Abstract
Introduction:
Pulmonary arterial hypertension (PAH) is a serious and often fatal complication of systemic lupus erythematosus (SLE). Because the diagnosis of PAH often is made years after symptom onset, early diagnostic strategies are essential. Doppler echocardiography currently is considered the noninvasive screening test of choice for evaluating pulmonary hypertension.
Aim:
Screening for asymptomatic pulmonary hypertension in systemic lupus erythematosus patients using Doppler echocardiography, and correlating it with inflammatory parameters of the disease.
Patients and methods:
Doppler echocardiography was performed in 74 patients with systemic lupus erythematosus over one year (66 adult and 8 juvenile), adult SLE included 57 patients with adult-onset and 9 patients with childhood-onset. Pulmonary hypertension was diagnosed if the peak systolic pressure gradient at the tricuspid valve was more than 30 mmHg. All patients were subjected to full history taking, rheumatological examination, laboratory studies and chest x-ray.
Results:
In seventy four SLE patients, the pulmonary hypertension was detected in 8 patients (10.8%), 7 adult-onset SLE patients (aged from 19 to 30 years) and 1 juvenile SLE (aged 12 years). The range of pulmonary artery systolic pressure was 34–61.2 mmHg (43.19 ± 9.28). No significant differences between patients with and those without pulmonary hypertension as regard clinical features. Significantly higher frequencies of rheumatoid factor and anti-cardiolipin antibodies were found in patients with pulmonary hypertension versus those without (P = 0.02, P = 0.008 respectively). Positive rheumatoid factor and ACL were significantly associated with occurrence of PAH in SLE (P = 0.007, P = 0.006 respectively). No significant correlations were found between pulmonary artery pressure, disease duration, SLE Disease Activity Index (SLEDAI), ESR, and anti-ds DNA.
Conclusion:
Patients with SLE have an increased risk of pulmonary arterial hypertension. Echocardiography should be used as a screening tool in patients at high risk for development of pulmonary hypertension. Positive anti-cardiolipin antibodies and rheumatoid factor were significant predictors of pulmonary hypertension in our study.
Keywords: systemic lupus erythematosus, pulmonary hypertension, echocardiography, screening
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown origin characterized by inflammation of multiple organs (kidneys, brain, heart, liver, lungs, joints, muscles, skin, etc.).1
The kidney has usually been considered the most frequently affected organ in systemic lupus erythematosus, the heart and the pulmonary vessels, however, may also be seriously involved.2
Pulmonary involvement is relatively frequent in adult patients rather than children.3 Pulmonary hypertension is the most severe forms of lupus associated pulmonary involvement, although they occur infrequently in children with SLE.4
Although several mechanisms are involved in pathogenesis of pulmonary hypertension in SLE, the real causes are yet unknown. The hypothesis of pulmonary vasculitis, with deposits of immunocomplexes and complements on the pulmonary artery walls, thromboembolic blockage in pulmonary vessels, possibly related to antibodies (anticardiolipin antibody and lupus anticoagulant), and vasospasms, are suggested by a greater frequency of Raynaud’s phenomenon in these patients.5
The prevalence of pulmonary arterial hypertension (PAH) in patients with lupus is largely unknown, but has been reported to approximate 6%–15% in adult patients, in whom it is most commonly associated with Raynaud’s phenomenon.6
There have been very few studies addressing the prevalence of PAH in childhood-onset SLE, which is reported to approximate 4%–8% using transthoracic echocardiography.7
The determination of the real prevalence and magnitude of pulmonary hypertension in SLE has been difficult over the years due to the fact that the only method with sufficient sensitivity for determining pulmonary pressures and confirming the existence of pulmonary hypertension has been the catheterization of the right chambers with direct measurement of pulmonary pressures. The advent of two-dimensional Doppler echocardiography, a method thought to be of similar sensitivity as right chamber catheterization for measuring pulmonary pressure, allowed the study of a greater number of patients, with or without symptoms.5
Pulmonary hypertension is often silent, with the development of clinical symptoms often signaling the presence of advanced disease, associated with poor survival. This has prompted the need for methods of detecting PAH, including annual echocardiographic screening of asymptomatic individuals with systemic autoimmunity.8
The sensitivity and specificity of Doppler echocardiography (DE) for estimating pulmonary artery pressure are 0.79 to 1.0 and 0.68 to 0.98, respectively.9 DE can evaluate the degree of PH, prognosticating variables (RA and RV enlargement, pericardial effusion, RV ejection fraction), and other potential causes of PH (left ventricular systolic/diastolic dysfunction, valvular heart disease, and intracardiac shunting).10
The aim of this study was to screen for asymptomatic pulmonary hypertension in systemic lupus erythematosus using Doppler echocardiography, and to correlate it with inflammatory parameters of the disease.
Patients and Methods
Study population
This is a cross-sectional study conducted on SLE patients attending outpatient clinic of Rheumatology and Rehabilitation Department at Minia university Hospital, Egypt, over one year. All patients were females and met the 1982 American College of Rheumatology revised criteria for SLE11 with no overlap syndrome. None of the patients were previously treated with medication correlated to PAH. Informed consent was obtained from all patients before entering the study. Seventy four female SLE patients were recruited, 66 adults (aged from 19 to 45 years), and 8 children (aged from 11 to 15 years). All patients were asymptomatic for pulmonary hypertension.
Clinical evaluation
All the patients were subjected to a thorough history taking, general examination, and rheumatological evaluation which included the assessment of SLE Disease Activity Index (SLEDAI).12
Laboratory studies
ESR was measured using Westergren method and Rheumatoid factor was performed using Latex fixation test. Antinuclear antibody (ANA), anti-double stranded DNA antibody (anti-dsDNA), and IgG anti-cardiolipin antibodies (ACL antibodies) were assayed by immuno-fluorescence technique and ELISA. Other routine laboratory tests as complete blood picture, renal function test, urinalysis (for pyuria, hematuria, casts), and 24-hour urine for protein were done for all patients.
Chest x-ray
A-P and lateral views were done for all patients to exclude evidence of parenchymal lung disease.
Echocardiographic evaluation
Two-dimensional (2D), M-mode, color flow mapping and spectral Doppler echocardiography were done using a Vivid 3 Expert GE echocardiography machine (GE vingmed ultrasound, Horten, Norway, model vivid 3 Expert), with a 5 MHz transducer for children patients. Patients were examined in the parasternal long and short axis, and four chamber projections. Special attention was given to the detection of tricuspid regurgitation.
To estimate pulmonary artery systolic pressure (PASP), the maximum transtricuspid pressure gradient was calculated using the simplified Bernoulli equation.13,14 The estimate of right atrial pressure, 10 mmHg, was added to the pressure gradient to calculate the right ventricular systolic pressure, which was considered equal to the PASP in the absence of right ventricular outflow obstruction. The Doppler recordings were reviewed by at least one echocardiographer who had no previous knowledge of the patient. Right ventricular dilatation was defined as the ventricular area greater than the left ventricular area obtained by planimetry from the apical four-chamber view. Right ventricular dilatation with flattening of the ventricular septum was considered indicative of right ventricular overload. Pulmonary hypertension was diagnosed if the peak systolic pressure gradient at the tricuspid valve was more than 30 mmHg.
The severity of PH is classified as: mild (PASP < 45 mmHg), moderate (PASP from 45 to 59 mmHg) or severe (PASP > 60 mmHg).13
Accordingly, patients enrolled in our study were divided into two groups:
Group I: Patients with PAH.
Group II: Patients without PAH.
Statistical analysis
Statistical analysis was undertaken using an SPSS 11 database. Quantitative variables such as pulmonary function tests were represented by mean ± SD. Qualitative variables, such as symptoms, age, sex were represented by numbers and percentages. Student’s t-test (t) was used when comparing the means of quantitative variables. Chi-squared test (x2) was used to compare frequencies (numbers and percentages) for qualitative variables. Correlations between variables were assessed with Pearson test. Odds ratios (OR) and 95% confidence intervals (95% CIs) were calculated. Linear regression analysis was used to examine the predictors contributing to PAH in SLE. Differences were considered significant at P < 0.05.
Results
Demographic data of all SLE patients are shown in (Table 1)
Table 1.
Demographic data of all SLE patients.
|
SLE patients (n = 74) |
|||
|---|---|---|---|
|
Adult SLE (n = 66) |
Juvenile SLE (n = 8) | ||
| Adult-onset SLE (n = 57) | Childhood-onset SLE (n = 9) | ||
| Age (yr) | |||
| Range | 19–45 | 19–23 | 11–15 |
| Mean ± SD | 28.14 ± 7.10 | 20.78 ± 1.39 | 13.56 ± 1.64 |
| Duration of the disease (yr) | |||
| Range | 0.3–16 | 3–12 | 0.1–4 |
| Mean ± SD | 4.21 ± 3.46 | 5.89 ± 2.67 | 1.61 ± 1.74 |
| Age at onset of the disease (yr) | |||
| Range | 17–37.5 | 11–16 | 10–14.9 |
| Mean ± SD | 23.93 ± 5.51 | 14.89 ± 1.83 | 11.95 ± 2.03 |
Comparison of patients with and without pulmonary arterial hypertension
According to Doppler echocardiographic findings, PAH was detected in 8 patients (10.8%) out of seventy four SLE patients. PASP ranged from 34 mmHg to 61.2 mmHg (43.19 ± 9.28). SLE patients were classified into two groups:
Group I: included 8 patients who had PAH: 7 adult-onset SLE (aged from 19 to 30 yr) and 1 juvenile SLE (aged 12 years).
Group II: included: 66 patients who had no PAH (50 adult-onset SLE, 9 childhood-onset SLE and 7 juvenile SLE).
Comparison of patients with and without PAH to find if there’s any association of PAH with inflammatory, SLE-related or other risk factors for pulmonary arterial hypertension had showed that, no significant differences between the two groups regarding, demographic and clinical features (Table 2), while laboratory parameters had significantly higher frequencies of rheumatoid factor and anti-cardiolipin antibodies in patients with PAH (P = 0.02, P = 0.008 respectively) as in (Table 3).
Table 2.
Comparison of demographic and clinical features in SLE patients with pulmonary arterial hypertension versus those without.
| SLE patients with PAH (N = 8, 10.8%) | SLE patients without PAH (N = 66, 89.2%) | P value# | |
|---|---|---|---|
| Age (yr) | |||
| Range | 12–30 | 11–45 | 0.4 |
| Mean ± SD | 23.50 ± 5.95 | 25.93 ± 8.14 | |
| Age at onset of the disease (yr) | |||
| Range | 11.7–29 | 10–37.5 | 0.9 |
| Mean ± SD | 21.53 ± 5.97 | 21.54 ± 6.76 | |
| Duration of the disease (yr) | |||
| Range | 0.3–6 | 0.1–16 | 0.05 |
| Mean ± SD | 1.98 ± 1.86 | 4.39 ± 3.42 | |
| Fever | 2 (25%) | 25 (37.9%) | 0.7 |
| Malar rash | 7 (87.5%) | 54 (81.8%) | 1 |
| Photosensitivity | 6 (75%) | 51 (77.3%) | 1 |
| Alopecia | 6 (75%) | 42 (63.6%) | 0.7 |
| Oral ulcers | 5 (62.5%) | 48 (72.7%) | 0.7 |
| Raynaud’s phenomenon | 5 (62.5%) | 18 (27.3%) | 0.1 |
| Arthralgia | 7 (87.5%) | 63 (95.5%) | 0.4 |
| Arthritis | 5 (62.5%) | 51 (77.3%) | 0.4 |
| Myalgia | 5 (62.5%) | 46 (69.7%) | 0.7 |
| Pleurisy | 0 | 11 (16.7%) | 0.6 |
| Renal affection | |||
| Proteinuria | 2 (25%) | 34 (51.5%) | 0.1 |
| Casts | 1 (12.5%) | 24 (36.4%) | 0.1 |
| Eye affection | 0 | 1 | 1 |
| SLEDAI | |||
| Range | 6–24 | 0–39 | 0.2 |
| Mean ± SD | 12.50 ± 5.55 | 17.18 ± 8.93 |
Note:
Chi-squared or Student’s t-test.
Abbreviations: PAH, pulmonary arterial hypertension; SLEDAI, SLE disease activity index.
Table 3.
Comparison of laboratory features in SLE patients with pulmonary arterial hypertension versus those without.
| SLE patients with PAH (N = 8, 10.8%) | SLE patients without PAH (N = 66, 89.2%) | P value# | |
|---|---|---|---|
| Hb (gm %) | |||
| Range | 8.3–12.5 | 6.0–13.4 | 0.2 |
| Mean ± SD | 10.91 ± 1.26 | 10.22 ± 1.55 | |
| WBC (mm3) | |||
| Range | 3.6–5.8 × 103 | 2.2–10.7 × 103 | 0.6 |
| Mean ± SD | 4.81 × 103 ± 0.78 | 5.18 × 103 ± 2.13 | |
| Platelets (mm3) | |||
| Range | 227–398 × 103 | 74–467 × 103 | 0.6 |
| Mean ± SD | 284.25 × 103 ± 63.34 × 103 | 270.64 × 103 ± 81.23 × 103 | |
| ESR (mm/h) | |||
| Range | 34–115 | 16–143 | 0.2 |
| Mean ± SD | 86.25 ± 34.02 | 70.80 ± 31.25 | |
| +ve Rheumatoid factor | 4 (50%) | 8 (12.1%) | 0.02* |
| +ve ANA | 8 (100%) | 64 (97%) | 1 |
| +ve Anti-ds DNA | 3 (37.5%) | 39 (59.1%) | 0.3 |
| +ve ACL | 7 (87.5%) | 24 (36.4%) | 0.008** |
| Serum urea (mg/dl) | |||
| Range | 14–126 | 10–187 | 0.4 |
| Mean ± SD | 37.25 ± 36.55 | 49.11 ± 35.73 | |
| Serum creatinine (mg/dl) | |||
| Range | 0.5–7.5 | 0.1–9.7 | 0.6 |
| Mean ± SD | 1.53 ± 2.42 | 1.18 ± 1.49 | |
| Urine examination | |||
| Proteinuria (gm/24 h) | 0–12 | 0–15.6 | 0.8 |
| 1.59 ± 4.21 | 1.39 ± 2.53 | ||
| Casts | |||
| Granular | 1 (12.5%) | 12 (18.2%) | 0.5 |
| Hyaline | 0 | 6 (9.1%) | |
| H&G | 0 | 6 (9.1%) | |
| Pyuria | 3 (37.5%) | 25 (37.9%) | 1 |
| Hematuria | 1 (12.5%) | 18 (27.3%) | 0.7 |
Notes:
Significant P < 0.05;
highly significant P < 0.01;
chi-squared or Student’s t-test.
Abbreviations: PAH, pulmonary arterial hypertension; Hb, haemoglobin; WBCs, white blood cells; ESR, erythrocyte sedimentation rate; ANA, antinuclear antibody; ACL, anti cardiolipin antibodies.
No signs of vasculitis, anti-phospholipid syndrome and/or lung affection were found in patients with PAH.
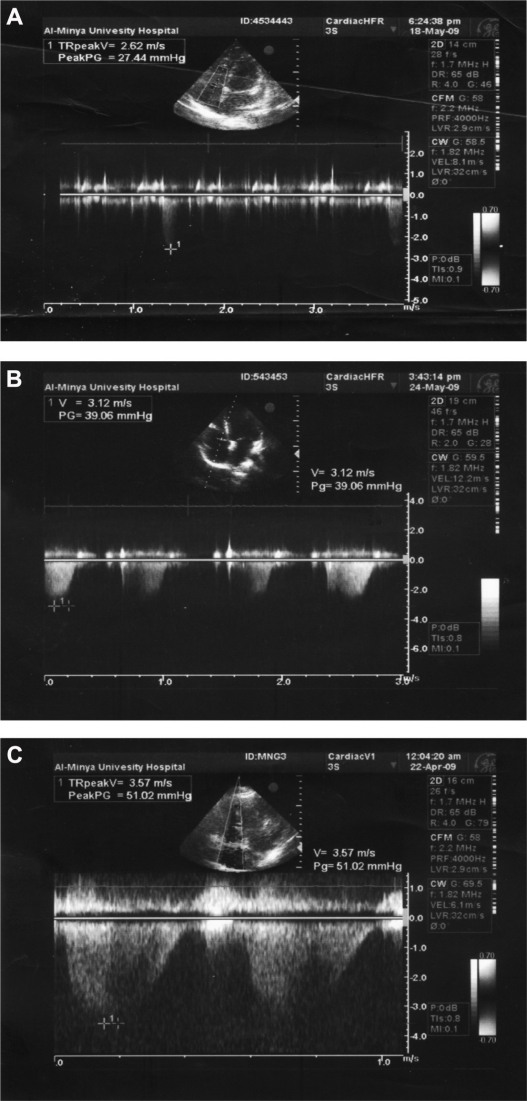
Pulmonary artery pressure was differed significantly between the two groups subset (P < 0.001). The degree of pulmonary arterial hypertension was mild in 5, moderate in 2, and severe in one patient as shown in (Table 4). Different degrees of pulmonary hypertension as found in our patients are represented in (Fig. 1A–C).
Table 4.
Comparison of ECHO features in SLE patients with pulmonary arterial hypertension versus those without.
| SLE patients with PAH (N = 8, 10.8%) | SLE patients without PAH (N = 66, 89.2%) | P value# | |
|---|---|---|---|
| Pulmonary artery systolic pressure (mmHg) | |||
| Range | 34–61.02 | 22–29 | <0.001*** |
| Mean ± SD | 43.19 ± 9.28 | 26.19 ± 1.89 | |
| Degree of Pulmonary hypertension | |||
| Mild | 5 (62.5%) | – | – |
| Moderate | 2 (25%) | – | |
| Severe | 1 (12.5%) | – | |
| Right ventricular dilatation | 1 (12.5%) | – | – |
| Valvular thickening: | 3 (37.5%) | 11 (16.7%) | 0.2 |
| – Mitral thickening | 3 (37.5%) | 9 (13.6%) | 0.1 |
| – Aortic thickening | 2 (25%) | 5 (7.6%) | 0.2 |
| Degree of tricuspid regurge | |||
| Mild | 1 (12.5%) | 18 (27.3%) | <0.001*** |
| Moderate | 5 (62.5%) | – | – |
| Severe | 2 (25%) | – | – |
| Mitral stenosis | 0 | 0 | – |
| Mitral regurgitation | 3 (37.5%) | 11 (16.7%) | 0.2 |
| Aortic stenosis | 0 | 0 | – |
| Aortic regurgitation | 0 | 0 | – |
| Mild pericardial effussion | 2 (25%) | 12 (18.2%) | 0.6 |
| Ejection fraction | |||
| Range | 58%–68% | 48%–80% | 0.6 |
| Mean ± SD | 61.75% ± 3.49% | 62.98% ± 7.05% |
Notes:
Chi-squared or Student’s t-test;
very highly significant P < 0.001.
Abbreviation: PAH, pulmonary arterial hypertension.
Figure 1.
(A) 24 years old female with SLE duration 6 years. PASP = 37.44 mmHg, mild degree. (B) 19 years old female with SLE duration 1 year. PASP = 49.06 mmHg, moderate degree. (C) 22 years old female with SLE duration 2.5 years. PASP = 61.02 mmHg, severe degree.
Odds ratio of possible risk factors for development of PAH in SLE patients are shown in (Table 5).
Table 5.
Possible risk factors that predict pulmonary arterial hypertension in SLE patients.
| Risk factors | Odds ratio | 95% CI | P value# |
|---|---|---|---|
| Renal disease | 0.28 | 0.05–1.48 | 0.06 |
| Raynaud’s phenomenon | 4.44 | 0.96–20.53 | 0.08 |
| Positive rheumatoid factor | 7.25 | 1.5–34.87 | 0.007** |
| Positive ACL | 12.25 | 1.42–105.64 | 0.006** |
Notes:
Linear regression analysis;
highly significant P < 0.01.
Linear regression (stepwise method) was performed to evaluate all clinical and laboratory parameters. Among all factors, both rheumatoid factor and ACL positivity were significantly associated with occurrence of PAH in SLE (P = 0.007, P = 0.006 respectively) (Table 5).
No significant correlations were found between pulmonary artery pressure, disease duration, SLE-DAI, ESR, and anti-ds DNA.
Table 6 summarizes clinical data, laboratory features and PASP in SLE patients with PAH.
Table 6.
Cases of SLE patients with pulmonary arterial hypertension.
| No. | Age (yr) | Duration of the disease (yr) | Extra-pulmonary manifestations | Laboratory investigations | PASP (mmHg), degree of PAH |
|---|---|---|---|---|---|
| 1 | 24 | 6 | Malar rash, alopecia, oral ulcers, arthralgia | ESR 34 mm/h +ve ANA +ve ACL Pyuria, hematuria |
37.44, mild |
| 2 | 22 | 2.5 | Malar rash, photosensitivity, oral ulcers, Raynaud’s phenomenon | ESR 34 mm/h +ve RF +ve ANA +ve DNA +ve ACL |
61.02, severe |
| 3 | 26 | 1 | Alopecia, Raynaud’s phenomenon, arthralgia, myalgia, arthritis | ESR 90 mm/h +ve ANA +ve DNA +ve ACL Pyuria, casts, proteinuria |
34, mild |
| 4 | 30 | 1 | Malar rash, photosensitivity, alopecia, Raynaud’s phenomenon, oral ulcers, arthralgia, myalgia, arthritis | ESR 115 mm/h +ve RF +ve ANA +ve ACL |
50, moderate |
| 5 | 25 | 3 | Malar rash, photosensitivity, alopecia, Raynaud’s phenomenon, arthralgia, myalgia | ESR 89 mm/h +ve ANA +ve DNA Pyuria, proteinuria |
39, mild |
| 6 | 19 | 1 | Malar rash, photosensitivity, alopecia, Raynaud’s phenomenon, fever, arthralgia, arthritis | ESR 98 mm/h +ve RF +ve ANA + ve ACL |
49.06, moderate |
| 7 | 30 | 1 | Malar rash, photosensitivity, alopecia, oral ulcers, arthralgia, myalgia, arthritis | ESR 115 mm/h +ve ANA +ve ACL |
36, mild |
| 8 | 12 | 0.3 | Malar rash, photosensitivity, oral ulcers, arthralgia, myalgia, arthritis | ESR 115 mm/h +ve RF +ve ANA +ve ACL |
39, mild |
Discussion
Systemic lupus erythematosus is an autoimmune disease with multiple organ involvement. Part of these patients represents variable degrees of pulmonary hypertension.15 Pulmonary hypertension in lupus patients has been described since 1973.16–18 The prevalence rate of PAH in SLE patients ranges from 0.5% to 14%.19,20 In a serial study of 28 patients with SLE, the prevalence of PH measured by echocardiogram increased from 14% to 43% with 5 years of follow-up.21
Prabu et al22 provides important data on the prevalence of PAH in a large cohort of SLE patients. The prevalence of PAH was found to be 4.2%, which was based on echocardiographic screening. Moreover, only 3 of the 12 patients had severe disease [systolic pulmonary arterial pressure (PAP) >40 mmHg]. These results are of particular interest, because the cohort, in contrast to other reports, has a community, non-tertiary background and seems not biased by the patient selection, and therefore might very well be considered as representative of the general SLE population.
Yeh et al7 reported the prevalence of PAH in juvenile SLE patients which range from 4% to 8% using transthoracic echocardiography. Yet, there are no studies using Doppler echocardiography as a screening tool for detection of asymptomatic pulmonary hypertension in juvenile SLE patients.
Doppler echocardiography is the most widely available technology among the noninvasive imaging methods. Echocardiography provides both estimates of pulmonary artery pressure and an assessment of cardiac structure and function. These features justify its application as the most commonly used screening tool in patients with suspected PAH.9
In the present study, Doppler echocardiography was used as a screening tool for detection of asymptomatic pulmonary hypertension in SLE patients. PAH was found in 8 of 74 SLE female patients (10.8%), they were 7 adult-onset SLE (aged from 19 to 30 yr) and 1 juvenile SLE (aged 12 years).
Asherson et al23 have reported that, patients with SLE-PH are predominantly women of child-bearing potential aged from 18 to 40 years with a 10 to 1 ratio of female over male.
Foïs et al24 reported that systolic PAP > 35 mmHg in 12 (13%) out of 93 SLE patients, 10 women and 2 men.
In the present study, Raynaud’s phenomenon was found in 5 adult-onset SLE patients with pulmonary hypertension (62.5%). In agreement with our results Haas20 reported that up to 58% of 105 patients (most of whom were female) with both PH and SLE had Raynaud’s phenomenon. Asherson et al25 found Raynaud’s phenomenon in 50% of adult SLE raising the possibility of vasospasm as an aetiological factor. However Asherson et al23 reported that 75% of patients with SLE-PH have Raynaud’s phenomenon, which is higher than the expected rate of 25% among all patients with SLE.
The striking correlation between the occurrence of Raynaud’s phenomenon and SLE-PH suggests that pulmonary arterial vasospasm may also be involved in the pathogenesis of SLE-PH. Raynaud’s phenomenon is part of a systemic vascular response that includes a decrease in size of the pulmonary capillary bed, which may in turn result in muscular necrosis and secondary inflammation.26
No signs of vasculitis were found in any case in the present study. However, Asherson et al23 have reported that one-third of patients with pulmonary hypertension in their study had evidence of peripheral cutaneous vasculitis, livedo reticularis, and digital gangrene.
In the present study, SLE patients with PAH showed statistically significant higher number of cases with positive rheumatoid factor (RF) (50%) and positive anticardiolipin antibodies (87.5%) in comparison to SLE patients without PAH (P = 0.02, P = 0.008 respectively). All SLE patients with PAH were positive for ANA. In agreement with our results Asherson et al and Wilson et al reported that patients with SLE-PH are universally positive for ANA. Antibodies to ribonuclear protein (RNP) and rheumatoid factor (RF) are often present in SLE-PH, although no pathogenic role has been postulated. The prevalence of RF has been reported to be as high as 50% to 80% in SLE-PH. The frequency of PH in patients with SLE and positive antiphospholipid antibodies is considerably higher than in patients with SLE and negative antiphospholipid antibodies (83% versus 25%).23,27
Lian et al28 have found positive rheumatoid factor in 46.3% and positive anticardiolipin antibodies in 53.7% of patients with SLE-PH. However, Foïs et al24 have reported a very high frequency of anticardiolipin antibodies (75%) in their patients and they suggested that these autoantibodies might facilitate the formation of microthrombi in the pulmonary vasculature and thereby contribute to sPAP elevation, but the relationship between them and PH in SLE remains controversial.
In our SLE patients with PAH, Doppler echocardiography detected elevated pulmonary artery systolic pressure in 8 patients (10.8%), being mild in 5, moderate in 2, and severe in one patient, right ventricular dilatation in 1 patient, (12.5%), valvular thickening in 3 patients, (37.5%), tricuspid regurge (mild in 1, moderate in 5, and severe in 2 patients), and even mild pericardial effusion in 2 patients, (25%) before symptom onset.
Lian et al28 reported that, Raynaud’s phenomenon, anticardiolipin antibodies, and anti-U1RNP were associated with increased odds ratio (OR = 3.204, OR = 3.753, OR = 5.393 respectively), and were considered as significant independent predictors of PAH in SLE (P = 0.01, P = 0.004, P = 0.001 respectively). But in the current study, Raynaud’s phenomenon, rheumatoid factor, and anti-cardiolipin antibodies were associated with increased risk for PAH (OR = 4.44, OR = 7.25, OR = 12.25 respectively). While only positive rheumatoid factor and positive ACL were significantly associated with occurrence of PAH in SLE (P = 0.007, P = 0.006 respectively) in the linear regression analysis.
Study findings by Robbins et al29 indicate that the duration of SLE does not correlate with the development of PAH, although many patients with SLE develop PAH within the first 5 years. These findings coincided with our results, as the duration of SLE was not correlated with PAH and SLE patients with PAH had shorter disease duration (0.3–6 yr) in comparison to SLE patients without PAH (0.1–16 yr), but the difference not statistically significant.
SLE disease related risk factors, the disease activity score (SLEDAI), ESR, anti-ds DNA and renal affection in SLE patients enrolled in the present study did not differ significantly between patients with PAH and those without, and they weren’t correlated with PAH. In agreement with our results, Asherson et al23 and Robbins et al29 reported that PH was unrelated to the severity or activity of SLE such as high anti ds-DNA and/or grossly elevated ESR, and can occur when non-pulmonary disease activity is quiescent.
Conclusion
In our SLE patients, Doppler echocardiography could detect elevated pulmonary artery systolic pressure in 10.8%, degree of pulmonary hypertension, right ventricular dilatation, valvular thickening, tricuspid regurge, and even mild pericardial effusion before symptom onset. SLE patients with PAH showed significant higher number of cases with positive rheumatoid factor (50%) and positive anticardiolipin antibodies (87.5%) in comparison to SLE patients without PAH.
Echocardiography should be performed routinely once a year (as a screening tool) and consideration should be given to screening SLE patients with positive anti-cardiolipin antibodies, and rheumatoid factor; as they were significant predictors of pulmonary hypertension in SLE.
Further study is required to determine if patients with asymptomatic pulmonary hypertension will benefit from therapy in terms of morbidity and mortality.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Pisetsky DS, Gilkeson G, St Clair W. Systemic lupus erythematosus—Diagnosis and treatment. Med Clin North Am. 1997;81:113–28. doi: 10.1016/s0025-7125(05)70507-1. [DOI] [PubMed] [Google Scholar]
- 2.Falcão CA, Lucena N, Alves IC, Pessoa AL, Godoi ET. Lupus Carditis Arq Bras Cardiol. 2000;74(1):55–71. [PubMed] [Google Scholar]
- 3.Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine. 1997;76(3):192–202. doi: 10.1097/00005792-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ciftci E, Yalcinkaya F, Ince E, et al. Pulmonary involvement in childhood-onset systemic lupus erythematosus: a report of five cases. Rheumatology (Oxford) 2004;43(5):587–91. doi: 10.1093/rheumatology/keh120. [DOI] [PubMed] [Google Scholar]
- 5.Simonson JS, Schiller NB, Petri M, Hellmann DB. Pulmonary hypertension in systemic lupus erythematosus. J Rheumatol. 1989;16:918–25. [PubMed] [Google Scholar]
- 6.Swigris JJ, Fischer A, Gilles J, Meehan RT, Brown KK. Pulmonary and thrombotic manifestations of systemic lupus erythematosus. Chest. 2008;133:271–80. doi: 10.1378/chest.07-0079. [DOI] [PubMed] [Google Scholar]
- 7.Yeh TT, Yang YH, Lin YT, Lu CS, Chiang BL. Cardiopulmonary involvement in pediatric systemic lupus erythematosus: a twenty year retrospective analysis. J Microbiol Immunol Infect. 2007;40:525–31. [PubMed] [Google Scholar]
- 8.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 9.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):S14–34. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 10.Selimovic N, Rundqvist B, Bergh CH, et al. Assessment of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2007;26:927–34. doi: 10.1016/j.healun.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 12.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 13.Yock PG, Popp RL. Noninvasive evaluation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 14.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–6. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen LJ, Chang HC, Lu LY, et al. Prolonged survival after single lung transplantation for pulmonary hypertension secondary to systemic lupus erythematosus. J Chin Med Assoc. 2004;67:248–51. [PubMed] [Google Scholar]
- 16.Cummings P. Primary pulmonary hypertension and SLE. N Engl J Med. 1973;288:1078–9. doi: 10.1056/NEJM197305172882018. [DOI] [PubMed] [Google Scholar]
- 17.Charoenpan P, Sukumalchantra Y, Ayuthya WIN. Pulmonary hypertension in SLE: a case report. J Med Ass Thai. 1977;60:670–5. [PubMed] [Google Scholar]
- 18.Perez HD, Kramer N. Pulmonary hypertension in systemic lupus erythematosus: report of four cases and review of literature. Semin Arth Rheum. 1981;11(1):177–81. doi: 10.1016/0049-0172(81)90098-6. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez O, Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary hypertension secondary to connective tissue disease. Thorax. 1999;54:273–7. doi: 10.1136/thx.54.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas C. Pulmonary hypertension associated with systemic lupus erythematosus. Bull Acad Natl Med. 2004;188(6):985–7. [PubMed] [Google Scholar]
- 21.Winslow TM, Ossipov MA, Fazio GP, et al. Five-year follow-up study of the prevalence and progression of pulmonary hypertension in systemic lupus erythematosus. Am Heart J. 1995;129:510–5. doi: 10.1016/0002-8703(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 22.Prabu A, Patel K, Yee CS, et al. Prevalence and risk factors for pulmonary arterial hypertension in patients with lupus. Rheumatology (Oxford) 2009;48:1506–11. doi: 10.1093/rheumatology/kep203. [DOI] [PubMed] [Google Scholar]
- 23.Asherson RA, Higenbottam TW, Dinh Xuan AT, Khamashta MA, Hughes GR. Pulmonary hypertension in a lupus clinic. Experience with twenty-four patients. J Rheumatol. 1990;17(10):1292–8. [PubMed] [Google Scholar]
- 24.Foïs E, Guern VL, Dupuy A, Humbert M, Mouthon L, Guillevin L. Noninvasive assessment of systolic pulmonary artery pressure in systemic lupus erythematosus: retrospective analysis of 93 patients. Clinical and Experimental Rheumatology. 2010;28:836–41. [PubMed] [Google Scholar]
- 25.Asherson RA, Mackworth-young CG, Boey ML, et al. Pulmonary hypertension in systemic lupus erythematosus. Brit Med J. 1983;287(6398):1024–5. doi: 10.1136/bmj.287.6398.1024-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naslund MJ, Pearson TA, Ritter JM. A documented episode of pulmonary vasoconstriction in systemic sclerosis. Johns Hopkins Med J. 1981;148:78–80. [PubMed] [Google Scholar]
- 27.Wilson L, Tomita T, Braniecki M. Fatal pulmonary hypertension in identical twins with systemic lupus erythematosus. Hum Pathol. 1991;22:295–7. doi: 10.1016/0046-8177(91)90164-k. [DOI] [PubMed] [Google Scholar]
- 28.Lian F, Chen D, Wang Y, et al. Clinical features and independent predictors of pulmonary arterial hypertension in systemic lupus erythematosus. Rheumatol Int. 2011 doi: 10.1007/s00296-011-1880-4. [DOI] [PubMed] [Google Scholar]
- 29.Robbins IM, Gaine SP, Schilz R, Tapson VF, Rubin LJ, Loyd JE. Epoprostenol for treatment of pulmonary hypertension in patients with systemic lupus erythematosus. Chest. 2000;117:14–8. doi: 10.1378/chest.117.1.14. [DOI] [PubMed] [Google Scholar]