Abstract
Water channel aquaporin-4 (AQP4) is the most abundant water channel in the rodent brain and is mainly expressed in cerebral areas involved in central osmoreception and osmoregulation. The neurohypophysis is the release site of hypothalamic neurohormones vasopressin and oxytocin, which are involved in the regulation of the water balance. The authors investigated the cellular and subcellular distribution of AQP4 in the mouse neurohypophysis before and after chronic osmotic stimulation, using immunofluorescence microscopy and immunoperoxidase electron microscopy. They showed that AQP4 was abundant in the mouse hypophysis, mainly in the neural lobe. AQP4 was discontinuously distributed along pituicytes plasma membranes, in the dense neurosecretory granules and microvesicles of nerve endings and fibers, and along the luminal and abluminal membranes of fenestrated capillary endothelial cells. After chronic osmotic stimulation, AQP4 immunolabeling was enhanced. Taken together, these results suggest that AQP4 could be involved in the pituicyte sensor effect during osmoregulation, the modification and/or maturation mechanism of neurosecretory granules during neurohormone release, and the blood perfusion of the hypophysis.
Keywords: aquaporin-4, neurohypophysis, mouse, osmoregulation, oxytocin, salt loading, vasopressin
Water homeostasis is crucial for life. In the brain, physiological neuronal activity is accompanied by ion fluxes and accumulation of neurotransmitters, which produce osmotically driven water flux between different cellular and extracellular compartments (Nicchia et al. 2003). Many brain diseases, including stroke, head trauma, tumors, and infections, result in brain edema, a consequence of changes in water homeostasis, leading to morbidity and even mortality. To maintain appropriate water homeostasis, water pores are present in cell plasma membranes and indeed are ubiquitous in tissues and organisms. These membrane pore proteins, aquaporins (AQPs), are a family of small molecular water channels (Agre 1997; Takata et al. 2004). To date, six AQP subtypes have been described in the rodent brain: AQP1, AQP3, AQP4, AQP5, AQP8, and AQP9 (Badaut et al. 2002; Nagelhus et al. 1998; Nielsen et al. 1997; Venero et al. 1999; Venero et al. 2001; Verkman 2005). AQP4 is the most abundant (Hasegawa et al. 1994; Jung et al. 1994; Frigeri et al. 1995; Nagelhus et al. 2004). It is strongly expressed in highly vascularized areas and in some areas involved in osmosensation and systemic osmoregulation, including the supraoptic and paraventricular nuclei of the neuroendocrine hypothalamus, the subfornical organ, the median eminence, and accessory nuclei (for reviews, see Badaut et al. 2002; Amiry-Moghaddam and Ottersen, 2003). Members of the aquaporin family, particularly AQP4, and their physiological roles in the brain and involvement in brain disorders have been extensively described (Amiry-Moghaddam et al. 2004; Badaut et al. 2001; Frigeri et al. 2001).
In the neuroendocrine hypothalamus, arginine-vasopressin (AVP) and oxytocin (OT) are the two major neuropeptides synthesized by magnocellular neurons of the supraoptic (SON) and the paraventricular nuclei (PVN). Axons of these neurons run through the median eminence to the neural lobe of the hypophysis, where AVP and OT are secreted into the blood circulation (Scharrer 1967). AVP and, to a lesser extent, OT are involved in the regulation of the water balance. Indeed, changes in extracellular fluid osmolality modulate homeostatic responses that affect the sodium balance and water balance. Hyperosmolality promotes the release of vasopressin to enhance water reabsorption in the kidney and increases the rate of natriuresis from the kidney by the release of oxytocin into the bloodstream (for reviews, see Antunes-Rodrigues et al. 2003; Bourque 2008). Accordingly, the release of these neuropeptides is regulated by the osmotic pressure of extracellular fluid through modulation of the electrical activity of OT and AVP neurons (Bourque and Oliet 1997; Bourque et al. 1994). The expression pattern of AQP4 within these hypothalamic magnocellular nuclei suggests that these water channels are involved in the transmission of osmotic pressure variations (Wells 1998). All published immunohistochemical studies are consistent with AQP4 being present in glial cells, especially in astrocyte membranes of perivascular end-foot processes (Nielsen et al. 1997; Badaut et al. 2000; Frigeri et al. 2001; Nagelhus et al. 2004). Thus, glial cells may act as Verney’s hypothalamic “vesicular osmometer” (Verney 1947), conveying information about water homeostasis (in blood or in the extracellular space) to the magnocellular neurons by transcellular water movements between the two cell types (Wells 1998).
After chronic physiological osmotic stimulation, such as that associated with lactation, and after chronic salt loading, there is an increase of AVP and OT synthesis within hypothalamic magnocellular neurons and an increase of their release into blood at the neurohypophysis (Arima et al. 1999; Dai and Yao 1995); the neurohypophysis exhibits a structural plasticity similar to that of the neuroendocrine hypothalamus (Hatton 1988, 1997; Theodosis and MacVicar 1996). Indeed, this structural plasticity of the neurohypophysis is translated as a reorganization of the magnocellular axon terminals and adjacent pituicytes (Matsunaga et al. 1999; Miyata et al. 1999, 2001; Wittkowski 1998; Wittkowski and Brinkmann 1974; Tweedle and Hatton 1980).
After the discovery of AQP4 in the median eminence (Frigeri et al. 1995) suggesting that osmotic modulation may also occur with neurosecretory axons, Wells (1998) suggested that AQP4, or a related aquaporin, may be present in the neurohypophysis. However, as far as we are aware, although there are data on the occurrence of AQP4 in the neurohypophysis of rats (Kuwahara et al. 2010; Pocsai et al. 2010), there are still no data available concerning AQP4 ultrastructural distribution in the neural lobe of the mouse pituitary gland and its changes before and after an osmotic stress. Therefore, we examined the cellular and subcellular distribution of AQP4 in the neurohypophysis in control and salt-loaded adult mice.
Materials and Methods
Animal and Maintenance Conditions
All experiments were performed in accordance with French and European legal requirements (decree 87-848). Experiments were performed on 8-week-old male C57BL6 mice (supplied by Janvier, France). Animals were housed under controlled conditions and provided with food and water ad libitum. Control animals were given free access to water, and experimental animals were supplied with drinking water containing 2% NaCl, ad libitum, for 8 days.
Fluorescent Double Immunohistochemistry
Three control mice and three salt loaded mice were used to perform fluorescent double immunohistochemistry. Mice were deeply anesthetized by intraperitoneal injection of sodium pentobarbital (25 mg/kg; Sanofi Santé Animale, Libourne, France) and transcardially perfused with saline solution (NaCl 0.9%) and then 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The hypophysis was immediately removed, postfixed in the same fixative for 2 hr at 4°C, cryoprotected in a 20% sucrose/0.05 M PB solution overnight at 4°C, and sectioned sagittally using a cryostat (20 µm). All sections were used immediately for immunofluorescent immunohistochemistry.
Sections of hypophysis were permeabilized and blocked by incubation for 1 hr with 0.05 M PBS (pH 7.4), 1% BSA, and 0.1% Triton X-100, then incubated overnight at room temperature with both a polyclonal rabbit antibody against AQP4 (1:500; Chemicon, Temecula, CA) and the monoclonal mouse anti-AVP antibody (1:5000; Robert et al. 1985) or the monoclonal mouse anti-OT (1:2000; Chemicon) diluted in PBS, 1% BSA, and 0.1% Triton X-100. The sections were washed and then incubated for 2 hr at room temperature with biotinylated anti-rabbit IgG antibodies (1:250; Vector Laboratories, Burlingame, CA) and then for 2 hr with a mixture of streptavidin Cy3 conjugate (1:250; Vector Laboratories), FITC-conjugated anti-mouse secondary antibodies (1:250; Vector Laboratories), and Hoëchst dye (1:1000; Sigma Aldrich, Saint-Quentin Fallavier, France). We checked the specificity of the double-immunofluorescence staining by carrying out experiments in which one of the two primary antibodies was omitted.
Fluorescence was observed with a Zeiss Axioskop 2 Plus fluorescence microscope (Carl Zeiss AG; Oberkochen, Germany), and images were captured with a Zeiss AxioCam HRc digital camera and AxioVision version 4.1 software (Carl Zeiss AG).
Electron Microscope Immunocytochemistry
Three control mice and three salt-loaded mice were used for electron microscopy study. Mice were anesthetized as described above and perfused with a saline solution and then a solution containing 0.2% glutaraldehyde and 4% paraformaldehyde in 0.1 M PB, pH 7.4. The hypophysis was immediately removed, postfixed in the same fixative solution for 4 hr at 4°C, embedded in 5% agar in water, and sectioned sagittally using a vibratome (50 µm).
Sections of hypophysis were incubated with 0.006% H2O2 (Sigma Aldrich) for 15 min at room temperature to exhaust and block endogenous peroxidase activity. Nonspecific bindings were blocked by incubation of sections for 1 hr with 0.05 M PBS (pH 7.4) and 1% BSA, and then sections were incubated overnight with a polyclonal rabbit antibody against AQP4 (1:500; Chemicon) at room temperature. The sections were washed and then were incubated for 2 hr at room temperature with biotinylated anti-rabbit IgG antibodies (1:250; Vector Laboratories). The sections were washed in PBS, incubated for 1 hr at room temperature with a peroxidase-labeled Avidin-Biotin Complex kit (1:100 avidin and 1:100 biotin; Vector Laboratories) in PBS, and rinsed in PBS. Horseradish peroxidase activity was then revealed by incubating the sections in 0.05 M Tris buffer (pH 7.6) containing 0.05% DAB (3,3′-diaminobenzidine; Sigma Aldrich) and 0.006% H2O2 (Sigma Aldrich) for 15 min at room temperature; the reaction was stopped by washing in 0.05 M Tris buffer (pH 7.6).
Then, labeled sections were washed in 0.1 M sodium cacodylate buffer, postfixed in 1% OsO4 and 0.2 M sodium cacodylate buffer (v/v) for 1 hr, dehydrated through an ascending alcohol series, and embedded in Araldite resin (Polysciences, Inc., Warrington, PA) polymerized at 60°C for 48 hr. Labeled areas were selected, glued onto Araldite blocks, and sectioned using a Reichert Ultracut ultramicrotome (Leica Microsystems, Germany). Ultrathin sections were put on nickel grids, contrasted with uranyl salts, and then observed with a Zeiss transmission electron microscope EM912.
We checked the specificity of the staining by omitting the first antibody during immunostaining procedures. All control sections did not show nonspecific staining even at the electron microscopic level.
Results
Distribution of Aquaporin-4 in the Mouse Hypophysis
We analyzed the distribution of aquaporin-4 (AQP4) in the C57BL6 mouse hypophysis neural lobe. We compared its distribution with those of the neuropeptides OT and AVP by double-fluorescence immunohistochemistry. OT and AVP immunoreactivities were detected only in the neural lobe and were absent from both the intermediate and anterior hypophysis lobes in control mice (Fig. 1A,C). AVP immunolabeling was intense and confined to the central area of the neural lobe, which corresponds to AVP-containing nerve fibers and endings of magnocellular neurons in the neuroendocrine hypothalamus (Fig. 1A). OT immunolabeling mainly appeared as clusters at the periphery of the neural lobe, in regions corresponding to OT nerve fibers and endings of hypothalamic magnocellular neurons (Fig. 1C). AQP4 immunoreactivity was found in both anterior and neural lobes of the hypophysis, including AVP- and OT-immunopositive areas. AQP4 staining was more intense in the neural than in the anterior lobe (Fig. 1E,G). No immunolabeling for AQP4 was observed in the intermediate lobe (Fig. 1E,G). The co-immunodetection of AQP4 and AVP (Fig. 1I) or OT (Fig. 1K) in control mice evidenced AQP4 both in cells identified by the presence of nuclei and also in AVP- and OT-containing structures (Fig. 1I,K).
Figure 1.
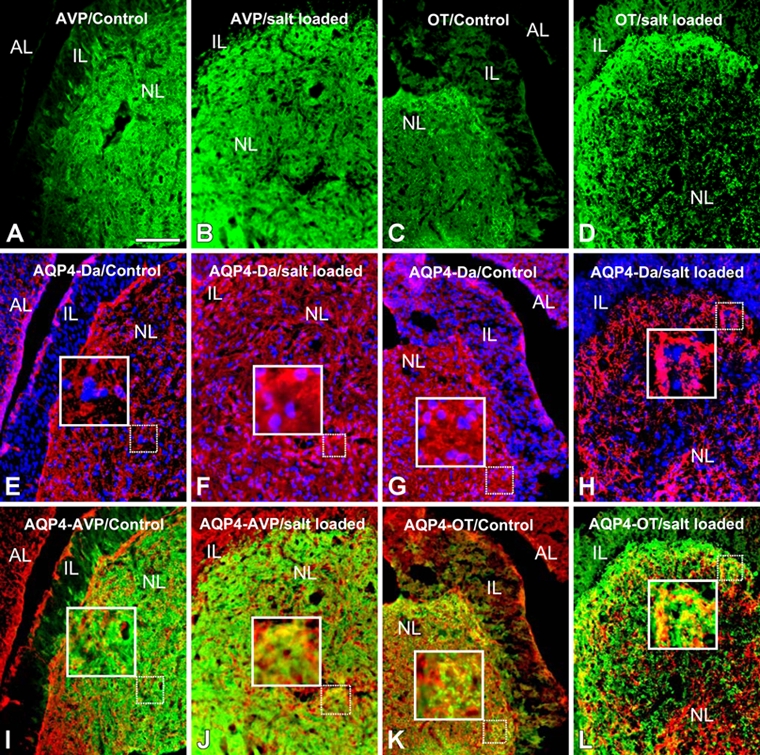
Double immunohistochemical detection of arginine-vasopressin (AVP; green)/aquaporin-4 (AQP4; red) (A, B, E, F, I, J) and oxytocin (OT; green)/AQP4 (red) (C, D, G, H, K, L) in the mouse hypophysis before salt loading (A, E, I, C, G, K) and after 8 days of salt loading (B, F, J, D, H, L). The hypophysis is composed of three parts: the anterior lobe (AL), the intermediate lobe (IL), and the posterior or neural lobe (NL). In control mouse neurohypophysis, AQP4 immunolabeling colocalizes with cells (Hoëchst-stained nuclei) (E, G) and also with AVP- and OT-containing structures (I, K). No staining for AQP4 was detected in the intermediate lobe (E, G). AQP4 immunolabeling in the neural lobe (F, H), particularly in AVP- and OT-positive areas (J, L), was more intense after 8 days of salt loading than in controls. AQ, aquaporin 4; VP, vasopressin; Da, DAPI, 4,6-diamidino-2-phenylindole dihydrochloride. Scale bar: 100 µm.
We next studied mice subjected to an osmotic stimulation (8 days of salt loading). Immunohistochemistry revealed that the distributions of AVP, OT, and AQP4 in salt-loaded mice (Fig. 1B,D,F,H) were similar to those in control mice (Fig. 1A,C,E,G); however, staining was more intense in the salt-loaded mice, particularly for AQP4 in the neural lobe (Fig. 1F,H), and in AVP- and OT-containing elements (Fig. 1J,L).
Ultrastructural Organization of the Mouse Neurohypophysis before and after Salt Loading
In control mice, pituicytes were easily recognized by the presence of large nuclei and characteristic lipid bodies in clear cytoplasm (Fig. 2A). Nerve fibers from hypothalamic magnocellular neurons, containing many neurosecretory granules, were numerous and in tight contact with pituicyte plasma membranes (Fig. 2A). After 8 days of salt loading, the nuclei and cytoplasm of pituicytes became denser (Fig. 2B). Around pituicytes, nerve terminals exhibited less dense neurosecretory vesicles but more abundant clear vesicles (Fig. 2B). Around capillaries in control animals, the perivascular space was small with few nerve terminals containing microvesicles, forming electron-dense clusters (Fig. 3A). After salt loading, the perivascular space was swollen (Fig. 3B) and occupied by large nerve terminals rich in neurosecretory granules and microvesicles; also, there were no pituitary processes between them (Fig. 3B). Outside the perivascular space, nerve terminals containing numerous microvesicles were tightly adjacent, and there were no pituicyte processes between them (Fig. 3B).
Figure 2.
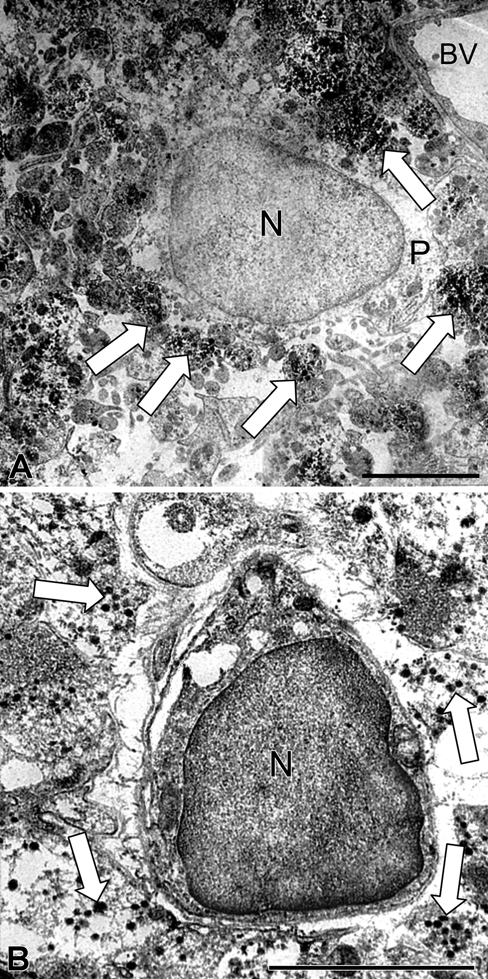
Ultrathin section of the neurohypophysis showing aquaporin-4 (AQP4) immunostaining in a pituicyte and its surroundings in control (A) and 8-day salt-loaded mice (B). In the control (A), pituicytes present a large nucleus and clear cytoplasm. Their plasma membrane is in tight contact with numerous labeled nerve fibers from hypothalamic magnocellular neurons, which contain many labeled neurosecretory granules (arrow). In the salt-loaded mouse (B), the nucleus and cytoplasm of pituicytes are denser, and nerve terminals around pituicytes exhibit fewer dense labeled neurosecretory vesicles (arrow) but more abundant clear vesicles. BV, blood vessel; N, nucleus; P, pituicytes. Scale bar: 2 µm.
Figure 3.
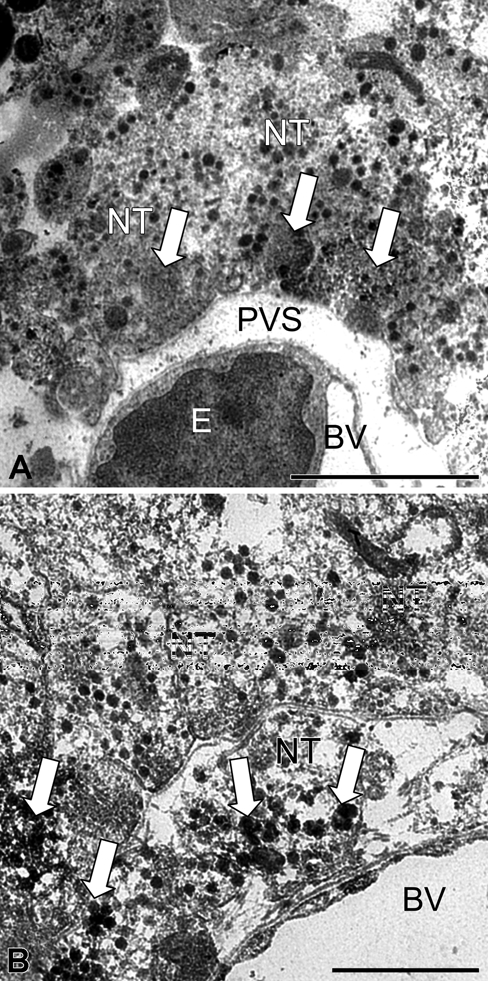
Ultrathin section of the neurohypophysis showing aquaporin-4 (AQP4) immunolabeling in a blood vessel and its surroundings in control (A) and 8-day salt-loaded mice (B). In the control (A), the perivascular space is small with few nerve terminals containing labeled microvesicles, forming electron-dense clusters (arrow). In the salt-loaded mouse (B), the perivascular space is swollen and occupied by large nerve terminals rich in labeled neurosecretory granules and microvesicles (arrow). BV, blood vessel; E, endothelial cell; NT, nerve terminal; PVS, perivascular space. Scale bar: 2 µm (A); 1 µm (B).
Subcellular AQP4 Distribution in the Mouse Neurohypophysis before and after Salt Loading
In control mice, AQP4 immunolabeling was detected along the plasma membranes of pituicytes. The plasma membrane of cell bodies and processes exhibited significant but discontinuous staining (Fig. 4A,D-F). Many of these labeled membrane segments were in close contact with nerve terminals and fibers (Fig. 4D-F). Neurosecretory granules and microvesicles contained in nerve terminals (Fig. 4B) and nerve fibers, including Herring cores, were labeled (Fig. 4C). Faint AQP4 immunolabeling was observed in the endothelium of capillaries (Fig. 5B). A significant staining was detected on portions of nerve terminal membranes, which were in contact with the basal lamina surrounding capillaries (Fig. 5C). This labeling was discontinuous on membranes of large nerve terminals and more intense and continuous on small ones. Neurosecretory granules and microvesicles of these nerve terminals were also labeled (Fig. 5D).
Figure 4.
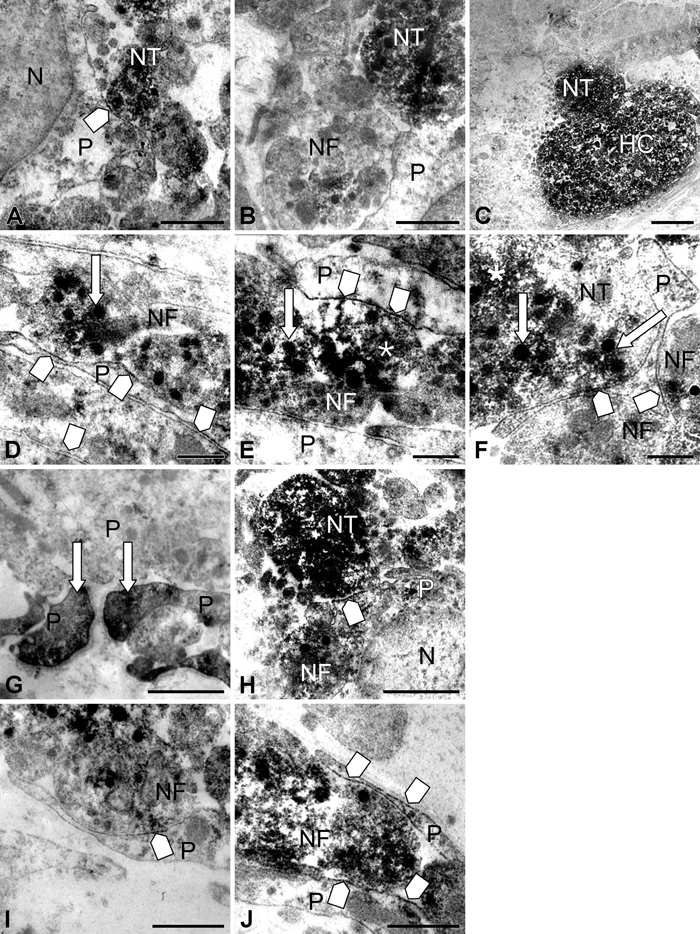
High magnifications of immunoperoxidase staining in and around pituicytes in control (A-F) and 8-day salt-loaded mice (G-J). In the control, discontinuous labeling on pituicyte plasma membranes (square arrow, A) is observed; these labeled membrane segments were often in close contact with nerve terminals and fibers (square arrow, D-F), in which granules (arrow) and microvesicles (asterisk) were immunopositive. Neurosecretory granules and microvesicles contained in nerve terminals (B) and nerve fibers, including Herring cores, were labeled (C). In 8-day salt-loaded mice, immunolabeling for aquaporin-4 (AQP4) was observed on processes of pituicytes (G, arrow). AQP4 was detected along the plasma membrane of pituicytes, as in controls, but the staining was more intense (square arrow, H-J). N, nucleus; NT, nerve terminal; P, pituicytes; NF, nerve fiber; HC, Herring core. Scale bar: 1 µm (A-B, G-H); 2 µm (C); 0.5 µm (D-F, I-J).
Figure 5.
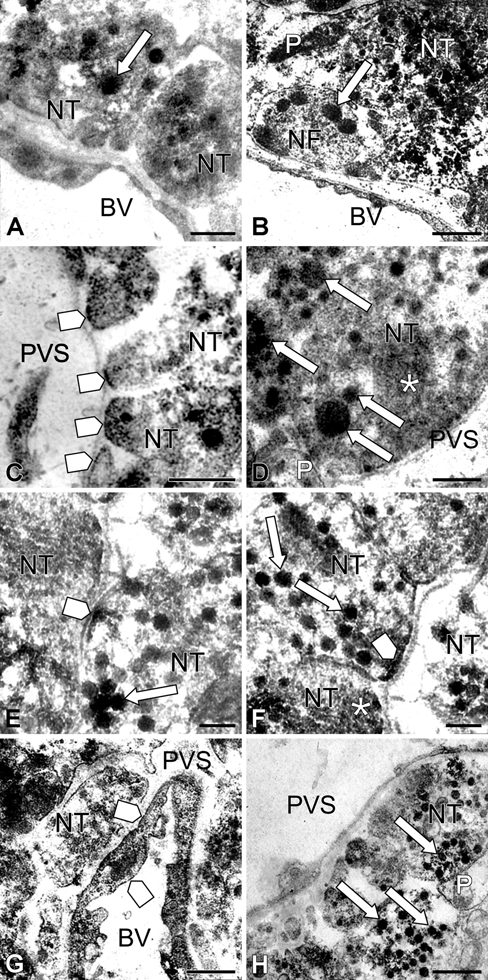
High magnifications of immunoperoxidase staining in and around blood vessels in control (A-D) and 8-day salt-loaded mice (E-H). In the control, a light aquaporin-4 (AQP4) immunolabeling is observed near blood vessel endothelia (arrow, A, B). More substantial staining is seen on portions of nerve terminal membranes in contact with the basal lamina surrounding capillaries (square arrow, C). Neurosecretory granules (arrow) and microvesicles (asterisk) of these nerve terminals are also labeled (D). In salt-loaded mice, AQP4 immunolabeling is observed on plasma membranes of nerve terminals (square arrow, E and F), in neurosecretory granules (arrow, E-H), and in microvesicle clusters (asterisk, F). AQP4 is also found in luminal and abluminal membranes of endothelial cells (square arrow, G). BV, blood vessel; NF, nerve fiber; NT, nerve terminal; P, pituicytes; PVS, perivascular space. Scale bar: 1 µm (B, G, H); 0.5 µm (A, C, D); 0.2 µm (E, F).
After salt loading, a similar pattern of AQP4 distribution along the plasma membrane of pituicytes was observed, but the staining was more intense (Fig. 4G-J). AQP4 immunolabeling was detected on plasma membranes of nerve terminals (Fig. 5E,F) and in membranes of neurosecretory granules and microvesicles (Fig. 5E,F). Furthermore, AQP4 was also found on luminal and abluminal membranes of endothelial cells (Fig. 5G).
Discussion
The neurohypophysis is composed of axons and nerve terminals from hypothalamic magnocellular neurons, glial cells named pituicytes, and fenestrated capillaries. Nerve terminals are aligned along the basal lamina surrounding capillaries or the perivascular space. They contain a dense population of neurosecretory granules, including dense-cored granules in which are stored the neurohormones vasopressin and oxytocin (Tweedle and Hatton 1980; Wittkowski and Brinkmann 1974). The neural lobe of the hypophysis is directly connected to the paraventricular and supraoptic nuclei of the neuroendocrine hypothalamus. These hypothalamic structures are involved in osmoregulation and water homeostasis and undergo chemical and structural modifications during chronic osmotic stimulation, such as that caused by salt loading. Salt loading can disturb ionic and osmotic homeostasis involving transmembrane water fluxes through aquaporin water channels (for review, see Antunes-Rodrigues et al. 2003; Nagelhus et al. 1998). High salt loading alters the expression and localization of glial aquaporin-4 in the rat retina, inducing intracellular edema in the neural retina (Qin et al. 2009).
The water channel AQP4 has been described in many cerebral areas, particularly in areas implicated in osmoregulation, including the glial limitans, ependymal lining system, the subfornical organ, and the supraoptic and paraventricular nuclei of the hypothalamus (Badaut et al. 2002; Jung et al. 1994; Venero et al. 1999). All immunological studies are consistent with AQP4 being present in astrocytes and ependymal cells but absent from neurons. In the rodent brain, AQP4 is present on astrocytic end-feet in contact with the basal lamina surrounding the endothelial cells of brain microvessels and appears to be involved in brain homeostasis and in central plasma osmolarity regulation. Perivascular glial processes and glia limitans are important sites for water flux (Nielsen et al. 1997; Rash et al. 1998).
The aim of this study was to describe the distribution of AQP4 throughout the neural lobe of the adult mouse hypophysis, using immunofluorescence microscopy and immunoperoxidase electron microscopy. We subjected mice to salt loading with the aim of stimulating the neuroendocrine hypothalamus. This paradigm is known to induce an osmoregulatory response without stimulating the hypothalamic-pituitary-adrenal stress axis. In the first day of salt loading, AVP release is increased. This is accompanied by a progressive elevation of AVP synthesis, but plasmatic osmolality remains high. After 8 days of salt loading, the osmoregulatory response reaches a steady-state level, with increased AVP synthesis and release, and a normal osmolality level is restored (Dai and Yao 1995; Arima et al. 1999).
We show that AQP4 is abundant in the mouse hypophysis, mainly in the neural lobe, as recently described in the rat pituitary gland (Kuwahara et al. 2010; Pocsai et al. 2010). By contrast, the intermediate lobe does not contain AQP4. We evidence AQP4 channels in pituicyte plasma membranes and in AVP- and OT-containing nerve terminals of the neurohypophysis. The distribution of AVP and OT immunoreactivity that we observed in the neurohypophysis in control mice was consistent with previous reports in mouse (Castel and Hochman 1976; Zhang et al. 2002) and in rat (Van Leeuwen and Swaab 1977; Van Leeuwen et al. 1979). AQP4 was discontinuously distributed along pituicyte plasma membranes. The labeled parts of membranes were in close contact with nerve terminals and fibers. After salt loading, the staining was more intense. This implicates AQP4 water channels in neurohypophyseal neuroglial interactions affecting water homeostasis not only during pathologies such as brain edema but also under physiological conditions. Indeed, pituicytes appear to be key elements in the osmoregulation process (Hussy 2002; Hussy et al. 2000): These cells are sensitive to osmolar changes and have been recently described as osmotic sensors (Rosso and Mienville 2009). Possibly, AQP4 in pituicyte plasma membranes is involved in this sensor effect during osmoregulation. In addition to a role in water homeostasis according to the osmotic status, pituicytes modulate neurohormone output (Rosso and Mienville 2009). Neurohormone output may be locally controlled by the amino acid taurine, which is produced by pituicytes (Miyata et al. 1997; Hussy et al. 2001) and by astrocytes in the supraoptic nuclei (Nagelhus et al. 1994; Deleuze et al. 1998). Taurine is a ubiquitous osmolyte involved in the regulation of cell volume and is also a regulator of vasopressin release in the hypothalamo-neurohypophyseal system (Hussy et al. 2001; Niermann et al. 2001; Rosso et al. 2004). Thus, water balance and neurohormone release in the neurohypophysis are processes that are closely interconnected.
We reveal AQP4 in the membranes of dense neurosecretory granules and microvesicles localized within nerve terminals and nerve fibers in the neural lobe of the hypophysis. After salt loading, AQP4 immunolabeling was enhanced, and nerve terminals contained fewer dense neurosecretory vesicles and more abundant clear vesicles. Our findings suggest that AQP4 in the mouse neurohypophysis may be involved in the modification and/or maturation mechanism of neurosecretory granules during neurohormone release. Most aquaporin channels are in the cell plasma membrane, but some (e.g., AQP1) have also been found in the membranes of regulated secretory pathway granules in the exocrine pancreas (Cho et al. 2002) and the parotid gland (Matsuki et al. 2005). AQP1 has been described in dense-core secretory granule membranes in bovine neural, intermediate, and anterior lobes of the pituitary; this study also demonstrated that AQP1 is important for maintaining secretory function and granule biogenesis and hence normal levels of hormone secretion in pituitary endocrine cells (Arnaoutova et al. 2008). Dense-core secretory granules are key organelles for the secretion of hormones by endocrine cells in response to stimulation. Peptide hormones are synthesized as larger precursors at the rough endoplasmic reticulum, inserted into the endoplasmic reticulum cisternae, and subsequently transported to the Golgi apparatus. The precursors are stored and packaged into immature secretory granules that bud off from the trans-Golgi network. Maturation of immature secretory granules involves several steps. Immature secretory granules in endocrine/exocrine cells decrease in size, and the contents undergo condensation, concomitant with the efflux of Na+, K+, CL−, and water from the granules during maturation. The mature secretory granules become electron dense and are stored within the cell, and they release their content by exocytosis upon stimulation (Wong et al. 1991). It has also been reported that water enters into dense granules, inducing their swelling during exocytosis (Cho et al. 2002). Thus, water channels on granules may be necessary for water efflux during maturation and water influx and vesicle swelling during exocytosis. Note that in neurons, water channels AQP1 and AQP6 are associated with synaptic vesicles and participate in their swelling during exocytosis (Jeremic et al. 2005).
The hypophysis is a circumventricular organ; the blood-brain barrier does not prevent it releasing, into the blood circulation, the neurohormones AVP and OT present in the neurosecretory granules of the hypothalamic magnocellular neuron terminals. Nerve terminals are in close contact with the basal lamina surrounding fenestrated capillaries. In osmotically stimulated animals, these terminals are enlarged and more numerous than in controls (Tweedle and Hatton 1987; Miyata et al. 2001), facilitating hormonal release (Hatton 1997). The luminal and abluminal membranes of fenestrated capillary endothelial cells exhibited AQP4 immunolabeling, implicating these water channels in maintaining hypophyseal blood perfusion. These are consistent with those described previously in the literature (Nagelhus et al. 1998; Amiry-Moghaddam and Ottersen 2003).
In conclusion, we demonstrate that AQP4 is expressed in the hypophysis neural lobe in adult mice. This water channel presents a particular distribution pattern, being present in pituicytes, fenestrated capillary endothelial cells, and nerve fibers and terminals of hypothalamic magnocellular neurons. Its co-localization with vasopressin and oxytocin in nerve fibers and terminals of magnocellular neurons appears to have functional significance: Sodium balance and water balance are the basic mechanisms of osmoregulation of extracellular fluid (for reviews, see Antunes-Rodrigues et al. 2003; Bourque 2008). Thus, water homeostasis in the neurohypophysis may be maintained by regulatory processes that, by control of aquaporin-4 expression and distribution, induce and organize water movements. The movement of water may lie at the heart of the mechanism of osmoreception, as well as AVP and OT secretion.
Acknowledgments
We are very grateful to Prof. Jacques Taxi and Danièle Raison for their help with electron microscopy.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) received no financial support for the research and/or authorship of this article.
References
- Agre P. 1997. Molecular physiology of water transport: aquaporin nomenclature workshop. Mammalian aquaporins. Biol Cell. 89:255-257 [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Frydelund DS, Ottersen OP. 2004. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 129:997-1008 [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Ottersen OP. 2003. The molecular basis of water transport in the brain. Nat Neurosci Rev. 4:991-1001 [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J, De Castro M, Elias LLK, Valença MM, McCann SM. 2003. Neuroendocrine control of body fluid metabolism. Physiol Rev. 84:169-208 [DOI] [PubMed] [Google Scholar]
- Arima H, Kondo K, Kakiya S, Nagasaki H, Yokoi H, Yambe Y, Murase T, Iwasaki Y, Oiso Y. 1999. Rapid and sensitive vasopressin heteronuclear RNA responses to changes in plasma osmolality. J Neuroendocrinol. 11:337-341 [DOI] [PubMed] [Google Scholar]
- Arnaoutova I, Cawley NX, Patel N, Kim T, Rathod T, Peng Loh Y. 2008. Aquaporin 1 is important for maintaining secretory granule biogenesis in endocrinecells. Mol Endocrinol. 22:1924-1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaut J, Hirt L, Granziera C, Bogousslavsky J, Magistretti PJ, Regli L. 2001. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 21:477-482 [DOI] [PubMed] [Google Scholar]
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. 2002. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 22:367-378 [DOI] [PubMed] [Google Scholar]
- Badaut J, Nehlig A, Verbavatz J-M, Stoeckel ME, Freund-Mercier MJ, Lasbennes F. 2000. Hypervascularization in the magnocellular nuclei of the rat hypothalamus: relationship with the distribution of aquaporin-4 and markers of energy metabolism. J Neuroendocrinol. 12:960-969 [DOI] [PubMed] [Google Scholar]
- Bourque CW. 2008. Central mechanisms of osmosensation and systemic osmoregulatioon. Nat Rev Neurosci. 9:519-531 [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SR. 1997. Osmoreceptors in the central nervous system. Ann Rev Physiol. 59:601-619 [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SR, Richard D. 1994. Osmoreceptors, osmorecaption, and osmoregulation. Front Neuroendocr. 15:231-274 [DOI] [PubMed] [Google Scholar]
- Castel M, Hochman J. 1976. Ultrastructural immunohistochemical localization of vasopressin in the hypothalamic-neurohypophysial system of three murids. Cell Tissue Res. 174:69-81 [DOI] [PubMed] [Google Scholar]
- Cho S-J, Sattar A, Jeong E-H, Satchi M, Cho JI, Dash S, Mayes MS, Stromer MH, Jena BP. 2002. Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc Natl Acad Sci U S A. 99:4720-4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai WJ, Yao T. 1995. Effects of dehydration and salt-loading on hypothalamic vasopressin mRNA level in male and female rats. Brain Res. 676:178-182 [DOI] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. 1998. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol. 507:463-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. 1995. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 108:2993-3002 [DOI] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Nico B, Quondamatteo F, Herken R, Roncali L. 2001. Aquaporin-4 deficiency in skeltal muscle and brain of dystrophic mdx mice. Faseb J. 15:90-98 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. 1994. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 269:5497-5500 [PubMed] [Google Scholar]
- Hatton GI. 1988. Pituicytes, glia and control of terminal secretion. J Exp Biol. 139:67-79 [DOI] [PubMed] [Google Scholar]
- Hatton GI. 1997. Function-related plasticity in the hypothalamus. Annu Rev Neurosci. 20:375-397 [DOI] [PubMed] [Google Scholar]
- Hussy N. 2002. Glial cells in the hypothalamo-neurohypophysial system: key elements of the regulation of neuronal electrical and secretory activity. Prog Brain Res. 139:95-112 [DOI] [PubMed] [Google Scholar]
- Hussy N, Bres V, Rochette M, Duvoid A, Alonso G, Dayanithi G. 2001. Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. J Neurosci. 15:7110-7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Desarmenien MG, Moos FC. 2000. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 62:113-134 [DOI] [PubMed] [Google Scholar]
- Jeremic A, Cho WJ, Jena BP. 2005. Involvement of water channels in synaptic vesicle swelling. Exp Biol Med. 230:674-680 [DOI] [PubMed] [Google Scholar]
- Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. 1994. Molecular characterization of an aquaporin cDNA from brain candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci U S A. 91:13052-13056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara S, Maeda S, Ardiles Y, Jun J, Tanaka K, Hayakawa T, Seki M. 2010. Immunohistochemical localization of aquaporin-4 in the rat pituitary gland. J Vet Med Sci. 72:1307-1312 [DOI] [PubMed] [Google Scholar]
- Matsuki M, Hashimoto S, Shimono M, Murakami M, Fujita-Yoshigaki J, Furuyama S, Sugiya H. 2005. Involvement of aquaporin-5 water channel in osmoregulation in parotid secretory granules. J Memb Biol. 203:119-126 [DOI] [PubMed] [Google Scholar]
- Matsunaga W, Miyata S, Kiyohara T. 1999. Redistribution of MAP2 immunoreactivity in the neurohypophysial astrocytes of adult rats during dehydration. Brain Res. 820:7-17 [DOI] [PubMed] [Google Scholar]
- Miyata S, Furuya K, Nakai S, Bun H, Kiyohara T. 1999. Morphological plasticity and rearrangement of cytoskeletons in pituicytes cultured from adult rat neurohypophysis. Neurosci Res. 33:299-306 [DOI] [PubMed] [Google Scholar]
- Miyata S, Matsushima O, Hatton GI. 1997. Taurine in rat posterior pituitary: localization in astrocytes and selective release by hypoosmotic stimulation. J Comp Neurol. 19:513-523 [PubMed] [Google Scholar]
- Miyata S, Takamatsu H, Maekawa S, Matsumoto N, Watanabe K, Kiyohara T. 2001. Plasticity of neurohypophysial terminals with increased hormonal release during dehydration: ultrastructural and biochemical analyses. J Comp Neurol. 434:413-427 [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Amiry-Moghaddam M, Lehmann A, Ottersen OP. 1994. Taurine as an organic osmolyte in the intact brain: immunocytochemical and biochemical studies. Adv Exp Med Biol. 359:325-334 [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Mathiisen TM, Ottersen OP. 2004. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 129:905-913 [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Veruki RT, Haug FM, Laake JH, Nielsen S, Agre P. 1998. Aquaporin-4 water channel protein in rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci. 18:2506-2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia GP, Frigeri A, Liuzzi GM, Svelto M. 2003. Inhibition of aquaporin-4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. Faseb J. 17:1508-1510 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. 1997. Specialized membrane domains for water transport in glial cells: high resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 17:171-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermann H, Amiry-Moghaddam M, Holthoff K, Witte OW, Ottersen OP. 2001. A novel role of vasopressin in the brain: modulation of activity-dependent water flux in the neocortex. J Neurosci. 21:3045-3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocsai K, Bagyura Z, Kalman M. 2010. Components of the basal lamina and dystrophin-dystroglycan complex in the neurointermediate lobe of rat pituitary gland: different localizations of beta-dystroglycan, dystrobrevins, alpha1-syntrophin, and aquaporin-4. J Histochem Cytochem. 58:463-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Fan J, Ye X, Xu G, Liu W, Da C. 2009. High salt loading alters the expression and localization of glial aquaporins in rat retina. Exp Eye Res. 89:88-94 [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CD, Agre P, Nielsen S. 1998. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci U S A. 95:11981-11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert FR, Léon-Henri BP, Chapleur-Chateau MM, Girr MM, Burlet AL. 1985. Comparison of three immunoassays in the screening and characterization of monoclonal antibodies against arginine-vasopressin. J Neuroimmunol. 9:205-220 [DOI] [PubMed] [Google Scholar]
- Rosso L, Mienville JM. 2009. Pituicyte modulation of neurohormone output. Glia. 57:235-243 [DOI] [PubMed] [Google Scholar]
- Rosso L, Peteri-Brunback B, Poujeol P, Hussy N, Mienville J-M. 2004. Vasopressin-induced taurine efflux from rat pituicytes: a potencial negative feedback for hormone secretion. J Physiol. 554:731-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharrer B. 1967. The neurosecretory neuron in neuroendocrine regulatory mechanisms. Am Zool. 7:161-169 [DOI] [PubMed] [Google Scholar]
- Takata K, Matsuzaki T, Tajika Y. 2004. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. 39:1-83 [DOI] [PubMed] [Google Scholar]
- Theodosis DT, MacVicar B. 1996. Neurone-glia interactions in the hypothalamus and pituitary. Trends Neurosci. 19:363-367 [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. 1980. Evidence for dynamic interactions between pituicytes and neurosecretory axons in the rat. Neuroscience. 5:661-671 [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. 1987. Morphological adaptability at neurosecretory axonal endings on the neurovascular contact zone of the rat neurohypophysis. Neuroscience. 20:241-246 [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, de Raay C, Swaab DF, Fisser B. 1979. The localization of oxytocin, vasopressin, somatostatin and luteinizing hormone releasinghormone in the rat neurohypophysis. Cell Tissue Res. 202:189-201 [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, Swaab DF. 1977. Specific immunoelectronmicroscopic localization of vasopressin and oxytocin in the neurohypophysis of the rat. Cell Tissue Res. 177:493-501 [DOI] [PubMed] [Google Scholar]
- Venero JL, Vizuete ML, Ilundáin AA, Machado A, Echevarria M, Cano J. 1999. Detailled localization of aquaporin-4 messenger RNA in the CNS: preferential expression in periventricular organ. Neuroscience. 94:239-250 [DOI] [PubMed] [Google Scholar]
- Venero J, Vizuete ML, Machado A, Cano J. 2001. Aquaporins in the central nervous system. Prog Neurobiol. 63:321-336 [DOI] [PubMed] [Google Scholar]
- Verkman AS. 2005. More just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 118:3225-3232 [DOI] [PubMed] [Google Scholar]
- Verney EB. 1947. The antidiuretic hormones and the factors which determine its release. Proc Royal Soc. 35:25-106 [PubMed] [Google Scholar]
- Wells T. 1998. Vesicular osmometers, vasopressin secretion and aquaporin-4: a new mechanism for osmoreception? Mol Cell Endocrinol. 136:103-107 [DOI] [PubMed] [Google Scholar]
- Wittkowski W. 1998. Tanicytes and pituicytes: morphological and functional aspects of neuroglial interaction. Microsc Res Tech. 41:29-42 [DOI] [PubMed] [Google Scholar]
- Wittkowski W, Brinkmann H. 1974. Changes of extent of neurovascular contacts and number of neuroglial synaptoid contacts in the pituitary posterior lobe of dehydrated rats. Anat Embryol Berl. 146:157-165 [DOI] [PubMed] [Google Scholar]
- Wong JG, Izutsu KT, Robinovitch MR, Iversen JM, Cantino ME, Johnson DE. 1991. Microprobe analysis of maturation-related elemental changes in rat parotid secretory granules. Am J Physiol. 261:1033-1041 [DOI] [PubMed] [Google Scholar]
- Zhang BJ, Kusano K, Zerfas P, Lacangelo A, Young WS, Gainer H. 2002. Targeting of green fluorescent protein to secretory granules in oxytocin magnocellular neurons and its secretion from neurohypophysial nerve terminals in transgenic mice. Endocrinology. 143:1036-1046 [DOI] [PubMed] [Google Scholar]


