Abstract
Treated glioblastoma patients survive from 6 to 14 months. In the first part of this review, we describe glioma origins, cancer stem cells and the genomic alterations that generate dysregulated cell division, with enhanced proliferation and diverse response to radiation and chemotherapy. We review the pathways that mediate tumour cell proliferation, neo-angiogenesis, tumor cell invasion, as well as necrotic and apoptotic cell death. Then, we examine the ability of gliomas to evade and suppress the host immune system, exhibited at the levels of antigen recognition and immune activation, limiting the effective signaling between glioma and host immune cells.
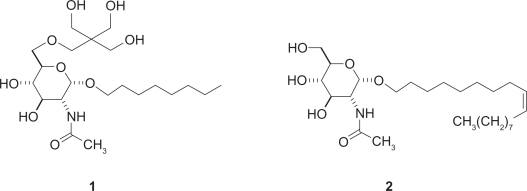
The second part of the review presents current therapies and their drawbacks. This is followed by a summary of the work of our laboratory during the past 20 years, on oligosaccharide and glycosphingolipid inhibitors of astroblast and astrocytoma division. Neurostatins, the O-acetylated forms of gangliosides GD1b and GT1b naturally present in mammalian brain, are cytostatic for normal astroblasts, but cytotoxic for rat C6 glioma cells and human astrocytoma grades III and IV, with ID50 values ranging from 200 to 450 nM. The inhibitors do not affect neurons or fibroblasts up to concentrations of 4 μM or higher.
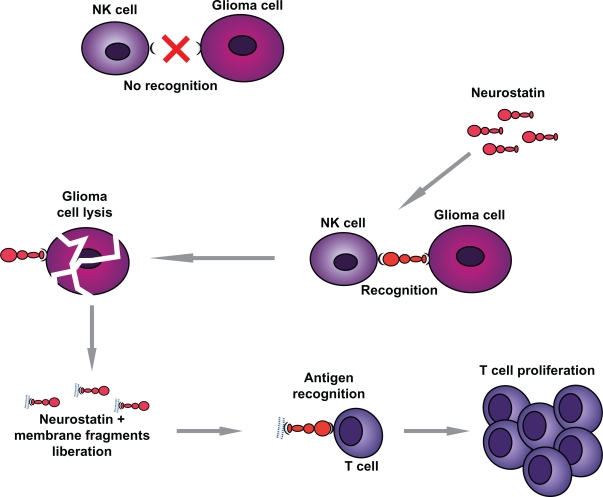
At least four different neurostatin-activated, cell-mediated antitumoral processes, lead to tumor destruction: (i) inhibition of tumor neovascularization; (ii) activation of microglia; (iii) activation of natural killer (NK) cells; (iv) activation of cytotoxic lymphocytes (CTL). The enhanced antigenicity of neurostatin-treated glioma cells, could be related to their increased expression of connexin 43. Because neurostatins and their analogues show specific activity and no toxicity for normal cells, a clinical trial would be the logical next step.
Keywords: glioma, immunosuppression, chemotherapy, neurostatin, antigen presentation
Glioma Genomics
Central nervous system tumors
Gliomas belong to a group of diverse tumors that affect the brain and spinal cord, known as central nervous system neoplasms. A brain tumor is a mass of abnormal cells in the brain that have grown and multiplied in an uncontrolled fashion. Brain tumors developing from the various types of cells that make up the brain, are called primary brain tumors. Brain tumors are usually confined to the brain itself and only rarely spread to other parts of the body. Approximately 50% of all primary brain tumors originate from the specialized neural cells called glial cells and are called gliomas. Other types of glial cells, susceptible to develop primary brain tumors, include oligodendrocytes and ependymal cells. Primary brain tumors that develop from astrocytes are referred to as astrocytomas and they are the most common gliomas. In their fourth edition of the World Health Organization (WHO) classification of tumours of the central nervous system, published in 2007, astrocytomas, were classified depending on their growth rate and their likelihood to spread (infiltrate) to nearby brain tissue, into the following four types or grades (Fig. 1):
Grade I = Pilocytic Astrocytoma - This is a slow-growing astrocytoma that usually does not spread to other parts of the central nervous system.
Grade II = Low-Grade Astrocytoma - This is also a relatively slow-growing type of astrocytoma, but grows faster than pilocytic astrocytoma (Grade I). It may or may not invade the surrounding normal brain tissue and tends to recur after treatment.
Grade III = Anaplastic Astrocytoma - This malignant astrocytoma grows faster than grade II astrocytoma. Invades normal brain tissue and recurs after treatment.
Grade IV = Glioblastoma Multiforme (GBM). This is the most malignant and fastest growing astrocytoma. Several different cell types can be observed in the tumor under a microscope, including astrocytes and oligodendrocytes. Areas of necrosis can also be observed at the center of the tumor. GBM invades very rapidly normal brain tissue.
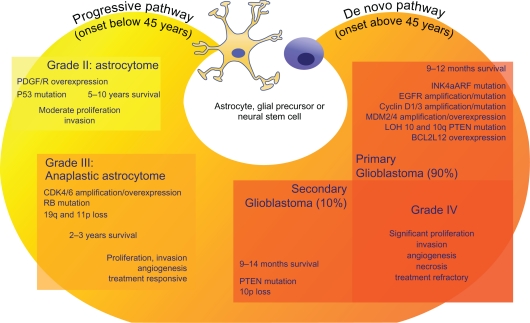
Figure 1.
Chromosomal and genetic abnormalities involved in glioblastoma. The figure shows the relationships between survival, pathobiology, and the molecular lesions that lead to the formation of primary (de novo) and secondary (progressive) glioblastomas. Grade IV gliomas are histologically indistinguishable, occur in different age groups and present distinct genetic alterations affecting similar pathways. Thus, inactivation of p53 function may be due to direct mutation in progressive GBMs, or INK4aARF mutation/decrease in expression or MDM2 amplification in de novo GBMs. Similarly, activation of the PI3K pathway can be achieved by several cooperative mechanisms, including EGFR amplification and mutation, as well as PTEN mutation, although underexpression of PTEN in the absence of mutation is frequently seen as well.
About 22,000 people were diagnosed with a malignant (cancerous) primary brain tumor in the United States in 2010. GBM, the most common type of primary malignant brain tumor in adults, accounted for 25% of all cases. GBM is most common in adults 50 to 70 years of age and accounts for less than 10% of childhood brain tumors. It is more frequent in males than females, by an approximate ratio of 3:2.
Although malignant brain tumours make up only about 1.5 percent of all forms of cancer, GBM is almost always fatal. We are not much better at treating them than we were 5 or 10 years ago. GBM have small, microscopic extensions in the brain, that cannot be removed surgically without sacrificing a large amount of normal brain tissue. Even then, unseen tumour cells are left behind. Therefore, the role of surgery is limited to: (1) obtaining biopsy tissue to characterize the tumour and (2) removing as much of the tumour as can be done safely, without causing further neurological damage. Surgery alone cannot cure these tumours.
Researchers have learned a great deal about the molecular and genetic events involved in the transformation of a “normal” cell to a “malignant” or cancerous cell.1 Brain tumors, like other types of cancers, are caused by genetic mutations, some inherited and other acquired, ie, developing after exposure to risk factors, such as smoking or chemicals, that cause damage to the genetic material of the cells. Despite extensive research to identify major risk factors, it appears that most primary brain tumors develop for no apparent reason. Radiation therapy to the head for the treatment of other types of cancers, is currently the only established risk factor for developing a primary brain tumor. For example, children with leukemia, who receive radiation therapy to the brain as part of their treatment, are at risk of developing a brain tumor later in life.
Most people who develop a primary brain tumor do not have a family history of brain tumors, ie, inherited mutations do not appear to play a major role in the development of brain cancer. With the exception of exposure to ionizing radiation during radiation therapy to the head for the treatment of other types of cancers, there is no clear-cut association between exposure to other environmental risk factors and the development of brain tumors. It appears that most primary brain tumors develop for no apparent reason, and the role in the development of primary brain tumors of environmental factors, genetic factors, and certain types of viruses, continues to be investigated.
Although the exact cause remains elusive, there is growing evidence that only a minor population of cells in primary brain tumors (GBM, medulloblastoma, and ependymoma), are capable of forming a tumor when orthotopically transplanted into immunocompromised mice.2
Cancer stem cells
There is no clear-cut association between exposure to environmental risk factors and the development of brain tumors. The link between exposure to certain chemicals (eg, vinyl chloride), petroleum products, and chemicals used in the production of synthetic rubber, has been suspected but not proven, as a risk factor for brain tumors. More recently, the expansion of wireless cellular telephones has raised the concern about a possible link between radiofrequency exposure from cellular phones and the development of brain tumors. Research in this area is ongoing, but an association between the use of cellular phones and brain tumors has not been found to date. Exposure to electromagnetic fields from high-tension wires has also been suspected as a risk factor for brain tumors. However, most studies have concluded that there is no strong evidence clearly proving an association.
With the exception of exposure to ionizing radiation during radiation therapy to the head for the treatment of other types of cancers, there is no clear-cut association between exposure to environmental risk factors and the development of brain tumors. Also, most primary brain tumors are developped by people who do not have a brain tumor family history, ie, inherited mutations do not appear to play a major role. It appears that most primary brain tumors develop for no obvious reason. Although the exact cause remains elusive, it appears that only a minor population of the cells in solid tumors, including primary brain tumors (GBM, medulloblastoma, and ependymoma), are capable of forming a tumor when orthotopically transplanted into an immunocompromised mouse.2 The concept of brain cancer stem cells (CSC)3 is based on the observation that only a small fraction of primary leukemic cells are capable of initiating and sustaining clonogenic growth and inducing leukemia in immunocompromised mice.4,5 Importantly, these leukemic subclones share cell surface markers (CD43+, CD38−) with “normal” hematopoietic stem cells (HSCs), while the progeny of these leukemic clones, the blast cells, often express more differentiated lymphoid or myeloid lineage markers and are not capable of producing leukemic disease. At present it is unclear whether CSC derive from a normal stem cell compartment or from a more differentiated progenitor, that dedifferentiates into a stem cell-like state. The identification of the “cell of origin” remains an area of active research for both hematological malignancies and solid tumors.2,6–14
The CSC hypothesis was independently proposed for GBM15 and pediatric gliomas.16 There were two critical findings in these studies. First, from a variety of primary CNS tumors (including GBM, medulloblastoma, ganglioglioma, ependymoma, and pilocytic astrocytomas), only a minor population of cells, identified in cell cultures, was able to self-renew and form clonogenic neurospheres (Fig. 2). These self-renewing brain tumor cells were identified15 by the expression of the cell surface marker CD133+ (prominin 1, PROM1, 1%–35% of total population). In contrast, the CD133− population failed to proliferate and remained as an adherent monolayer and expressed mature lineage specific markers. Second, CD133+ tumor neurospheres under neural stem cell (NSC) culture conditions, expressed the stem cell marker Nestin and, upon exposure to serum, differentiated into a mixed population of neurons (Tuj1+), astrocytes (GFAP+), and oligodendrocytes (PDGFR+), which mirrored the mixed cell types found in the original patient’s tumor. These observations supported a hierarchical CSC hypothesis, suggesting that only CD133+ brain tumor cells can self-renew and undergo lineage-specific differentiation.
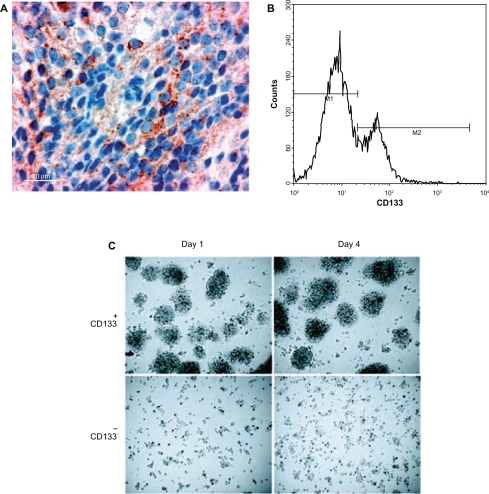
Figure 2.
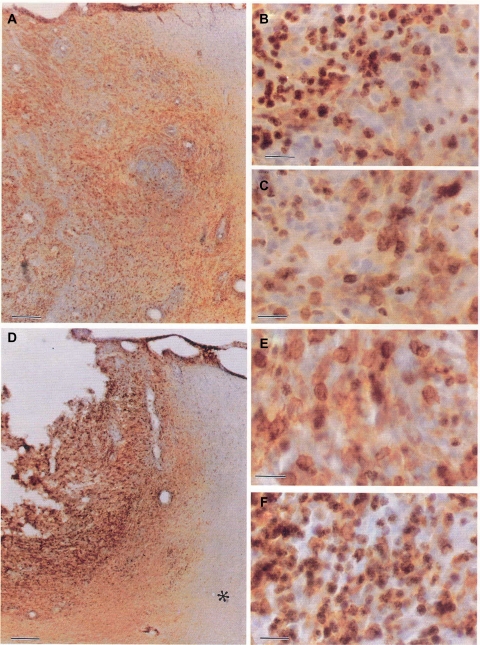
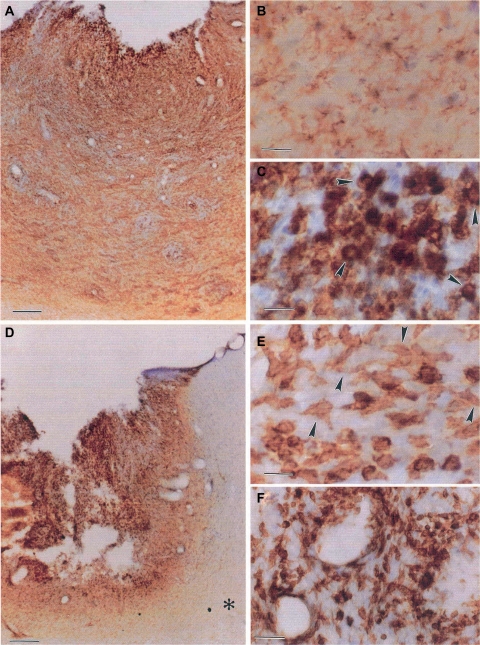
Cancer Stem cells. CD133+ tumor cells show marked stem cell features. (A) CD133 immunohistochemistry shows plasma membrane staining in cells scattered within a medulloblastoma. Brain tumor stem cell from both medulloblastomas and pilocytic astrocytomas immunostained for CD133. (B) flow cytometry histogram in medulloblastoma tumor cells, the first peak representing cells negative for CD133 expression, and the second peak representing CD133 positive cells. (C) CD133+ tumor cells proliferated in culture as nonadherent spheres, whereas CD133− tumor cells adhered to culture dishes, did not proliferate and did not form spheres.
Subsequently, it was shown that FACS-sorted CD133+ cells had enhanced tumor-forming ability (as few as 100 implanted cells were able to produce orthotopic tumors) following in vitro expansion.2 In contrast, CD133− cells failed to form tumors, even following injection of a much larger cell innoculum. The orthotopic tumors mirrored the original tumor heterogeneity, with CD133+ cells forming a minor fraction and the CD133− cells failing to form tumors on serial transplantation. These data suggest that loss of CD133 expression reflects an “irreversible” loss of cellular ability to propagate a tumor. Whether CD133+ cells are only important for tumor initiation and are less critical for tumor progression, will require a genetic strategy similar to that used to monitor skin stem cells in vivo, using a doxycyline-inducible H2B-eGFP reporter tag, to permit selection of CD133+ cells over time.17
Cancer-forming ability in vivo is very much increased in CD133+ cells for GBM2,18,19 and colon cancer.10,20 There are, however, a number of reports suggesting a less clear distinction between the ability of CD133+ and CD133− cells to form orthotopic tumors.21–24 Thus, Beier et al23 reported that CD133− cells isolated from primary GBM tumors were as capable of forming orthotopic tumors as CD133+ cells, whereas under the same conditions none of the secondary GBM tumors (zero of seven) produced viable neurosphere cultures.23 The same authors also reported that in 4 of 11 primary GBM tumors, CD133− cells grew as an adherent monolayer, yet were able to produce orthotopic tumors. Similarly, CD133− primary GBM tumor cells, maintained as an adherent monolayer by addition of serum to stem cell culture media, were also able to produce highly infiltrative orthotopic tumors.22 These data indicate that even brief ex vivo manipulations may alter the molecular and phenotypic properties of freshly isolated tumor cells and complicate the conclusions that can be drawn from this type of experiments, pointing to the need for studies using directly isolated tumor cells from fresh specimens and immediate implantation into immunocompromised mice. While the GBM-stem cell idea is in its infancy and many questions remain, its potential for our understanding of tumor development and therapy design and selection is exciting indeed. Tumour relapse often occurs after conventional therapy, whereas therapy specific for cancer stem cells will lead to complete tumour regression (Fig. 3).
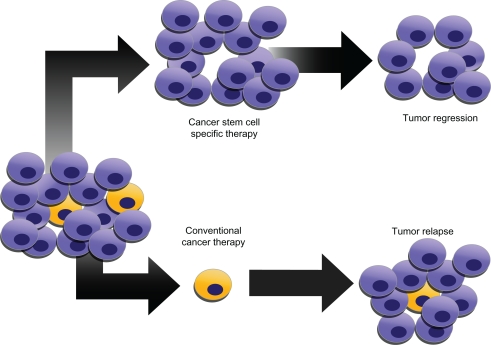
Figure 3.
Conventional vs. specific glioma therapies. Cancer stem cells (CSCs) may generate tumors through processes of self-renewal and differentiation. CSCs may persist in tumors as a distinct population and cause relapse and metastasis, giving rise to new tumors. Because CSCs form a very small proportion of the tumor, conventional chemotherapies may not necessarily act specifically on the stem cells, killing differentiated or differentiating cells, that form the bulk of the tumor, whereas CSCs may remain untouched and cause a relapse.
Genomic alterations in clinical GBM subtypes
The Cancer Genome Atlas (TCGA) Research Network was established to generate the catalogue of genomic abnormalities driving tumorigenesis. TCGA provided a detailed view of the genomic changes in a large GBM cohort containing 206 patient samples.24 Sequence data of 91 patients and 601 genes were used to describe the mutational spectrum of GBM (Fig. 1), confirming previously reported TP53 and RB1 mutations and identifying GBM-associated mutations in such genes as PIK3R1, NF1, and ERBB2. Projecting copy number and mutation data on the TP53, RB, and receptor tyrosine kinase pathways, showed that the majority of GBM tumors harbor abnormalities in all of these pathways, suggesting that this is a core requirement for GBM pathogenesis. Human cancer cells typically harbour multiple chromosomal aberrations, nucleotide substitutions and epigenetic modifications that drive malignant transformation. This analysis provides new insights into the roles of ERBB2, NF1 and TP53, uncovers frequent mutations of the phosphatidylinositol-3-OH kinase regulatory subunit gene PIK3R1, and provides a network view of the pathways altered in the development of glioblastoma. Furthermore, integration of mutation, DNA methylation and clinical treatment data reveals a link between DNA methyltransferase promoter methylation and a hypermutator phenotype consequent to mismatch repair deficiency in treated glioblastomas, an observation with potential clinical implications.
Thirty heterozygous deletions in NF1 were observed among the sample set of 206 cases, 6 of which also harbour point mutation.24 Some samples also exhibited loss of expression without evidence of genomic alteration. Overall, at least 47 of the 206 patient samples (23%) harboured somatic NF1 inactivating mutations or deletions, definitively addressing NF1’s relevance to sporadic human glioblastoma. It was concluded that NF1 is a human glioblastoma suppressor gene.
EGFR is frequently activated in primary glioblastomas. Variant III deletion of the extracellular domain (‘vIII mutant’) has been the most commonly described event, in addition to extracellular domain point mutations and cytoplasmic domain deletions.24 Here, high-resolution genomic and exon-specific transcriptomic profiling readily detected vIII and carboxy-terminal deletions with correspondingly altered transcripts. Among the 91 glioblastoma cases with somatic mutation data, 22 harboured focal amplification of wild-type EGFR with nopoint mutation, 16 had point mutations in addition to focal amplification, and 3 had EGFR point mutations but no amplification. Collectively, EGFR alterations were observed in 41 of the 91 sequenced samples.24
ERBB2 mutation has previously been reported in only one glioblastoma tumour.24 In the TCGA cohort, 11 somatic ERBB2 mutations in 7 of 91 samples were validated, including 3 in the kinase domain and 2 involving V777A, a site of recurrent missense and in-frame insertion mutations in lung, gastric and colon cancers. The remaining eight mutations (including seven missense and one splice-site mutation) occurred in the extracellular domain of the protein, similar to somatic EGFR substitutions in glioblastoma. Unlike in breast cancers, focal amplifications of ERBB2 were not observed in glioblastomas.
Various somatic mutations of the PI(3)K complex are relevant in human glioblastoma. PI3Ks catalyze the mitogen-stimulated phosphorylation of phosphatidylinositol-4,5 bisphosphate [PtdIns(4,5)P2] to produce PtdIns(3,4,5)P3. The PI(3)K complex consists of a catalytically active protein, p110a, encoded by PIK3CA, and a regulatory protein, p85a, encoded by PIK3R1. Frequent activating missense mutations of PIK3CA have been reported in multiple tumour types, including glioblastoma.25,26 These mutations occur primarily in the adaptor binding domain (ABD) as well as the C2 helical and kinase domains. Indeed, PIK3CA somatic nucleotide substitutions were detected in 6 of the 91 sequenced samples.24 Apart from the four mutations already reported in the COSMIC database,27 two novel in-frame deletions were detected in the adaptor binding domain of PIK3CA (‘L10 del’ and ‘P17 del’). Those deletions may disrupt interactions between p110a and its regulatory subunit, p85a.28
Somatic mutations in the genes IDH1 and IDH2
Sequencing of matched tumor and normal gene samples led to the unexpected finding of somatic point mutations in the genes for two isocitrate dehydrogenase isoenzymes, IDH1 and IDH2. The IDH enzymes play a key role in cellular metabolism, catalyzing the conversion of isocitrate to α-ketoglutarate and generating NADPH from NADP in the process. Mutated IDH1 was found in 12% of glioblastoma multiforme samples analyzed29 and mutations at arginine 132 (R132) of IDH1 were found in more than 80% of secondary GBMs. These mutations strongly reduced the ability of the enzyme to convert isocitrate to α-ketoglutarate, compared with the wild-type enzyme and further kinetic analyses revealed a dramatically reduced affinity for isocitrate in the mutants. On examining gliomas negative for IDH1 mutations, recurrent somatic mutations of IDH2 at the analogous R172 residue were identified.30,31 Not only were the IDH1 and IDH2 mutations frequent, but studies by several laboratories established that the mutation in IDH1 occurred early in glioma progression.32 Notably, the mutations affected only one allele of the IDH locus (of the two alleles of either IDH1 or IDH2, but not both in the same tumor), which is puzzling considering that they are selected for early in tumorigenesis.
The IDH enzymes play a key role in cellular metabolism. The crystal structure of IDH134 predicts that the amino acid substitutions at the R132 position will impair the interaction of the enzyme with its isocitrate substrate, Zhao and colleagues34 evaluated the in vitro enzymatic activities of three tumor-derived IDH1 mutants and observed that they had a more than 80% reduced ability to convert isocitrate to α-ketoglutarate, compared with the wild-type enzyme. Kinetic analyses revealed a dramatically reduced affinity for isocitrate in the three mutants. As IDH1 functions as a homodimeric complex, Zhao et al34 isolated IDH1 dimers expressed from the R132H mutant and wild-type genes introduced into Escherichia coli. Three dimer combinations were identified, the wildtype: R 132H heterodimer exhibited only 4% of the wild-type dimer enzyme activity, while R132H:R132H homodimers were almost completely inactive.
What are the metabolic consequences of IDH1 mutations? Using the U-87MG human glioblastoma cell line, Zhao et al34 demonstrated a concomitant reduction in cellular α-ketoglutarate levels after knocking down endogenous IDH1. Because α-ketoglutarate is required by prolylhydroxylases, enzymes that hydroxylate and promote the degradation of hypoxia-inducible factor 1α (HIF-1α), the intracellular levels of HIF-1α were also reduced. Zhao et al34 showed that when wild-type IDH1 was knocked down by RNA interference, HIF-1α was elevated, and when IDH1 was over-expressed, HIF-1α levels were reduced. HIF-1α is a component of HIF-1, a transcription factor that regulates the expression of genes related to glucose metabolism, angiogenesis, and other signaling pathways, by sensing low cellular oxygen levels. Using quantitative PCR to measure the transcripts of three known HIF-1 target genes—glucose transporter 1 (Glut1), vascular endothelial growth factor (VEGF), and phosphoglycerate kinase (PGK1)—Zhao et al34 demonstrated induced expression of these genes as a consequence of either the knockdown of wild-type IDH1 or the expression of the IDH1 R132H mutant. On staining glioma samples for HIF-1α, the tumors with previously identified R132H mutations showed a statistically stronger staining signal than those without mutations. Thus, the function of mutated IDH1 was reduced and the down-stream impact of that reduced function (the consequential upregulation of HIF-1α) contributed to the cell’s progression to cancer, indicating that a likely function of IDH1 is that of a tumor suppressor gene and that IDH2 may have a similar role.35
Building on the initial characterizations of IDH1 mutations in gliomas, Dang et al36 took a metabolomics-based approach to identify additional changes in metabolite levels when an IDH1 mutation was present. 36 They found 2-hydroxyglutarate to be the only metabolite with significantly increased abundance in cells expressing the R132H mutant IDH1. The increase in 2-hydroxyglutarate resulted from the NADPH-dependent reduction of α-ketoglutarate by mutant IDH1, a new function enabled by the mutation at R132. The authors demonstrated a similar gain of function for the R132C, R132L and R132S mutations. Their X-ray crystallographic studies showed that the R132H mutation in IDH1 results in the formation of an active site distinct from that of the wild-type enzyme. With the aim of improving diagnostic efficacy, Dang et al36 examined 12 GBM tumors with various R132 mutations in IDH1, and found 2-hydroxyglutarate levels 100-fold greater or more than in tumors with wild-type IDH1; the measured decrease in α-ketoglutarate was, however, not statistically different in mutant versus wild-type IDH1 tumors. This finding indicates that in the clinic, detecting patients with increased 2-hydroxyglutarate levels would identify GBMs with IDH1 mutations, predicting an overall longer survival time. Indeed, since secondary GBMs develop from lower-grade gliomas, therapeutic inhibition of 2-hydroxyglutarate production might slow the transition time to GBM development, offering an improved survival benefit as a result.
Mardy’s laboratory used a whole-genome shotgun approach to sequence tumor genomes. In the second case of acute myeloid leukemia sequenced, they discovered an IDH1 R132 mutation that was subsequently found in about 8% of our 187 banked acute myeloid leukemia patient samples, showing that this mutation was not restricted to gliomas.37 A subsequent study by Gross et al38 examined an additional 145 acute myeloid leukemia biopsies, identifying 11 IDH1 R132 mutant samples.38 Four IDH1-mutant primary samples had relapse samples that also carried the IDH1 mutation. Acute myeloid leukemia cells carrying the R132 mutant of IDH1 were found by gas chromatography-mass spectrometry to have 2-hydroxyglutarate levels around 50-fold greater than in samples with wild-type IDH1. Similarly, higher 2-hydroxyglutarate levels were detected in sera from patients positive for the IDH1 R132 mutation. Two wild-type IDH1 samples had elevated 2-HG levels and were found to be carrying IDH2 R172 mutations, the first report of these in acute myeloid leukemia. Because of the apparent predominance in acute myeloid leukemia of the IDH1 R132C mutation over R132H (which is more predominant in gliomas), Gross et al38 looked at the kinetics of the R132C mutant enzyme. The R132C enzyme showed a dramatic loss of affinity for isocitrate (resulting in a reduction in KM) and a drop of more than six orders of magnitude in net efficiency (Kcat/KM) of isocitrate metabolism.
Another recent study has extended our understanding of IDH mutations and their detection. Ward et al39 have shown that the gain of function seen in the IDH1 R132 mutants (that is, the ability to reduce α-ketoglutarate) is also found in the IDH2 R172K mutant. Metabolic profiling of cells expressing IDH2 R172K revealed an approximately 100-fold increase in intracellular 2-hydroxyglutarate compared with cells overexpressing wild-type IDH2, and this finding was extended to leukemia cells carrying the IDH2 R172K mutation. Ward et al39 also screened acute myeloid leukemia samples with normal cytogenetics but unknown IDH mutational status for increased levels of 2-hydroxyglutarate, and then evaluated the mutational status based on the result of the screening assay. In this test, 2-hydroxyglutarate measurement was found to predict mutational status with high accuracy.39
This work, aiming to characterize the impact of IDH mutations on tumor cell biology, has led to the conclusion that all mutations discovered so far, enable a gain of function in α-ketoglutarate reduction with a concomitant increase in the tumor-specific metabolite, or oncometabolite, 2-HG. Although the contribution of 2-hydroxyglutarate to tumor cell biology remains speculative, Ward et al39 noted that all IDH mutation-containing tumor types identified so far (leukemias and gliomas) are distinguished by proliferation of a relatively undifferentiated cell population. In this context, the effect of 2-hydroxyglutarate on the tumor and its microenvironment is to block cellular differentiation.39
DNA methyltransferase methylation and mismatch repair in treated glioblastomas
Tumour cell-derived gelatinases (matrix metalloproteinase-2, matrix metalloproteinase-9, MGMT) can be considered prime factors in glioma invasiveness: their expression correlates with the progression and the degree of malignancy.40 Alkylating agents are the most widely used chemotherapeutic agents to treat GBM. Among the chemotherapeutic compounds used in its treatment, temozolomide (TMZ), a cytotoxic alkylating agent, has shown activity in recurrent glioblastoma.41–44 Epigenetic silencing of O6-methylguanine-DNA-methyltransferase (MGMT) gene by promotor methylation was among the strongest predictors of survival in the European-Canadian study for newly diagnosed GBM.45 Patients with tumors harboring MGMT promoter methylation clearly benefit most from combined RT/TMZ. Cancer-specific DNA methylation of CpG dinucleotides located in CpG islands within the promoters of 2,305 genes, was measured relative to normal brain DNA.24 The promoter methylation status of MGMT, a DNA repair enzyme that removes alkyl groups from guanine residues,46 is associated with glioblastoma sensitivity to alkylating agents.47,48 Among the 91 sequenced cases, 19 samples were found to contain MGMT promoter methylation (including 13 of the 72 untreated cases and 6 of the 19 treated cases; see below, chemotherapy).24 When juxtaposed with somatic mutation data, an intriguing relationship between the hypermutator phenotype and MGMT methylation status emerged in the treated samples. Specifically, MGMT methylation was associated with a profound shift in the nucleotide substitution spectrum of treated glioblastomas. Among the 13 treated samples without MGMT methylation, 29% (29 out of 99) of the validated somatic mutations occurred as GNC to ANT transitions in CpG dinucleotides (characteristic of spontaneous deamination of methylated cytosines), and a comparable 23% (23 out of 99) of all mutations occurred as GNC to ANT transitions in non-CpG dinucleotides. In contrast, in the six treated samples with MGMT methylation, 81% of all mutations (146 out of 181) turned out to be of the GNC to ANT transition type in non-CpG dinucleotides, whereas only 4% (8 out of 181) of all mutations were GNC to ANT transition mutations within CpGs. That pattern is consistent with a failure to repair alkylated guanine residues caused by treatment. In other words, MGMT methylation shifted the mutation spectrum of treated samples to a preponderance of GNC to ANT transition at non-CpG sites. Notably, the mutational spectra in the mismatch repair (MMR) genes themselves reflected MGMT methylation status and treatment consequences. All seven mutations in MMR genes found in six MGMT methylated, hypermutated (treated) tumours occurred as GNC to ANT mutations at non-CpG sites, whereas neither MMR mutation in non-methylated, hypermutated tumours was of this characteristic. Hence, MMR deficiency and MGMT methylation together, in the context of treatment, exerted a powerful influence on the overall frequency and pattern of somatic point mutations in glioblastoma tumours, an observation of potential clinical importance. In some Phase II clinical trials, the combined therapy with marimastat (MT), a broad spectrum matrix metalloproteinase inhibitor, plus other chemotherapeutic agents, compared to conventional therapy for glioma, has provided promising antitumor effects, although musculoskeletal toxicity was observed.49,50
Mitogenic Pathways in Glioma
Cell cycle dysregulation and enhanced glioma cell proliferation
Cell cycle regulatory genes have a great importance for glioma growth, as underscored by the frequent mutations of these genes in cellular proliferation and senescence. The RB and p53 pathways, that govern the G1-to-S-phase transition in the cell cycle, suffer inactivating mutations in GBM (Fig. 4). The absence or misfunction of these guardians of the cell cycle, renders tumoral cells susceptible to cell division driven by constitutively active mitogens, such as phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK).
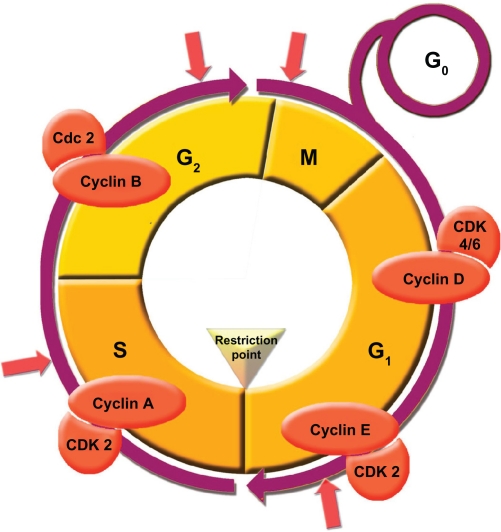
Figure 4.
Cell division checkpoints. The cell cycle proceeds by a defined sequence of events that ensures that complete and accurate replicas of the genome reach daughter cells. To monitor this process, cells are equipped with checkpoints at various stages of the cycle. DNA damage activates at least 3 checkpoints, that arrests cell cycle: in G1/S (G1) checkpoint, intra-S phase checkpoint, and G2/M checkpoint. Perturbation of DNA replication by drugs that interfere with DNA synthesis, DNA lesions, or obstacles on DNA, activate the DNA replication checkpoint, that arrests cell cycle at G2/M transition, until DNA replication is complete. Additional checkpoints, such as the morphogenesis checkpoint, detect abnormality in cytoskeleton and arrests the cell cycle at the G2/M transition.
The Rb pathway
In quiescent cells, hypophosphorylated RB blocks proliferation by binding and sequestering the E2F family of transcription factors, which prevent the transactivation of genes essential for progression through the cell cycle.51 Upon mitogenic stimulation, the activation of the MAPK cascade leads to the induction of cyclin D1 and its association with the cyclin-dependent kinases CDK4 and CDK6, as well as the degradation of the CDK2/cyclin E inhibitor, p27 Kip1.52–54 These activated CDK complexes in turn phosphorylate RB, enabling E2F transactivation of its direct transcriptional targets governing S-phase entry and progression55,56 (Fig. 4).
Gliomas circumvent RB-mediated cell cycle inhibition through any of several genetic alterations. The Rb1 gene, which maps to chromosome 13q14, is mutated in nearly 25% of high-grade astrocytomas and the loss of 13q typifies the transition from low- to intermediate-grade gliomas.57,58 Moreover, amplification of the CDK4 gene on chromosome 12q13–14 accounts for the functional inactivation of RB in 15% high-grade gliomas, and CDK6 is also amplified but at a lower frequency.59,60 RB activity is also frequently lost through the inactivation of a critical negative regulator of both CDK4 and CDK6, p16Ink4a.61 This gene is one of two transcripts generated at the CDKN2A locus on chromosome 9p21 (in addition to p14 ARF, p14 alternate reading frame), which is predominantly inactivated by allelic loss or hypermethylation in 50%–70% of high-grade gliomas and about 90% of cultured glioma cell lines.62–66 Consistent with its role as an important glioma tumor suppressor, p16Ink4a is also a critical inhibitor of progenitor cell renewal in the subventricular zone of aging mice.67 The importance of the inactivation of the RB pathway in glioma progression is evidenced by the near-universal, and mutually exclusive, alteration of RB pathway effectors and inhibitors, in both primary and secondary GBM.68,69 However, numerous in vitro and in vivo assays have demonstrated that the neutralization of this pathway alone is insufficient to abrogate cell cycle control, to the extent needed for cellular transformation. Therefore, other important cell cycle regulation pathways probably complement their activities in preventing gliomagenesis.70–78
The p53 pathway
The p53 tumor suppressor prevents the propagation of cells with unstable genomes, predominantly by halting the cell cycle in the G1 phase or instigating a program of apoptosis or proliferative arrest.79 P53 achieves these ends primarily through its function as a transcription factor: upon being post-translationally modified by various genotoxic and cytotoxic stress-sensing agents, p53 is stabilized, then binds and transcriptionally regulates the promoters of more than 2500 potential effector genes.80,81 The best characterized of these effectors is the transcriptional target CDNK1A, that encodes the protein for the CDK2 inhibitor p21.82,83 Although this gene has not been found to be genomically altered in gliomas, its expression is frequently abrogated by p53 functional inactivity, as well as by mitogenic signaling through the PI3K and MAPK pathways.
The p53 pathway is almost invariably altered in sporadic gliomas. Loss of p53, through either point mutations that prevent DNA binding or loss of chromosome 17p, is a frequent and early event in the pathological progression of secondary GBM.84,85 The importance of p53 in gliomagenesis is also underscored by the increased incidence of gliomas in Li-Fraumeni syndrome, a familial cancer-predisposition associated with germline p53 mutations.86,87 This genetic linkage has been reinforced by a glioma-prone condition in mice engineered with a p53 mutation commonly observed in Li-Fraumeni,88 as well as in p19 ARF-null mice.89
The finding that a second promoter drives an alternatively spliced transcript at the CDKN2A locus prompted the discovery of an additional tumor suppressor gene that is inactivated at this locus.90 The second protein encoded by CDKN2A, p14 ARF, was subsequently shown to be an important accessory to p53 activation, under conditions of oncogenic stress due to neutralization of the p53 ubiquitin ligase, MDM2.91–94 This oncogene was originally found amplified in a spontaneously transformed murine cell line, and later discovered to be a key negative regulator of p53 during normal development and in tumorigenesis.95–102 Concordantly, the chromosomal region containing MDM2, 12q14-15, was amplified in about 10% of primary GBM, the majority of which contained intact p53.59 The discovery of the MDM2-related gene, MDM4 (chromosome 1q32), which inhibits p53 transcription and enhances the ubiquitin ligase activity of MDM2, prompted the finding that the p53 pathway is also inactivated by the amplification of MDM4 in 4% of GBM with neither TP53 mutation nor MDM2 amplification.103–106 Additionally, the tumor suppressor gene CHD5 (chromodomain helicase DNA-binding domain 5), which maps to chromosome 1p36 and is therefore, frequently hemizygously deleted in those human gliomas that have 1p loss, has been shown to maintain p53 levels by facilitating expression of p19 Arf (mouse p14 Arf ortholog), and thus presents an additional mechanism for inactivation of this critical pathway.68
Mitogenic signaling pathways
Many mitogens and their specific membrane receptors are present in overactive form in gliomas. Proliferation of normal cells requires activation of mitogenic signaling pathways through diffusible growth factor binding, cell–cell adhesion, and/or contact with extracellular matrix (ECM) components. These signals are transduced intracellularly by transmembrane receptors that typically activate the PI3K and MAPK signaling pathways. In contrast, tumor cells acquire genomic alterations that greatly reduce their dependence on exogenous growth stimulation, enabling their inappropriate cell division, survival, and motility through the constitutive activation of these pathways. While gliomas overcome the normal impositions on the control of mitogenic signaling through multiple mechanisms, activation of receptor tyrosine kinases (RTKs), discussed in detail below, appears to be the predominant mechanism.
MAPK
The MAPK pathway can transduce proliferation signals by both integrins and receptor tyrosine kinases (RTKs). Integrins are membrane-bound extracellular matrix (ECM) receptors, that mediate the interaction between the ECM and the cytoskeleton. Upon adhesion to ECM, integrins bind cytoplasmic anchor proteins that coordinate the binding of integrins to actin filaments, thus creating a focal adhesion complex.
Multiple molecules of focal adhesion kinase (FAK) cluster at these complexes and become activated by crossphosphorylation, whereupon FAK activates a signal transduction cascade that leads to extracellular signal regulated kinase (ERK) phosphorylation. This takes place either through activation of Ras, recruiting the adaptor protein Grb2 and the Ras guanine nucleotide exchange factor SOS to phospho-FAK at the plasma membrane, or through Src-dependent phosphorylation of p130Cas.107–109 Ras-GTP, in turn, phosphorylates Raf kinase, which phosphorylates MEK, which phosphorylates ERK. The phosphorylated kinase enters the nucleus and phosphorylates nuclear transcription factors, that induce the expression of genes promoting cell cycle progression, such as cyclin D1 (Fig. 4). RTKs activate the MAPK pathway when activated by growth factor signaling, mutation, or overexpression. RTK activation results in receptor dimerization and cross-phosphorylation, creating binding sites for adaptor protein complexes such as Grb2/SOS, which in turn activates Ras. Whereas mutated forms of Ras, constitutively activated, are found in about 50% of all human tumors, few Ras mutations have been found in gliomas. However, high levels of active Ras-GTP are found in advanced astrocytomas,110 suggesting that a more relevant mechanism for MAPK-dependent mitogenic signaling in GBM is through inappropriate activation of RTKs and/or integrins.
PI3K/PTEN/AKT
Class I kinases (PI3Ks) catalyze the mitogen-stimulated phosphorylation of phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2] to produce Ptd Ins(3,4,5)P3. This creates docking sites for a multitude of signaling proteins containing domains capable of binding either PtdIns(3,4,5)P3 itself or the 5-dephosphorylated product, PtdIns(3,4)P2.111,112 Class IA PI3Ks are heterodimers, recruited to activated RTKs and adaptor proteins via their regulatory subunit, of which there are five isoforms encoded by three genes: p85 (PIKR2), p55 (PIKR3), and p50 (PIK3R1).
Because the regulatory subunits appear functionally equivalent, class IA PI3Ks are currently defined by their catalytic isoforms p110, encoded by the PIK3CA, PIK3CB, and PIK3CD genes, respectively. 112 Evidence for the importance of p110 in transformation, derives from the discovery of a vPIK3CA oncogene in avian sarcoma virus with potent transforming activity in chicken embryo fibroblasts.113 PIK3CA gain-of-function point mutants have been detected in a variety of cancers, including malignant gliomas such as GBM, in which the frequency of mutation has been cited in some studies to be as high as 15%.25,26 Elevated expression of the PIK3D gene has also been reported in GBM.114,115
The p110 subunits can be activated by binding both p85 and GTP-bound Ras.116,117 Recently, the study of mice bearing a p110 point mutant unable to bind Ras, revealed that this interaction is essential both, for normal development and for Ras-driven tumorigenesis, as assessed both by transformation of mouse embryonic fibroblasts by H-Ras and by using a mouse model of K-ras-induced lung adenocarcinomas.118
The action of class I PI3K enzymes is directly antagonized by the PtdIns(3,4,5) P3 3-phosphatase encoded by the PTEN gene, located at 10q23.3.119–121 PTEN is a major tumor suppressor that is inactivated in 50% of highgrade gliomas by mutations or epigenetic mechanisms, each resulting in uncontrolled PI3K signaling in these tumors.114,122 In mouse models, brain-specific inactivation of PTEN caused overgrowth of the mouse brain and aberrant proliferation of astrocytes, both in vivo and in vitro.123 An elegant mouse model of astrocytoma has been developed in which the Rb family of proteins are inactivated by GFAP-directed expression of SV40 T antigen.78 In this model system, PTEN inactivation was associated with increased angiogenesis—a close parallel to the progression of high-grade glioma in humans, coincident with loss of PTEN.78,124 While regulation of PI3K signaling is critical for controlling cell growth and survival, a number of recent studies have pointed to additional levels at which PTEN may act to suppress transformation and tumor progression. Differentiated and quiescent cells harbor high levels of nuclear PTEN, which appears to fulfill important roles in the maintenance of genomic integrity, through centromere stabilization and promotion of DNA repair.124 Importantly, a number of PTEN point mutations found in familial cancer predisposition syndromes have no effect on enzyme activity, but instead lie within sequences important for regulating PTEN localization. Analysis of such mutants has confirmed that aberrant sequestration of PTEN into either the nucleus or the cytoplasm compromises its tumor suppressor function.125,126
The phosphoinositide-dependent kinase (PDK1) and Akt/PKB (the cellular homolog of a viral oncoprotein), are two of the many signaling proteins recruited to the membrane and activated by binding to PtdIns(3,4,5)P3, required for tumorigenesis in PTEN+/− mice and for growth of PTEN−/− embryonic stem (ES) cells as tumors in nude mice. In response to PI3K activation, PDK1 and the mammalian target of rapamycin mTOR, acting in the rapamycin-insensitive TORC2 complex, activate Akt via phosphorylation of two key residues, T308 and S473.127,128 Assessment of the phosphorylation status of these residues is often the method of choice for monitoring PI3K pathway activity in cell lines and primary tumors, including GBM samples, 85% of which have been reported to display activated Akt.129 In addition to aberrant PI3K signaling, there are a number of other possible mechanisms by which Akt activation may become dysregulated in GBM. PHLPP (PH domain leucine-rich repeat protein phosphatase), which dephosphorylates S473, is expressed at very low levels in certain GBM cell lines, as is CTMP (C terminal modulator protein), which binds to Akt and inhibits its phosphorylation.130–132 PIKE-A, a small GTPase highly expressed in GBMs and glioma cell lines, binds directly to phosphorylated Akt and enhances its anti-apoptotic function.133,134
Akt phosphorylates many proteins involved in the regulation of cell growth, proliferation, metabolism, and apoptosis. A recent study on v-H-ras-induced transformation of MEFs and skin carcinogenesis, indicates that activation of mTOR in the rapamycin-sensitive TORC1 complex via inhibition of the TSC2 tumor suppressor, is a key pro-oncogenic function of Akt.135 Because H-ras mutation is seldom seen in human tumors, it will be important to determine whether Akt/TSC/TORC1 signaling is similarly required downstream from glioma-relevant perturbations, such as EGFR mutation and overexpression and/or PTEN loss. Evidence that this may indeed be the case is provided by the efficacy of PI-103, a small molecule inhibitor of both p110 and mTOR, which potently blocks the growth of glioma cell lines and of U87EG-FRvIII xenografts following subcutaneous injection in nude mice, without discernable toxicity to the animals.136 The use of TSC2−/− cells, which display constitutive phosphorylation of the TORC1 substrates S6 K1 and 4E-BP1, revealed the existence of a negative feedback loop, whereby inhibitory phosphorylation of the insulin receptor substrate (IRS-1) by S6 K1 causes a reduction in Akt activation.137–140 Treatment of glioma cells with TORC1-specific inhibitors, such as rapamycin, disrupts such feedback control, resulting in increased Akt activity.136 Dual inhibition of PI3K and TORC1 by PI-103 overcomes these problems and likely explains its increased efficacy. In addition, phosphorylation of the FOXO transcription factors by Akt, which promotes their exclusion from the nucleus, reduces the expression of a number of important target genes, including the CDK inhibitors p21 WAF1/CIP1 and p27KIP1 (both of which are also directly targeted by Akt) and the RB family member p130.141–143 Recent data on context-specific actions of FOXO on different cell types and tissues, suggest the need to validate these FOXO targets in glioma.144
PI3K-MAPK-p53-RB pathway interactions
PI3K, MAPK, p53, and RB pathways are often considered as distinct entities, but there is significant cross-talk among them. Such cross-talk reinforces the inappropriate regulation of any single pathway perturbation. For example, because p53 enhances PTEN transcription and represses the expression of p110,145,146 the loss of p53 in cells with constitutively active RTK signaling, can further potentiate PI3K pathway activation. Therapies aimed at reactivating p53 in GBM, may be compromised by MAPK and PI3K intervention in the activity of p53 and its effectors. MAPK signaling activates c-myc, which binds the miz-1 transcriptional repressor to block p21 gene induction,147,148 while Akt impacts on p53 function by phosphorylation of Mdm2,149–151 in addition to the direct inhibition of p21. Moreover, these pathways can negate each other: p53 can inhibit activated FOXOs by inducing the expression of the kinase SGK1, which phosphorylates and exports FOXOs from the nucleus.152 Conversely, FOXOs can inhibit p53 transcriptional activity, by increasing its association with nuclear export receptors that translocate it to the cytoplasm.153 The recent finding that Sprouty 2, a gene involved in suppression of Ras signaling during oncogene-induced senescence, is also a direct transcriptional target of FOXO, emphasizes the complexity of cross-talk that exists between the Ras/MAPK and PI3K pathways.144,154 The complicated interplay among these critical molecules highlights the need for detailed dissection of aberrant pathways in each tumor, to accurately guide the choice of combination therapies that can simultaneously target multiple pathways.
Receptor tyrosine kinases (RTKs)
Gliomas may activate receptor-driven pathways by different mechanisms: overexpression of both ligands and receptors leading to an autocrine loop, genomic amplification, and/or mutation of the receptor leading to constitutive activation in the absence of ligand. The EGF and platelet-derived growth factor (PDGF) pathways play important roles in both CNS development and gliomagenesis, and targeted therapy against these potentially critical signaling pathways is currently under vigorous basic and clinical investigation.
EGFR
EGFR gene amplification occurs in −40% of all GBMs, and the amplified genes are frequently rearranged.155–159 An EGFR mutant allele with deletion of exons 2–7 (known variously as EGFRvIII, EGFR, or EGFR*) occurs in 20%–30% of all human GBM (and in 50%–60% of those that have amplified wild-type EGFR), making it the most common EGFR mutant.160,161 EGFRvIII is a highly validated glioma target as evidenced by the capacity of activated EGFR mutants to enhance tumorigenic behavior of human GBM cells by reducing apoptosis and increasing proliferation162–165 and to malignantly transform murine Ink4a/Arfnull neural stem cells (NSCs) or astrocytes in the mouse brain.70,74 Thus, EGFR has been a prime target for therapeutic intervention in GBM with small molecule kinase inhibitors, antibody-based immunotherapy and immunotoxins,166–169 and, more recently, small interfering RNA (siRNA)-directed neutralization of either wild-type EGFR or the unique junction present in the EGFRvIII allele.170,171
Transcriptional profiles of GBM with EGFR overexpression have revealed distinct gene expression profiles that have enabled classification of molecular subgroups among phenotypically undistinguishable tumors.172 Along similar lines, immunohistochemical studies have demonstrated that GBM could be stratified according to PI3K pathway activation status and that these activation profiles are associated with EGFRvIII expression and PTEN loss.173 Such efforts to stratify patients appear to be important in the optimal deployment of small molecule EGFR inhibitors as only a small fraction of GBM patients show meaningful responses to such agents.174,175 Thus far, in responsive cases, patients with coexpression of EGFRvIII176 or wild-type EGFR,177 together with PTEN presence or low Akt activation levels in their GBM cells, exhibited the most favorable outcomes to EGFR inhibitors. In accordance with findings of multiple activated pathways in GBM, addition of the mTOR inhibitor, rapamycin, has been shown to enhance the sensitivity of PTEN-deficient tumor cells to the EGFR kinase inhibitor, erlotinib.178–180 Consistent with enhanced apoptosis resistance by EGFRvIII, activated EGFR has also been shown to confer radio- and chemo-resistance to GBM cells.181,182 These experimental observations and the capacity of EGFR inhibitors to sensitize GBM cells to radiation and chemotherapeutic agents,168,183,184 predict improvent of therapeutic outcome by disruption of EGFR function at the time of ionizing radiation and subsequent chemotherapy, instead of at the time of recurrence.185 These results, coupled with the identification of EGFR-activating ectodomain mutations in 14% of GBMs conveying sensitivity to erlotinib,186 are beginning to detail tumor molecular profiles and therapeutic regimens that will best benefit tumor patients with EGF receptor and downstream pathway genetic lesions.
PDGF receptor (PDGFR)
In addition to the EGFR signaling axis, PDGFR and its ligands, PDGF-A and PDGF-B, are expressed in gliomas, particularly in highgrade tumors, and strong expression of PDGFR occurs in proliferating endothelial cells in GBM.187–190 PDGF-C and PDGF-D, which require proteolytic cleavage for activity, are also frequently expressed in glioma cell lines and in GBM tissues.191 In contrast to EGFR, amplification or rearrangement of PDGFR is much less common, and a relatively rare oncogenic deletion mutation of PDGFR (loss of exons 8 and 9) has been described192 that, similar to EGFRvIII, is constitutively active and enhances tumorigenicity. Given the tumoral coexpression of PDGF and PDGFR, autocrine and paracrine loops may be the primary means by which this growth factor axis exerts its effects. Supportive evidence for a paracrine circuitry initiated by PDGF-B secretion, that enhances glioma angiogenesis, has been shown through stimulation of endothelial cells displaying PDGFR, in part, to express VEGF.193 Besides glial precursor cells, neural stem cells (NSCs) in the adult subventricular zone have been shown to express PDGFR and PDGF could stimulate these NSCs to form glioma-like lesions in the mouse.194 Furthermore, mice transgenic for neural progenitor PDGF-B expression resulted in the formation of oligodendrogliomas and elevation of PDGF-B levels increased overall tumor incidence,195,196 suggesting that targeted therapy against this pathway could have therapeutic potential.197 To this end, an orally active kinase inhibitor of the 2-phenylaminopyrimidine class such as STI571 (imatinib mesylate, Gleevec) has been shown to be a potent inhibitor of these oncogenic loops198,199 and, when combined with hydroxyurea in a phase II study, has been shown to achieve durable anti-tumor activity in some patients with recurrent GBM.200 In contrast, when used alone, imatinib has minimal activity on malignant glioma.201
RTK coactivation and cooperation
An explanation for the failure of EGFR and PDGFR inhibitors to elicit significant clinical outcomes, is that additional RTKs may cooperate to provide a signaling threshold that prevents the inhibition of mitogenic and survival signals through the inactivation of any single RTK. This hypothesis is supported by work that demonstrates that multiple RTKs, in addition to EGFR and PDGFR, are activated simultaneously in primary GBM patients.202 Oncogenic signaling, survival, and anchorage-independent growth, were not fully abrogated until cell lines with endogenous coactivation of RTKs were treated with pharmacological agents or siRNAs targeting at least three different receptors. The significant cross-talk among mitogenic pathways, serves to reinforce the inappropriate regulation of any single pathway perturbation. Importantly, these effects were observed irrespective of PTEN status, indicating that the presence of a tumor suppressor may not be a critical determinant of therapeutic success, as long as upstream signaling effectors are sufficiently inhibited. The discovery of receptor coactivation or cooperation suggests that tumor RTK profiling may be an important step in the development of a personalized GBM therapeutic regimen. Glioma cells engineered to overexpress EGFRvIII to levels observed in GBM caused increased c-MET phosphorylation, that was dependent on the kinase activity and levels of this mutant EGFR.203 The cross-talk between two receptors could be targeted with specific inhibitors to both, resulting in enhanced cytotoxicity of EGFRvIII-expressing cells, compared with either compound alone. It appears that the initially disappointing clinical trials using RTK-targeted agents in GBM should be reanalyzed with respect to the RTK activation profiles of responders and nonresponders and that, when selecting combination inhibitor regimens in future trials, RTK coactivation should be taken into account.
Cell Death, Migration and Angiogenesis
Apoptosis
A hallmark feature of malignant glioma cells is an intense resistance to death-inducing stimuli, such as radiotherapy and chemotherapy. This biological property has been linked to genetic alterations of key regulatory molecules involved in mitogenic signaling, most prominently RTKs and the PI3K–PTEN–Akt signaling axis, as well as regulatory and effector molecules residing in classical cell death networks of both extrinsic (death receptor-mediated) and intrinsic (mitochondria-dependent) apoptosis signaling pathways.
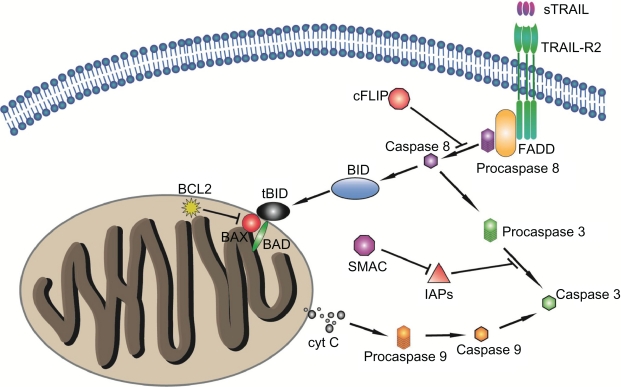
The “death receptors” are cell surface molecules that, upon binding their cognate ligands, recruit adapter molecules to provide a molecular scaffold for the autoproteolytic processing and activation of caspases.204 The most important death receptor systems include TNFR1 (DR1/CD120a), TRAILR1 (DR4/APO-2), TRAILR2 (DR5/KILLER/TRICK2), and CD95 (DR2/Fas/APO-1) (Fig. 5). Several lines of evidence support important roles for these death receptors in glioma pathogenesis. First, various human glioma cell lines and primary glioma-derived cell cultures are sensitive to death ligand-mediated apoptosis in vitro and in xenograft model systems in vivo.205–210 Second, expression levels of these death receptors and in particular of their corresponding (antagonistic) decoy receptors, seem to correlate with susceptibility of glioma cells to death ligand-induced apoptosis. A prominent example is the decoy receptor for CD95 ligand (CD95L), the soluble decoy receptor 3 (DcR3). It is expressed on malignant glioma cell lines, and its expression pattern correlates with the grade of malignancy in human glioma specimens. 211 Interestingly, infiltration of CD4+ and CD8+ T cells and microglia/macrophages was significantly decreased in DcR3-driven xenografts, suggesting that glioma cells escaped CD95L-dependent immune-cytotoxic attack by expressing a decoy receptor, that neutralized CD95L by preventing its interaction with the receptor.211
Figure 5.
The TNF-related apoptosis-inducing ligand, TRAIL. Also designated CD253, this protein, with homology to other members of the tumor necrosis factor superfamily, binds to the death receptors TRAIL-R2 and TRAIL-R1 and causes caspase-8-dependent apoptosis. Caspase-8 activates downstream effector caspases, including procaspase-3, -6, and -7, leading to activation of specific kinases.
The TRAIL death receptor system (Fig. 5), has considerable interest as a specific inducer of cancer cell apoptosis, as its expression has been positively correlated with survival of patients with primary GBM.212 In this regard, loco-regional administration of TRAIL inhibited growth of human glioma cell xenografts,213 and acted synergistically with chemotherapeutic drugs,208,210 in part through up-regulation of TRAIL-R2 and Bak protein and down-regulation of the caspase-8-specific inhibitor cFLIPs.214,215 In addition, peptides derived from the second mitochondria-derived activator of caspases (Smac), a potent antagonist of members of the IAP family of caspase inhibitors, acted synergistically with TRAIL to induce tumor cell apoptosis in vitro and in vivo, without demonstrable neurotoxicity.216 Mechanistically, these peptides abrogate IAP-binding activity and, consequently inhibition of effector caspase-9, caspase-3, and caspase-7 activity downstream from mitochondrial membrane disintegration. This underscores the importance of post-mitochondrial caspase activation for apoptosis propagation in glioma cell lines and its validity as a therapeutic target.216
The role of the Bcl-2 family in gliomagenesis and active cell suicide (apoptosis) has also been extensively studied. The apoptosis regulators Bcl-2, BH (Bcl homology) are a family of evolutionarily related proteins that govern mitochondrial outer membrane permeabilization and can be either pro-apoptotic (Bax, BAD, Bak and Bok) or anti-apoptotic (including Bcl-2 proper, Bcl-xL, and Bcl-w, among a total of 25 Bcl-2 family genes known to date). The members of the Bcl-2 family share one or more of the four characteristic domains of homology entitled the BH domains (BH1 through BH4). The BH domains are crucial for function and deletion of these domains by cloning affects survival/apoptosis. The anti-apoptotic Bcl-2 proteins, such as Bcl-2 and Bcl-xL, conserve all four BH domains. The Bcl-2 family has a general structure that consists of a hydrophobic helix surrounded by amphipathic helices.217 Many members of the family have transmembrane domains. The site of action for the Bcl-2 family is fundamentally the outer mitochondrial membrane.218–220 Apoptogenic factors within the mitochondria (cytochrome c, Smac/Diablo homolog, Omi) if and when released, activate the executioners of apoptosis, the caspases.
On the mechanistic level, classical anti-apoptotic Bcl-2 family members (BAK, BAD, BID, BAX, BCL-XL, MCL-1) modulate apoptosis by preserving mitochondrial membrane integrity and preventing the release of cytochrome C, the caspase cascade and the apoptotic program.221 On the clinical level, tumor grade correlates with the expression of anti-apoptotic Bcl-2 proteins (BCL-2 and MCL-1).222,223 In general, Bcl-2 regulation is shifted toward an anti-apoptotic balance during the transition from initial to recurrent GBM.224 Additionally, Bcl-XL is up-regulated by overexpression of EGFRvIII in glioma cells and this upregulation confers resistance to the chemotherapeutic agent cisplatin.181 In addition to their classical roles, Bcl2 family members may contribute to gliomagenesis through enhancement of migration and invasion, by altering the expression of metaloproteinases and their inhibitors.225–227 Due to their central role and importance in apoptosis signaling, neutralization of antiapoptotic Bcl-2 proteins by antisense technology,228 small molecules that block BcL2 interactions with other families,229 or by viral-mediated delivery of select proapoptotic members,230 may represent promising future avenues of therapeutic intervention.
Necrosis
GBM cells are highly resistant to therapeutic apoptotic stimuli. However, they exhibit a paradoxical propensity for extensive cellular necrosis, which is the most prominent form of spontaneous cell death in GBM. It shows as foci of micronecrosis, surrounded by broad hypercellular zones contiguous with normal tissue or parenchymal infiltrates.231,232 Important causes of necrosis are limited blood supply and anoxia, due to microthrombotic processes. These are the molecular bases for necrosis that, in the context of high apoptotic therapy resistance, has recently come into focus with the discovery and characterization of the Bcl2-like 12 (Bcl2 L12) protein.
Bcl2L12 is a potent inhibitor of the post-mitochondrial apoptosis signal transduction, that is significantly overexpressed in primary GBMs.233 Bcl2L12 is a proline-rich protein characterized by a C-terminal 14-amino-acid sequence with significant homology with BH2 domain, found in several members of the Bcl-2 protein family.234 Overexpression of Bcl2L12 in primary cortical astrocytes inhibited apoptosis, and its RNAi-mediated knockdown sensitized human glioma cell lines to drug-induced apoptosis and reduced tumor formation in an orthotopic transplant model in vivo.233 The anti-apoptotic actions of Bcl2L12 relate significantly to its capacity to neutralize effector caspase activity downstream from mitochondrial dysfunction and apoptosome activity, probably by interacting specifically with effector caspase-7.233 These activities of Bcl2L12 are highly relevant to the necrotic process, considering that suppression of caspase activity downstream from mitochondria redirects the death program from apoptosis to necrosis (reviewed in),235 indicating that post-mitochondrial caspase activation acts as a molecular switch between apoptotic and necrotic cell death paradigms.235
In support of this model, germline deletion of postmitochondrial apoptosis signaling components, such as the caspase activator Apaf-1, or blockade of effector caspase maturation by pan-specific caspase inhibitors, results in decreased apoptosis yet causes increased necrosis.235 Mechanistically, oxidative phosphorylation and consequently intracellular ATP levels, decrease due to extensive cytochrome C release and mitochondrial dysfunction, rendering cells unable to maintain ion homeostasis and provoking cellular edema, dissolution of organelles, and plasma membranes. 235 That apoptosis and necrosis signaling pathways are interconnected, is evidenced by the ability of enforced Bcl2L12 expression to provoke necrotic cell morphology, evidenced by substantial plasma membrane disintegration and enhanced nuclear and subcellular organelle swelling in apoptosis-primed astrocytes.233 Therefore, up-regulation of Bcl2L12 as a novel regulator of the apoptosis/necrosis balance in glial cells, may represent an important event in malignant glioma pathogenesis.
Angiogenesis
GBMs are among the most highly vascularized solid tumors. Microvascular hyperplasia is the defining histopathological phenotype of both primary and secondary GBM. It consists of proliferating endothelial cells, emerging from normal parent microvessels as microaggregates (glomeruloid bodies), accompanied by stromal elements, including pericytes and basal lamina.236 Microvascular density, a measure of microvascular proliferation, is an independent prognostic factor for adult gliomas.237,238 The idea that angiogenesis is rate limiting for tumor growth, and therefore a rational therapeutic target, is strongly supported by animal studies that have shown that angiogenesis is vital for macroscopic solid tumor growth.239
One common feature in the transition from low-grade or anaplastic astrocytomas to secondary GBM, is a dramatic increase in microvascular proliferation (Fig. 1). An equivalently robust microvasculature proliferation phenotype is observed in primary GBM. Since there are marked genomic differences between primary and secondary GBM,240 it is likely that different genetic programs converge on a final common angiogenesis pathway, involving hypoxia-inducible factor (HIF) and non-HIF-dependent downstream effectors, including positive (VEGF, PDGF, bFGF, IL-8, SDF-1) and negative (thrombospondin1, thrombospondin2, endostatin, tumstatin, interferons) regulators of this process.241 A comprehensive understanding of the molecular mechanisms driving angiogenesis in GBM will be necessary for the rational development and deployment of anti-angiogenesis therapies. It is becoming increasingly evident that tumor-associated angiogenesis is not simply a physiological adaptation to hypoxia as a result of an increasing tumor cell mass. Rather it appears to be the result of critical genetic mutations that activate a transcriptional program for angiogenesis, with local tumor oxygen status further modifying this response. The relative contributions of these two mechanisms are not yet fully defined, but it is likely that both may operate to different extents in different tumors or even in different regions of the same tumor. Several experimental studies have shown that key glioma-relevant mutations—including those in the PTEN, EGFR, and CMYC genes—may act as an “angiogenic switch” by stabilizing HIF-1 or one of its downstream targets, VEGF.242–245 The distinction between microvascular proliferation being an adaptive response to hypoxia, or it being an epiphenomenon of critical genetic mutations that also activate a cascade of proangiogenesis pathways, has clinical and therapeutic importance.
Another issue, are the functional consequences of tumor angiogenesis with respect to tissue perfusion.246 Tumor microvessels are highly tortuous, with sluggish flow and diminished gradient for oxygen delivery, increasing susceptibility to thrombosis and microhemorrhages. 247 Thus, GBM microvasculature proliferation may provide little increase in oxygen/nutrient delivery, paradoxically contributing to exacerbate a metabolic mismatch between “supply and demand,” leading to progressive hypoxia and eventual necrosis. This scenario is supported by the experience with anti-angiogenesis drugs, where their limited clinical benefit seems to be the result of “pruning” immature vessel growth and allowing “normalization” of the pre-existing vasculature.248 In addition to the poor vascular architecture, endothelial cells associated with tumor vessels fail to form tight junctions and have few associated pericytes or astrocytic feet, compromising the integrity of the BBB, increasing interstitial edema. Interstitial edema may further compromise regional blood flow and exacerbate tumor hypoxia, leading to areas of necrosis. In addition to these mal-adapted biophysical properties of GBM microvasculature, specific genetic mutations in GBM probably contribute to compromised tumor bioenergetics, specifically the shift in energy production from oxidative phosphorylation to glycolysis.249,250 These interrelated mechanisms lead to a level of metabolic demand that exceeds the ability of the cerebrovascular system to maintain adequate blood flow to prevent hypoxia and necrosis.
Anti-angiogenesis therapies
The hypothesis that interruption of blood supply to the tumor will cause the regression or dormancy of the tumor, has led to the development of several drugs, that target multiple steps in angiogenesis (Table 1). Three approaches, in advanced stages of clinical testing, aim to target VEGF/VEGFR signaling pathways: (1) monoclonal antibodies against VEGF or its receptor(s);251–253 (2) small molecule inhibitors of VEGFR-2 tyrosine kinase activity,254 and (3) soluble decoy receptors created from VEFGR1 receptor, that selectively inhibit VEGF.239 Two new approaches, one of them targeting V3 and V5 integrin receptors on endothelial cells255 and another using umbilical cord blood stem cells256 are also being tested as anti-angiogenesis therapy for GBM. Inhibition of glioma angiogenesis by human umbilical cord blood stem cells (hUCBSC), has been tested in vitro and in nude mice. Downregulation of FAK gene is correlated with downregulation of many angiogenesis-related genes, including Ang1, VEGFA and Akt. Neovascularization and intracranial tumor growth of glioma cells in athymic mice was inhibited by hUCBSC in vivo. Similar to in vitro results, downregulation of FAK, VEGF and Akt molecules was observed, leading to inhibition of angiogenesis in hUCBSC-treated mice brains. Therefore, hUCBSC have the potential to inhibit growth of glioma both in vitro and in vivo.256
Table 1.
Examples of targeted therapies for cancer.
| Drug in clinical use | Commercial name | Drug target | Cancer type |
|---|---|---|---|
| Bevacizumab | Avstin | VEGF | Colorectal, breast, lung, renal |
| Bortezomib | Velcade | Proteasome | Myeloma, lymphoma |
| Colecoxib | Onsenal | COX 2 | Adenomatous polyposis |
| Erlotinib | Treceva | ||
| Gefitinib | Iressa | ||
| Cetuximab | Erbitux | EGFR | Colrectal, lung, head and neck |
| Panitumumab | Vectibix | ||
| RAD 001 | Certican | ||
| Temirolimus | Torticel | mTOR | Renal |
| Imatinib | Gleevec | ||
| Dasatinib Nilotinib |
Sprycel Tasigna |
PDGFR, BCR ABL, cKIT | Leukemia, gastrointestinal |
| Sorafenib Sunitinib |
Nexavar Tasigna |
VEGFR, RAF, cKIT, PDGFR | Renal, hepatic |
| Topotecan Irinotecan | Hycamtin Camptosar | Topoisomerase I | Multiple cancer types |
| Trastuzumab | Herceptin | ERBB2 | |
| Lapatinib | Tykerb | HER2, EGFR | |
| Tamoxifen | Nolvadex | ERα | |
| Exemestane | Aromasin | Aromatase cytochrome P450 | Breast |
| Anastrozole | Arimidex | ||
| Letrozole | Femara | ||
| Rituximab | MabThera | ||
| Tositumomab | Bexxar | CD20 | Lymphoma |
Abbreviations: BCR-ABL, fusion protein of breakpoint cluster region and tyrosin kinase ABL 1; CD20, B-cell phosphoprotein CD20; c-KIT, tyrosine kinase cKIT; COX-2, cyclooxygenase 2; EGFR, epidermal growth factor receptor; ERα, estrogen receptor α; ERBB2, erythroblastic leukemia viral oncogene homolog 2; HER _epidermal growth factor receptor 2; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; RAF, small GTPase RAF; VEGF, VEGFR, vascular endothelial growth factor, receptor.
Clinical studies that used anti-angiogenesis drugs as “single” agents to treat GBM, have shown little efficacy. This may reflect the fact that these drugs have no direct effect on the pre-existing stable microvasculature that may be co-opted to support tumor growth, especially at the infiltrating tumor edge. Recent data, however, suggest that anti-angiogenesis drugs may be more effective when combined with cytotoxic therapy (Table 1). Recently a phase II study of bevacizumab (Avastin; Genentech, Inc.),253 a recombinant humanized monoclonal antibody targeting VEGF, plus irinotecan (CPT-11) in patients with recurrent high-grade gliomas, reported dramatic rates (63%) of radiographic response and a near doubling of 6 month and median progression free survival (PFS) in patients with GBM (30% and 20 week, compared with historical controls of 15% and 9 week). The therapeutic benefits in the setting of combination therapy (radiation and/or conventional chemotherapy) could be attributed to: (1) improved drug delivery, because of improved blood flow; (2) improved drug penetration into the tumor, because of reduced interstitial pressure, and/or (3) improved radiation/chemotherapy response, as a result of reducing tumor hypoxia. Hypoxia is well known to create radiation resistance and reduce efficacy of chemotherapies.257 Overall, the clinical data for the anti-angiogenic drugs, when used in combination with radiation or conventional chemotherapies, are encouraging. The possibility that anti-angiogenic drugs may enhance intratumoral concentration of conventional chemotherapeutics, raises the intriguing possibility that these drugs may improve the efficacy profile of some available antimitotics. A possible mechanism for such synergy could be enhanced drug delivery, although off-target drug effects and/or poorly understood pharmacological mechanisms remain possible. The full benefit of anti-angiogenesis will derive from an improved understanding of the molecular basis of tumor angiogenesis, how tumor cell metabolism drives angiogenesis versus cooptation of normal brain microvascular networks, and definition of those patients that are likely to benefit from various types of anti-angiogenic therapies operating on different levels of the process.
GBM can be roughly separated into an angiogenic component, and an invasive or migratory component. Although the latter component seems insensitive to anti-angiogenic therapy, it is of major importance for disease progression and survival. Clinical symptoms seem to be tempered by anti-angiogenic treatment, but tumour invasion continues. Unfortunately, current imaging modalities are affected by antiangiogenic treatment too, making it very hard to define tumour margins, as shown by MRI, biopsy and autopsy of bevacizumab-treated patients. Moreover, while treatment of other tumour types may be improved by combining chemotherapy with anti-angiogenic drugs, inhibiting angiogenesis in GBM may antagonise the efficacy of chemotherapeutic drugs by normalising the blood-brain barrier function.258 In summary, although angiogenesis inhibition is of considerable value for symptom reduction in GBM patients, lack of proof of a true antitumour effect has raised concerns about the place of this type of therapy in the treatment of GBM.
Tumor cell invasion
Infiltration throughout the brain is prominent in low-and high-grade malignant glioma259 and is the principal reason for the failure of surgical cure. In more than 90% of the cases, the recurrent tumor develops immediately adjacent to the resection margin or within several centimetres of the resection cavity. Invasion by glioma cells into regions of normal brain is driven by a process involving cell interactions with the ECM and with adjacent cells, as well as biochemical processes supportive of proteolytic degradation of ECM and active cell movement. These processes bear a striking resemblance to the robust intrinsic migration potential of glial cells during embryogenesis.260
The most frequent route of invasion of glial tumor cells is along white matter tracts and basement membranes of blood vessels. Whether this route offers a path of least resistance or there are biochemical substrates that mediate adhesion and promote migration, or both, is unclear. Invasion and migration of glial tumors differs from other tumors where local spread is very limited and dissemination occurs hematogenously or via the lymphatic system. In fact, glioma cells lack the ability to penetrate the basement membrane of blood vessels,261 and cells gaining access to the blood through a disrupted blood vessel within the tumor are unable to establish robust tumor growth outside the CNS. The molecular basis for this curious inability of glioma cells to metastasize outside of the CNS is not known and warrants further investigation.
Several genes involved in glioma invasiveness have been identified, including members of the family of metalloproteases (MMP) and their endogenous tissue inhibitors (TIMPs). Expression of MMP-2 and, to a lesser extent, MMP-9 correlate with invasiveness, proliferation and prognosis in astrocytomas.262 Other non-MMP proteases, including urokinase-type plasminogen activator (uPA)263–265 and cysteine proteases (eg, cathepsinB),266 are elevated in high-grade malignant gliomas.267 Despite these findings, the role of proteases in glioma invasion remains unclear, since low-grade astrocytomas infiltrate diffusely throughout the brain, despite their relatively normal levels of proteases.
Integrins, especially V3 complexes, are elevated in GBM and appear to be relevant to processes of glioma invasion and angiogenesis.268 Several studies have also reported potential novel glioma invasion genes. Invasion inhibitory protein 45 (IIp45), a potential tumor suppressor gene on chromosome 1p36, is frequently down-regulated in GBMs. Its product inhibits invasion through the binding of IGFBP2.269 In contrast, IGFBP2 promotes invasion in GBM by up-regulating a panel of genes involved in invasion, one of which is MMP-2.270 Other proteins are overexpressed in invasive areas of GBM, such as angiopoietin-2, which in addition to its involvement in angiogenesis also plays a role in inducing tumor cell infiltration by activating MMP-2.271 Ephrin receptors and their ligands, the ephrins, mediate neurodevelopmental processes such as axon guidance and cell migration and regulate migration and invasion of glioma. Compared with low grade astrocytoma or normal brain, GBMs migratory tumor cells overexpress EphB2,271 which has been linked to poor survival.272
Other novel invasion- and migration-associated genes have been identified using oligonucleotide microarray technology273,274 on RNA isolated by laser-captured microdissection of cryostat sections from human glioma biopsy tumor cores and invasive edges. These genes include P311, a 68-amino-acid polypeptide described in embryonic neuronal migration; death-associated protein 3 (DAP3), which conferred protection from Fas-induced, ionizing radiation-induced, and streptonigrin-induced cell death;275 and FN14, which encodes a cell surface receptor for the tumor necrosis factor super-family member named TWEAK, all of which modulate glioma cell migration and apoptosis.276–278
Since migrating glioma cells show increased levels of phosphorylated Akt, PI3K inhibitors have been tested experimentally on these cells, resulting in a decrease in migration and increased apoptosis sensitivity.279 In conjunction with this, a PTEN mutation has been implicated in an invasive phenotype, not only as contributing to deregulated PI3K signaling, but also in its ability to stabilize E-cadherin and modulate cell matrix adhesion complexes.280 These findings highlight the multitude of ways in which glioma adopt a broad spectrum of the tumor phenotypes, ranging from aberrant cell proliferation to invasion and resistance to apoptosis.
Glioma Chemotherapy, Immunoresistance
Glioma, blood-brain barrier and immunoresistance
Untreated, GBM patients survive less than 6 months. Even after intensive therapy, combining gross total resection, radiation, and chemotherapy, the mean survival time is only 14 months. The lack of effectivity of standard therapies is due to several reasons, including robust tumour cell proliferation, neo-angiogenesis, genetic instability, and immunosuppression. A factor that contributes to GBM malignancy is its high degree of genetic instability, that generates cellular heterogeneity. The various cell types in a glioma population do not respond equally to radiation and chemotherapy, causing further relapses. In addition, chemotherapy has generally been unsuccessful because of poor drug delivery. The presence of active efflux transporters in the blood-brain barrier (BBB), prevents systemically administered drugs from entering the brain, highlighting the need for new comprehensive strategies to overcome this functional obstacle.
A most important and lethal property of gliomas is their immune invisibility. Malignant brain gliomas are able to evade and suppress the immune system. Glioma evasion from the immune system occurs at different levels of antigen recognition and immune activation.281–284 First, by limiting effective signaling between glioma and host immune cells, glioma cells evade immune detection. An important component of this efficient immune escape is the complexity of the self-sustaining glioma microenvironment. Gliomas generate several immunosuppressive mechanisms (Fig. 6) that acting simultaneously, create positive feedback loops that enhance their effects.281–284
Figure 6.
Strategies used by glioma cells to evade host immune system. Glioma inhibited T-cell activation and proliferation by interfering with antigen presentation. Antigen presenting cells (APC) expressed MHC and B7 costimulatory molecules. The interaction via T-cell receptors permitted to identify antigens as self or foreign. When T cells identify non-self-antigens, they activate and proliferate, mounting an immune response. This interaction is disrupted by tumors, that cause downregulation of MHC expression. Loss of MHC molecules blocks the cross talk between tumor cells and the immune cells responding to the tumor. Downregulation of the costimulatory molecules, B7.1 and B7.2, induce T-cell anergy. Upregulation of inhibitory B7 molecules, such as B7-H1, or death signals, such as FasL, cause T-cell apoptosis.
In order to evade immune detection, many glioma cells express low levels of human leukocyte antigens (HLA; major histocompatibility complex, MHC) or express defective MHC.285 A recent report by Facoetti and colleagues286 described that approximately 50% of 47 glioma samples had lost MHC type I antigen. Among these, 80% showed evidence of selective loss of HLA-A2 antigen. It should be noted that loss of HLA type I antigen was more common with higher grade tumors, suggesting a role of deficient antigen presentation in glioma progression. Inhibition of antigen presentation by microglia and macrophages in the tumor microenvironment, also contributes to the tumors’ ability to escape immune detection (Fig. 6). The presence of glioma cells caused monocytes to reduce their phagocytic activity in vitro.287 In addition, microglia found within glioma tissue appeared deficient in proper antigen presentation for cytotoxic and helper T-cell activation.288 Schartner and colleagues289 demonstrated that MHC-II induction by stimulation was significantly less in microglia and infiltrating macrophages derived from gliomas than in those isolated from normal brain.
The CNS is considered immune privileged relative to other organs, by the virtue of the BBB restricting the migration of immune cells and cytokines into the brain, the absence of a lymphatic drainage system, the presence of a high concentration of immunosuppressive factors, and the lack of major histocompatibility complex (MHC) molecule expression in normal CNS cells. However, newer data indicate that the CNS is a perfectly adequate environment for immune responses, as evidenced by the presence of both humoral and cell-mediated CNS immunity.290–292 In addition, lymphocytes have been shown to traffic to normal brain (both naive lymphocytes and activated T cells)293 by crossing the BBB without antigen specificity.293–295 Furthermore, many types of lymphocytes appear in the CNS during illness, such as infection or autoimmune processes.296–298
Many tumors, including GBM, create an immunosuppressive local environment to shield themselves from the body’s normal immune response. The immune microenvironment created by GBM likely plays a much larger role in immune evasion than the general BBB, which is typically compromised by the tumor. The strategies used by GBM to evade the immune system include : (1) aberrant antigen recognition, leading to insufficient immune cell activation, (2) promotion of suppressor immune cells, inducing T-cell tolerance or apoptosis, (3) upregulation of co-inhibitory molecules, (4) secretion of immune inhibiting molecules, (5) recruitment of suppressor immune cells, and (6) activation of immunosuppressive pathways.
Glioma-induced abnormalities in antigen recognition and immune cell activation
One mechanism by which GBM evades the immune system is by preventing normal antigen recognition, a process orchestrated by the major histocompatibility complex (MHC). The MHC, known in humans as human leukocyte antigen (HLA), displays fragmented pieces of self or non-self-antigens on the surface of host antigen presenting cells. Normally, T cells interact with MHC via T-cell receptor molecules, to determine if the antigen is self or foreign. A second signal, the costimulatory signal, is also required for T cells to become fully activated. If this process occurs properly, T cells will react appropriately to foreign peptides and ignore self-peptides (Fig. 6).
Parney and colleagues299 found that most GBM expressed low levels of class I MHC and no class II MHC. These data were supported by Lampson’s finding that class I MHC could be upregulated in gliomas after interferon γ (IFNγ) exposure in vitro. 300 There are several reasons why class I and class II MHC molecules are not expressed on the glioma cell surface. First, gliomas have been reported to express immunoinhibitory factors, such as transforming growth factor β (TGF-β)301 and prostaglandin E2 (PGE2),302 that downregulate class II MHC on glioma cells (Fig. 6). Second, most GBM lesions express mutated class I HLA molecules. Loss of HLA class I antigen significantly correlates with tumor grade3 and with tumors refractory to immunotherapy.303 The components of the antigen processing machinery (APM) were also investigated, and tapasin expression was found to be downregulated in GBM lesions. Tapasin is an endoplasmic reticulum (ER) molecule uniquely dedicated to tether HLA class I molecules, jointly with the chaperone calreticulin (Crt) and the oxidoreductase ERp57, to the transporter associated with antigen processing (TAP).304 These changes seem to be linked with mutations of HLA class I antigen expression, and significantly correlate with the clinical course of the disease. Mutations in HLA class I antigen and in APM components may provide a mechanism for GBM to escape immune recognition and killing by cytotoxic T lymphocytes (CTLs). These findings emphasize the need to monitor expression of HLA class I antigen and APM components in GBM lesions, when selecting patients for T-cell–based immunotherapy treatment.
Co-stimulatory molecules
Co-stimulation of T-cells is necessary for their proliferation, differentiation, and survival. Activation of T cells without co-stimulation may lead to T-cell anergy, T-cell deletion, or development of immune tolerance. CD28, one of the best characterized costimulatory molecules expressed by T cells, interacts with CD80 (B7.1) and CD86 (B7.2) on the membrane of antigen presenting cells (APCs). Besides expressing low levels of MHC peptide (Fig. 6), cancer cells downregulate the co-stimulatory molecules required for activating a proper immune response. Lack of T-cell co-stimulation is another mechanism used by GBM to avoid immune surveillance. So far, B7 molecule expression has been found to be absent from glioma cells.305 In addition, peripheral blood T cells from patients with glioma typically show a high degree of anergy to GBM antigens that results from the absence of co-stimulatory molecules (see Fig. 6). The receptors for the co-stimulatory molecules on tumor-infiltrating APCs are downregulated. Human glioma-infiltrated microglia or macrophages (GIMs) completely lack CD80/CD40 expression and show minimal CD86 expression, which could explain their inability to properly activate naive T cells.306 GIMs from brain tumors in intracranial RG2 glioma-bearing rodents, responded differently to general activators, such as CpG oligodeoxynucleotides (CpG ODN) and IFNg/lipopolysaccharide (LPS), when compared with GIMs from normal brain. CpG ODN induced the upregulation of B7 molecules but had little effect on MHC-II expression, whereas IFNg/LPS had the opposite effect. Both upregulations were significantly lower in tumor-associated GIMs, in comparison with GIMs from normal brain. Further studies are necessary to understand if these diminished effects result from local GBM immunocompromising environment, abnormal signaling, or mutated receptor expression on the tumor-infiltrating GIMs.289
The B7 costimulatory family includes activating and inhibiting molecules that regulate immune response positively and negatively. Among the latter group, B7-H1, one of the newly identified B7 family members, provides negative signals that control and suppress T-cell responses.307 The regulation of B7-H1 seems to be pivotal in shaping the immune response to tumors, because it can exert costimulatory and immune regulatory functions.308 Although B7-H1 has been shown to mediate tumor evasion by binding to programmed death-1 (PD-1) receptor, additional counter receptors can also control the functions of B7-H1.309 Human and rodent cancer cells and immune cells in the cancer microenvironment, have been shown to upregulate expression of inhibitory B7 molecules. Analysis of multiple glioma cell lines and human specimens have also shown high levels of B7-H1 (Fig. 1D).305,310 This high level of expression reduces glioma cell immunogenicity by suppressing T-cell cytokine production and activation. A study by Parsa and colleagues311 demonstrates a potential relationship between B7-H1 and the phosphatase and tensin homolog-phosphatidylinositol 3-kinase (PTENPI3K) pathway. The loss of PTEN and the activation of the PI3K-pathway, leading to elevated post-transcriptional expression of B7-H1, is a new mechanism of immunoresistance mediated by B7-H1, further demonstrating the importance of this molecule in tumor evasion of immune surveillance. 311 B7-H1 correlates with the malignancy grade of gliomas.312 These studies demonstrate the potential benefits of using neutralizing antibodies specific for B7-H1 and PD-1 in the treatment of patients with malignant brain tumors.
Glioma-induced deregulation of cell-mediated immunity
Many studies of patients harboring glioma, performed during the past 3 decades, revealed that these individuals exhibit a broad suppression of cell mediated immunity (Fig. 6). The immune cells from GBM patients appear to behave in a manner reminiscent of autoimmune diseases, showing cutaneous anergy to common bacterial antigens,313 lymphopenia,314 impaired antibody production, and abnormal delayed-type hypersensitivity response to common recall antigens or neoantigens in vivo.314,315 It seems that the lymphocytes of patients with GBM present intrinsic cellular abnormalities that render potentially reactive T cells unresponsive. Peripheral blood lymphocytes (PBLs) from patients with GBM did not proliferate or proliferated minimally in response to mitogen stimulation in vitro. Elliott and colleagues showed that PBLs obtained from patients with GBM have approximately 6 times fewer phytohemagglutinin (PHA)-responsive cells than PBLs from normal subjects.315 These lymphocytes failed to expand into a pool of proliferating cells in vitro. In addition, the supernatant fluids of PHA-stimulated lymphocytes obtained from patients, showed a substantial reduction of interleukin-2 (IL-2) and IFNγ, compared to lymphocytes obtained from normal donors. Moreover, T cells obtained from patients with GBM were unable to offer helper activity in allogeneic pokeweed mitogen cultures in vitro.316–318 This comprehensive depression in cellular immune function was not typical of head trauma, or other tumors of the brain. Hence, it must be the complex GBM tumor microenvironment that compromised T-cell compartments and their functions.
In addition to alterations in the intrinsic activation pathways in T cells, GBM also induced accumulation of immunosuppressive cells in its microenvironment. GBM promoted impaired immunocompetence, using normal immunosuppressive mechanisms involving enhanced proliferation of the regulatory T cells (Treg). Tregs play an indispensable role in maintaining immunological unresponsiveness to self-antigens and in suppressing excessive immune responses, deleterious to the host. In vivo depletion of Treg cells caused severe autoimmune disease, which could be reversed by reconstitution.319 Moreover, the regression of tolerogenic tumors after depletion of Treg cells has been observed in vivo.320
Fecci and colleagues321 reported an unbalanced ratio between CD41 T cells and Treg cells in GBM. Although both fractions were greatly reduced in patients with malignant glioma, Treg cells often represented most of the CD4 population. It is well known that Tregs can inhibit T-cell activation and proliferation by downregulating IL-2 and IFNγ production in the target cells.286,289,305,319,322,323 This would also explain the shift from TH1 to TH2 cytokines, which propagate the regulatory phenotype. As a demonstration of this, depletion of Tregs in vitro reestablishes the normal CD4 functions of the T cells isolated from patients with GBM and reverses the cytokine production to the TH1 type.321 Tumor tolerance induced by Tregs is common in solid tumors other than GBM. In addition to Treg cells, there are other suppressive cell types in the tumor microenvironment. Recruitment of suppressive myeloid cells, such as regulatory dendritic cells (DCs), characterized by indoleamine-pyrrole 2,3 dioxygenase expression324 and myeloid-derived suppressor cells at the tumor site, is another way of inhibiting immune responses.325
Glioma therapies: combined surgery, radiotherapy and chemotherapy
Any disease with an incidence of less than 50/100000 is defined as an orphan disease. There are some 5,000 orphan diseases and about 8% of the population are affected by these disorders. Orphan diseases are, by and large, disregarded by pharmaceutical companies, as the predictable financial incentive is small. Glioblastoma multiforme (GBM), the most common primary tumors of the central nervous system (CNS) in adults,326 with an incidence of about 5 per 100,000 belongs to this group of diseases.327 GBM, WHO grade IV, accounts for approximately 50% of all glial tumor types and shows a median survival of less than one year.326,328
Radiation, introduced some 30 years ago, was the first addition to surgery to treat glioma. The techniques have changed, so that radiation can be directed much more precisely at the tumour, sparing uninvolved brain tissue. However, the value of radiation alone is quite limited, extending life only for a few months.
Chemotherapy, involving various agents over the years, has been added next. The current drug of choice is temozolomide (Temodar),329,330 an oral drug that alters tumoral DNA, inducing tumour cell death. However, intense temozolamide chemotherapy, increased the mutational load within the cancer genome 17-fold in comparison to untreated GBM cells.29 We have already mentioned anti-angiogenesis therapy. Recently, bevacizumab, a monoclonal antibody against VEGF331 used to treat other types of cancer (Avastin), has earned FDA approval for use in brain tumours. Avastin affects tumour blood vessels, rather than the tumour cells251,332 and it is still too early to say whether this therapy will be a significant advance. While therapy directed against tumour blood vessels may limit tumoral growth, its efficacy against the invading tumour cells is unclear.333 The latest recruit to the anti-glioma army is a neuronal micro RNA, mR-326.334 MicroRNA-326 was downregulated in gliomas via decreased expression of its host gene. Transfection of microRNA-326 into both established and stem cell-like glioma lines was cytotoxic.
Unfortunately, chemotherapy can also contribute to inhibit the immune response to the tumor. Thus, temozolomide can cause CD41 lymphopenia,335 which may negatively affect immunotherapeutic approaches that use a CD41 T-cell response. The current state of glioma therapy, in the best medical centers, include the conventional approaches of surgery, radiation and chemotherapy. However, this multimodal treatment still offers a poor prognosis for GBM patients. The therapeutic challenge now is to block the aberrant and complex individual signaling network present in most GBM. Given the extreme adaptability of GBM cells, the therapeutic challenge has to lead to tumor cell death, preventing sublethal hits of tumor cells leading to the growth of more malignant clonal cell populations. This crucial requirement explains the limitations of antiproliferative and antiangiogenetic approaches as therapies. Notwithstanding, blocking simultaneously proliferation and angiogenesis can be a powerful additive pro-apoptotic approach.
Glioblastoma subtypes and treatments adjusted to genomic abnormalities
The diagnosis of brain tumors has been based on a complete clinicopathological assessment. This approach has permitted the distinction of different grades within categories of the same tumor type, such as astrocytomas, that have predictive value in determining clinical outcome. It has become evident from genetic and patient outcome studies, that subgroups are present within each grade. Large-scale gene expression profile studies in glioblastoma have demonstrated that transcriptional profiles reflect the underlying tumor biology. This can be used to predict tumor classification (eg, being a surrogate for pathological grading), patient outcome, and response to treatment. An additional outcome of these investigations has been to realize that each tumor is unique in its expression profile, and therefore biology, suggesting that medicine needs to become more personalized. Although that is a distant goal, it was clear from these studies that it was already possible to cluster the profiles from glioblastoma patients into molecular subtypes defined by combinations of genes that were over- or underexpressed within each group. The Cancer Genome Atlas Network (CGAN) was established to generate a comprehensive catalogue of genomic abnormalities driving tumorigenesis. CGAN provided a detailed view of the genomic changes in a large GBM cohort, containing 206 patient samples. Sequence data of 91 patients and 601 genes were used to describe the mutational spectrum of GBM, confirming previously reported TP53 and RB1 mutations and identifying GBM-associated mutations in such genes as phosphatidyl inositol kinase regulatory subunit (PIK3R1), neurofibromatosis type-1 (NF1), and erythroblastic leukemia viral oncogene homolog (ERBB2).24 Based on gene expression and integrated multidimensional genomic data, GBM were classified into Proneural, Neural, Classical, and Mesenchymal subtypes, establishing patterns of somatic mutations and DNA copy number. The classical, mesenchymal, and proneural types depended on aberrations and gene expression changes of epidermal growth factor receptor (EGFR), NF1, and PDGFRA/IDH1 (platelet derived growth factor receptor alpha/isocitrate dehydrogenase 1), respectively. Gene signatures of normal brain cell types showed a strong relationship between subtypes and neural lineages. The various subtypes differred in their response to aggressive therapy, the classical subtype receiving the greatest benefit and the proneural subtype showing no benefit.413 The work of Verhaak et al413 expanded on previous glioblastoma classification studies, associating known subtypes with specific alterations in NF1 and PDGFRA/IDH1 and identifying two additional subtypes, one of which is characterized by EGFR abnormalities and wild-type p53. In addition, subtypes had specific differentiation characteristics that, combined with data from mouse studies, suggested a link to alternative cells of origin.
To gain insight into the biological meaning of the subtypes, Verhaak et al336 used the data from the brain transcriptome database of Cahoy et al,337 to define gene sets associated with neurons, oligodendrocytes, astrocytes, and cultured astroglial cells. These mature cells may be of interest both, for their primary associations with tumor subtypes, as well as for the inherent signatures retained from progenitor cells. Using these four gene sets, a single-sample gene set enrichment analysis score was calculated for all samples.338 The enrichment score indicates the closeness in expression of a sample to the expression pattern of a gene set. In their exploratory analysis, Verhaak et al336 observed a number of patterns associating each subtype with expression patterns from purified murine neural cell types. The proneural class was highly enriched with the oligodendrocytic signature but not the astrocytic signature, whereas the classical group was strongly associated with the murine astrocytic signature. The neural class showed association with oligodendrocytic and astrocytic differentiation, but also showed strong enrichment for genes differentially expressed by neurons. The mesenchymal class was strongly associated with the cultured astroglial signature. Interestingly, the majority of immortalized cell lines evaluated showed expression patterns most similar to the mesenchymal subtype. Additionally, well-described microglia markers, such as CD68, PTPRC (protein tyrosine phosphatase receptor type C), and TNF, were highly expressed in the mesenchymal class and the set of murine astroglial samples. Together, these data provide a framework for investigating targeted herapies.
Concerns about anti-angiogenic and targeted therapies in GBM patients
GBM growth can be roughly separated into an angiogenic component, and an invasive or migratory component. Although the latter component seems inert to anti-angiogenic therapy, it is of major importance for disease progression and survival.258 Although clinical symptoms are tempered by anti-angiogenic treatment, tumour invasion continues. GBM patients benefit greatly from angiogenesis inhibition, because it reduces cerebral oedema and intracranial pressure. However, on its own, anti-angiogenesis cannot be considered as an effective anti-tumour treatment. Multiple angiogenesis inhibitors have been therapeutically validated in preclinical cancer models, and several in clinical trials. Páez-Ribes et al reported339 that angiogenesis inhibitors targeting the VEGF pathway, showed antitumor effects in mouse models of pancreatic neuroendocrine carcinoma and glioblastoma. However, those inhibitors concomitantly elicited tumor adaptation and progression to stages of greater malignancy, with heightened invasiveness and in some cases increased lymphatic and distant metastasis. Increased invasiveness is also seen after genetic ablation of the Vegf-A gene, substantiating the results of the pharmacological inhibitors. The realization that potent angiogenesis inhibition can alter the natural history of tumors by increasing invasion and metastasis, has important implications for the development of enduring antiangiogenic therapies and warrants further investigation.
The invasive and migratory components of GBM are not affected by anti-angiogenic therapy. Although anti-angiogenic treatment relieves clinical symptoms, tumour invasion continues. Unfortunately, antiangiogenic treatment affects current imaging modalities, making it harder to define tumour margins.258 Moreover, while treatment of other tumour types may be improved by combining chemotherapy with anti-angiogenic drugs, inhibiting angiogenesis in GBM may antagonize the efficacy of chemotherapeutic drugs, by normalizing the blood-brain barrier. Thus, angiogenesis inhibition is of considerable value in GBM patients for symptom reduction, but lack of proof of a true antitumour effect raises concerns about the place of this type of therapy in the treatment of GBM.
Gefitinib and erlotinib are small molecule inhibitors belonging to the newly named “targeted therapies”, designed to inhibit the epidermal growth factor receptor (EGFR) tyrosine kinase.340 Clinical trials have shown their effectiveness for the treatment of patients with advanced non-small cell lung cancer. They have been considered as relatively safe agents, common adverse reactions including mild and reversible diarrhea and skin rash. However, Yan et al341 reported that two cases of brain metastasis from non-small cell lung cancer, developed brain hemorrhage after gefitinib therapy.
Available antiproliferative and antiangiogenetic therapies show clear limitations below, we review the work of our laboratory during the past 20 years. It has led to the purification of a brain glycolipid, named neurostatin, that shows antiproliferative and antiangiogenic activity two orders of magnitude better than temozolomide (Table 2). Experimental glioma treated with neurostatin became detectable to the immune system, allowing for its eradication.
Table 2.
Inhibitory activity (ID50, mM) of O-acetylated (Compounds 2–5) and O-butyrylated (compounds 6–9) compounds on U373MG human astrocytoma and C6 rat glioma cell lines.
| Compounda | ID50 (μM) on U373 cells | ID50 (μM) on C6 cellsb |
|---|---|---|
| TMZc | 401.20 ± 15.30 | 434.92 ± 18.65 |
| GD1b | >50 | >50 |
| O-AcGD1b | 2.00 ± 0.45 | 0.23 ± 0.12 |
| Bi-O-AcGD1b | 2.54 ± 0.41 | 1.65 ± 0.35 |
| Tri-O-AcGD1b | 2.97 ± 0.52 | 3.56 ± 0.74 |
| Tetra-O-Ac GD1b | 2.93 ± 0.49 | 4.01 ± 0.56 |
| O-ButGD1b | 0.83 ± 0.34 | 0.32 ± 0.17 |
| Bi-O-ButGD1b | 1.50 ± 0.46 | 1.31 ± 0.39 |
| Tri-O-ButGD1b | 2.46 ± 0.52 | 1.74 ± 0.44 |
| Tetra-O-ButGD1b | 2.57 ± 0.59 | 1.88 ± 0.61 |
Notes:
All O-acetylated and O-butyrylated compounds were generated from ganglioside GD1b (compound 1);
EGF was used as the mitogen for human U373MG or rat C6 cells. Data are expressed as mean ± SD of ID50 of four independent experiments performed in quadriplicate;
Temozolomide (TMZ) is the best glioma inhibitor clinically available.
Making Glioma Immunodetectable
Gangliosides, growth factor receptors and tumoral growth
Gangliosides are complex glycosphingolipid components of the mammalian plasma membrane, containing sialic acid. They were discovered by Ernst Klenk342 and named on the basis of their isolation properties and high concentration in ganglion cells. Since 1956, when Svennerholm first showed the structural complexity of gangliosides,343 his nomenclature based on the number of the sialic acid(s) and their chromatographic mobility has been commonly used. The structure of a representative ganglioside, GM1, is illustrated, along with similar representations of other gangliosides discussed in this review (Fig. 7). Since their discovery in the 1940s, gangliosides have been associated with a number of biological processes, such as growth, differentiation, and toxin uptake. Hypotheses about regulation of these processes by gangliosides are based on indirect observations and lack a clear definition of their mechanisms within the cell. The first insights were provided when a reduction in cell proliferation in the presence of gangliosides was attributed to inhibition of the epidermal growth factor receptor (EGFR). Since that initial finding most, if not all, growth factor receptors have been described as regulated by gangliosides.344 The effects of gangliosides on growth factor receptors may be understood based on three models: fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and EGFR (Table 3). In the FGFR model, gangliosides can modulate ligand binding; in the second, PDGFR, gangliosides can regulate receptor dimerization; and finally, in the EGFR, gangliosides may affect receptor activation state and subcellular localization. These three models may be extended to all growth factor receptors, bearing in mind that the three models may not be mutually exclusive. Gangliosides may not act independently of well-established mechanisms of receptor regulation, such as clathrin-coated pit internalization and ubiquitination, but gangliosides may contribute to these functions and to signal transduction pathways. Gangliosides probably have a role in diverse biological structures that affect directly the duration of the signal and the localization in the membrane of the growth factor receptor. For this purpose, the plasma membrane is organized into microdomains (lipid rafts) of unique ganglioside composition. Gangliosides were the first tumor-associated antigens described, and changes in cellular ganglioside composition were associated with altered growth properties.345 In other experiments, exogenous administration of gangliosides to Neuro-2A neuroblastoma cells stimulated neurite sprouting and enhanced axonal elongation.346 Therefore, gangliosides are involved in controlling both, growth and differentiation, by modulating growth factor receptor activity.347 The original finding demonstrated that cell growth stimulated by fibroblast growth factor (FGF) was suppressed by exogenous addition of saturating amounts of gangliosides, that inhibited FGF receptor activity. The PDGFR348 and EGFR349 activities were also regulated by gangliosides, and the number of growth factor receptors found to be modulated by gangliosides has been growing since 1990. References to studies of growth factor receptor activities regulated by gangliosides are provided in Table 3. Gangliosides can now be appreciated for their structural role in the organization of plasma membrane lipid microdomains, as well as for their role in regulating growth factor receptor signaling processes.350
Figure 7.
General structure of a ganglioside. The structure of ganglioside GM1 is shown in detail. The Svenerholm shorthand is used: G, ganglioside; M, monosialo; D, disalo; and T, trisialo. The carbohydrate backbone consist of the following sugars: Gluc, glucose; Gal, galactose; GalNac, N-acetylgalactosamine; and NANA, N-acetyl Neuraminic acid (sialic acid). The ceramide (Cer) is composed of sphingosine substituted in the amino group by long-chain fatty acid, typically stearic (C18), or C16, C20, or C22.
Table 3.
Effects of gangliosides on growth factor receptors.
| Growth factor | Ganglioside | References |
|---|---|---|
| FGFR | GM1 | 426, 426–430 |
| GM2 | 431 | |
| GM3 | 422, 432, 433 | |
| GD1b | 426 | |
| GT1b | 426 | |
| PDGFR | GM1 | 423, 434–440 |
| GM2 | 434, 438–440 | |
| GM3 | 423, 434, 438, 442 | |
| GD1a | 434, 438–440 | |
| GD3 | 434 | |
| GT1b | 434, 438–440 | |
| EGFR | GM1 | 424, 436, 437, 444–447 |
| GM2 | 444, 445, 447 |
Notes: Effects of gangliosides on growth factor activity. Gangliosides have been associated with a number of biological processes, such as growth, differentiation, and toxin uptake. Regulation of these processes by gangliosides are based on indirect observations and lack a clear definition of their mechanisms within the cell.
Natural and synthetic regulators of glial proliferation
The number of glial cells in the mammalian brain remains stationary throughout adulthood,351,352 thanks to the concomitant presence of specific mitogens and mitogen inhibitors.353 Definite evidence for the existence in brain of specific inhibitors of astroblast division was presented by Nieto-Sampedro354 and soon confirmed.355 The inhibitor had epitopes in common with both, the carbohydrate moiety of the epidermal growth factor receptor (EGFR) and with human blood groups. Determination of its structure was facilitated by realizing its glycolipidic nature,356 which led to preparation of brain ganglioside extracts. The inhibitor was purified to homogeneity, using a combination of conventional and high performance ion-exchange chromatographies. A combination of bidimensional nuclear magnetic resonance (NMR), MALDI-TOF mass spectrometry and biochemical studies, permitted us to conclude that the inhibitor was O-acetylated GD1b.357 This very scarce ganglioside was called neurostatin, attending to its source and biological activity. It inhibited the proliferation in culture of both primary astroblasts and glioma cells (both rodent and human) at nanomolar concentrations, both in defined medium or in the presence of 10% foetal calf serum. Synthetic oligosaccharide analogues of neurostatin inhibited the division of astroblast, glioma and neuroblastoma cells in culture358,359 and promoted the destruction in vivo of an experimental rat brain glioma.360 Compounds like neurostatin probably arrest glioma growth by direct antimitotic action on the tumoral cells, affecting lipid raft microdomains and interfering with multiple signals regulating cell cycle progression (Fig. 4). Neurostatin-like compounds may also act indirectly, by activating CD4 and CD8 positive immune cells. Neurostatins may be the new type of chemotherapeutic agent that will permit glioma eradication.
Glioma growth inhibition by a synthetic tetrasaccharide, TS4
The synthetic tetrasaccharide α-D-GalNAc-(1–3)-β-D-Gal-(1–4)-[α-L-Fuc-(1–3)]-β-D-GlcMe (TS4), structurally related to blood groups, was an analogue of the carbohydrate moiety of neurostatin (Fig. 9). It was the best first generation synthetic inhibitor of astroblast and astrocytoma growth, designed based on its immunological properties before neurostatin structure was known.358,359 TS4 inhibited the proliferation of C6 rat glioma cells in culture, as well as the growth of brain tumors formed after intracerebral transplantation of C6 cells.360 As expected from the cytostatic action of TS4 on glioma cells in culture, TS4-treated tumors were substantially smaller than controls. This action was probably mediated by two plasma membranes C-lectin type proteins of apparent molecular weight 250 kDalton and a 150 kDalton (isolated by affinity chromatography on immobilized TS4 from both C6 cells and brain tissue; Díaz-Mauriño and Nieto-Sampedro, unpublished). TS4-like or blood group-like cell surface molecules, together with their binding proteins, define a cell adhesion system involved in the control of cell division. These cell adhesion systems interact with tyrosine kinase pathways at more than one level, mediating growth or its inhibition.361–363 Interaction of soluble TS4 with cell surface glycan binding proteins, mimicked cell-cell contact, and evoked inhibition of cell proliferation and tumor growth.
Figure 9.
Structures of neurostatin (O-acetyl GD1b) and O- butanoyl-GD1b. O-acetyl-GD1b (neurostatin) is a natural, GD1b derived, inhibitor of astroblast and astrocytoma division. Its structure and scarcity limits its stability and availability. Therefore, we prepared O-acetylated and O-butyrylated GD1b by chemical synthesis.
TS4 binding to glioma cells made them immunovisible
Because TS4 was cytostatic for C6 cells in culture, finding that TS4-treated tumors were smaller than controls was expected. However, in addition, the tumors appeared necrotic. Tumor appearance suggested that the tumors had grown comparatively large and later had been destroyed (Figs. 10 and 11). C6 glioma cells originated from Wistar rats and, when transplanted on isogenic hosts, proliferated unhindered and migrated without causing vasculature disruption. The effects of TS4 treatment in vivo went beyond interference with cell division and caused transformed cell apoptosis and tumor destruction. Probably, TS4 binding activated indirectly other tumour toxicity mechanism/s. At least four cell-mediated actions, not mutually exclusive, could lead to tumor destruction: (i) inhibition of tumor neovascularization; (ii) activation of microglia; (iii) activation of natural killer (NK) cells and iv) activation of cytotoxic lymphocytes (CTL).360 The enhanced immunogenicity of TS4-treated glioma cells seemed related to their increased expression of connexin 43, observed in glioma cell cultures treated with the oligosaccharide.361,362
Figure 10.
CD-8 immunoreactive cells in and around a brain glioma. Saline-treated C6 glioma tumours, 21 days following transplantation of 105 cells into brain. (A–C) The tumors were infiltrated by CD8-positive cells of two types: (B), small, intensely staining, and (C), larger, less immunoreactive. (D), Tumors treated with TS4 were smaller than saline-treated controls and their appearance was necrotic. (E, F) CD8-positive cells in TS4-treated tumors were more numerous, stained more intensely, and were larger than similar cells in controls. High numbers of CD8-positive cells were observed infiltrating the tumor, forming a gradient of decreasing immunoreactivity towards adjacent tisue (star). The sections werecounter stained with cresyl violet. Magnification bars: A, D, 230 μm; B, C, E, F, 23 μm.
Figure 11.
Infiltration of glioma by microglia or macrophages (GIMs). OX-42 (CD 11b) immunoreactive cells infiltrating C6 glioma tumors, 21 days after transplantation of C6 cells (104 cells/2 μl) into the parietal cortex. The tumors were treated during the 14 days previous to sacrifice with: (A), saline solution; (D), TS4 solution in saline. Immunoreactivity was intense in and near the tumor, sharply decreasing with distance (D, star). (B) Immunoreactive cells in normal tissue had the morphology of ramified microglia. (C), In saline-treated tumors, infiltrating immunoreactive cells were round (arrowheads), whereas (E), most CD11b-positive cells in TS4-treated rats were slightly elongated (arrowheads) and, frequently, particularly in cells near blood capillaries, had short processes (F). Sections were counterstained with cresyl violet. Bars: A, D, 230 μm; B, E, 23 μm; F, 47 μm.
Although CD8 positive (CD8+) cells with lymphocyte morphology infiltrated C6 tumors, both TS4-treated as well as untreated, they did not prevent the growth of untreated glioma controls. Therefore, simple recruitment of extra lymphocytes, not observed after TS4 infusion into normal brain, was not involved in TS4-induced tumor destruction. Two types of infiltrating CD8+ cells were observed which, judging by their size and morphology, may be NK cells and CTLs (Fig. 10).360 Activation of NK cells requires activator binding to the NK receptor protein, NKR-P1 and TS4-like oligosaccharides are ligands of this protein.364,365 The oligosaccharide activators must be presented as lipid or pseudolipid micelles,364 but this manner of presentation could be mimicked by TS4 bound to its C lectin receptors on the surface of C6 cells. The specificity of NKR-P1 and of the TS4 receptor lectins is such, that each binds preferently to sugar sequences at opposite ends of the TS4 molecule (Fig. 13). Hence, TS4 bound to lectin receptor on C6 membranes could still associate to NKR-P1, leading to NK cell activation and glioma destruction.
Figure 13.
Cell adhesion systems interact with tyrosine kinase pathways at more than one level, mediating growth or its inhibition. Interaction of soluble neurosttin or analogues with cell surface glycan binding proteins, mimick cell-cell contact, and evoke inhibition of cell proliferation and tumor growth.
Intercellular junctions, connexin 43 expression and glioma growth
Transfection of C6 cells with cDNA coding for the gap junction protein Cx43, caused decreased proliferation of glioma cells.361,362 Transfected cells showed molecular disturbances in the IGF system,366 which made the tumor cells detectable by host CD8+ cytotoxic lymphocytes.367 The possibility that TS4 could enhance Cx43 expression by glioma cells, was tested by treating equal numbers of exponentially growing C6 cells with either, TS4 solution (final concentration 1 mg/ml or 1.4 mM), or with the same volume of PBS. TS4-treated cells showed complete arrest of cell division, but maintained viability. Controls, continued to grow normally. Northern blots of RNA from control and TS4-treated cells, performed using a Cx43 cDNA probe, indicated a specific increase in expression of Cx43 mRNA in TS4-treated cells. The increase in Cx43 mRNA expression, ranged from 2.5 to 2.9-fold in four different experiments (average ±17%, n = 4). Cx43 expression in vivo, in tumors treated with TS4 solution (20 mg/ml) during 14 days, could not be observed by in situ hybridization with the same probe, as treated tumor cells were not viable.
New synthetic glioma inhibitors
The synthesis of the tetrasaccharide TS4 was rather long and labour-intensive. To overcome this limitation, we designed a simpler synthesis of analogues exhibiting the main structural features of TS4.368–371 An octyl N-acetylglucosaminide derivative with a pentaerythritol chain at position 6 (Compound 1, Chart 1) inhibited the growth of a neuroectodermic tumor implanted in rats and, when loaded on a slow-delivery polymer disk, caused the destruction of cultured human astroblastoma, obtained after surgical biopsy.369 Compound 1 also inhibited the division of human U-373 glioma cells in culture, although with a modest ID50 value (43 mM). A variety of di- and monosaccharides were readily synthesized and evaluated as inhibitors of neural tumor growth. In order to get compounds with improved activity, a new series of monosaccharides was obtained by systematic modification of the substituents at positions 1, 2, 3, and 6 of the glucosamine backbone, and tested as inhibitors of proliferation on rat (C6) and human (U-373) glioma.370 The results obtained indicated that the activity was increased by a long hydrocarbon chain at position C-1 of the glucosamine backbone, the most inhibitory compound being the oleyl glycoside 2 (Chart 1). To obtain information about its mode of action, metabolite changes in C6 glioma cells were analyzed after treatment with glycoside 2, using high-resolution magic angle spinning (HR-MAS) 1H NMR.371 The data obtained from the 1H NMR spectra of the different experiments suggest that glycoside 2 inhibited cell division (IC50 approx. 10 μM) by inhibiting de novo synthesis of fatty acids. At higher concentrations (above 40 μM), a significant ratio of cell death occurred through apoptosis.
Chart 1.
A long hydrocarbon chain at position C-1 of the glucosamine backbone of synthetic glycolipids increased their inhibitory activity.
Neurostatins, natural brain antimitotics
Neurostatin, purified from rat and bovine brain extracts,356,357 had epitopes in common with both, the carbohydrate moiety of the epidermal growth factor receptor (EGFR) and with human blood groups.354,355 This very low abundance modified ganglioside, like its synthetic oligosaccharide analogues, inhibited in culture the division of astrocytes, glioma and neuroblastoma cells354,359 and promoted, in vivo, the immune destruction of an experimental rat brain glioma.360 Determination of the precise structure of neurostatin required the purification of comparatively large amounts of the molecule. The fractionation of the amounts of brain tissue required was facilitated by preparation of ganglioside extracts.371 Neurostatin was purified from such extracts, using a combination of conventional and high performance ion-exchange and reverse phase chromatographies.357 The purification to homogeneity of neurostatin permitted the determination of the inhibitor structure, combining bidimensional nuclear magnetic resonance (NMR), MALDI-TOF mass spectrometry and biochemical studies. We concluded that neurostatin was GD1b, 9-O-acetylated on the outer sialic acid residue (Fig. 9). The acetylated ganglioside inhibited the proliferation in culture of both primary astroblasts and glioma cells (both rat and human) at nanomolar concentrations, either in defined medium or in the presence of 10% foetal calf serum.357
The O-acetylated forms of gangliosides GD3, GT3, GD1b, GT1b and GQ1b are present in neural tisue, at developmentally regulated concentrations.372–374 The expression of O-acetyl-GD3 and O-acetyl-GT3 is particularly high during the O2A precursor proliferative stage.374 GD3 is overexpressed in many types of tumors,375 whereas O-acetylated GD3 is present in fast growing tumors, such as melanoma.376 In contrast, pure neurostatin was cytostatic for astrocytes and glial-derived cell lines, several of them capable of malignant growth. Thymidine incorporation into rat C6 glioma cells or human astrocytomas U-373 and U-118, was inhibited in a concentration-dependent manner, like for TS4 (Fig. 8), but with IC50 values ranging from 200 to 450 nM (Table 2). In contrast, primary rat fibroblasts or the fibroblast line 3T3, mouse neuroblastoma N2A or human neuroblastoma SH-SY5Y, were not affected, suggesting that the antimitotic activity of neurostatin addressed selectively cells of glial lineage. Neurostatin inhibited glial proliferation, regardless of whether or not the cells required mitogens to enter division. Thus, it inhibited proliferation of human U-373 astrocytoma, a cell line probably autocrine and capable of dividing in serum-free or growth factor-free culture medium.
Figure 8.
Dose-response of the inhibition by TS4 of C6 glioma cell division. Inhibition of glioma cell growth, promoted by 10% fetal calf serum, was measured as incorporation of tritiated thymidine into cultured cells and correlated with the proportion (%) of viable cells determined by counting cell number. The concentration of TS4 that inhibitedby 50% [3H] Thy incorporation (ID50) was 110 μM. At the maximal concentration of TS4 tested in vitro (4 mg/ml; 102% + 9% inhibition), 96% of the cells were viable (not shown).
New semi-synthetic inhibitors
When ganglioside GD1b was O-acetylated in its outer sialic acid, it became the potent inhibitor of astroblast and astrocytoma division, we called neurostatin.356,357 Because neurostatin showed specificity for cells of astroglial lineage, it was a suitable candidate for treatment of CNS astrocytomas.356,357 However, the need to prepare and purify neurostatin from brain extracts, limited the availability and purity of the compound.357,377 Moreover, the O-acetyl group of neurostatin was very labile to hydrolysis under physiological pH conditions. 357 These limitations prevented the application of the compound to in-vivo studies and made its prospective clinical use virtually impossible. In order to overcome these limitations, we proposed to use a semi-synthetic alternative to brain neurostatin, a method that permitted to obtain neurostatin in larger amounts.378 We hypothesized that an O-acyl aliphatic chain longer than acetyl (ie, butyryl), may lead to neurostatin analogues more resistant to hydrolysis that neurostatin itself, also preventing the degradation of the natural compound by specific enzymes. Gangliosides O-Ac GD1b (Neurostatin; Galβ1–3GalNAcβ1–4[9-O-Ac Neu5Ac 2–8Neu5Ac α 2–3]Galβ1-4Glcβ1-1′-ceramide) and O-But GD1b (Galβ1–3GalNAcβ1–4[9-O-But Neu5Acα2–8Neu5Acα2–3]Galβ1-4Glcβ1–1′-ceramide) were obtained by chemical O-acetylation or O-butyrylation of GD1b,378,379 using a modification for gangliosides380 of the original method of Ogura et al.381 Ganglioside GD1b (125 μg) was dissolved in dimethylsulfoxide (DMSO; 12.5 μl) and treated with either trimethyl orthoacetate (TMOA; Sigma-Aldrich) or trimethyl orthobutyrate (TMOB; Sigma-Aldrich, St Louis, MO) in 500 molar excess, in the presence of p-toluensulfonic acid (0.0125 mg; Sigma-Aldrich) as catalyst. The reaction mixture was maintained for 8 hours in the darkness at 18–21 °C and acylation was stopped by addition of methanol (1 ml). The mixture was desalted by reverse phase filtration382 and O-substituted gangliosides were purified by preparative thin layer chromatography.383
Both, O-Ac GD1b and O-But GD1b (Fig. 9), had similar inhibitory activities on rat C6 glioma cells (ID50, 230 and 320 nM, respectively) and rat C6 glioma cells transfected with green fluorescent protein (C6-GFP). Inhibition of division of C6 cells promoted by EGF and analyzed by flow cytometry, showed that the inhibitors maintained a large proportion (60%) of the glioma cells in the resting G0 phase, compared to 47% in the absence of inhibitor (Fig. 12). Inhibition of glioma proliferation occurred without toxic or inhibitory effects for fibroblasts or neuroblasts, at the maximal concentration tested (10 mM).378 Therefore, use in vivo was possible. The anti-tumoral activity in-vivo of compounds O-Ac GD1b and O-But GD1b was tested on two glioma models: (i) a glioma xenografts in Foxn1nu/nu nude mice; (ii) intracranial glioma transplanted in rats (C6 or C6-GFP).378
Figure 12.
Neurostatins inhibit experimental glioma growth in nude mice. (A) Growth of C6 glioma cells xenografted on nude mice. Effects of GD1b, O-Ac GD1b and O-But GD1b. When tumors reached the required size (120 mm3; day 12), they were treated with PBS or with the gangliosides GD1b, O-Ac GD1b or O-But GD1b (8 mg/kg animal in PBS, in 5 injections from day 12 to 24). Tumor growth was evaluated determining the tumor volume every three days. (B) Representative images of tumor appearance at the end of the experiment (day 33). Treatment with PBS was the vehicle control and GD1b was used as inactive ganglioside control.
Notes: Daily growth statistical differences: *P < 0.05 vs. PBS; **P < 0.01 vs. PBS; +P < 0.05 vs. GD1b; ++P < 0.01 vs. GD1b. Overall growth statistical differences: #P < 0.05 vs. PBS; ##P < 0.01 vs. PBS; &P < 0.05 vs. GD1b; &&P < 0.01 vs. GD1b, calculated by ANOVA followed by posthoc Tukey test.
Female Foxn1nu/nu nude mice were subcutaneously injected in the right flank with a suspension of 3 × 106 C6 cells in serum-free DMEM medium. Treatment with inhibitors was started when palpable tumors reached a volume of about 120 mm3. The animals were treated by intratumoral injection (8 μg/kg animal in PBS in 5 injections, from day 12 to day 24) of O-Ac GD1b and O-But GD1b, and the tumor volume was compared to those of controls injected either GD1b or PBS (vehicle). Tumor dimensions were measured every 3 days, and tumor volume was calculated as (width)2 × length × π/6. Tumor volume index was calculated using the difference between the measured volume and the volume measured at the start of the treatment. Additionally, the time-course of growth was adjusted to a straight line to compare the slopes (tumor growth rate) after the various treatments (Fig. 12). The animals were sacrificed 33 days after tumor implantation and tumor progression evaluated. Two independent experiments were carried out (n = 8). Neither, the PBS solvent nor the parent ganglioside GD1b, had any inhibitory effect on tumor growth. On the other hand, one single intratumoral injection of low concentration of chemically substituted O-Ac GD1b and O-But GD1b, inhibited tumor growth significantly (Fig. 12A), when compared to the vehicle (PBS) and the parent compound, GD1b, from day 27 after cell implantation until the end of the experiment (day 33). O-But GD1b showed the highest inhibitory activity (Fig. 12A and B), reducing tumor growth by 49% at day 33 compared with the PBS control and 45% when compared with the unmodified ganglioside (GD1b). O-Ac GD1b also inhibited the tumor growth, but it was less effective than O-But GD1b (Fig. 12A and B). The overall tumor growth rate was assessed comparing the slopes of the time-course of tumor growth: compared to controls (PBS and GD1b), both O-acyl compounds significatively reduced tumor growth rate.378
Mechanisms of Growth Inhibition
Direct inhibition of glioma proliferation
Growth factor signal transduction pathways, often upregulated in brain tumors, may contribute to oncogenesis through autocrine and paracrine mechanisms. The Ras signaling pathway is frequently overactive in gliomas. Receptor tyrosine kinase inhibitors, antireceptor monoclonal antibodies and antisense oligonucleotides, are approaches under investigation to regulate aberrant growth factor signaling pathways in brain tumors.384 Inhibitors of tyrosine-kinase receptors, that inhibit the Ras signaling pathway, also inhibit the growth of malignant gliomas. Cytokines PDGF and EGF play important roles in glial development and oncogenesis.155,385,386 The EGF receptor (EGFR) is mainly expressed in glioblastoma multiforme,155 while the receptor for PDGF (PDGFR) is expressed in most types of gliomas.385 PDGFR-A and PDGFR-B expression were observed in highly proliferating tumor cells, as well as in endothelial cells.385,387 Both growth factor signal transduction pathways are involved in cancer stem cell proliferation and glioma growth, but we do not know how neurostatin or TS4 interfere with them. The tetrasaccharide had receptors on the plasma membranes of neural cells. Two C-lectin type proteins, of apparent molecular weight 250 kDalton and a 150 kDalton, respectively, were isolated by affinity chromatography of solubilized plasma membranes from either C6 cells or from brain tissue on immobilized TS4.388 TS4-like molecules or membrane-bound neurostatin, together with their receptor proteins, define a cell adhesion system that may be involved in cell division control (Fig. 13). Cell adhesion systems interact with tyrosine kinase pathways at more than one level, mediating signaling of growth or its inhibition.361–363,389 Interaction of soluble TS4 or neurostatin with cell surface glycan binding proteins, mimicked cell-cell contact, and evoked inhibition of cell proliferation. Such inhibition was also observed as a significant decrease in the respose to mitogen, ie, diminution of the number of cells incorporating bromodeoxyuridine (BrdU), or showing inhibited expression of phosphohistone H3 (pHH3), a mitosis marker that is phosphorylated in the late G2 phase of the cell division cycle. Valle Argos et al390 observed that tumors treated with O-Ac GD1b or O-But GD1b incorporated 48% or 65% less BrdU, and expressed three times less pHH3, than tumors treated with PBS. The transition of G1 to phase S is regulated by cyclin D1, kinase CDK6 and the cell cycle inhibitors p21 and p27. The expression of both cyclin D1 and CDK6 was reduced 1.7 and 2.8 times, respectively, in tumors treated with neurostatin, and 3 and 3.4 times if the tratment was with O-But GD1b. On the other hand, the cell cycle inhibitor p27 was overexpressed after treatment with neurostatin (5-fold) or O-But GD1b (6.4-fold). Similar increases were observed for p21 inhibitor.390 Tumoral growth inhibition occurred with concomittant activation of pro-apoptotic proteases, such as caspase-3.
Indirect, immune cell-mediated glioma destruction
C6 glioma cells originate from Wistar rats and, when transplanted into isogenic hosts, proliferate unhindered and migrate without causing vasculature disruption. Because TS4 was cytostatic for C6 cells in culture, it was expected to find that TS4-treated tumors would be smaller than controls (Fig. 2C). However, in addition, the tumors appeared necrotic. Tumor appearance suggested that the tumors had grown comparatively large and, at a latter time, had been destroyed (Figs. 10 and 11). Destruction of tumors treated with neurostatin or TS4, suggested that the glycocompounds activated indirect toxicity mechanism/s. At least four cell-mediated actions, not mutually exclusive, may lead to tumor destruction: (i) activation of natural killer (NK) cells; (ii) activation of cytotoxic lymphocytes (CTL); activation of microglia, and (iii) inhibition of tumor neovascularization. Each of them will be briefly considered.
CD8+ cells with lymphocyte morphology infiltrated all C6 tumors, both TS4-treated as well as untreated, but did not prevent the growth of controls. Tumor destruction induced by TS4 did not involve simple recruitment of extra lymphocytes, since it was not observed after TS4 infusion into normal brain. Two types of infiltrating CD8+ cells were observed which, judging by their size, may be NK cells and CTLs (Fig. 10). Activation of NK cells requires activator binding to NKR-P1, and TS4-like oligosaccharides are ligands of this protein.364,365 The oligosaccharide activators must be presented as lipid or pseudolipid micelles,364 but this manner of presentation could be mimicked by TS4 bound to its C lectin receptors on the surface of C6 cells. The specificities of NKR-P1 and that of the TS4 receptor lectins are such, that each could bind preferently to sugar sequences at opposite ends of the TS4 molecule. Hence, TS4 bound to lectin receptor on C6 membranes could still associate to NKR-P1, leading to NK cell activation and glioma destruction.
Various reports suggest that TS4-induced Cx43 expression could mediate CTL activation. Stable C6 transfectants overexpressing Cx43, showed inhibition of proliferation391,392 and decreased tumorigenicity,393 probably related to improved immune response.285 The link between Cx43 expression and increased tumor immunogenicity, appears to be the IGF-I autocrine system. C6 transfectants overexpressing Cx43, showed altered expression of IGF-I-binding proteins (IGFBP)366 and transfection of C6 with antisense IGF-I cDNA also led to loss of tumorigenicity.394,395 Antisense IGF-I cDNA transfected cells expressed reduced levels of growth factor, were not tumorigenic and, in addition, transplantation of transfectant cells caused the regression of tumors formed by wild type C6 cells. CD8+ cytotoxic lymphocytes that had previously ignored the tumor, were activated by transfectant cells.394 It is conceivable that a similar chain of events could be triggered by TS4 infusion. Increased expression of Cx43 by TS4 treated C6 cells, would also lead to enhanced immunogenicity, cytotoxic lymphocyte activation and tumor destruction.
It has been reported that reactive microglia surround experimental tumors formed by rat glioma cells, and that tumors are infiltrated by numerous microglia-derived macrophages.396 In agreement with these observations, we found numerous OX-42 (CD 11b) positive microglial cells both surrounding and infiltrating C6 tumours (Fig. 11). It is well established that microglia are efficient antigen-presenting cells, and both Morioka et al391 and ourselves have shown, using different experimental models, that these brain cells react strongly to tumoral growth. The number of reactive microglia/macrophages infiltrating the tumour increased 5 to 10-fold in neurostatin-treated tumours.397
Finally, the cytostatic activity of TS4 and neurostatin was not limited to neural cells. Proliferation of other cell types such as endothelial cells or fibroblasts was also inhibited, though at concentrations much higher.398 Therefore, at the high TS4 concentrations used in vivo, endothelial cell division, and hence tumor vascularization, might have been inhibited. This would help to inhibit tumor growth and possibly lead to tumor necrosis.
The biological role of blood group carbohydrates has never been established, but the results reported in previous papers suggested that they may be involved in controlling abnormal cell proliferation.355,359 Neurostatin and TS4-like analogues, in which glucose was replaced by N-acetyl-glucosamine, or where N-acetyl-galactosamine was replaced by N-acetylneuraminic (as in sialyl Lewis X, SiLex), had similar antimitotic properties.399 Sialic acid containing glycoproteins are common markers of transformed cells400 and SiLex has been found in tumors401 and in the sera of cancer patients.402 It has been proposed that interaction of SiLex with the endothelial selectin ELAM-1 mediates extravasation of tumoral cells and, hence, metastasis.403–406 Both fucose and sialic acid are absolute requirements for ELAM-1 binding,406 but the effect of substituting sialic acid for GalNAc has not been tested. Neurostatin, TS4 and analogues could be also antimetastatic, if used at concentrations capable of competing for binding to the natural ligand of the selectin.
Glioma immunotherapy
The possibility of harnessing the potency and specificity of the immune system to destroy tumours, underlies the growing interest in cancer immunotherapy of our and other groups.407–409 The compromised viability of glioma cells after binding neurostatin or analogues, like TS4, may engage the immune system, presumably by attracting numerous microglia-derived macrophages or dendritic cells to glioma debris. Thus, glioma antigens would cease to be cryptic and its presentation to NK and T cells would follow, leading to glioma destruction.
Another approach has used dendritic cells (DCs) to present tumor-associated antigens (TAA) thereby, generating tumor-specific immunity.410–412 DCs are extremely potent antigen-presenting cells, specialized in inducing activation and proliferation of CD8 cytotoxic T lymphocytes (CTL) and helper CD4+ lymphocytes.413 This unique property has prompted their application in therapeutic cancer vaccination. In the design and conduct of DC-based immunotherapy trials, several important considerations influence induction of a successful protective response.414 First is the source of tumor antigen that can be loaded onto DC. In case of unknown tumor antigens, the source of antigen is, by necessity, a tumor cell lysate, apoptotic tumor cells, whole tumor–derived RNA, or tumor-derived exosomes.415 Second, it is important the way in which DCs are activated, because immature DCs can tolerize the antitumoral response.416 Other important variables are dose, frequency, timing, and route of administration.417–422 Taking into account all these variables, most studies have shown that injection of mature tumor antigen-treated autologous DCs into tumor-bearing hosts, induced protective and therapeutic antitumor immunity in experimental animals and, for some malignancies, in patients.422,423 Mice receiving dendritic cells treated with tumor lysate before tumor implantation, demonstrated protective antitumor immunity with prolonged survival (3 months) and even resisted a second tumor challenge. Tumor protection was associated with strong tumor-specific cytotoxic T-lymphocyte responses. Adoptive transfer of splenocytes or purified CD8 T lymphocytes transferred tumor protection to unimmunized mice in vivo. When given after tumor implantation in a therapeutic setting, pulsed dendritic cells prevented malignant mesothelioma growth. However, with higher tumor load and delayed administration after tumor implantation, dendritic cells were not effective.407 Nearly twenty years of experimental immunotherapy for malignant glioma have yielded important insights in the mechanisms governing glioma immunology. However, although still considered promising, it is clear that immunotherapy, on its own, does notrepresent the magic bullet in glioma therapy.
Genetically engineered models of glioma
There is little debate on the importance of murine models for advancing our understanding of the complex biology of gliomas. Various types of in vivo model systems have been developed and utilized, including traditional orthotopic xenotransplants with established human glioma cell lines and, more recently, with primary human glioma cells enriched for surface expression of CD133.2,424 There is great interest in the further development of the CD133 primary tumor model system as this appears to be superior in recapitulating the diffuse infiltrative nature of the primary human disease. Whether the CD133 primary tumor system will prove to be a more accurate biological model or be more predictive in drug testing than xenotransplant models with established cell lines is an area of current investigation.
In recent years, important advances have been made in the construction of genetically engineered mouse (GEM) models, harboring glioma-relevant mutations or combinations of mutations. In several cases, such GEMs predictably develop gliomas with many of the features of the human disease.1,14,425,426 Given the experimentally tractable nature of the mouse, these glioma-prone GEM models are beginning to shed light on a number of key issues such as, for example, the glioma cells of origin,427 the ordering of mutations and whether such events underlie various glioma subtypes428 and the cooperative and epistatic relationship of such mutations. The complex heterotypic interactions between the evolving tumor cell and the host microenvironment, among other issues central to the problem of gliomagenesis, may also be approached with GEMs. With further refinement, there is now increasing evidence that these GEM model systems will provide an additional vantage with which to test the timing, dosing, and combination of drugs in the pipeline and assist in the development of drug response biomarkers.124,429 These models are ideal for investigating the biological mechanisms underlying tumorigenesis and for the functional validation of candidate genes identified through large-scale genomic analysis of tumor specimens. The need for accurate models is perhaps most acute in preclinical testing, where experimental data often determine the fate of a drug in development. Although additional study is needed, it is widely anticipated that refined GEM models of glioma should enable the identification of tumor maintenance genes and the testing of agents targeting such mission critical lesions, thereby identifying key targets, the best agent, and the right patient population ie, genotype; Sharpless and Depinho.430 Thus, GEM models may permit culling of ineffective drugs and improve the design of trials for those entering phase I/II clinical trials. In addition, the availability of refined GEM models that evolve through stages may help define the tumor grade where an agent or combination of agents may be most effective.
Micro RNA and glioma growth
MicroRNAs (miRNAs) are short single stranded RNA molecules, that serve as master regulators of gene expression in a sequence-specific fashion. miRNAs bind to 3’untranslated regions (UTRs) of mRNAs and affect the translation and/or stability of that mRNA, leading to a reduction in the levels of protein. Tumors analyzed by miRNA profiling have exhibited significantly distinct miRNA signatures compared to normal cells from the same tissue.431 The abnormal levels of miRNAs in tumors have important pathogenetic consequences. Some miRNAs are over-expressed in tumors and act as oncogenes, promoting tumor aggravation by down-regulating tumor suppressors.432 Thus, the miR-17- miR-92 cluster in T-cell acute lymphoblastic leukemia, reduces the level of the transcription factor E2F1; miR-21 in lung cancer cells downregulates the tumor-inhibiting factor PTEN; and miR-125b is an important repressor of p53, inhibiting p53-induced apoptosis in human neuroblastoma cells.433 On the other hand, tumors lost miRNAs generally participate in oncogene overexpression. For example, the let-7 family represses Ras and Myc oncogenes in cancers,434 and the miR-15-miR-16-1 cluster down-regulates Bcl-2 and induces apoptosis in a leukemic cell line model.435
miR-26b is one of the miRNAs involved in the response to hypoxia, a well documented tumor microenvironment factor. A recent study confirmed that the expression of miR-26b was changed in several human cancer cell lines including glioma cells.436 miRNA profile analyses revealed that miR-26b was one of the significantly decreased miRNAs in glioma cells compared to normal brain tissues.436 However, the role of miR-26b in glioma development has not been well documented and little is known about its target genes. Additionally, the effect of abnormal expression of miR-26b on tumor grade needs to be addressed. Erythropoietin-producing hepatocellular (EPH) receptors and their Ephrin ligands constitute the largest sub-family of receptor tyrosine kinases (RTKs), which are involved in many biological processes and play important roles in disease and development. 437 To date, fourteen Eph receptors have been found in mammals. They were divided into two distinct classes, A and B, based on the sequence homology of their extracellular domains. More recently, EphA receptors and their corresponding ligands have been implicated in numerous malignancies. 438 Among them, EphA2 and ephrinA1 are the most widely studied with respect to development, tumorigenesis, angiogenesis, and metastasis, and they may represent potential therapeutic targets because of their diverse functions in several types of cancer. Activation of the EphA2 receptor tyrosine kinase by ephrinA1 ligands plays important roles in cellular signal transduction. 439 EphA2 is functionally altered in a number of cancers and has potential roles in the regulation of cancer cell growth, survival, migration, invasion, and angiogenesis.440–442 Increased expression of EphA2 has been demonstrated in most cancers of epithelial origin, like breast,443 ovarian,441,444,445 prostate,445 melanoma,446 esophageal,447–449 lung carcinomas450 and brain.451,452 Immunohistochemical analysis has revealed that EphA2 was strongly overexpressed in 90% of GBM patient tumors,451 85% of prostate adenocarcinomas and 76% of ovarian cancers. 442 Furthermore, the frequent over-expression of EphA2 in human cancers correlates with poor prognosis and increases metastatic potential.442 In epithelial cells, ectopic expression of EphA2 has been shown to result in a malignant phenotype in both in vitro and in vivo experiments.443 EphA2 has been proposed as an attractive target for developing novel anticancer therapeutic agents. Wu et al453 studied, using real-time PCR analysis, the expression of miR-26b in glioma cells and the tissues from glioma patients of defined grades. Proliferation, migration, and invasion were analyzed to confirm the effects of miR-26b in glioma cells. The regulation by miR-26b of EphA2 was confirmed by the experiments of luciferase analysis, Western blotting, and Vasculogenic mimicry (VM) network formation. They found that ectopic expression of miR-26b in U251 and C6 glioma cells resulted in diminished proliferation, migration and invasion activity, accompanied by a low level expression of EhpA2. VM formation was also abolished in glioma cells transfected with the miR-26b duplex. This study provides evidence that miR-26b acts as an antioncogene in glioma cells and is an important negative regulator of the EphA2 gene.
Future directions
The progress and depth of understanding of the biology and genetics of glioma, together with truly manipulable experimental models, now offer real opportunities for the development of effective therapies. Despite significant gaps in our understanding, a wealth of information now exists about the clinical and biological behavior of the tumors, the genetic pathways involved in gliomagenesis, the nature of the disease and how its heterogeneity contributes to its untractability. The therapeutic resistance is a hallmark of their malignancy, which raises the question of which genetic alterations should be targeted as drivers of tumor maintenance, which could be ignored because they are initially needed for tumor establishment, and which drive the glioma stem cell niche, thus providing a reservoir from which therapeutically resistant cells can emerge. To fully understand the relevance of this niche in driving therapeutic resistance, many critical questions remain to be answered, including whether CD133+ cells are equivalent to the actively proliferating tumor cells seen in routine histological analysis, or whether they represent a quiescent population activated by ex vivo manipulations. It is also not yet clear whether there is a prognostic correlation between CD133+ and patient outcome, and whether CD133+ cells are selectively spared by radiation and chemotherapeutic drugs.
Our ability to isolate and culture neural and CSCs, astrocytes and oligodendrocytes and the creation of faithful models of this disease, coupled to enormous advances in genomic characterization of gliomas and functional validation of causative mutations, offer the very real prospect of rapid and thorough preclinical testing of compounds and other agents to directly answer the relevant questions. By identifying the weaknesses of the tumor, useful treatments for patients with these devastating diseases will become a reality. Thus, Chirasani et al431,454 reported very recently that endogenous neural precursor cells perform an anti-tumour response by specifically targeting stem-like brain tumour cells. Neurospheres of neural precursor cells constitutively release in vitro, bone morphogenetic protein-7 (BMP-7) and induce canonical bone morphogenetic protein signalling in stem-like glioblastoma cells. Exposure of human and murine stem-like brain tumour cells to neurosphere-derived BMP-7 induces tumour stem cell differentiation, attenuates stem-like marker expression and reduces self-renewal and the ability for tumour initiation. Neurosphere derived or recombinant BMP-7 reduces glioblastoma expansion from stem-like cells by down-regulating the transcription factor Olig2. In vivo, large numbers of BMP-7-expressing neural precursors encircle brain tumours in young mice, induce canonical BMP signalling in stem-like glioblastoma cells and can thereby attenuate tumour formation. This anti-tumour response is strongly reduced in older mice. The results of Kettenmann and Glass groups431,454 indicate that endogenous neural precursor cells protect the young brain from glioblastoma by releasing BMP-7, which acts as a paracrine tumour suppressor, repressing proliferation, self-renewal and tumour-initiation of stem-like glioblastoma cells.
The main goal of any drug approach has to be induction of apoptosis of tumor cells given the hyper-mutability of GBM as a response to the therapeutic challenge. Antiangiogenic, antiproliferative, and antiinvasive strategies represent adjuvant strategies. Although treatment of glioma may be improved by combining chemotherapy with anti-angiogenic drugs,258 inhibiting angiogenesis in GBM may antagonise the efficacy of chemotherapeutic drugs by normalising the function of the blood-brain barrier. Although it is unlikely that a single magic bullet will cure GBM and it is likely that multiple drug approaches may be needed, neurostatin and its analogues look like the experimental compounds closest to the magic bullet: a single compound inhibits division of both tumoral and endothelial cells, while making the tumor immunovisible and engaging the immune system to fight it. The development of enzymatic methods for the synthesis of neurostatin and related compunds in high yield,432,455 will make possible continuous intratumoral injection of high concentrations of a chemotherapeutic agent capable of inducing multimodal glioma destruction. Together with new advances in surgical treatment and radiotherapy,433,456 neurostatin chemotherapy will significantly prolong life of a reasonably quality.
Abbreviations
- Akt/PKB
a serine/threonine protein kinase that plays a key role in cell proliferation, apoptosis, transcription and cell migration;
- APCs
antigen presenting cells;
- APM
antigen processing machinery;
- BBB
blood-brain barrier;
- Bcl-2
B-cell lymphoma 2;
- CSC
cancer stem cells;
- CTL
cytotoxic lymphocyte;
- DC
dendritic cells;
- ECM
extracellular matrix;
- EGFR
epidermal growth factor receptor;
- ERK
Extracellular Receptor Kinase;
- FAK
focal adhesion kinase;
- c-FLIP
cellular FLICE inhibitory protein;
- GBM
glioblastoma multiforme;
- GEM
genetically engineered mouse;
- GIM
glioma-infiltrated microglia or macrophages;
- HLA
human leukocyte antigens;
- IGFBP
insulin-like growth factor binding protein;
- IAP
inhibitor of apoptosis protein;
- IDH
isocitrate dehydrogenase;
- IFN
interferon;
- LPS
lipopolysaccharide;
- MALDI-TOF-MS
matrix-assisted laser desorption ionisation time-of-flight mass spectrometry;
- MAPK
mitogen-activated protein kinases;
- MHC
major histocompatibility complex;
- MGMT
methylguanine-DNA methyltransferase;
- MMR
mismatch repair;
- NF1
neurofibromatosis type-1;
- NK
natural killer cells;
- NMR
nuclear magnetic resonance;
- PBL
peripheral blood lymphocytes;
- PD
programmed death;
- PDGF
platelet derived growth factor;
- PIK
phosphatidylinositol kinase;
- PTEN
phosphatase and tensin homolog;
- TRAIL
(TNF)-related apoptosis-inducing ligand;
- Tre.g.
regulatory T cell;
- RTKs
receptor tyrosine kinases;
- Smac
second mitochondria-derived activator of caspases;
- TAA
tumor-associated antigens;
- TNF
tumor necrosis factor;
- TRAIL
TNF-related apoptosis inducing ligand;
- TS4
synthetic tetrasaccharide;
- VEGF
vascular endothelial growth factor. The nomenclature system of Svennerholm was followed for ganglioside abbreviations.
Acknowledgments
This work was supported in part by spanish grants from the SESCAM, the FISCAM and the Interministerial Comittee for Science and Technology.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes and Dev. 2007;21:2683. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 6.Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci. 2003;100(Suppl 1):11842. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. New Engl J Med. 2005;353:811. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci. 2007;104:973. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100:3983. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 14.Weissenberger J, Steinbach JP, Malin G, Spada S, Rülicke T, Aguzzi A. Development and malignant progression of astrocytomas in GFAP-v-src transgenic mice. Oncogene. 1997;14:2005. doi: 10.1038/sj.onc.1201168. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821. [PubMed] [Google Scholar]
- 16.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci. 2003;100:15178. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 19.Piccirillo SG, Reynolds BA, Zanetti N, et al. Nature. 2006;444:761. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 20.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006b;66:7843. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 22.Sakariassen PØ, Prestegarden L, Wang J, et al. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci. 2006;103:16466. doi: 10.1073/pnas.0607668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X, Shen G, Yang X, Liu W. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67:3691. doi: 10.1158/0008-5472.CAN-06-3912. [DOI] [PubMed] [Google Scholar]
- 25.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 26.Gallia GL, Rand V, Siu IM, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4:709. doi: 10.1158/1541-7786.MCR-06-0172. [DOI] [PubMed] [Google Scholar]
- 27.http://www.sanger.ac.uk/genetics/CGP/cosmic/
- 28.Huang CH, Mandelker D, Schmidt-Kittler O, et al. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science. 2007;318:1744. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- 29.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–74. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Zhao J, Xu Z, et al. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004;279:33946. doi: 10.1074/jbc.M404298200. [DOI] [PubMed] [Google Scholar]
- 34.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mardis ER. Cancer genomics identifies determinants of tumor biology. Genome Biol. 2010;11:211. doi: 10.1186/gb-2010-11-5-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225.475. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabelloni P, Da Pozzo E, Bendinelli S, et al. Inhibition of metalloproteinases derived from tumours: new insights in the treatment of human glioblastoma. Neuroscience. 2010;168:514. doi: 10.1016/j.neuroscience.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 41.Brada M, Judson I, Beale P, et al. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br J Cancer. 1999;81:1022. doi: 10.1038/sj.bjc.6690802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrad CA, Milosavljevic VP, Yung WK. Advances in chemotherapy for brain tumors. Neurol Clin. 1995;3:795. [PubMed] [Google Scholar]
- 43.Hirose Y, Berger MS, Pieper RO. Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 2001;61:1957. [PubMed] [Google Scholar]
- 44.Levin VA, Leibel SA, Gutin PH. In: Cancer: principles of oncology. De Vita VT Jr, Hellman S, Rosenberg SA, editors. Philadelphia, PA: Lippincott-Raven; 2001. pp. 2100–61. [Google Scholar]
- 45.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 46.Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167. doi: 10.1016/s0079-6603(08)60879-x. [DOI] [PubMed] [Google Scholar]
- 47.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. New Engl J Med. 2000;343:1350. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 48.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl J Med. 2005;352:997. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 49.Groves MD, Puduvalli VK, Hess KR, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20:1383. doi: 10.1200/JCO.2002.20.5.1383. [DOI] [PubMed] [Google Scholar]
- 50.Levin VA, Phuphanich S, Yung WK, et al. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J Neuro Oncol. 2006;78:295. doi: 10.1007/s11060-005-9098-5. [DOI] [PubMed] [Google Scholar]
- 51.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 52.Albanese C, Johnson J, Watanabe G, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 53.Lavoie JN, L’Allemain G, Brunet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 54.Aktas H, Cai H, Cooper GM. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 56.Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci. 2004;117:2173. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- 57.James CD, Carlbom E, Dumanski JP, et al. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988;48:5546. [PubMed] [Google Scholar]
- 58.Henson JW, Schnitker BL, Correa KM, et al. The retinoblastoma gene is involved in malignant progression of astrocytomas. Ann Neurol. 1994;36:714. doi: 10.1002/ana.410360505. [DOI] [PubMed] [Google Scholar]
- 59.Reifenberger G, Reifenberger J, Ichimura K, Meltzer PS, Collins VP. Amplification of multiple genes from chromosomal region 12q13–14 in human malignant gliomas: preliminary mapping of the amplicons shows preferential involvement of CDK4, SAS, and MDM2. Cancer Res. 1994;54:4299. [PubMed] [Google Scholar]
- 60.Costello JF, Plass C, Arap W, et al. Cyclin-dependent kinase 6 (CDK6) amplification in human gliomas identified using two-dimensional separation of genomic DNA. Cancer Res. 1997;57:1250. [PubMed] [Google Scholar]
- 61.Serrano M, Hannon GJ, Beach DA. new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 62.Jen J, Harper JW, Bigner SH, et al. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994;54:6353. [PubMed] [Google Scholar]
- 63.Schmidt EE, Ichimura K, Reifenberger G, Collins VP. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994;54:6321. [PubMed] [Google Scholar]
- 64.Merlo A, Herman JG, Mao L, et al. 5’ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nature Med. 1995;1:686. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- 65.Costello JF, Berger MS, Huang HS, Cavenee WK. Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res. 1996;56:2405. [PubMed] [Google Scholar]
- 66.Fueyo J, Gomez-Manzano C, Bruner JM, et al. Hypermethylation of the CpG island of p16/CDKN2 correlates with gene inactivation in gliomas. Oncogene. 1996;13:1615. [PubMed] [Google Scholar]
- 67.Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagchi A, Papazoglu C, Wu Y, et al. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128:459. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 69.Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150. [PubMed] [Google Scholar]
- 70.Holland EC, Hively WP, DePinho RA, Varmus HEG. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes and Dev. 1998;12:3675. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holland EC, Hively WP, Gallo V, Varmus HE. Modeling mutations in the G1 arrest pathway in human gliomas: overexpression of CDK4 but not loss of INK4a-ARF induces hyperploidy in cultured mouse astrocytes. Genes and Dev. 1998;12:3644. doi: 10.1101/gad.12.23.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formational. Cancer Res. 2001;61:3556. [PubMed] [Google Scholar]
- 73.Sonoda Y, Ozawa T, Hirose Y, et al. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956. [PubMed] [Google Scholar]
- 74.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 75.Huang ZY, Baldwin RL, Hedrick NM, Gutmann DH. Astrocyte-specific expression of CDK4 is not sufficient for tumor formation, but cooperates with p53 heterozygosity to provide a growth advantage for astrocytes in vivo. Oncogene. 2002;21:1325. doi: 10.1038/sj.onc.1205206. [DOI] [PubMed] [Google Scholar]
- 76.Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62:5551. [PubMed] [Google Scholar]
- 77.Uhrbom L, Kastemar M, Johansson FK, Westermark B, Holland EC. Cell type-specific tumor suppression by Ink4a and Arf in Kras-induced mouse gliomagenesis. Cancer Res. 2005;65:2065. doi: 10.1158/0008-5472.CAN-04-3588. [DOI] [PubMed] [Google Scholar]
- 78.Xiao A, Wu H, Pandolfi PP, Louis DN, Van Dyke T. Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell. 2002;1:157. doi: 10.1016/s1535-6108(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 79.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nature Rev Cancer. 2002;2:594. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 80.Hoh J, Jin S, Parrado T, Edington J, Levine AJ, Ott J. The p53MH algorithm and its application in detecting p53-responsive genes. Proc Natl Acad Sci. 2002;99:8467. doi: 10.1073/pnas.132268899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine AJ, Hu W, Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13:1027. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 82.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 83.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 84.Louis DN. The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol. 1994;53:11. doi: 10.1097/00005072-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Louis DN, Cavenee W. Cancer: Principles and practice of oncology. In: Devita VT, Hellman S, editors. Lippincott-Raven Philadelphia. 1997. p. 2013. [Google Scholar]
- 86.Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 87.Srivastava S, Zou ZQ, Pirollo K, Blattner W, Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990;348:747. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- 88.Olive KP, Tuveson DA, Ruhe ZC, et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ. Tumor spectrum in ARF-deficient mice. Cancer Res. 1999;59:2217. [PubMed] [Google Scholar]
- 90.Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 91.Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci. 1998;95:8292. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pomerantz J, Schreiber-Agus N, Liégeois NJ, et al. The Ink4a tumor suppressor gene product, p19 Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell. 1998;92:713. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 93.Stott FJ, Bates S, James MC, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fakharzadeh SS, Trusko SP, George DL. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991;10:1565. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 97.Oliner JD, Pietenpol JA, Thiagalingam S, et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 98.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 99.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in Mdm2-deficient mice by deletion of p53. Nature. 1995;378:203. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 100.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 101.Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 102.Honda R, Yasuda H. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene. 2000;19:1473. doi: 10.1038/sj.onc.1203464. [DOI] [PubMed] [Google Scholar]
- 103.Shvarts A, Steegenga WT, Riteco N, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. G. EMBO J. 1996;15:5349. [PMC free article] [PubMed] [Google Scholar]
- 104.Riemenschneider MJ, Büschges R, Wolter M, et al. Amplification and overexpression of the MDM4 (MDMX) gene from 1q32 in a subset of malignant gliomas without TP53 mutation or MDM2 amplification. Cancer Res. 1999;59:6091. [PubMed] [Google Scholar]
- 105.Gu J, Kawai H, Nie L, et al. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J Biol Chem. 2002;277:19251. doi: 10.1074/jbc.C200150200. [DOI] [PubMed] [Google Scholar]
- 106.Linares LK, Hengstermann A, Ciechanover A, Müller S, Scheffner M. HdmX stimulates Hdm2-mediated ubiquitination and degradation of p53. Proc Natl Acad Sci. 2003;100:12009. doi: 10.1073/pnas.2030930100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 108.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 110.Guha A, Feldkamp MM, Lau N, Boss G, Pawson A. Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene. 1997;15:2755. doi: 10.1038/sj.onc.1201455. [DOI] [PubMed] [Google Scholar]
- 111.Vanhaesebroeck B, Leevers SJ, Ahmadi K, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 112.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 113.Chang HW, Aoki M, Fruman D, et al. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 114.Knobbe CB, Reifenberger G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3’-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13:507. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci. 2006;103:1289. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodriguez-Viciana P, Warne PH, Dhand R, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442. [PMC free article] [PubMed] [Google Scholar]
- 118.Gupta S, Ramjaun AR, Haiko P, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 119.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 120.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 121.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 122.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 123.Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 124.Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172. doi: 10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- 125.Denning G, Jean-Joseph B, Prince C, Durden DL, Vogt PK. A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene. 2007;26:3930. doi: 10.1038/sj.onc.1210175. [DOI] [PubMed] [Google Scholar]
- 126.Trotman LC, Wang X, Alimonti A, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mora A, Komander D, van Aalten DM, Alessi DR. Semin. PDK1, the master regulator of AGC kinase signal transduction. Cell Dev Biol. 2004;15:161. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 128.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84:941. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 130.Maira SM, Galetic I, Brazil DP, et al. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science. 2001;294:374. doi: 10.1126/science.1062030. [DOI] [PubMed] [Google Scholar]
- 131.Knobbe CB, Reifenberger J, Blaschke B, Reifenberger G. Hypermethylation and transcriptional downregulation of the carboxyl-terminal modulator protein gene in glioblastomas. J Natl Cancer Inst. 2004;96:483. doi: 10.1093/jnci/djh064. [DOI] [PubMed] [Google Scholar]
- 132.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 133.Ahn JY, Hu Y, Kroll TG, Allard P, Ye K. PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci. 2004;101:6993. doi: 10.1073/pnas.0400921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Knobbe CB, Trampe-Kieslich A, Reifenberger G. Genetic alteration and expression of the phosphoinositol-3-kinase/Akt pathway genes PIK3CA and PIKE in human glioblastomas. Neuropathol Appl Neurobiol. 2005;31:486. doi: 10.1111/j.1365-2990.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 135.Skeen JE, Bhaskar PT, Chen CC, et al. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 136.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harrington LS, Findlay GM, Gray A, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 139.Riemenschneider MJ, Betensky RA, Pasedag SM, Louis DN. AKT activation in human glioblastomas enhances proliferation via TSC2 and S6 kinase signaling. Cancer Res. 2006;66:5618. doi: 10.1158/0008-5472.CAN-06-0364. [DOI] [PubMed] [Google Scholar]
- 140.Shah OJ, Hunter T. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol. 2006;26:6425. doi: 10.1128/MCB.01254-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 142.Kops GJ, Medema RH, Glassford J, et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22:2025. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seoane J, Le HV, Shen L, Anderson SA, Massagué J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 144.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stambolic V, MacPherson D, Sas D, et al. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 146.Singh B, Reddy PG, Goberdhan A, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes and Dev. 2002;16:984. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Herold S, Wanzel M, Beuger V, et al. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol Cell. 2002;10:509. doi: 10.1016/s1097-2765(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 148.Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 149.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nature Cell Biol. 2001;3:245. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 150.Shin I, Yakes FM, Rojo F, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nature Med. 2002;8:1145. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 151.Feng J, Tamaskovic R, Yang Z, et al. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem. 2004;279:35510. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 152.You H, Jang Y, You-Ten AI, et al. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc Natl Acad Sci. 2004;101:14057. doi: 10.1073/pnas.0406286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci. 2006;103:9051. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Courtois-Cox S, Genther Williams SM, Reczek EE, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 156.Libermann TA, Razon N, Bartal AD, Yarden Y, Schlessinger J, Soreq H. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984;44:753. [PubMed] [Google Scholar]
- 157.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164. [PubMed] [Google Scholar]
- 158.Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci. 1992;89:2965. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. IARC Press; Lyon, France: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci. 1990;87:8602. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383. [PubMed] [Google Scholar]
- 162.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci. 1994;91:7727. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nagane M, Coufal F, Lin H, Bögler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079. [PubMed] [Google Scholar]
- 164.Huang HS, Nagane M, Klingbeil CK, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 165.Narita Y, Nagane M, Mishima K, Huang HJ, Furnari FB, Cavenee WK. Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidylinositol 3-kinase/Akt pathway in glioblastomas. Cancer Res. 2002;62:6764. [PubMed] [Google Scholar]
- 166.Lorimer IA, Wikstrand CJ, Batra SK, Bigner DD, Pastan I. Immunotoxins that target an oncogenic mutant epidermal growth factor receptor expressed in human tumors. Clin Cancer Res. 1995;1:859. [PubMed] [Google Scholar]
- 167.Mishima K, Johns TG, Luwor RB, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61:5349. [PubMed] [Google Scholar]
- 168.Nagane M, Narita Y, Mishima K, et al. Human glioblastoma xenografts overexpressing a tumor-specific mutant epidermal growth factor receptor sensitized to cisplatin by the AG1478 tyrosine kinase inhibitor. J Neurosurg. 2001;95:472. doi: 10.3171/jns.2001.95.3.0472. [DOI] [PubMed] [Google Scholar]
- 169.Jungbluth AA, Stockert E, Huang HJ, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci. 2003;100:639. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fan QW, Weiss WA. RNA interference against a glioma-derived allele of EGFR induces blockade at G2M. Oncogene. 2005;24:829. doi: 10.1038/sj.onc.1208227. [DOI] [PubMed] [Google Scholar]
- 171.Kang CS, Zhang ZY, Jia ZF, et al. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13:530. doi: 10.1038/sj.cgt.7700932. [DOI] [PubMed] [Google Scholar]
- 172.Mischel PS, Shai R, Shi T, et al. Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene. 2003;22:2361. doi: 10.1038/sj.onc.1206344. [DOI] [PubMed] [Google Scholar]
- 173.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3’-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742. [PubMed] [Google Scholar]
- 174.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 175.Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11:7841. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 176.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. New Engl J Med. 2005;353:2012. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 177.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 178.Fan QW, Specht KM, Zhang C, Goldenberg DD, Shokat KM, Weiss WA. Combinatorial efficacy achieved through two-point blockade within a signaling pathway-a chemical genetic approach. Cancer Res. 2003;63:8930. [PubMed] [Google Scholar]
- 179.Goudar RK, Shi Q, Hjelmeland MD, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101. [PubMed] [Google Scholar]
- 180.Wang MY, Lu KV, Zhu S, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 181.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci. 1998;95:5724. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62:4307. [PubMed] [Google Scholar]
- 183.Stea B, Falsey R, Kislin K, et al. Time and dose-dependent radiosensitization of the glioblastoma multiforme U251 cells by the EGF receptor tyrosine kinase inhibitor ZD1839 (‘Iressa’) Cancer Lett. 2003;202:43. doi: 10.1016/j.canlet.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 184.Lammering G, Hewit TH, Holmes M, et al. Inhibition of the type III epidermal growth factor receptor variant mutant receptor by dominant-negative EGFR-CD533 enhances malignant glioma cell radiosensitivity. Clin Cancer Res. 2004;10:6732. doi: 10.1158/1078-0432.CCR-04-0393. [DOI] [PubMed] [Google Scholar]
- 185.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nature Rev Cancer. 2006;6:876. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 186.Lee JC, Vivanco I, Beroukhim R, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213. [PubMed] [Google Scholar]
- 188.Plate KH, Breier G, Farrell CL, Risau W. Platelet-derived growth factor receptor-beta is induced during tumor development and upregulated during tumor progression in endothelial cells in human gliomas. Lab Invest. 1992;67:529. [PubMed] [Google Scholar]
- 189.Westermark B, Heldin CH, Nister M. Platelet-derived growth factor in human glioma. Glia. 1995;15:257. doi: 10.1002/glia.440150307. [DOI] [PubMed] [Google Scholar]
- 190.Di Rocco F, Carroll RS, Zhang J, Black PM. Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery. 1998;42:341. doi: 10.1097/00006123-199802000-00080. [DOI] [PubMed] [Google Scholar]
- 191.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62:3729. [PubMed] [Google Scholar]
- 192.Clarke ID, Dirks PB. A human brain tumor-derived PDGFR-alpha deletion mutant is transforming. Oncogene. 2003;22:722. doi: 10.1038/sj.onc.1206160. [DOI] [PubMed] [Google Scholar]
- 193.Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 195.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes and Dev. 2001;15:1913. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 197.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 198.Kilic T, Alberta JA, Zdunek PR, et al. Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res. 2000;60:5143. [PubMed] [Google Scholar]
- 199.Hägerstrand D, Hesselager G, Achterberg S, et al. Characterization of an imatinib-sensitive subset of high-grade human glioma cultures. Oncogene. 2006;25:4913. doi: 10.1038/sj.onc.1209497. [DOI] [PubMed] [Google Scholar]
- 200.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 201.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12:4899. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 202.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 203.Huang PH, Mukasa A, Bonavia R, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci. 2007;104:12867. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weller M, Frei K, Groscurth P, Krammer PH, Yonekawa Y, Fontana A. Anti-Fas/APO-1 antibody-mediated apoptosis of cultured human glioma cells. Induction and modulation of sensitivity by cytokines. J Clin Invest. 1994;94:954. doi: 10.1172/JCI117462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Roth W, Fontana A, Trepel M, Reed JC, Dichgans J, Weller M. Immunochemotherapy of malignant glioma: synergistic activity of CD95 ligand and chemotherapeutics. Cancer Immunol Immunothe. 1997;44:55. doi: 10.1007/s002620050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shinoura N, Yoshida Y, Sadata A, et al. Apoptosis by retrovirus- and adenovirus-mediated gene transfer of Fas ligand to glioma cells: implications for gene therapy. Hum Gene Ther. 1998;9:1983. doi: 10.1089/hum.1998.9.14-1983. [DOI] [PubMed] [Google Scholar]
- 208.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847. [PubMed] [Google Scholar]
- 209.Maleniak TC, Darling JL, Lowenstein PR, Castro MG. Adenovirus-mediated expression of HSV1-TK or Fas ligand induces cell death in primary human glioma-derived cell cultures that are resistant to the chemotherapeutic agent CCNU. Cancer Gene Ther. 2001;8:589. doi: 10.1038/sj.cgt.7700348. [DOI] [PubMed] [Google Scholar]
- 210.Röhn TA, Wagenknecht B, Roth W, et al. CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene. 2001;20:4128. doi: 10.1038/sj.onc.1204534. [DOI] [PubMed] [Google Scholar]
- 211.Roth W, Isenmann S, Nakamura M, et al. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759. [PubMed] [Google Scholar]
- 212.Kuijlen JM, Mooij JJ, Platteel I, et al. TRAIL-receptor expression is an independent prognostic factor for survival in patients with a primary glioblastoma multiforme. J Neurooncol. 2006;78:161. doi: 10.1007/s11060-005-9081-1. [DOI] [PubMed] [Google Scholar]
- 213.Roth W, Isenmann S, Naumann U, et al. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265:479. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- 214.LeBlanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death receptor—induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nature Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 215.Song JH, Song DK, Pyrzynska B, Petruk KC, Van Meir EG, Hao C. TRAIL triggers apoptosis in human malignant glioma cells through extrinsic and intrinsic pathways. Brain Pathol. 2003;13:539. doi: 10.1111/j.1750-3639.2003.tb00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nature Med. 2002;8:808. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 217.Reed JC, Zha H, Aime-Sempe C, Takayama S, Wang HG. Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death. Adv Exp Med Biol. 1996;406:99. [PubMed] [Google Scholar]
- 218.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 219.Fesik SW, Shi Y. Structural biology. Controlling the caspases. Science. 2001;294:1477. doi: 10.1126/science.1062236. [DOI] [PubMed] [Google Scholar]
- 220.Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta. 2006;1762:191. doi: 10.1016/j.bbadis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 221.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 222.Weller M, Malipiero U, Aguzzi A, Reed JC, Fontana A. Protooncogene bcl-2 gene transfer abrogates Fas/APO-1 antibody-mediated apoptosis of human malignant glioma cells and confers resistance to chemotherapeutic drugs and therapeutic irradiation. J Clin Invest. 1995;95:2633. doi: 10.1172/JCI117965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Krajewski S, Krajewska M, Ehrmann J, et al. Immunohistochemical analysis of Bcl-2, Bcl-X, Mcl-1, and Bax in tumors of central and peripheral nervous system origin. Am J Pathol. 1997;150:805. [PMC free article] [PubMed] [Google Scholar]
- 224.Strik H, Deininger M, Streffer J, et al. BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radiochemotherapy. J Neurol Neurosurg Psychiatr. 1999;67:763. doi: 10.1136/jnnp.67.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wick W, Wagner S, Kerkau S, Dichgans J, Tonn JC, Weller M. BCL-2 promotes migration and invasiveness of human glioma cells. FEBS Lett. 1998;440:419. doi: 10.1016/s0014-5793(98)01494-x. [DOI] [PubMed] [Google Scholar]
- 226.Wick W, Grimmel C, Wild-Bode C, Platten M, Arpin M, Weller M. Ezrin-dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL-2 and transforming growth factor-beta2. J Neurosci. 2001;21:3360. doi: 10.1523/JNEUROSCI.21-10-03360.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wick W, Wild-Bode C, Frank B, Weller M. BCL-2-induced glioma cell invasiveness depends on furin-like proteases. J Neurochem. 2004;91:1275. doi: 10.1111/j.1471-4159.2004.02806.x. [DOI] [PubMed] [Google Scholar]
- 228.Julien T, Frankel B, Longo S, et al. Antisense-mediated inhibition of the bcl-2 gene induces apoptosis in human malignant glioma. Surg Neurol. 2000;53:360. doi: 10.1016/s0090-3019(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 229.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nature Rev Cancer. 2005;5:876. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 230.Naumann U, Schmidt F, Wick W, et al. Adenoviral natural born killer gene therapy for malignant glioma. Hum Gene Ther. 2003;14:1235. doi: 10.1089/104303403767740777. [DOI] [PubMed] [Google Scholar]
- 231.Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R. Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery. 2002;51:2. doi: 10.1097/00006123-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 232.Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest. 2004;84:397. doi: 10.1038/labinvest.3700070. [DOI] [PubMed] [Google Scholar]
- 233.Stegh AH, Kim H, Bachoo RM, et al. Bcl2 L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes and Dev. 2007;21:98. doi: 10.1101/gad.1480007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Scorilas A, Kyriakopoulou L, Yousef GM, Ashworth LK, Kwamie A, Diamandis EP. Molecular cloning, physical mapping, and expression analysis of a novel gene, BCL2 L12, encoding a proline-rich protein with a highly conserved BH2 domain of the Bcl-2 family. Genomics. 2001;72:217. doi: 10.1006/geno.2000.6455. [DOI] [PubMed] [Google Scholar]
- 235.Nicotera P, Melino G. Regulation of the apoptosis-necrosis switch. Oncogene. 2004;23:2757. doi: 10.1038/sj.onc.1207559. [DOI] [PubMed] [Google Scholar]
- 236.Stiver SI, Tan X, Brown LF, Hedley-Whyte ET, Dvorak HF. VEGF-A angiogenesis induces a stable neovasculature in adult murine brain. J Neuropathol Exp Neurol. 2004;63:841. doi: 10.1093/jnen/63.8.841. [DOI] [PubMed] [Google Scholar]
- 237.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77:362. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 238.Birlik B, Canda S, Ozer E. Tumour vascularity is of prognostic significance in adult, but not paediatric astrocytomas. Neuropathol Appl Neurobiol. 2006;32:532. doi: 10.1111/j.1365-2990.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 239.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Rev Drug Discov. 2007;6:273. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 240.Maher EA, Brennan C, Wen PY, et al. Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 2006;66:11502. doi: 10.1158/0008-5472.CAN-06-2072. [DOI] [PubMed] [Google Scholar]
- 241.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 242.Watnick RS, Cheng YN, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3:219. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 243.Blum R, Jacob-Hirsch J, Amariglio N, Rechavi G, Kloog Y. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1alpha, causing glycolysis shutdown and cell death. Cancer Res. 2005;65:999. [PubMed] [Google Scholar]
- 244.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes and Dev. 2006;20:2527. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vogel J, Gehrig M, Kuschinsky W, Marti HH. Massive inborn angiogenesis in the brain scarcely raises cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:849. doi: 10.1097/01.WCB.0000126564.89011.11. [DOI] [PubMed] [Google Scholar]
- 247.Kaur B, Tan C, Brat DJ, Post DE, Van Meir EG. Genetic and hypoxic regulation of angiogenesis in gliomas. J Neurooncol. 2004;70:229. doi: 10.1007/s11060-004-2752-5. [DOI] [PubMed] [Google Scholar]
- 248.Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 249.Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 250.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 251.Norden AD, Drappatz J. Antiangiogenic therapies for high-grade glioma. Nature Reviews Neurology. 2009;5:610. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 252.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 253.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 254.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dasari VR, Kaur K, Velpula KK, et al. Downregulation of Focal Adhesion Kinase (FAK) by cord blood stem cells inhibits angiogenesis in glioblastoma. Aging. 2010;2:791. doi: 10.18632/aging.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev Cancer. 2003;3:721. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 258.Verhoeff JJ, van Tellingen O, Claes A, et al. Oncerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lefranc F, Brotchi J, Kiss RJ. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. Clin Oncol. 2005;23:2411. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 260.Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 261.Bernstein JJ, Woodard CA. Glioblastoma cells do not intravasate into blood vessels. Neurosurgery. 1995;36:124. doi: 10.1227/00006123-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 262.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003;20:65. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
- 263.Landau BJ, Kwaan HC, Verrusio EN, Brem SS. Elevated levels of urokinase-type plasminogen activator and plasminogen activator inhibitor type-1 in malignant human brain tumors. Cancer Res. 1994;54:1105. [PubMed] [Google Scholar]
- 264.Yamamoto M, Sawaya R, Mohanam S, et al. Expression and localization of urokinase-type plasminogen activator in human astrocytomas in vivo. Cancer Res. 1994a;54:3656. [PubMed] [Google Scholar]
- 265.Yamamoto M, Sawaya R, Mohanam S, et al. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas. Cancer Res. 1994b;54:5016. [PubMed] [Google Scholar]
- 266.McCormick D. Secretion of cathepsin B by human gliomas in vitro. Neuropathol Appl Neurobiol. 1993;19:146. doi: 10.1111/j.1365-2990.1993.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 267.Uhm JH, Dooley NP, Villemure JG, Yong VW. Mechanisms of glioma invasion: role of matrix-metalloproteinases. Can J Neurol Sci. 1997;24:3. doi: 10.1017/s0317167100021028. [DOI] [PubMed] [Google Scholar]
- 268.Kanamori M, Vanden Berg SR, Bergers G, Berger MS, Pieper RO. Integrin beta3 overexpression suppresses tumor growth in a human model of gliomagenesis: implications for the role of beta3 overexpression in glioblastoma multiforme. Cancer Res. 2004;64:2751. doi: 10.1158/0008-5472.can-03-3354. [DOI] [PubMed] [Google Scholar]
- 269.Song SW, Fuller GN, Khan A, et al. IIp45, an insulin-like growth factor binding protein 2 (IGFBP-2) binding protein, antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl Acad Sci. 2003;100:13970. doi: 10.1073/pnas.2332186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang H, Wang H, Shen W, et al. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315. [PubMed] [Google Scholar]
- 271.Hu B, Guo P, Fang Q, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci. 2003;100:8904. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Liu F, Park PJ, Lai W, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 273.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 274.Tatenhorst L, Senner V, Püttmann S, Paulus W. Regulators of G-protein signaling 3 and 4 (RGS3, RGS4) are associated with glioma cell motility. J Neuropathol Exp Neurol. 2004;63:210. doi: 10.1093/jnen/63.3.210. [DOI] [PubMed] [Google Scholar]
- 275.Kissil JL, Cohen O, Raveh T, Kimchi A. Structure-function analysis of an evolutionary conserved protein, DAP3, which mediates TNF-alpha- and Fas-induced cell death. EMBO J. 1999;18:353. doi: 10.1093/emboj/18.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Taylor GA, Hudson E, Resau JH, Vande Woude GF. Regulation of P311 expression by Met-hepatocyte growth factor/scatter factor and the ubiquitin/proteasome system. J Biol Chem. 2000;275:4215. doi: 10.1074/jbc.275.6.4215. [DOI] [PubMed] [Google Scholar]
- 277.Mariani L, McDonough WS, Hoelzinger DB, et al. Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res. 2001;61:4190. [PubMed] [Google Scholar]
- 278.Wiley SR, Winkles JA. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev. 2003;14:241. doi: 10.1016/s1359-6101(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 279.Joy AM, Beaudry CE, Tran NL, et al. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- 280.Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E. The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J Cell Biol. 2001;155:1129. doi: 10.1083/jcb.200105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Taniguchi Y, Ono K, Yoshida S, Tanaka R. Antigen-presenting capability of glial cells under glioma-harboring conditions and the effect of glioma-derived factors on antigen presentation. J Neuroimmunol. 2000;111:177. doi: 10.1016/s0165-5728(00)00361-1. [DOI] [PubMed] [Google Scholar]
- 282.Albesiano E, Han JE, Lim M. Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am. 2010;21:17. doi: 10.1016/j.nec.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 283.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am. 2010;21:31. doi: 10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 284.Parney I, Hao C, Petruk K. Glioma immunology and immunotherapy. Neurosurgery. 2000;46:778. doi: 10.1097/00006123-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 285.Yang I, Kremen TJ, Giovannone AJ, et al. Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg. 2004;100:310. doi: 10.3171/jns.2004.100.2.0310. [DOI] [PubMed] [Google Scholar]
- 286.Facoetti A, Nano R, Zelini P, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11:8304. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 287.Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009;110:572. doi: 10.3171/2008.7.JNS08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Flügel A, Labeur MS, Grasbon-Frodl EM, Kreutzberg GW, Graeber MB. Microglia only weakly present glioma antigen to cytotoxic T cells. Int J Dev Neurosci. 1999;17:547. doi: 10.1016/s0736-5748(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 289.Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia. 2005;51:279. doi: 10.1002/glia.20201. [DOI] [PubMed] [Google Scholar]
- 290.Sandberg-Wollheim M, Zweiman B, Levinson AI, Lisak RP. Humoral immune responses within the human central nervous system following systemic immunization. J Neuroimmunol. 1986;11:205. doi: 10.1016/0165-5728(86)90004-4. [DOI] [PubMed] [Google Scholar]
- 291.Bernheimer H, Lassmann H, Suchanek G. Dynamics of IgG+, IgA+, and IgM+ plasma cells in the central nervous system of guinea pigs with chronic relapsing experimental allergic encephalomyelitis. Neuropathol Appl Neurobiol. 1988;14:157. doi: 10.1111/j.1365-2990.1988.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 292.Levi-Strauss M, Mallat M. Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J Immunol. 1987;139:2361. [PubMed] [Google Scholar]
- 293.Hickey WF, Kimura H. Graft-vs.-host disease elicits expression of class I and class II histocompatibility antigens and the presence of scattered T lymphocytes in rat central nervous system. Proc Natl Acad Sci USA. 1987;84:2082. doi: 10.1073/pnas.84.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 295.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 296.Sampson JH, Archer GE, Ashley DM, et al. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proc Natl Acad Sci USA. 1996;93:10399. doi: 10.1073/pnas.93.19.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gordon LB, Nolan SC, Cserr HF, Knopf PM, Harling-Berg CJ. Growth of P511 mastocytoma cells in BALB/c mouse brain elicits CTL response without tumor elimination: a new tumor model for regional central nervous system immunity. J Immunol. 1997;159:2399. [PubMed] [Google Scholar]
- 298.Gordon FL, Nguyen KB, White CA, Pender MP. Rapid entry and downregulation of T cells in the central nervous system during the reinduction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;112:15. doi: 10.1016/s0165-5728(00)00341-6. [DOI] [PubMed] [Google Scholar]
- 299.Parney IF, Farr-Jones MA, Chang LJ, Petruk KC. Human glioma immunobiology in vitro: implications for immunogene therapy. Neurosurgery. 2000;46:1169. doi: 10.1097/00006123-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 300.Lampson LA. Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Microsc Res Tech. 1995;32:267. doi: 10.1002/jemt.1070320402. [DOI] [PubMed] [Google Scholar]
- 301.Zuber P, Kuppner MC, De Tribolet N. Transforming growth factor-beta 2 down-regulates HLA-DR antigen expression on human malignant glioma cells. Eur J Immunol. 1988;18:1623. doi: 10.1002/eji.1830181023. [DOI] [PubMed] [Google Scholar]
- 302.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother. 1997;20:165. [PubMed] [Google Scholar]
- 303.Chang CC, Campoli M, Ferrone S. HLA class I defects in malignant lesions: what have we learned? Keio J Med. 2003;52:220. doi: 10.2302/kjm.52.220. [DOI] [PubMed] [Google Scholar]
- 304.Momburg F, Tan P. Tapasin-the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol Immunol. 2002;39:217. doi: 10.1016/s0161-5890(02)00103-7. [DOI] [PubMed] [Google Scholar]
- 305.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462. [PubMed] [Google Scholar]
- 306.Hussain SF, Heimberger AB. Immunotherapy for human glioma: innovative approaches and recent results. Expert Rev Anticancer Ther. 2005;5:777. doi: 10.1586/14737140.5.5.777. [DOI] [PubMed] [Google Scholar]
- 307.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 308.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 309.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wilmotte R, Burkhardt K, Kindler V, et al. B7-homolog 1 expression by human glioma: a new mechanism of immune evasion. Neuroreport. 2005;16:1081. doi: 10.1097/00001756-200507130-00010. [DOI] [PubMed] [Google Scholar]
- 311.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 312.Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. 2009;11:757. doi: 10.1215/15228517-2009-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mahaley MS, Jr, Brooks WH, Roszman TL, Bigner DD, Dudka L, Richardson S. Immunobiology of primary intracranial tumors. Part 1: studies of the cellular and humoral general immune competence of brain-tumor patients. J Neurosurg. 1977;46:467. doi: 10.3171/jns.1977.46.4.0467. [DOI] [PubMed] [Google Scholar]
- 315.Young HF, Sakalas R, Kaplan AM. Inhibition of cell-mediated immunity in patients with brain tumors. Surg Neurol. 1976;5:19. [PubMed] [Google Scholar]
- 316.Elliott LH, Brooks WH, Roszman TL. Cytokinetic basis for the impaired activation of lymphocytes from patients with primary intracranial tumors. J Immunol. 1984;132:1208. [PubMed] [Google Scholar]
- 317.Ausiello CM, Palma C, Maleci A, et al. Cell mediated cytotoxicity and cytokine production in peripheral blood mononuclear cells of glioma patients. Eur J Cancer. 1991;27:646. doi: 10.1016/0277-5379(91)90235-6. [DOI] [PubMed] [Google Scholar]
- 318.Roszman TL, Brooks WH, Steele C, Elliott LH. Pokeweed mitogen-induced immunoglobulin secretion by peripheral blood lymphocytes from patients with primary intracranial tumors. Characterization of T helper and B cell function. J Immunol. 1985;134:1545. [PubMed] [Google Scholar]
- 319.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 320.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 321.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 322.Camara NO, Sebille F, Lechler RI. Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T cell activation. Eur J Immunol. 2003;33:3473. doi: 10.1002/eji.200323966. [DOI] [PubMed] [Google Scholar]
- 323.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 324.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 325.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 326.Newton HB. Primary brain tumors: review of etiology, diagnosis and treatment. Am Fam Physician. 1994;49:787. [PubMed] [Google Scholar]
- 327.Newton HB. Molecular neuro-oncology and the development of targeted therapeutic strategies for brain tumors. Part 3: brain tumor invasiveness. Expert Rev Anticancer Ther. 2004;4:105. doi: 10.1586/14737140.4.5.803. [DOI] [PubMed] [Google Scholar]
- 328.Davis FG, McCarthy BJ. Current epidemiological trends and surveillance issues in brain tumors. Expert Rev Anticancer Ther. 2001;1:395. doi: 10.1586/14737140.1.3.395. [DOI] [PubMed] [Google Scholar]
- 329.Stevens MF, Hickman JA, Stone R, et al. Antitumor imidazotetrazines. 1. Synthesis and chemistry of 8-carbamoyl-3-(2-chloroethyl)imidazo[5, 1-d]-1,2,3,5-tetrazin-4(3 H)-one, a novel broad-spectrum antitumor agent. J Med Chem. 1984;27:196. doi: 10.1021/jm00368a016. [DOI] [PubMed] [Google Scholar]
- 330.Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045;M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47:5846. [PubMed] [Google Scholar]
- 331.Chen HX, Gore-Langton RE, Cheson BD. Clinical trials referral resource: Current clinical trials of the anti-VEGF monoclonal antibody bevacizumab. Oncology. 2001;15:1017, 1020, 1023. [PubMed] [Google Scholar]
- 332.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 333.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kefas B, Comeau L, Floyd DH, et al. The neuronal microRNA miR-326 acts in a feedback loop with notch and has therapeutic potential against brain tumors. J Neurosci. 2009;29:15161. doi: 10.1523/JNEUROSCI.4966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Guha A, Mukherjee J. Advances in the biology of astrocytomas. Curr Opin Neurol. 2004;17:655. doi: 10.1097/00019052-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 336.Verhaak RG, Hoadley KA, Purdom E, et al. Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Reguart N, Cardona AF, Rosell R. Role of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancer. Cancer Manag Res. 2010;2:143. doi: 10.2147/cmar.s5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Yan DF, Yan SX, Yang JS, et al. Hemorrhage of brain metastasis from non-small cell lung cancer post gefitinib therapy: two case reports and review of the literature. BMC Cancer. 2010;10:49. doi: 10.1186/1471-2407-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Klenk E. Uber die ganglioside, eine neue Gruppe von zuckerhaltigen Gehirnlipoiden. Z Physiol Chem. 1942;273:76–86. [Google Scholar]
- 343.Svennerholm L. Composition of gangliosides from human brain. Nature. 1956;177:524. doi: 10.1038/177524b0. [DOI] [PubMed] [Google Scholar]
- 344.Miljan EA, Bremer EG. Regulation of growth factor receptors by gangliosides. Sci STKE. 2002 Nov 26;(160) doi: 10.1126/stke.2002.160.re15. [DOI] [PubMed] [Google Scholar]
- 345.Hakomori S. Traveling for the glycosphingolipid path. Glycoconj J. 2000;17:627. doi: 10.1023/a:1011086929064. [DOI] [PubMed] [Google Scholar]
- 346.Roisen FJ, Bartfeld H, Nagele R, Yorke G. Ganglioside stimulation of axonal sprouting in vitro. Science. 1981;214:577. doi: 10.1126/science.7291999. [DOI] [PubMed] [Google Scholar]
- 347.Bremer EG, Hakomori S. GM3 ganglioside induces hamster fibroblast growth inhibition in chemically-defined medium: ganglioside may regulate growth factor receptor function. Biochem Biophys Res Commun. 1982;106:711. doi: 10.1016/0006-291x(82)91769-7. [DOI] [PubMed] [Google Scholar]
- 348.Bremer EG, Hakomori S, Bowen-Pope DF, Raines E, Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984;259:6818. [PubMed] [Google Scholar]
- 349.Bremer EG, Schlessinger J, Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986;261:2434. [PubMed] [Google Scholar]
- 350.Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990;265:18713. [PubMed] [Google Scholar]
- 351.Sturrock RR. Cell division in the normal central nervous system. Adv Cell Neurobiol. 1982;3:3. [Google Scholar]
- 352.Korr H. Proliferation and cell cycle parameters of astrocytes. In: Fedoroff S, Vernadakis A, editors. Astrocytes. Vol. 3. Acad. Press Inc. Ltd; London: 1986. p. 77. [Google Scholar]
- 353.Nieto-Sampedro M, Saneto RP, de Vellis J, Cotman CW. The control of glial populations in brain: changes in astrocyte mitogenic and morphogenic factors in response to injury. Brain Res. 1985;343:320. doi: 10.1016/0006-8993(85)90750-4. [DOI] [PubMed] [Google Scholar]
- 354.Nieto-Sampedro M. Astrocyte mitogen inhibitor related to EGF receptor. Science. 1988;240:1784. doi: 10.1126/science.3289118. [DOI] [PubMed] [Google Scholar]
- 355.Nieto-Sampedro M, Broderick JT. A soluble brain molecule related to epidermal growth factor receptor is a mitogen inhibitor for astrocytes. J Neurosci Res. 1989;22:28. doi: 10.1002/jnr.490220105. [DOI] [PubMed] [Google Scholar]
- 356.Abad-Rodríguez J, Vallejo-Cremades M, Nieto-Sampedro M. Control of glial number: Purification from mammalian brain extracts of an inhibitor of astrocyte division. Glia. 1998;22:160. doi: 10.1002/(sici)1098-1136(199806)23:2<156::aid-glia7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 357.Abad-Rodríguez J, Bernabe M, Romero-Ramírez L, Vallejo-Cremades M, Fernández-Mayoralas A, Nieto-Sampedro M. Purification and structure of neurostatin, an inhibitor of astrocyte division from mammalian brain. J Neurochem. 2000;74:2547. doi: 10.1046/j.1471-4159.2000.0742547.x. [DOI] [PubMed] [Google Scholar]
- 358.Santos-Benito FF, Nieto-Sampedro M, Fernández-Mayoralas A, Martin-Lomas M. Synthesis of oligosaccharide inhibitors of neural cell division. Carbohydrate Res. 1992;230:185. doi: 10.1016/s0008-6215(00)90521-4. [DOI] [PubMed] [Google Scholar]
- 359.Santos-Benito FF, Fernández-Mayoralas A, Martín-Lomas M, Nieto-Sampedro M. Inhibition of proliferation of normal and transformed neural cells by blood group-related oligosaccharides. J Expt Med. 1992;176:915. doi: 10.1084/jem.176.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nieto-Sampedro M, Bailón C, Fernández-Mayoralas A, Martín-Lomas M, Mellström B, Naranjo JR. Experimental brain glioma: growth arrest and destruction by a blood group-related tetrasaccharide. J Neuropathol Exper Neurol. 1996;55:169. doi: 10.1097/00005072-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 361.Zhu D, Caveney S, Kidder GM, Naus CCG. Transfection of C6 glioma cells with connexin 43 cDNA: analysis of expression, intercellular coupling, and cell proliferation. Proc Nat Acad Sci USA. 1991;88:1883. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhu D, Kidder GM, Caveney S, Naus CC. Growth retardation in glioma cells cocultured with cells overexpressing a gap junction protein. Proc Nat Acad Sci USA. 1992;89:10218. doi: 10.1073/pnas.89.21.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yarden Y, Harari I, Schlessinger J. Purification of an active EGF receptor kinase with monoclonal antireceptor antibodies. J Biol Chem. 1985;260:315. [PubMed] [Google Scholar]
- 364.Bezouska K, Yuen CT, O’Brien J, et al. Oligosaccharide ligands for NKR-P1 protein activate NK cells and cytotoxicity. Nature. 1994;372:150. doi: 10.1038/372150a0. [DOI] [PubMed] [Google Scholar]
- 365.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Ann Rev Immunol. 1993;11:613. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 366.Bradshaw SL, Naus CC, Zhu D, Kidder GM, D’Ercole AJ, Han VK. Alterations in the synthesis of insulin-like growth factor binding proteins and insulin-like growth factors in rat C6 glioma cells transfected with a gap junction connexin43 cDNA. Regul Pept. 1993;48:99. doi: 10.1016/0167-0115(93)90339-a. [DOI] [PubMed] [Google Scholar]
- 367.Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML, Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259:94. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- 368.Aguilera B, Romero-Ramírez L, Abad-Rodríguez J, Corrales G, Nieto-Sampedro M, Fernández-Mayoralas A. Novel disaccharides inhibitors of human glioma cell division. J Med Chem. 1998;41:4599. doi: 10.1021/jm980365i. [DOI] [PubMed] [Google Scholar]
- 369.Fernández-Mayoralas A, de la Figuera N, et al. Central Neural Tumor Destruction by Controlled Release of a Synthetic Glycoside Dispersed in a Biodegradable Polymeric Matrix. J Med Chem. 2003;46:1286. doi: 10.1021/jm025620k. [DOI] [PubMed] [Google Scholar]
- 370.García-Álvarez I, Corrales G, Doncel-Pérez E, Muñoz A, Nieto-Sampedro M, Fernández-Mayoralas A. Design and Synthesis of Glycoside Inhibitors of Glioma and Melanoma Growth. J Med Chem. 2007;50:364. doi: 10.1021/jm0611556. [DOI] [PubMed] [Google Scholar]
- 371.García-Álvarez I, Garrido L, Doncel-Pérez E, Nieto-Sampedro M, Fernández-Mayoralas A. Detection of Metabolite Changes in C6 Glioma Cells Cultured with Antimitotic OleylGlycoside by 1H MAS NMR. J Med Chem. 2009;52:1263. doi: 10.1021/jm8012807. [DOI] [PubMed] [Google Scholar]
- 372.Mendez-Otero R, Schlosshauer B, Barnstable CJ, Constantine-Paton M. A developmentally regulated antigen associated with neural cell and process migration. J Neurosci. 1988;8:564. doi: 10.1523/JNEUROSCI.08-02-00564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Igarashi M, Waki H, Saito S, Komiya Y, Ando S. Characteristics of gangliosides including O-acetylated species in growth cone membranes at several developmental stages in rat forebrain. Brain Res Developl Brain Res. 1994;78:17–24. doi: 10.1016/0165-3806(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 374.Farrer RG, Quarles RH. GT3 and its O-acetylated derivative are the principal A2B5-reactive gangliosides in cultured O2A lineage cells and are down-regulated along with O-acetyl GD3 during differentiation to oligodendrocytes. J Neurosci Res. 1999;57:371. [PubMed] [Google Scholar]
- 375.Zeng G, Li DD, Gao L, et al. Alteration of ganglioside composition by stable transfection with antisense vectors against GD3-synthase gene expression. Biochemistry. 1999;6:8762. doi: 10.1021/bi9906726. [DOI] [PubMed] [Google Scholar]
- 376.Birkle S, Ren S, Slominski A, Zeng G, Gao L, Yu RK. Down-regulation of the expression of O-acetyl-GD3 by the O-acetylesterase cDNA in hamster melanoma cells: effects on cellular proliferation, differentiation, and melanogenesis. J Neurochem. 1999;72:954. doi: 10.1046/j.1471-4159.1999.0720954.x. [DOI] [PubMed] [Google Scholar]
- 377.Romero-Ramirez L, Nieto-Sampedro M. Inhibiting human astrocytoma growth: Structure-activity relationships in neurostatin related glycolipids. J Med Chem. 2004;47:4983. doi: 10.1021/jm049816r. [DOI] [PubMed] [Google Scholar]
- 378.Valle-Argos B, Gómez-Nicola D, Nieto-Sampedro M. Synthesis and characterization of neurostatin-related compounds with high inhibitory activity of glioma growth. Eur J Med Chem. 2010;45:2034. doi: 10.1016/j.ejmech.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 379.Gómez-Nicola D, Valle-Argos B, Suardíaz M, Taylor JS, Nieto-Sampedro M. Role of IL-15 in spinal cord and sciatic nerve after chronic constriction injury: regulation of macrophage and T-cell infiltration. J Neurochem. 2008;107:1741. doi: 10.1111/j.1471-4159.2008.05746.x. [DOI] [PubMed] [Google Scholar]
- 380.Hubl U, Ishida H, Kiso M, Hasegawa A, Schauer R. Studies on the specificity and sensitivity of the influenza C virus binding assay for 9-O-acetylated sialic acids and its application to human melanomas. J Biochem. 2000;127:1021. doi: 10.1093/oxfordjournals.jbchem.a022693. [DOI] [PubMed] [Google Scholar]
- 381.Ogura H, Furuhata K, Sato S, Anazawa K, Itoh M, Shitori Y. Synthesis of 9-O-acyl- and 4-O-acetyl-sialic acids. Carbohydr Res. 1987;167:77. doi: 10.1016/0008-6215(87)80269-0. [DOI] [PubMed] [Google Scholar]
- 382.Williams MA, McCluer RH. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980;35:266. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- 383.Gómez-Nicola D, Doncel-Perez E, Nieto-Sampedro M. Regulation by GD3 of the proinflammatory response of murine microglia mediated by Interleukin-15. J Neurosci Res. 2006;83:754. doi: 10.1002/jnr.20777. [DOI] [PubMed] [Google Scholar]
- 384.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 1: Growth factor and Ras signaling pathways. Expert Rev Anticancer Ther. 2003;3:595. doi: 10.1586/14737140.3.5.595. [DOI] [PubMed] [Google Scholar]
- 385.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:321. [PubMed] [Google Scholar]
- 386.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 387.Lafuente JV, Adán B, Alkiza K, Garibi JM, Rossi M, Cruz-Sánchez FF. Expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor-beta (PDGFR-beta) in human gliomas. J Mol Neurosci. 1999;13:177. doi: 10.1385/JMN:13:1-2:177. [DOI] [PubMed] [Google Scholar]
- 388.Diaz-Mauriño, Nieto-Sampedro, unpublished observations.
- 389.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 390.Valle-Argos B, Gomez-Nicola D, Nieto-Sampedro M. Neurostatin blocks glioma cell cycle progression by inhibiting EGFR activation. Mol Cell Neurosci. 2011;46:89. doi: 10.1016/j.mcn.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 391.Morioka T, Baba T, Black KL, Streit WJ. Inflammatory cell infiltrates vary in experimental primary and metastatic brain tumors. Glia. 1992;6:75. doi: 10.1227/00006123-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 392.Loewenstein WR. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979;560:1. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- 393.Naus CC, Bechberger JF, Caveney S, Wilson JX. Expression of gap junction genes in astrocytes and C6 glioma cells. Neurosci Lett. 1991;112:33. doi: 10.1016/0304-3940(91)90364-y. [DOI] [PubMed] [Google Scholar]
- 394.Klaunig JE, Ruch RJ. Role of inhibition of intercellular communication in carcinogenesis. Lab Invest. 1990;62:135. [PubMed] [Google Scholar]
- 395.Rose B, Mehta PO, Loewenstein WR. Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis. 1993;14:1073. doi: 10.1093/carcin/14.5.1073. [DOI] [PubMed] [Google Scholar]
- 396.Naus CC, Elisevich K, Zhu D, Belliveau DJ, Del Maestro RF. In vivo growth of C6 glioma cells transfected with connexin43 cDNA. Cancer Res. 1992;52:4208. [PubMed] [Google Scholar]
- 397.Valle-Argos B. Síntesis química, actividad antitumoral y modo de acción de la neurostatina y sus análogos sobre el crecimiento de gliomas. 2010. Ph D Dissertation. Madrid University.
- 398.Stoker MG. Transfer of growth inhibition between normal and virus-transformed cells: autoradiographic studies using marked cells. J Cell Sci. 1967;2:293. doi: 10.1242/jcs.2.3.293. [DOI] [PubMed] [Google Scholar]
- 399.Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987;17:1271. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- 400.Warren L, Fuhrer JP, Buck CA. Surface glycoproteins of normal and transformed cells: a difference determined by sialic acid and a growth-dependent sialyl transferase. Proc Nat Acad Sci USA. 1972;69:1838. doi: 10.1073/pnas.69.7.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Fukushima K, Hirota M, Terasaki PI, et al. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984;44:5279. [PubMed] [Google Scholar]
- 402.Iguro T, Wakisaka A, Terasaki PI, et al. Sialylated Lewis antigen detected in the sera of cancer patients. The Lancet. 1984;2:817. doi: 10.1016/s0140-6736(84)90747-5. [DOI] [PubMed] [Google Scholar]
- 403.Rice GE, Bevilacqua MP. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989;246:1303. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- 404.Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990;250:1132. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 405.Tiemeyer M, Swiedler SJ, Ishihara M, et al. Carbohydrate ligands for endothelial-leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:1138. doi: 10.1073/pnas.88.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Hegmans JP, Hemmes A, Aerts JG, Hoogsteden HC, Lambrecht BN. Immunotherapy of murine malignant mesothelioma using tumor lysate-pulsed dendritic cells. Am J Respir Crit Care Med. 2005;171:1168. doi: 10.1164/rccm.200501-057OC. [DOI] [PubMed] [Google Scholar]
- 407.Okada H. Brain tumor immunotherapy with type-1 polarizing strategies. Ann NY Acad Sci. 2009;1174:18. doi: 10.1111/j.1749-6632.2009.04932.x. [DOI] [PubMed] [Google Scholar]
- 408.Okada H, Kohanbash G, Zhu X, et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Chang DH, Dhodapkar MV. Dendritic cells and immunotherapy for cancer. Int J Hematol. 2003;77:439. doi: 10.1007/BF02986611. [DOI] [PubMed] [Google Scholar]
- 410.Berger TG, Schultz ES. Dendritic cell-based immunotherapy. Curr Top Microbiol Immunol. 2003;276:163. doi: 10.1007/978-3-662-06508-2_8. [DOI] [PubMed] [Google Scholar]
- 411.Banchereau J, Paczesny S, Blanco P, et al. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann NY Acad Sci. 2003;987:180. doi: 10.1111/j.1749-6632.2003.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 412.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 413.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nature Med. 2004;10:475. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 414.Markiewicz MA, Kast WM. Progress in the development of immunotherapy of cancer using ex vivo-generated dendritic cells expressing multiple tumor antigen epitopes. Cancer Invest. 2004;22:417. doi: 10.1081/cnv-200029072. [DOI] [PubMed] [Google Scholar]
- 415.Jonuleit H, Giesecke-Tuettenberg A, Tüting T, et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 416.McIlroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother. 2003;52:583. doi: 10.1007/s00262-003-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Goldman M. Immunotherapy based on dendritic cells: from experimentation to clinical development. Pathol Biol (Paris) 2003;51:74. doi: 10.1016/s0369-8114(03)00102-0. [DOI] [PubMed] [Google Scholar]
- 418.Svennerholm L. Composition of gangliosides from human brain. Nature. 1956;177:524–5. doi: 10.1038/177524b0. [DOI] [PubMed] [Google Scholar]
- 419.Morse MA, Mosca PJ, Clay TM, Lyerly HK. Dendritic cell maturation in active immunotherapy strategies. Expert Opin Biol Ther. 2002;2:35. doi: 10.1517/14712598.2.1.35. [DOI] [PubMed] [Google Scholar]
- 420.Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53:240. doi: 10.1007/s00262-003-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 422.Reay PA. Dendritic cells: immunological features and utilisation for tumour immunotherapy. Expert Opin Ther Targets. 2001;5:491. doi: 10.1517/14728222.5.4.491. [DOI] [PubMed] [Google Scholar]
- 423.Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9 L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94:299. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Uhrbom L, Hesselager G, Nister M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58:5275. [PubMed] [Google Scholar]
- 425.Tchougounova E, Kastemar M, Bråsäter D, Holland EC, Westermark B, Uhrbom L. Loss of Arf causes tumor progression of PDGFB-induced oligodendroglioma. Oncogene. 2007;26:6289. doi: 10.1038/sj.onc.1210455. [DOI] [PubMed] [Google Scholar]
- 426.Zhu Y, Guignard F, Zhao D, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7:356. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65:7429. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- 429.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 430.Chirasani SR, Sternjak A, Wend P, et al. Bone morphogenetic protein-7 release from endogenous neural precursor cells suppresses the tumourigenicity of stem-like glioblastoma cells. Brain. 2010;133:1961. doi: 10.1093/brain/awq128. [DOI] [PubMed] [Google Scholar]
- 431.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 432.Hammond SM. MicroRNAs as tumor suppressors. Nat Genet. 2007;39:582. doi: 10.1038/ng0507-582. [DOI] [PubMed] [Google Scholar]
- 433.Hyun S, Lee JH, Jin H, et al. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139:1096. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 434.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 435.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Gaur A, Jewell DA, Liang Y, et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 437.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 438.Walker-Daniels J, Hess AR, Hendrix MJ, Kinch MS. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Salaita K, Nair PM, Petit RS, et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 441.Lu C, Shahzad MM, Wang H, et al. EphA2 overexpression promotes ovarian cancer growth. Cancer Biol Ther. 2008;7:1098. doi: 10.4161/cbt.7.7.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301. [PubMed] [Google Scholar]
- 444.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 445.Walker-Daniels J, Coffman K, Azimi M, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 446.Easty DJ, Bennett DC. Protein tyrosine kinases in malignant melanoma. Melanoma Res. 2000;10:401. doi: 10.1097/00008390-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 447.Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology. 1997;65:195. doi: 10.1159/000164123. [DOI] [PubMed] [Google Scholar]
- 448.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 449.Xu F, Zhong W, Li J, et al. Predictive value of EphA2 and EphrinA-1 expression in oesophageal squamous cell carcinoma. Anticancer Res. 2005;25:2943. [PubMed] [Google Scholar]
- 450.Cercone MA, Schroeder W, Schomberg S, Carpenter TC. EphA2 receptor mediates increased vascular permeability in lung injury due to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L856. doi: 10.1152/ajplung.00118.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 452.Li X, Wang Y, Wang Y, et al. Expression of EphA2 in human astrocytic tumors: correlation with pathologic grade, proliferation and apoptosis. Tumour Biol. 2007;28(3):165. doi: 10.1159/000103010. [DOI] [PubMed] [Google Scholar]
- 453.Wu Ning, Zhao Xiangzhong, Liu Ming, et al. Role of MicroRNA-26b in Glioma Development and Its Mediated Regulation on EphA2. PLOS one. 2011;6(1):e16264. doi: 10.1371/journal.pone.0016264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Romero-Ramírez L, Nieto-Sampedro M. Inhibidores de la división de células tumorales. Spanish patent No. 201031552. Madrid October 2010.
- 455.Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67:279. doi: 10.1001/archneurol.2010.5. [DOI] [PubMed] [Google Scholar]
- 456.Gabelloni P, da Pozzo E, Bendinelli S, et al. Inhibition of metalloproteinases derived from tumours: new insights in the treatment of human glioblastoma. Neuroscience. 2010;168:514. doi: 10.1016/j.neuroscience.2010.03.064. [DOI] [PubMed] [Google Scholar]