis versus controls.
| Variables | RA cases (n = 30) | Controls (n = 20) | P | Sig | |
|---|---|---|---|---|---|
| Age (years) mean ± SD | 35.7 ± 7.6 (23–54) | 35.7 ± 7 | >0.05 | NS | |
| Gender | Male | 10/30 (33.3%) | 3 (15%) | >0.05 | NS |
| Female | 20/30 (66.7%) | 17 (85%) | |||
| Smoking | No | 24/30 (80%) | 18 (90%) | >0.05 | NS |
| Yes | 6/30 (20%) | 2 (10%) | |||
| Disease duration (mean ± SD) and range (months) | 8.6 ± 3.6 (2–12) | ||||
| Drug intake | NSAIDS only | 17/30 (56.6) | |||
| Combination of cortisone and DMARDS | 13/30 (43.3%) | ||||
| DAS-28 | Remission (≤2.4) | 5/30 (16.6%) | |||
| Mild disease activity (2.5–3.6) | 1/30 (3.3%) | ||||
| Moderate disease activity (3.7–5.5) | 17/30 (56.6%) | ||||
| Severe disease activity (>5.5) | 7/30 (23.3%) | ||||
| HAQ | 0–1 (mild disability) | 24/30 (80%) | |||
| >1–2 (moderate disability) | 5/30 (16.7%) | ||||
| >2–3 (severe disability) | 1/30 (3.3%) | ||||
| SENS score (mean ± SD) | 11.7 ± 9.7 (0–31.4) | ||||
| ESR mm/hour (mean ± SD) | 56.5 ± 31 (10–120) | 12 ± 4 | <0.001 | HS | |
Abbreviations: DAS-28, Disease Activity Scale-28; HAQ, Health Assessment Questionnaire; SENS, simple erosion narrowing score; ESR, erythrocyte sedimentation rate; DMARDS, disease-modifying antirheumatic drugs; NS, not statistically significant; HS, highly statistically significant; Sig, statistical significance; RA, rheumatoid arthritis.
Using a modified DAS-28, it was found that 5/30 RA patients (16.6%) were in remission, one patient (3.3%) was in a low disease activity, 17/30 patients (56.6%) were in a moderate disease activity, and 7/30 patients (23.3%) were in a high disease activity (Table 1). HAQ scores showed that 24/30 (80%) of the RA patients had mild functional disability (score 0–1), 5/30 (16.7%) had moderate disability (score 1–2), and one patient (3.3%) had severe disability (score 2–3, Table 1).
Radiological assessment of joint damage in RA patients showed a SENS score of 0–31.4 (11.7 ± 9.7), Table 1. On DEXA scanning, 15 (50%) RA patients had osteopenia and four (13.3%) had osteoporosis at the lumbar spine. In the control group, two (10%) had osteopenia at the lumbar spine, and none had osteoporosis. Fisher’s Exact test showed that RA patients had a significantly higher frequency of osteoporosis and osteopenia than controls (P < 0.05, Table 2).
Table 2.
Comparison of bone mineral density at three anatomical sites between patients with rheumatoid arthritis and controls.
| t score |
RA patients (n = 30) |
Controls (n = 20) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal BMD (n = 11) | Osteopenia (n = 15) | Osteoporosis (n = 4) | Normal BMD (n = 18) | Osteopenia (n = 2) | Osteoporosis (n = 0) | |||||||
| Lumbar spine (L1–L4) | 13/30 | 43.3% | 13/30 | 43.3% | 4/30 | 13.3% | 18/20 | 90% | 2/20 | 10% | 0 | 0% |
| Left radius | 22/30 | 73.3% | 7/30 | 23.3% | 1/30 | 3.3% | 20/20 | 100% | 0 | 0% | 0 | 0% |
| Left femur | 22/30 | 73.3% | 6/30 | 20% | 2/30 | 6.6% | 20/20 | 100% | 0 | 0% | 0 | 0% |
Notes: BMD: normal (t score > −1), osteopenia (t score ≤ −1.0 > −2.5), osteoporosis (t score ≤ −2.5). 50% of RA patients were found to have osteopenia while only 10% of controls were osteopenic (P < 0.05).
Abbreviations: BMD, bone mineral density; RA, rheumatoid arthritis.
Comparison of risk factors for osteoporosis between patients and controls revealed no statistically significant (P > 0.05) regarding age, gender, and smoking. However, routine laboratory tests showed a highly statistically significant difference in ESR (P < 0.001, Table 1) between the groups. Other routine laboratory tests, including kidney and liver function tests, and complete blood count did not show any statistically significant differences.
Osteoporosis was significantly more common in males than in females (P = 0.009) at all sites, ie, lumbar spine, femur, and forearm (P < 0.001). Furthermore, osteoporosis was significantly more common in patients treated with corticosteroids and DMARDs compared with those treated with NSAIDs alone (P = 0.004) and this was mainly in the t score for the lumbar spine and femur (P < 0.05). Osteoporosis was also more common in RA patients who smoked (2/6 [33.3%]) compared with those who did not smoke (2/24 [8.3%]) but the difference was not statistically significant (P = 0.2, Table 3).
Table 3.
Comparison of BMD between patients with rheumatoid arthritis having low versus high DAS score, HAQ, SENS, CRP.
| Normal BMD (n = 11) | Osteopenia (n = 15) | Osteoporosis (n = 4) | χ2/F | P | ||
|---|---|---|---|---|---|---|
| Age (mean + SD), years | 33.3 ± 6.4 | 36.3 ± 6.0 | 40.0 ± 14.6 | 1.2 | 0.3 | |
| NS | ||||||
| Disease duration (mean + SD), years | 8.5 ± 3.6 | 8.2 ± 4.0 | 10.2 ± 1.7 | 0.4 | 0.6 | |
| NS | ||||||
| Gender | Female (n = 20) | 9 (45%) | 11 (55%) | 0 | 9.4 | 0.009* |
| Males (n = 10) | 2 (20%) | 4 (40%) | 4 (40%) | HS | ||
| Drug intake | NSAIDS only (n = 17) | 7 (41.2%) | 10 (58.8%) | 0 (0%) | 11.1 | 0.004 |
| Corticosteroids and DMARDs (n = 13) | 4 (30.8%) | 5 (38.5%) | 4 (30.8%) | HS | ||
| Smoking | Smokers (n = 6) | 1 (16.7%) | 3 (50%) | 2 (33.3%) | 3.0 | 0.2 |
| Nonsmoker (n = 24) | 10 (41.7%) | 12 (50%) | 2 (8.3%) | NS | ||
| DAS-28 | Low DAS (n = 15) | 6 (54.5%) | 8 (53.3%) | 1 (25%) | 0.6 | 0.4 |
| High DAS (n = 15) | 5 (45.5%) | 7 (46.7%) | 3 (75%) | NS | ||
| HAQ | 0–1 (mild disability) | 4 (36.4%) | 5 (33.3%) | 0 | 10.5 | 0.02 S |
| >1–2 (moderate disability) | 7 (63.3%) | 7 (46.7%) | 1 (25%) | |||
| >2–3 (severe disability) | 0 | 3 (20%) | 3 (75%) | |||
| SENS score | Low SENS (n = 13) | 7 (63.6%) | 6 (40%) | 0 | 4.6 | 0.03 S |
| High SENS (n = 17) | 4 (36.4%) | 9 (60%) | 4 100% | |||
| CRP (mg/dL) | CRP-positive (n = 19) | 8 (73%) | 7 (47%) | 4 100% | 3.5 | <0.05 S |
| CRP-negative (n = 11) | 3 (27%) | 8 (53%) | 0 | |||
Note: DAS-28 score cutoff value was 4.35 and SENS score cutoff value was 11.6%.
Abbreviations: BMD, bone mineral density; DAS-28, Disease Activity Scale-28; HAQ, Health Assessment Questionnaire; SENS, simple erosion narrowing score; CRP, C-reactive protein; DMARDS, disease-modifying antirheumatic drugs; NS, not statistically significant; HS, highly statistically significant; S, statistically significant; RA, rheumatoid arthritis; NSAIDs, nonsteroidal anti-inflammatory drugs; SD, standard deviation.
By HAQ score, 3/4 (75%) RA patients with osteoporosis had severe disability (HAQ 3), while 7/15 (46.7%) patients with osteopenia had moderate disability (HAQ 2). Furthermore, 11/30 RA patients had normal BMD, of whom four with mild disability (HAQ 1), seven had moderate disability, and none had severe disability. This indicates that there was significant disability (increased HAQ score) consistent with lower BMD in subgroups of RA patients according to HAQ score and the difference was statistically significant (P = 0.02, Table 3). More RA patients with osteoporosis had an elevated DAS-28 score than those with normal BMD or osteopenia, but the difference was not statistically significant (P = 0.4, Table 3). Significantly more RA patients with osteoporosis had elevated SENS than those having normal BMD or osteopenia (P = 0.03, Table 3). Higher SENS were found in RA patients with high ESR than in those with low ESR, but the difference was not statistically significant (P = 0.7).
Four out of 19 CRP-positive RA patients had osteoporosis and no none of the CRP-negative RA patients had osteoporosis. Seven of the 19 CRP-positive patients were found to be osteopenic, ie, BMD was lower in CRP-positive RA patients than in CRP-negative patients, and the difference was statistically significant (P < 0.05, Table 3). However, on comparing RF-positive and CRP-negative RA patients with regard to BMD (t and z scores at different sites), there was no statistically significant difference (P > 0.05).
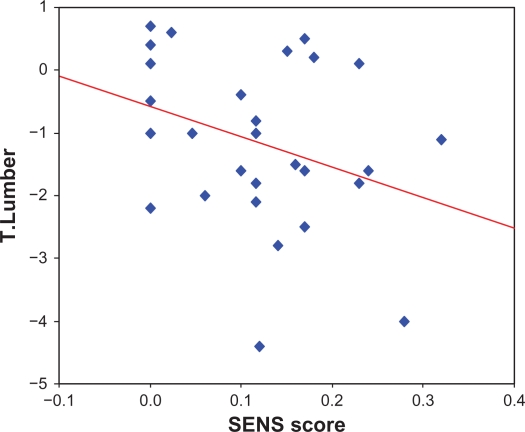
There was no statistically significant correlation was found between disease duration, ESR, DAS-score and BMD at any anatomical site (P > 0.05). However, there was a statistically significant negative correlation between SENS score and BMD at the lumbar spine (r = −0.054, P < 0.01). Moreover, we found a significant negative correlation between BMD and HAQ score (r = −0.425 and P = 0.019, Table 4 and Fig. 2).
Table 4.
Correlations between t scores, z scores, and various clinical parameters in patients with rheumatoid arthritis.
|
BMD t and z scores |
ESR |
Disease duration |
DAS score 28 |
SENS score |
HAQ |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| t score lumbar spine | −0.09 | >0.05 | −0.18 | >0.05 | −0.14 | >0.05 | −0.54 | >0.001 | −0.425 | >0.05 |
| t score radius | −0.007 | >0.05 | −0.15 | >0.05 | −0.07 | >0.05 | −0.18 | >0.05 | −0.251 | >0.05 |
| t score femur | −0.12 | >0.05 | −0.10 | >0.05 | −0.20 | >0.05 | −0.13 | >0.05 | −0.360 | 0.05 |
| z score lumbar spine | 0.11 | >0.05 | 0.011 | >0.05 | −0.08 | >0.05 | 0.15 | >0.05 | – | – |
| z score radius | 0.17 | >0.05 | 0.07 | >0.05 | −0.10 | >0.05 | −0.13 | >0.05 | – | – |
| z score femur | −0.01 | >0.05 | −0.02 | >0.05 | −0.24 | >0.05 | −0.19 | >0.05 | – | – |
Abbreviations: BMD, bone mineral density; DAS-28, Disease Activity Scale-28; HAQ, Health Assessment Questionnaire; SENS, simple erosion narrowing score; ESR, erythrocyte sedimentation rate.
Figure 2.
Significant negative correlation between t score at lumbar spine and SENS in patients with rheumatoid arthritis (r = −0.54, P < 0.001).
Discussion
Osteoporosis and fragility-related fractures are one of the most common complications seen in patients with RA and dramatically affect quality of life.11 The present study was designed to evaluate BMD changes in patients with recent-onset RA, as well as the effect of inflammation, mobility, and drugs (steroids and DMARDS) on these changes.
Our results support the association between early RA and osteoporosis. We found that 13.3% of our patients with early RA had osteoporosis, 50% had osteopenia, and 36.7% had normal BMD, while none of the control subjects had osteoporosis. Only two controls (10%) had osteopenia, and 18 control subjects (90%) had normal BMD. These findings are in agreement with those of Brand et al12 who reported that patients with RA have a higher risk of low BMD than normal age- and gender-matched populations. Similarly, a study reported by Kim et al13 showed an increased risk of osteoporotic fractures in RA patients in all age groups, regardless of gender, and at various anatomical sites compared with individuals without RA. In contrast, Curtis et al14 found that the proportion of their RA patients meeting t score criteria for osteoporosis (t score ≤ −2.5 at either the lumbar spine or femoral neck) was only 4%, and Yoon et al15 reported that 52% of their patients with early-onset RA had osteoporosis and 39% were classified as having osteopenia.
In the present study, we found that the most common site for osteoporosis in our RA patients was the lumbar spine (four patients [13.3%]), followed by the femur (two patients [6.6%]), with involvement of the forearm in only one patient (3.3%). We also found that only two of 20 (10%) control subjects had osteopenia which affected the lumbar spine. These findings are consistent with those of Güler-Yüksel et al16 who reported that the most common site for osteoporosis in patients with early RA was the spine (9%), followed by the total hip (4%), with 11% of cases occurring at either the spine or the hip. The proportion of patients with decreased BMD was 19% for the spine, 14% for the hip, and 25% for either the spine or the hip. Yoon et al15 showed that osteoporosis was slightly more prevalent at the lumbar spine than at the femur in women with RA who were younger than 60 years of age, but the difference was not statistically significant.
We found that 22/30 (73.3%) of our RA patients had normal BMD at the femoral neck, six (20%) had osteopenia, and two (6.6%) had osteoporosis. These findings are similar to that reported by Curtis et al14 who found that the majority of their RA patients had normal t scores at the femoral neck (t score > −1.0) while the remainder had femoral neck t scores < −1.0 but > −2.0, and none had osteoporosis. In our RA cohort, 43.3% had normal BMD, 43.3% had osteopenia, and 13.3% had osteoporosis at the lumber spine. Zhang et al17 found that 38.6% of their subjects had either osteopenia or osteoporosis at the lumbar spine, and 44.9% had either osteopenia or osteoporosis at the femoral neck.
Interestingly, in the present study, osteoporosis was more common in RA men (40%) than in women (0%) with RA, and this difference was highly statistically significant (P < 0.01). This might be due to the natural protective effect of estrogen hormone against the occurrence of osteoporosis Meanwhile, osteopenia were significantly (P < 0.01) more common in female RA patients (55%) than in males (40%). Güler-Yüksel et al16 found similar results, reporting reduced BMD in men with RA than in their female counterparts. However, the study reported by Yoon et al15 showed that when patients with RA were grouped by gender, the prevalence of osteoporosis in women was 57%, whereas men had a prevalence of only 28%.
Furthermore, our RA patients with osteoporosis were older and had a longer disease duration than those without osteoporosis, but the difference was not statistically significant (P > 0.05). These findings are similar to those of Sinigaglia et al18 who found that RA patients with spine or femoral osteoporosis had a significantly longer disease duration. Our results are also consistent with those of Güler-Yüksel et al16 who reported that RA patients with early, active, erosive disease and a positive rheumatoid factor had more aggressive joint disease and decreased BMD.
In the present study, we found a significantly lower BMD in RA patients who received steroids and DMARDS compared with those who received NSAIDS alone (P = 0.004). This might be explained by the fact that the introduction of NSAIDS as lone treatment for RA occurs very early, even before definitive diagnosis of the disease, ie, at a time when BMD would still be normal. However, we would expect normalization of BMD after controlling disease activity in patients with known RA who are treated with corticosteroids and DMARDS, given the widely recognized osteoporotic effect of corticosteroids. However, Curtis et al14 stated that there was no association between glucocorticoid use and BMD.
Additionally, we found that osteoporosis in RA patients was more common in smokers than in non-smokers, but the difference was not statistically significant (P = 0.2), and this finding was in agreement with that of Lorentzon et al19 who reported that smokers had significantly lower BMD on DEXA than nonsmokers.
We have also found that osteoporosis was significantly more common in CRP-positive than in CRP-negative RA patients (P < 0.05), but there was no significant difference in BMD between RF-positive and RF-negative patients (P > 0.05). Kinjo et al20 found that in patients with RA, reduced BMD tended to be seen with CRP > 1 mg/dL and a positive rheumatoid factor (RF) in female patients. However, none of these findings reached statistical significance. Kim et al14 also observed an increased risk of osteoporosis associated with a positive RF and elevated acute phase reactants, although this was not statistically significant. While Solomon et al,21 have found that BMD at the total hip was significantly lower in rheumatoid factor-positive women than in rheumatoid factor-negative women. However, values at the lumbar spine were almost identical for rheumatoid factor-positive and -negative women.
Regarding HAQ score, we have found that most of RA patients with osteoporosis have severe functional disability (HAQ 3) (P = 0.02). Similarly, Sinigaglia et al18 found that patients with spinal or femoral osteoporosis had a significantly higher HAQ score (P = 0.001).
In our study RA patients with osteoporosis were found to have a higher SENS, indicative of radiological damage than non osteoporotic RA patients In another way significantly lower BMD was found in RA patients with higher SENS than in those with a lower SENS (P = 0.03). These findings support the hypothesis of a common pathologic mechanism for generalized bone loss and localized radiographic joint damage in RA patients. Similar data were reported by Lodder et al22 who found significant radiological damage in RA patients with low BMD at the hip, in contrast with Güler-Yüksel et al16 who found that DAS, HAQ, and SENS scores were not correlated with BMD at the spine or hip in RA patients. A higher SENS in our RA patients was not correlated with RF status (P = 0.9). Courvoisier et al23 found that the radiographic Sharp score at 10 years was correlated significantly with baseline ESR, IgA, RF and baseline radiographic scores (including erosion, joint narrowing, and total scores).
We found a higher incidence of osteoporosis in patients with a high DAS-28 score compared with those with a low DAS-28 score, but the difference was not statistically significant (P = 0.4). This was in agreement with Brand et al,12 the DAS-28 score was not corrrelated with BMD. Similarly, Curtis et al14 reported that there were no associations of RA activity, severity, or erosions with BMD at either site on univariate or multivariate analysis.
Moreover, we found that there was no correlation between ESR and BMD at different sites (lumbar spine, r = −0.09; radius, r = −0.007; femur, r = −0.12, P > 0.05), while Cortet et al24 found that at femoral neck BMD was significantly inversely correlated with ESR, age and HAQ score. These differences might be due to different patients’ cohort as our RA patients have early RA and longer periods of follow up were needed to establish these possible correlations.
In our study, we found a statistically significant inverse correlation between SENS and BMD only- at the lumbar spine (r = −0.54, P < 0.001). This may be due to the fact that the vertebral bodies contain more trabecular bone than the hip, the trabecular bone is more metabolically active, so one might anticipate that perturbations in cytokines related to RA would affect BMD at the lumbar spine more dramatically than in the hip. In contrast, Solomon et al21 found that in RA there was a significant correlation between erosions and total hip BMD more than at the lumbar spine. Several potential explanations exist for this apparent discrepancy. It is possible that the inflammatory process underlying RA affects BMD at the hip more than at the lumbar spine. Also, total hip BMD may more closely relate to joint mobility and overall functional status than lumbar spine BMD.
Finally, we found that there was a significant positive correlation between HAQ and DAS-28 (P = 0.000) and between HAQ and SENS (P = 0.01). These were in accordance with Courvoisier et al23 who found that HAQ scores were strongly associated with disease activity parameters, as DAS-28. Only erosion score was weakly but significantly associated with HAQ score. Van der Heijde et al25 reported that HAQ scores tended to increase with increasing radiographic progression.
We concluded that BMD changes do occur early in patients with RA, they are not necessarly correlated with clinical disease activity, as it can occur in patients with mild disease activity. There was a significant correlation between radiographic damage and reduced bone mass. DEXA is an important diagnostic tool for detecting osteoporosis in patients with early RA, serving as a promising supplement to x-ray scoring methods.
Figure 1.
Comparison of bone mineral density between patients with rheumatoid arthritis and controls.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Chung SJ, Kwon YJ, Park MC, Park YB, Lee SK. The correlation between increased serum concentrations of interleukin-6 family cytokines and disease activity in rheumatoid arthritis patients. Yonsei Med J. 2011;52:113–20. doi: 10.3349/ymj.2011.52.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkateshan SP, Sidhu S, Malhotra S, Pandhi P. Efficacy of biologicals in the treatment of rheumatoid arthritis. a meta-analysis. Pharmacology. 2009;83(1):1–9. doi: 10.1159/000165777. [DOI] [PubMed] [Google Scholar]
- 3.Wijbrandts CA, Klaasen R, Dijkgraaf MGW, Gerlag DM, van Eck-Smit BLF, Tak PP. Bone mineral density in rheumatoid arthritis patients 1 year after adalimumab therapy: Arrest of bone loss. Ann Rheum Dis. 2009;68:373–6. doi: 10.1136/ard.2008.091611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizer J, Reed G, Onofrei A, Harisson MJ. Predictors of bone density testing in patients with rheumatoid arthritis. Rheumatol Int. 2009;29:897–905. doi: 10.1007/s00296-008-0804-4. [DOI] [PubMed] [Google Scholar]
- 5.Leib ES, Lewiecki EM, Binkley N, Hamdy RC. Official positions of the International Society for Clinical Densitometry. J Clin Densitom. 2004;7:1–6. doi: 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 6.Aletaha D, Neogi T, Silimana AJ, et al. 2010 Rheumatoid Arthritis classification criteria. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 7.Aletaha D, Smolen JS. The Simplified Disease Activity Index (SDAI) and the clinical disease activity index (CDAI): A review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8. [PubMed] [Google Scholar]
- 8.El Meidany Y, El Gaafary M, Ahmed I. Cross-cultural adaptation and validation of an Arabic Health Assessment Questionnaire for use in rheumatoid arthritis patients. Joint Bone Spine. 2003;70:195–202. doi: 10.1016/s1297-319x(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 9.Fries JF, Bloch DA, Sharp JT, et al. Assessment of radiographic progression in rheumatoid arthritis. Arthritis Rheum. 1986;29:1–9. doi: 10.1002/art.1780290101. [DOI] [PubMed] [Google Scholar]
- 10.Richards JS, Peng J, Amdur RL, et al. Dual energy X-ray absorptiometry and evaluation of osteoporosis self assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12:434–40. doi: 10.1016/j.jocd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Ranganathan P. Genetics of bone loss in rheumatoid arthritis role of vitamin D receptor polymorphisms. Rheumatology (Oxford) 2009;48:342–6. doi: 10.1093/rheumatology/ken473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand C, Lowe A, Hall S. The utility of clinical decision tools for diagnosing osteoporosis in postmenopausal women with rheumatoid arthritis. BMC Musculoskelet Disord. 2008;9:13. doi: 10.1186/1471-2474-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SY, Schneeweiss S, Liu J, et al. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R154. doi: 10.1186/ar3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis JR, Arora T, Donaldson M, et al. Skeletal health among African Americans with recent onset rheumatoid arthritis. Arthritis Rheum. 2009;61:1379–86. doi: 10.1002/art.24841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon J, Kwon SR, Lim MJ, et al. A comparison of three different guidelines for osteoporosis treatment in patients with rheumatoid arthritis in Korea. Korean J Intern Med. 2010;25:436–46. doi: 10.3904/kjim.2010.25.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Güler-Yüksel M, Bijsterbosch J, Goekoop-Ruiterman YP, et al. Bone mineral density in patients with recently diagnosed, active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1508–12. doi: 10.1136/ard.2007.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Redden DT, McGwin G, Jr, et al. Generalized bone loss as a predictor of three-year radiographic damage in African American patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2010;62:2219–26. doi: 10.1002/art.27510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinigaglia L, Nervetti A, Mela Q, et al. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol. 2000;27:2582–9. [PubMed] [Google Scholar]
- 19.Lorentzon M, Mellström D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab. 2007;92:497–503. doi: 10.1210/jc.2006-1294. [DOI] [PubMed] [Google Scholar]
- 20.Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in older adult patients with rheumatoid arthritis: An analysis of NHANES III. J Rheumatol. 2007;34:1971–5. [PubMed] [Google Scholar]
- 21.Solomon DH, Finkelstein JS, Shadick N, et al. The relationship between focal erosions and generalized osteoporosis in postmenopausal women with rheumatoid arthritis: The Osteoporosis in Rheumatoid Arthritis (OPiRA) Cohort Study. Arthritis Rheum. 2009;60:1624–31. doi: 10.1002/art.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodder MC, de Jong Z, Kostense PJ, et al. Bone mineral density in patients with rheumatoid arthritis: Relation between disease severity and low bone mineral density. Ann Rheum Dis. 2004;63:1576–80. doi: 10.1136/ard.2003.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courvoisier N, Dougados M, Cantagrel A, et al. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: A prospective study. Arthritis Res Ther. 2008;10:R106. doi: 10.1186/ar2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortet B, Flipo RM, Blanckaert F, Duquesnoy B, Marchandise X, Delcambre B. Evaluation of bone mineral density in patients with rheumatoid arthritis. Influence of disease activity and glucocorticoid therapy. Rev Rhum Engl Ed. 1997;64:451–8. [PubMed] [Google Scholar]
- 25.Van der Heijde D, Landewé R, van Vollenhoven R, Fatenejad S, Klareskog L. Level of radiographic damage and radiographic progression are determinants of physical function: A longitudinal analysis of the TEMPO trial. Ann Rheum Dis. 2008;67:1267–70. doi: 10.1136/ard.2007.081331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnett FC, Edworthy SM, Bloch DA. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 27.Prevoo ML, Van’T Hof MA, Kuper HH, Van Leeuwen MA, Van de Putte LB, Van Riel PL. Modified disease activity scores that include twenty-eight joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 28.Tuna H, Birtane M, Ekuklu G, Cermik F, Tuna F, Yonsei KS. Does quantitative tibial ultrasound predict low bone mineral density defined by dual energy x-ray absorptiometry? Med J. 2008;49:436–42. doi: 10.3349/ymj.2008.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Heijde D. How to read radiography according to the sharp Van der Heijde method. J Rheumatol. 1999;26:743–5. [PubMed] [Google Scholar]